Abstract
Activation of dopamine D2 receptors (D2R) modulates G protein/cAMP-dependent signaling and also engages Akt-GSK-3 signaling through D2R/β-arrestin 2 scaffolding of Akt and PP2A. This G protein-independent pathway may be important in mediating the antimanic effects of mood stabilizers and antipsychotics. The mood stabilizer lithium influences behavior and Akt/GSK-3 signaling in mice and many antipsychotics have been shown to more potently antagonize the activity of the β-arrestin-2 pathway relative to the G protein-dependent pathway. Cariprazine, an antipsychotic with potent D3R/D2R partial agonist activity and preferential binding to D3R, was investigated for its effects on the mediators of D2R pathways in vitro and its efficacy in animal models of mania. Effects on G protein-dependent activity were measured via inhibition of isoproterenol-induced cAMP production; effects on D2R/β-arrestin 2 signaling were determined using bioluminescence resonance energy transfer (BRET). Cariprazine was tested in vivo for antimanic-like activity, using the ouabain-induced hyperactivity model in rats. Cariprazine was more potent than aripiprazole in inhibiting isoproterenol-induced cAMP although both compounds showed similar maximum efficacy. In assays of D2R/β-arrestin 2-dependent interactions, cariprazine showed very weak partial agonist activity, unless the levels of receptor kinase were increased; as an antagonist it showed similar potency to haloperidol and ∼fivefold greater potency than aripiprazole. In an animal model of mania, cariprazine showed similar efficacy as lithium in attenuating the effects of ouabain-induced hyperactivity. In summary, the differential effects of cariprazine on D2R G protein and β-arrestin 2 mediators of signal transduction pathways could contribute to its potent antimanic-like activity.
Keywords: Animal models, antipsychotics, arrestin, dopamine receptors, protein kinases
Introduction
Cariprazine, a drug under development for schizophrenia and bipolar disorder, is a dopamine D3 and D2 receptor (D3R and D2R, respectively) partial agonist with preferential binding to D3R 2010. Dopaminergic activity has been associated with the regulation of locomotion, reward, emotion, and affect, while dysregulation of this system has been implicated in both bipolar disorder 2009 and schizophrenia 2012.
Classical dopaminergic physiological functions are mediated through five receptors with seven-transmembrane spanning G protein-coupled regions, which either enhance (D1 and D5 receptors) or inhibit (D2, D3, D4) cellular cyclic AMP (cAMP) production. The inhibitory pathway receptors can also modulate calcium and potassium channels through the activity of G protein βγ subunits (2000; 2005; 2011).
In addition, several lines of evidence suggest that the D2R (and to a lesser extent the D3R) can also act through a G protein-independent pathway to increase glycogen synthase kinase-3 (GSK-3) activity, via a complex consisting of β-arrestin 2, protein kinase B (Akt), and protein phosphatase 2A (PP2A) ,,. In this pathway, PP2A dephosphorylates and deactivates the kinase Akt, thereby enabling the subsequent dephosphorylation and activation of GSK-3 (for a review, see 2007a). It has been hypothesized that hyperactivation of GSK-3 is involved in both bipolar disorder 2010 and schizophrenia 2007.
In support of this hypothesis, mice that overexpress the GSK-3β subunit have a phenotype of hyperactivity similar to mice treated with amphetamine, and behavior akin to mania 2006. Moreover, mice overexpressing D2R in the striatal complex (presumably leading to an increased dopaminergic state) display cognitive and working memory deficits characteristic of mania and psychosis 2010. Lithium, a mood stabilizer commonly used in bipolar disorder, has been shown to inhibit GSK-3 both directly and through an indirect mechanism that destabilizes this D2R-mediated β-arrestin 2/Akt/GSK-3 signaling complex (2008; 2011).
The GSK-3 signaling pathway has also been implicated in the actions of antidepressants and antipsychotics ,. While antipsychotics have diverse and complex pharmacological actions, activity at dopamine D2R is believed to underlie their clinical efficacy 2012. Interestingly, although individual antipsychotics show differential effects on D2R-mediated G protein activation (ranging from inverse to partial agonism to antagonism), all antipsychotics display a common biased antagonism for the D2R/â-arrestin 2 interaction 2008.
As a D3R and D2R partial agonist, cariprazine may activate/inhibit both subtypes of dopamine receptors depending on the actual dopaminergic tone 2010. Although the role of D3R in the β-arrestin 2/Akt/GSK-3 signaling pathway has not been extensively studied, preliminary studies with D3R knockout mice suggest that the D3R plays a role in regulating and enhancing the sensitivity of the D2R 2007b. To investigate the effects of cariprazine on D3R/D2R-related signaling, we compared its activity with that of haloperidol (a typical antipsychotic) and aripiprazole (a dopamine D2 partial agonist atypical antipsychotic) in two in vitro assays: a G protein-mediated cAMP inhibition assay and an assay to measure the interaction between the D2R and β-arrestin 2. In addition, we tested the effect of cariprazine in an animal model of mania, inducing mania-like behavior via injection with ouabain, a potent inhibitor of the Na+/K+ transmembrane ATPase. Intracerebroventricular (i.c.v.) ouabain injection is among the best characterized models of mania, and is the only available model of bipolar disorder that adequately fulfills all major criteria required of an animal model 2007. Ouabain-injected rats have been shown to be sensitive to lithium and other antimanic agents such as carbamazepine and haloperidol (but not olanzapine) 2007. Additionally, the model has been used with novel agents such as memantine, which is predicted by the model to have antimanic efficacy 2011. Ouabain can also alter the phosphorylation state of Akt in some cell lines in vitro (,; 2012), as well as in vivo in rats, on the same time scale as its manic effects 2010, suggesting that GSK-3 signaling may be involved in this model system as well. More recently, a knockout mouse model, in which the animals are hemideficient for the alpha3 subunit of the Na+/K+ ATPase, has been shown to induce lithium-normalized manic-like behavior 2011. Nonetheless, animal models based on the single alterations that attempt to mimic a complex human illness such as bipolar disorder, need to be viewed with some caution.
