Abstract
Background
Lead is a toxic non-essential metal with widespread exposure starting in utero. Lead has been reclassified in 2004 by the International Agency for Research on Cancer Working Group from a “possible” to “probably” human carcinogen. Lead may be a facilitative or permissive carcinogen which means that lead may permit or augment the genotoxic effects of other exposures.
Methods
This population-based study in Wisconsin gathered survey data and home-collected urine specimens from 246 women, aged 20–69 years, with incident invasive breast cancer identified from the Wisconsin state registry and 254 age-matched control subjects from population lists from September 2004 to February 2005. We measured urinary lead concentrations by inductively-coupled plasma mass spectrometry, adjusted the values by specific gravity and conducted interviews by telephone to obtain information on known and suspected breast cancer risk factors.
Results
Women in the highest quartile of specific gravity-adjusted lead level (≥1.10 μg/L) had twice the breast cancer risk of those in the lowest quartile (<0.42 μg/L; OR = 1.99, 95% CI = 1.1 to 3.6) after adjustment for established risk factors. Excluding women who were currently taking nonsteroidal aromatase inhibitors (n=52), we did not observe any increased breast cancer risk after adjustment for established risk factors.
Conclusion
Our population-based case-control study suggests that lead exposure, as determined by specific gravity-adjusted urinary lead concentrations, is not associated with a significant increased risk for breast cancer.
Keywords: breast cancer, lead exposure, urine samples
Lead is a non-essential, toxic, and bioaccumulating metal. Although lead use dates to prehistoric times, widespread environmental lead contamination has become common only in the past 100-150 years. Anthropogenically mobilized lead now dominates modern-day human exposure as a result of past and continuing use in automobiles and aviation fuels and in a myriad of common uses (e.g., batteries, paints, solders, glazes, plastics). Arguably the degree of environmental lead contamination peaked in the mid-late twentieth century in developed counties, such as the