Materials and Methods
Drugs investigated
Cariprazine and aripiprazole were provided by Forest Research Institute (Forest Laboratories Inc., New York, NY); haloperidol and quinpirole were purchased from Sigma-Aldrich (St. Louis, MO).
In vitro assays
Cell culture and transient transfections
Human embryonic kidney HEK-293T cells (ATCC, Manassas, VA), were maintained in Dulbecco's Modification of Eagle's Medium supplemented with 10% fetal bovine serum (FBS; Sigma-Aldrich), 0.05 mg/mL of Gentamicin, and 2 mmol/L glutamine, in a 5% CO2 incubator. Cells were seeded at a density of 3 × 106 cells per 100 mm dishes, or at 500,000 cells/well in six-well plates, and then cultured for 24 h and transfected using calcium phosphate. Twenty-four hours post transfection, the cells were divided into 96-well clear bottom plates (Corning, Lowell, MA) in phenol-free Minimum Essential Medium (Gibco, Grand Island, NY) supplemented with 2% FBS, 2 mmol/L l-glutamine, and 0.05 mg/mL gentamicin. Assays were conducted 24 h after plating cells onto the 96-well plates.
Measurement of cAMP production
HEK-293T cells were transfected with the GloSensor™ cAMP construct (Promega, Madison, WI) and mouse D2R-long form (mD2LR) DNA 2008. On the day of the experiment, the cells were washed in Hank's Balanced Salt Solution (HBSS; Gibco, Grand Island, NY); then 25 μL of 25 mmol/L luciferin (Gold Biotechnology, St. Louis, MO) in HBSS was added to each well and incubated in the dark at room temperature for 2 h. Following incubation, the luciferin solution was aspirated and 80 μL HBSS was added, and dose–response curves were generated for quinpirole, aripiprazole, and cariprazine. Five minutes after the addition of each drug, isoproterenol (10−7 mol/L) was added to induce cAMP production for an additional five minutes. Luminescence generated from the GloSensor™ construct was measured with a Mithras LB940 Multimode Reader, using MikroWin 2000 software (Berthold Technologies, Oak Ridge, TN). All curves generated were normalized to the maximal response of isoproterenol, to control for expression differences between experiments.
Measurement of the interaction between the D2R and β-arrestin-2
Bioluminescence resonance energy transfer (BRET) assays were performed as described in 2008, with minor modifications. Briefly, mD2LR DNA was fused with that of Renilla luciferase (RLuc; the BRET donor, emission wavelength of 465–505 nm), and mouse β-arrestin-2 (mβ-arrestin-2) DNA was fused with that of enhanced yellow fluorescent protein (EYFP; the BRET acceptor, emission wavelength of 515–555 nm); the resulting mD2LR-RLuc and mβ-arrestin-2-EYFP transcripts were transfected into HEK-293T cells and cultured as described above. The ratio of BRET donor and acceptor was carefully controlled and experiments carried out as previously described 2008. Briefly, mD2LR-RLuc (0.2 μg DNA/100 mm dish) and 10 μg DNA/dish of mβ-arrestin-2 EYFP were transfected using calcium phosphate. Between experiments the mD2LR-RLuc expression as confirmed by RLuc emission did not vary more than 10%. Twenty-four hours after calcium phosphate transfection, the cells were detached and plated in phenol red-free medium containing 2% of FBS, 0.05 mg/mL of Gentamicin and 2 mmol/L glutamine in 96-well microplates (White OptiPlate, Perkin Elmer, Waltham, MA) at a density of 100,000 cells per well. After an additional 24 h, the medium was removed and replaced by phosphate buffer saline (PBS) containing calcium and magnesium and 0.003% (w/v) ascorbic acid before initiating the assay.
The BRET assay was initiated by adding 10 μL of the cell-permeable substrate specific for Renilla luciferase, coelenterazine h (Promega, Madison, WI), at a final concentration of 5 μmol/L. After 5 min, cariprazine, aripiprazole, and haloperidol, were added across seven decades of concentration (see Fig. 2) and assessed 5 min later. In antagonist assays the concentrations of cariprazine, aripiprazole, and haloperidol were added 1 min after the addition of the coelenterazine h substrate, quinpirole (final concentration 1 μmol/L) was added at 5 min, and the plate was assayed 10 min after the substrate addition. The ratio of EYFP emission to RLuc emission was measured using a Mithras LB940 with Mikrowin 2000 software. The BRET ratios derived were normalized to the maximal quinpirole response on mD2LR-RLuc.
Figure 2.
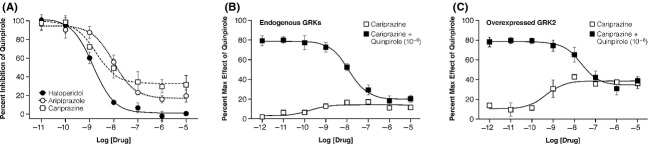
β-arrestin 2 antagonism. (A) Inhibition of quinpirole-induced β-arrestin 2/D2R complex formation by cariprazine, aripiprazole, and haloperidol. (B) Comparison of antagonism as well as agonism of β-arrestin 2 recruitment induced by cariprazine under endogenous complement of GPCR kinase (GRK). (C) Comparison of antagonism and agonism of β-arrestin 2 recruitment induced by cariprazine with overexpression of GRK2. Results shown are expressed relative to the effects of Quinpirole at 10−6 mol/L and are the averages of 3–4 different experiments, each run in triplicate.
Data analysis
Data analysis for cAMP assays was performed on curves normalized to the inhibition of isoproterenol-induced response, while BRET values were normalized to the maximal response of the full agonist, quinpirole. This allows for the most facile way to portray the partial agonist activity present for cariprazine and aripiprazole at the cAMP assay. For agonist assays, the dose–response curves were calculated using GraphPad Prism 5 using the nonlinear regression curve fit for the function Y = Bottom +(Top-Bottom)/{1 + 10^([LogEC50-X[)}, which is referred to as “log[agonist[ versus response” in the software. The errors present in the table were calculated from the curve fit. For antagonist assays, the dose–response curves were calculated using the nonlinear regression curve fit for the function Y = Bottom + (Top-Bottom)/{1 + 10^([X-LogIC50[)}, which is referred to as “log[inhibitor[ versus response” in the software. The IC50 values were used to calculate the KB values for haloperidol, aripiprazole, and cariprazine using the equation, KB = IC50/{(2 + ([A[/EC50)n)1/n − 1} where IC50 was calculated from the experimental curves, [A[ = concentration of quinpirole used, EC50 = the EC50 of quinpirole for each functional assay, and n = the hill coefficient (which had a value of 1 for BRET experiments).
Behavioral testing
Animals
All animal procedures were approved by the University of Louisville institutional animal studies committee. Adult male Sprague–Dawley rats (150–300 g; Harlan, Indianapolis, IN) were housed individually at 24–26°C in a 12 h:12 h, light:dark cycle; food and water were provided ad libitum. The animals were allowed to acclimate to the animal facility for 4–5 days after shipping. Cannulae were surgically implanted (i.c.v.) according to methodology reported previously 1995. During the conduct of the study an imbalance in sample size developed, with the number of rats per treatment group ranging from 3 to 8. All statistical analyses are for the final number of rats in each group. Throughout the studies, the animals were individually caged. Experiments on animals were conducted according to the Guide for the Care and Use of Laboratory Animals, as adopted and promulgated by the U.S. National Institutes of Health.
Ouabain-induced mania
Mania-like behavior was induced by single i.c.v. injection of 5 μL of 1 mmol/L ouabain (Sigma, St. Louis, MO) dissolved in artificial cerebrospinal fluid (aCSF) 4 days after the cannulation surgery. The aCSF consisted of 124 mmol/L NaCl, 5 mmol/L KCl, 3.7 mmol/L MgSO4, 23 mmol/L NaHCO3, and 0.75 mmol/L NaH2PO4, pH 7.4 1988. Open field activity was examined immediately after i.c.v. injection of ouabain and also at 7 days post injection.
Cariprazine was dissolved in 0.9% saline and administered at 0.06, 0.25, 0.5, and 1.0 mg/kg via intraperitoneal (i.p.) injection 1 h before i.c.v. injection of ouabain and daily thereafter for 7 days. Open field activity was assessed immediately following the i.c.v. injection and again after 7 days (the activity was noted 10–14 h after the last i.p. injection of cariprazine).
“Acute” lithium was administered via i.p. injection (6.75 mEq/kg) 1 h before i.c.v. injection of ouabain; whereas maintenance doses were added to rat chow (Harlan Teklad, Madison, WI), at 2.4 g/kg of food, for 7 days. This lithium dose is known to achieve behaviorally quantifiable levels of 1.01 ± 0.18 mmol/L in the rats 2003. Open field activity was examined 1 h and 18 h after lithium administration, and again on day 7. After an i.p. injection, lithium concentrations reach a maximum serum level by 0.5 h and maximum brain levels by 8 h 1976. Control animals received an i.c.v. injection of 5 μL aCSF and an i.p. injection of saline, of the same volume as cariprazine-treated animals.
Behavioral testing was performed in an open arena (86 × 86 cm) that was evenly divided into 16 squares (21.5 × 21.5 cm, marked on the floor). Open field activity was defined as the number of squares traversed in 30 min, assessed by a trained observer. Baseline open field activity was assessed before cannulae implantation, 4 days after shipping of the animal, to allow for acclimation to the testing arena.
Data analysis
The behavioral data were evaluated to determine whether acute or long-term cariprazine administration is capable of normalizing ouabain-induced hyperactivity. Raw data from each treatment group were evaluated and filtered, such that only data falling within the 95% confidence interval of the sample (assuming normal distribution) were retained for further analysis. Statistical evaluation (performed separately for the acute and the long-term experiments) utilized two-factorial analysis of variance (ANOVA), with i.p. drug treatment (including the Li-treated group) and ouabain treatment as between-groups factors. Student–Newman–Keuls test was used for post hoc analyses. Data are reported as means with standard error of mean (SEM).
Results
D2R-dependent effects on cAMP production and â-arrestin 2 interaction
To compare the effects of cariprazine with that of other atypical and typical antipsychotics, we assayed the effects of cariprazine, aripiprazole, and haloperidol in cell culture on G protein-mediated cAMP production and β-arrestin 2 recruitment.
Cariprazine was over sixfold more potent (EC50 = 1.4 nmol/L) than aripiprazole (EC50 = 9.2 nmol/L) in inhibiting isoproterenol-induced cAMP production in HEK-293 cells; however, both compounds displayed similar maximum effect (∼75% of efficacy, in comparison with the full D2R agonist quinpirole) (Fig.1A; Table1). In this assay, haloperidol demonstrated no intrinsic activity and instead behaved as a potent D2R antagonist (data not shown; 2008).
Figure 1.
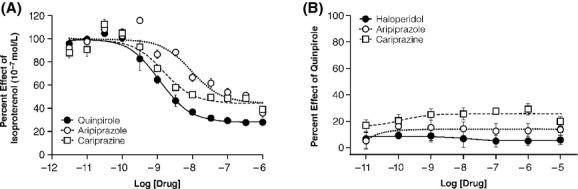
D2R agonist effects on cAMP production and â-arrestin recruitment. (A) pharmacological profile of D2R-mediated inhibition of G protein-dependent cAMP production by cariprazine, compared with the atypical antipsychotic aripiprazole and the selective D2R/D3R agonist quinpirole. (B) Lack of activity of haloperidol, a full antagonist, compared to aripiprazole and cariprazine, which are very weak partial agonists for â-arrestin 2 recruitment. Results shown are the average of 3–4 different experiments, each run in triplicates.
Table 1.
Activity of cariprazine in G protein and B-arrestin-2 signaling pathways
| Drug | G protein |
β−arrestin-2 |
||||
|---|---|---|---|---|---|---|
| Agonist activity |
Agonist activity |
Antagonist activity |
||||
| Emax (%) | EC50 (nmol/L) | Emax (%) | EC50 (nmol/L) | Emax (%) | KB (nmol/L) | |
| Quinpirole | 100 ± 2.2 | 1.1 ± 0.1 | 95.1 ± 4.7 | 109.1 ± 1.3 | Not determined | |
| Haloperidol | Not determined | Not determined | 101.2 ± 4.3 | 1.3 ± 0.5 | ||
| Aripiprazole | 77.7 ± 4.2 | 9.2 ± 0.1 | Not determined | 77.6 ± 5.1 | 8.7 ± 6.0 | |
| Cariprazine | 77.4 ± 2.8 | 1.4 ± 0.1 | 13.9 ± 1.2 | 0.2 ± 2.9 | 80.4 ± 2.6 | 1.6 ± 1.9 |
| Cariprazine/GRK2 | Not determined | 38.4 ± 2.3 | 0.5 ± 2.1 | 62.9 ± 2.9 | 11.1 ± 0.2 | |
Results show mean ± standard error (SE). Cariprazine/GRK2 denotes evaluation of cariprazine in cells overexpressing GRK2.
The same compounds were tested for their ability to promote the interaction of the mouse D2R with β-arrestin 2 using a BRET assay. While haloperidol showed no agonist activity, both aripiprazole and cariprazine showed very weak activity (∼10%) compared with the full agonist quinpirole in this BRET assay (Fig.1B; Table1). When tested for their ability to antagonize the effect of quinpirole in this assay, all three agents inhibited the ability of quinpirole to promote interactions between D2R and β-arrestin 2; cariprazine (KB = 1.6 nmol/L) was as potent as the classical D2R antagonist haloperidol (KB = 1.3 nmol/L) and more potent than aripiprazole (KB = 8.7 nmol/L) (Table1). Potentially reflecting their ability to act as D2R partial agonists and promote D2R/β-arrestin-2 interaction, the maximal antagonist activity of cariprazine and aripiprazole was significantly less than that of haloperidol) (Fig.2A). In order to more fully characterize the ability of cariprazine to function both as a weak partial agonist as well as an antagonist for the recruitment of â-arrestin-2 to D2R (Fig.2B), we performed assays with GRK2 overexpression, several folds over the endogenous levels (data not shown; Fig2C). Receptor kinase overexpression enhances D2R phosphorylation and enhances the efficacy for agonists. Figure2C clearly shows that cariprazine is capable of functioning as a stronger partial agonist in the presence of overexpressed kinase, however, the compound does maintain a potent antagonist effect (Table1). Under those conditions as would be expected, the extent of the antagonist effect of cariprazine is reduced to match the level of partial agonist activity of the compound.
Effect of cariprazine in the ouabain-induced rat mania model
To investigate the effects of cariprazine in an animal model of mania, we administered it to rats prior to injection with ouabain, a potent inhibitor of the Na+/K+ transmembrane ATPase. In the acute experiment cariprazine (0.06–1.0 mg/kg) or lithium was injected (i.p.) 1 h before the administration of ouabain; open field activity was assessed immediately after ouabain injection. A two-factorial ANOVA revealed a significant drug-treatment effect (F5,50 = 13.52, P < 0.001), a significant ouabain effect (F1,50 = 14.48, P < 0.001), and a significant drug–ouabain interaction (F5,50 = 2.41, P < 0.05). Post hoc analyses demonstrated that a single, acute i.c.v. injection (5 μL) of 1 mmol/L ouabain caused a significant increase in open field activity (number of squares traversed in 30 min) immediately after injection, compared with an injection of vehicle (aCSF) alone (mean ± SEM: 114.6 ± 14.33 and 60.6 ± 4.30, respectively; P < 0.01) (Fig.3). However, no significant effect of ouabain was found in cariprazine- or lithium-treated animals. A significant (P < 0.01) reduction in ouabain-induced hyperactivity was observed after acute i.p. administration of all doses of cariprazine (mean ± SEM: 0.06 mg/kg, 64.2 ± 3.88; 0.25 mg/kg, 72.7 ± 11.67; 0.50 mg/kg, 40.6 ± 5.32; 1.0 mg/kg, 19.5 ± 8.78) and lithium (40.4 ± 12.78), compared with ouabain injection alone (114.6 ± 14.33) (Fig.3). The highest cariprazine dose produced significant sedation (72% inhibition for cariprazine 1.0 mg/kg aCSF vs. saline aCSF; P < 0.05).
Figure 3.
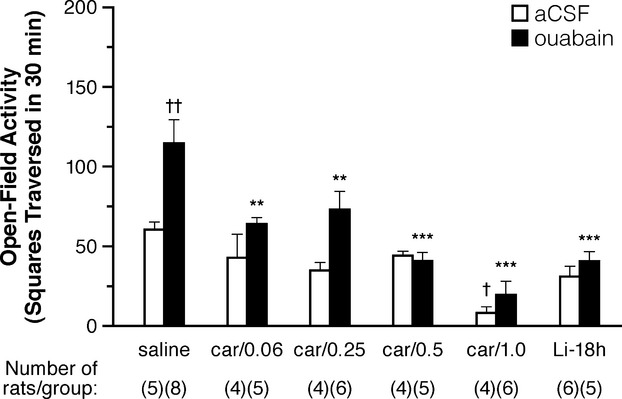
Open field activity immediately after injection with ouabain Acute administration of cariprazine inhibits ICV ouabain-induced motoric hyperactivity in rats. The open field activities were measured immediately after ouabain injection (1 h after cariprazine treatment), or 18 h after lithium treatment. aCSF indicates artificial cerebrospinal fluid (vehicle for ouabain injection); car/x, cariprazine/concentration (in mg/kg); Li-18h, lithium treatment, assessed 18 h after injection. †indicates P < 0.05; cariprazine/aCSF versus saline/aCSF ††indicates P < 0.01; saline/ouabain versus saline/aCSF **,***indicates P < 0.01 and P < 0.001, respectively; cariprazine/ouabain versus saline/ouabain.
To investigate the effects of longer term cariprazine treatment, we administered cariprazine (0.06–1.0 mg/kg) or lithium 1 h before the single-dose administration of ouabain and once daily for 7 days thereafter. A two-factorial ANOVA revealed no significant drug effects, but a significant ouabain effect (F1,52 = 7.39, P < 0.01) and drug–ouabain interaction (F5,52 = 5.30, P < 0.001). Post hoc analyses showed no significant differences between any two of the aCSF vehicle-treated groups, but a significant effect of ouabain (vs. aCSF vehicle) in the saline-treated group (198.4 ± 19.85 vs. 75.2 ± 20.87, respectively, P < 0.01) (Fig.4); there were no significant effects of ouabain (vs. aCSF vehicle) in the cariprazine- or lithium-treated groups. Administration of 1.0 mg/kg cariprazine i.p. for 7 days significantly decreased ouabain-induced hyperactivity (98.60 ± 23.05 vs. 198.4 ± 19.55; P < 0.05) (Fig.4). Treatment with 0.06 and 0.5 mg/kg cariprazine for 7 days, as well as 7 days of treatment with lithium, showed decreases in hyperactivity that bordered on significance (111.8 ± 22.94, 110.20 ± 19.18, and 130.1 ± 17.40, respectively; P < 0.08). Interestingly, in the 7-day dosing studies, there appeared to be a bimodal pattern of decreased hyperactivity, with higher and lower doses of cariprazine being more effective than the 0.25 mg/kg dose.
Figure 4.
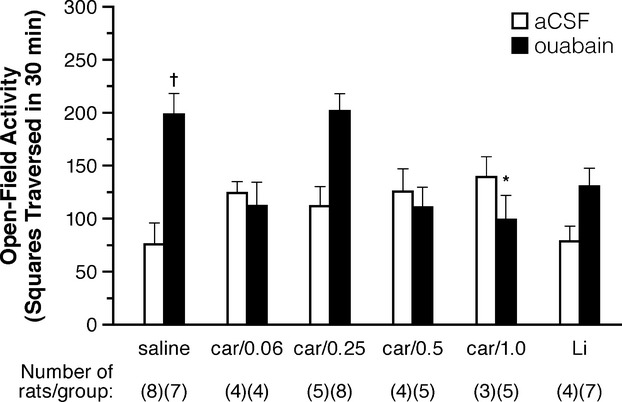
Open field activity 7 days after injection with ouabain. Administration of cariprazine for 7 days inhibits ICV ouabain-induced motoric hyperactivity in rats. The open field activities were measured 7 days after a single administration of ouabain. aCSF indicates artificial cerebrospinal fluid (vehicle for ouabain injection); car/x, cariprazine/concentration (in mg/kg); Li, lithium treatment (administered daily in food). †indicates P < 0.01; saline/ouabain versus saline/aCSF *indicates P < 0.05; cariprazine/ouabain versus saline/ouabain.
Discussion
The dopamine D2R has been classically shown to mediate its modulatory effects on synaptic transmission via signal transduction through Gi/o-mediated inhibition of cAMP 2008. In addition to this G protein-dependent pathway, several lines of evidence suggest that D2R can also engage a G protein-independent pathway, inhibiting the Akt/GSK-3 signaling cascade. This mode of signaling is mediated by a molecular complex involving the D2R, â-arrestin 2, Akt, and the multimeric protein phosphatase PP2A 2007a.
Various genetic and preclinical studies suggest that Akt and GSK-3 may be associated with the pathophysiology of schizophrenia (2010; 2010; 2010). In support of this hypothesis, 2004 have reported decreased Akt1 protein levels and GSK-3β phosphorylation in the lymphocytes and postmortem brain of individuals with schizophrenia. Haplotype variants in the Akt1 gene are positively associated with schizophrenia pedigrees (2004; 2005), and nonsymptomatic individuals carrying these haplotypes show similar deficits in lymphocyte Akt levels, as well as cognitive deficits associated with executive function 2008. In addition, Akt1 deficiency in mice affects neuronal morphology and predisposes them to impaired prefrontal function 2006, while transgenic overexpression of GSK-3β results in hyperactive behavior that models mania 2006. Furthermore, several antipsychotics have been shown to affect GSK-3 phosphorylation 2005, and the mood stabilizer lithium is also believed to inhibit GSK-3 activity, either directly, or by increasing phosphorylation/activation of Akt via destabilization of the D2R-mediated complex of β-arrestin-2/Akt/PP2A (2008; 2011). Hyperactivation of GSK-3 has also been hypothesized to be involved in bipolar disorder 2010.
In this study, cariprazine and aripiprazole acted as partial agonists for D2R/G protein-meditated cAMP production, but very weak agonists at the D2R/β-arrestin 2 interactions. Both compounds effectively antagonized the ability of the receptor to interact with β-arrestin 2. Interestingly, in the BRET-based assay used in our study to monitor D2R/β-arrestin 2 interactions, both cariprazine and aripiprazole exhibited only marginal agonist activities. However, aripiprazole has been shown to function as a partial agonist for â-arrestin-2 recruitment using assays that amplify the D2R/β-arrestin 2 signal 2011. As depicted in Figure2B and C a similar situation exists for cariprazine since in the presence of overexpression of the receptor kinase GRK2, the partial agonist activity of cariprazine is significantly enhanced. Interestingly, the potency of antagonism of quinpirole-mediated D2R/β-arrestin-2 interactions in this study correlated with the clinical efficacy of the three compounds in bipolar disorder, as suggested in a recent meta-analysis (cariprazine = haloperidol > aripiprazole) 2011. If the Akt/GSK-3 signaling pathway is important in some endophenotypes of schizophrenia and bipolar disorder, specifically targeting this pathway may represent a new therapeutic opportunity in both disorders. In addition, the activity of cariprazine at the D3R (2010; 2010) may enhance its response at the D2R 2007b. The current experiments did not include recombinant D3R expression in the model system so any potential D3R-mediated effects of cariprazine on the Akt/GSK-3 signaling would not be seen in these assays.
In our animal behavioral studies, acute administration of cariprazine attenuated ouabain-induced hyper-locomotor activity at all doses tested. Longer term administration of cariprazine at all doses except 0.25 mg/kg prevented ouabain-induced hyperactivity reaching statistical significance at the 1.0 mg/kg dosing level. The effects of cariprazine in both the acute and the 7-day studies were similar to the effects seen in animals treated with lithium. The acute administration of 1 mg/kg cariprazine alone significantly decreased open field activity (Fig.3), suggesting a possible sedating effect of acute administration at this dose.
Intracerebroventricular ouabain injection is a well-characterized model of mania that adequately fulfills multiple criteria required of an animal model 2007. Ouabain increases dopamine levels in the synapse (1998; 2007), and ouabain-induced hyperactivity is reduced with lithium and other antimanic agents (such as carbamazepine and haloperidol) 2007. Ouabain can also alter the phosphorylation state of Akt in some cell lines (,; 2012), as well as in vivo in rats 2010, although it should be noted that the effects of ouabain are actually contrary to what would be expected according to the Akt/GSK-3 model. According to this model, symptoms of mania 2010 and schizophrenia 2007 are caused by an overstimulation of the D2R, leading to the inactivation of Akt through dephosphorylation, followed by a dephosphorylation/hyperactivation of GSK-3 2007a. In the ouabain model of mania, however, i.c.v. injection of ouabain induces a dose-dependent increase in the immunoreactivity of phosphorylated Akt, and also leads to increased phosphorylation of downstream proteins GSK-3â, endothelial nitric oxide synthase, and Forkhead box protein O1 2010. Despite this discrepancy in the direction of Akt phosphorylation, it is possible that perturbations in the Akt/GSK-3 system in general may underlie the symptoms of mania and schizophrenia, in ways that are more complex than the current models predict.
While the ouabain paradigm is associated with several behavioral and neurobiological facets of mania, it primarily models the hyperactivity symptoms associated with mania. Bipolar mania, though, is a complex and multidimensional disease with a spectrum of symptoms that cannot be accurately evaluated in any single animal model 2011 Investigation of cariprazine in other behavioral models that represent additional mania symptom domains including sleep deprivation, aggression, reward-seeking behavior, and cognitive impairment is warranted 2011.
Phase III clinical trials of cariprazine have now been successfully completed in patients with schizophrenia and in patients with bipolar disorder , and the results from a recent meta-analysis of antimanic treatments suggest that cariprazine demonstrates robust clinical efficacy in patients with acute mania associated with bipolar disorder 2011. The effects of cariprazine on Akt/GSK-3 signaling may play a role in its antimanic efficacy.
Acknowledgments
This study was sponsored by grants from Forest Laboratories, Inc., and Gedeon Richter Plc. A Sponsored Research Agreement from Forest Laboratories to Duke University (MGC) supported the in vitro part of this work. Additional research, medical writing, and editorial assistance were provided by Michael L. Miller, Ph.D., and Adam Ruth, Ph.D., from Prescott Medical Communications, Chicago IL.
Glossary
Abbreviations
- aCSF
artificial cerebrospinal fluid
- Akt
protein kinase B
- ANOVA
analysis of variance
- BRET
bioluminescence resonance energy transfer
- D2R
dopamine 2 receptor
- D3R
dopamine 3 receptor
- EYFP
enhanced yellow fluorescent protein
- FBS
fetal bovine serum
- GSK-3
glycogen synthase kinase-3
- HBSS
Hank's Balanced Salt Solution
- HEK
human embryonic kidney
- mD2LR
mouse dopamine receptor D2-long form
- mβ-arrestin-2
mouse β-arrestin-2
- PBS
Phosphate Buffer Saline
- PP2A
protein phosphatase 2A
- RLuc
Renilla luciferase
Disclosures
Nika Adham is an employee of Forest Research Institute, a division of Forest Laboratories, Inc. Istvan Gyertyán and Béla Kiss are employees of Gedeon Richter Plc.
References
- Alimohamad H, Rajakumar N, Seah YH, Rushlow W. Antipsychotics alter the protein expression levels of beta-catenin and GSK-3 in the rat medial prefrontal cortex and striatum. Biol Psychiatry. 2005;57:533–542. doi: 10.1016/j.biopsych.2004.11.036. [DOI] [PubMed] [Google Scholar]
- Allen JA, Yost JM, Setola V, Chen X, Sassano MF, Chen M. Discovery of beta-arrestin-biased dopamine D2 ligands for probing signal transduction pathways essential for antipsychotic efficacy. Proc Natl Acad Sci USA. 2011;108:18488–18493. doi: 10.1073/pnas.1104807108. , et al. ( [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu JM, Gainetdinov RR. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev. 2011;63:182–217. doi: 10.1124/pr.110.002642. [DOI] [PubMed] [Google Scholar]
- Beaulieu JM, Gainetdinov RR, Caron MG. The Akt-GSK-3 signaling cascade in the actions of dopamine. Trends Pharmacol Sci. 2007a;28:166–172. doi: 10.1016/j.tips.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Beaulieu JM, Tirotta E, Sotnikova TD, Masri B, Salahpour A, Gainetdinov RR. Regulation of Akt signaling by D2 and D3 dopamine receptors in vivo. J Neurosci. 2007b;27:881–885. doi: 10.1523/JNEUROSCI.5074-06.2007. , et al. ( [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu JM, Marion S, Rodriguiz RM, Medvedev IO, Sotnikova TD, Ghisi V. A beta-arrestin 2 signaling complex mediates lithium action on behavior. Cell. 2008;132:125–136. doi: 10.1016/j.cell.2007.11.041. , et al. ( [DOI] [PubMed] [Google Scholar]
- Beaulieu JM, Gainetdinov RR, Caron MG. Akt/GSK3 signaling in the action of psychotropic drugs. Annu Rev Pharmacol Toxicol. 2009;49:327–347. doi: 10.1146/annurev.pharmtox.011008.145634. [DOI] [PubMed] [Google Scholar]
- Berk M, Dodd S, Kauer-Sant'anna M, Malhi GS, Bourin M, Kapczinski F. Dopamine dysregulation syndrome: implications for a dopamine hypothesis of bipolar disorder. Acta Psychiatr Scand. 2007;(Suppl):41–49. doi: 10.1111/j.1600-0447.2007.01058.x. , et al. ( [DOI] [PubMed] [Google Scholar]
- Boireau A, Meunier M, Imperato A. Ouabain-induced increase in dopamine release from mouse striatal slices is antagonized by riluzole. J Pharm Pharmacol. 1998;50:1293–1297. doi: 10.1111/j.2042-7158.1998.tb03348.x. [DOI] [PubMed] [Google Scholar]
- Changaris DG, Porter JL, Miller JJ, Levy RS. Des-Leu angiotensin I: biosynthesis and drinking response. Regul Pept. 1988;20:273–280. doi: 10.1016/0167-0115(88)90062-6. [DOI] [PubMed] [Google Scholar]
- Citrome L. Cariprazine in bipolar disorder: clinical efficacy, tolerability, and place in therapy. Adv Ther. 2013a;30:102–113. doi: 10.1007/s12325-013-0004-9. [DOI] [PubMed] [Google Scholar]
- Citrome L. Cariprazine in schizophrenia: clinical efficacy, tolerability, and place in therapy. Adv Ther. 2013b;30:114–126. doi: 10.1007/s12325-013-0006-7. [DOI] [PubMed] [Google Scholar]
- Cousins DA, Butts K, Young AH. The role of dopamine in bipolar disorder. Bipolar Disord. 2009;11:787–806. doi: 10.1111/j.1399-5618.2009.00760.x. [DOI] [PubMed] [Google Scholar]
- El-Mallakh RS, Harrison LT, Li R, Changaris DG, Levy RS. An animal model for mania: preliminary results. Prog Neuropsychopharmacol Biol Psychiatry. 1995;19:955–962. doi: 10.1016/0278-5846(95)00123-d. [DOI] [PubMed] [Google Scholar]
- El-Mallakh RS, El-Masri MA, Huff MO, Li XP, Decker S, Levy RS. Intracerebroventricular administration of ouabain as a model of mania in rats. Bipolar Disord. 2003;5:362–365. doi: 10.1034/j.1399-5618.2003.00053.x. [DOI] [PubMed] [Google Scholar]
- Emamian ES, Hall D, Birnbaum MJ, Karayiorgou M, Gogos JA. Convergent evidence for impaired AKT1-GSK3beta signaling in schizophrenia. Nat Genet. 2004;36:131–137. doi: 10.1038/ng1296. [DOI] [PubMed] [Google Scholar]
- Freyberg Z, Ferrando SJ, Javitch JA. Roles of the Akt/GSK-3 and Wnt signaling pathways in schizophrenia and antipsychotic drug action. Am J Psychiatry. 2010;167:388–396. doi: 10.1176/appi.ajp.2009.08121873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Payne RS, Schurr A, Hougland T, Lord J, Herman L. Memantine reduces mania-like symptoms in animal models. Psychiatry Res. 2011;188:366–371. doi: 10.1016/j.psychres.2010.12.030. , et al. ( [DOI] [PubMed] [Google Scholar]
- Ginovart N, Kapur S. Role of dopamine D(2) receptors for antipsychotic activity. Handb Exp Pharmacol. 2012:26–52. doi: 10.1007/978-3-642-25761-2_2. [DOI] [PubMed] [Google Scholar]
- Grunder G. Cariprazine, an orally active D2/D3 receptor antagonist, for the potential treatment of schizophrenia, bipolar mania and depression. Curr Opin Investig Drugs. 2010;11:823–832. [PubMed] [Google Scholar]
- Herman L, Hougland T, El-Mallakh RS. Mimicking human bipolar ion dysregulation models mania in rats. Neurosci Biobehav Rev. 2007;31:874–881. doi: 10.1016/j.neubiorev.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Hernandez-Lopez S, Tkatch T, Perez-Garci E, Galarraga E, Bargas J, Hamm H. D2 dopamine receptors in striatal medium spiny neurons reduce L-type Ca2 + currents and excitability via a novel PLC[beta[1-IP3-calcineurin-signaling cascade. J Neurosci. 2000;20:8987–8995. doi: 10.1523/JNEUROSCI.20-24-08987.2000. , et al. ( [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes OD, Kambeitz J, Kim E, Stahl D, Slifstein M, Abi-Dargham A. The nature of dopamine dysfunction in schizophrenia and what this means for treatment. Arch Gen Psychiatry. 2012;69:776–786. doi: 10.1001/archgenpsychiatry.2012.169. , et al. ( [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda M, Iwata N, Suzuki T, Kitajima T, Yamanouchi Y, Kinoshita Y. Association of AKT1 with schizophrenia confirmed in a Japanese population. Biol Psychiatry. 2004;56:698–700. doi: 10.1016/j.biopsych.2004.07.023. , et al. ( [DOI] [PubMed] [Google Scholar]
- Karam CS, Ballon JS, Bivens NM, Freyberg Z, Girgis RR, Lizardi-Ortiz JE. Signaling pathways in schizophrenia: emerging targets and therapeutic strategies. Trends Pharmacol Sci. 2010;31:381–390. doi: 10.1016/j.tips.2010.05.004. , et al. ( [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khundmiri SJ, Metzler MA, Ameen M, Amin V, Rane MJ, Delamere NA. Ouabain induces cell proliferation through calcium-dependent phosphorylation of Akt (protein kinase B) in opossum kidney proximal tubule cells. Am J Physiol Cell Physiol. 2006;291:C1247–C1257. doi: 10.1152/ajpcell.00593.2005. [DOI] [PubMed] [Google Scholar]
- Khundmiri SJ, Amin V, Henson J, Lewis J, Ameen M, Rane MJ. Ouabain stimulates protein kinase B (Akt) phosphorylation in opossum kidney proximal tubule cells through an ERK-dependent pathway. Am J Physiol Cell Physiol. 2007;293:C1171–C1180. doi: 10.1152/ajpcell.00535.2006. , et al. ( [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirshenbaum GS, Clapcote SJ, Duffy S, Burgess CR, Petersen J, Jarowek KJ. Mania-like behavior induced by genetic dysfunction of the neuron-specific Na+, K + -ATPase á3 sodium pump. Proc Natl Acad Sci USA. 2011;108:18144–18149. doi: 10.1073/pnas.1108416108. , et al. ( [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss B, Horvath A, Nemethy Z, Schmidt E, Laszlovszky I, Bugovics G. Cariprazine (RGH-188), a dopamine D(3) receptor-preferring, D(3)/D(2) dopamine receptor antagonist-partial agonist antipsychotic candidate: in vitro and neurochemical profile. J Pharmacol Exp Ther. 2010;333:328–340. doi: 10.1124/jpet.109.160432. , et al. ( [DOI] [PubMed] [Google Scholar]
- Koros E, Dorner-Ciossek C. The role of glycogen synthase kinase-3beta in schizophrenia. Drug News Perspect. 2007;20:437–445. doi: 10.1358/dnp.2007.20.7.1149632. [DOI] [PubMed] [Google Scholar]
- Lai WS, Xu B, Westphal KG, Paterlini M, Olivier B, Pavlidis P. Akt1 deficiency affects neuronal morphology and predisposes to abnormalities in prefrontal cortex functioning. Proc Natl Acad Sci USA. 2006;103:16906–16911. doi: 10.1073/pnas.0604994103. , et al. ( [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Jope RS. Is glycogen synthase kinase-3 a central modulator in mood regulation? Neuropsychopharmacology. 2010;35:2143–2154. doi: 10.1038/npp.2010.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Zhu W, Roh MS, Friedman AB, Rosborough K, Jope RS. In vivo regulation of glycogen synthase kinase-3beta (GSK3beta) by serotonergic activity in mouse brain. Neuropsychopharmacology. 2004;29:1426–1431. doi: 10.1038/sj.npp.1300439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Friedman AB, Zhu W, Wang L, Boswell S, May RS. Lithium regulates glycogen synthase kinase-3beta in human peripheral blood mononuclear cells: implication in the treatment of bipolar disorder. Biol Psychiatry. 2007;61:216–222. doi: 10.1016/j.biopsych.2006.02.027. , et al. ( [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masri B, Salahpour A, Didriksen M, Ghisi V, Beaulieu JM, Gainetdinov RR. Antagonism of dopamine D2 receptor/beta-arrestin 2 interaction is a common property of clinically effective antipsychotics. Proc Natl Acad Sci USA. 2008;105:13656–13661. doi: 10.1073/pnas.0803522105. , et al. ( [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee BP, Bailey PT, Pradhan SN. Temporal and regional differences in brain concentrations of lithium in rats. Psychopharmacology. 1976;48:119–121. doi: 10.1007/BF00423317. [DOI] [PubMed] [Google Scholar]
- Nicodemus KK, Law AJ, Radulescu E, Luna A, Kolachana B, Vakkalanka R. Biological validation of increased schizophrenia risk with NRG1, ERBB4, and AKT1 epistasis via functional neuroimaging in healthy controls. Arch Gen Psychiatry. 2010;67:991–1001. doi: 10.1001/archgenpsychiatry.2010.117. , et al. ( [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien WT, Huang J, Buccafusca R, Garskof J, Valvezan AJ, Berry GT. Glycogen synthase kinase-3 is essential for beta-arrestin-2 complex formation and lithium-sensitive behaviors in mice. J Clin Invest. 2011;121:3756–3762. doi: 10.1172/JCI45194. , et al. ( [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prickaerts J, Moechars D, Cryns K, Lenaerts I, van Craenendonck H, Goris I. Transgenic mice overexpressing glycogen synthase kinase 3beta: a putative model of hyperactivity and mania. J Neurosci. 2006;26:9022–9029. doi: 10.1523/JNEUROSCI.5216-05.2006. , et al. ( [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab SG, Hoefgen B, Hanses C, Hassenbach MB, Albus M, Lerer B. Further evidence for association of variants in the AKT1 gene with schizophrenia in a sample of European sib-pair families. Biol Psychiatry. 2005;58:446–450. doi: 10.1016/j.biopsych.2005.05.005. , et al. ( [DOI] [PubMed] [Google Scholar]
- Silva E, Soares-da-Silva P. New Insights into the Regulation of Na(+), K(+)-ATPase by Ouabain. Int Rev Cell Mol Biol. 2012;294:99–132. doi: 10.1016/B978-0-12-394305-7.00002-1. [DOI] [PubMed] [Google Scholar]
- Simpson EH, Kellendonk C, Kandel E. A possible role for the striatum in the pathogenesis of the cognitive symptoms of schizophrenia. Neuron. 2010;65:585–596. doi: 10.1016/j.neuron.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan HY, Nicodemus KK, Chen Q, Li Z, Brooke JK, Honea R. Genetic variation in AKT1 is linked to dopamine-associated prefrontal cortical structure and function in humans. J Clin Invest. 2008;118:2200–2208. doi: 10.1172/JCI34725. , et al. ( [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb CK, McCudden CR, Willard FS, Kimple RJ, Siderovski DP, Oxford GS. D2 dopamine receptor activation of potassium channels is selectively decoupled by Galpha-specific GoLoco motif peptides. J Neurochem. 2005;92:1408–1418. doi: 10.1111/j.1471-4159.2004.02997.x. [DOI] [PubMed] [Google Scholar]
- Yildiz A, Vieta E, Leucht S, Baldessarini RJ. Efficacy of antimanic treatments: meta-analysis of randomized, controlled trials. Neuropsychopharmacology. 2011;36:375–389. doi: 10.1038/npp.2010.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Henry BL, Geyer MA. Predictive animal models of mania: hits, misses and future directions. Br J Pharmacol. 2011;164:1263–1284. doi: 10.1111/j.1476-5381.2011.01318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu HS, Kim SH, Park HG, Kim YS, Ahn YM. Activation of Akt signaling in rat brain by intracerebroventricular injection of ouabain: a rat model for mania. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:888–894. doi: 10.1016/j.pnpbp.2010.04.010. [DOI] [PubMed] [Google Scholar]


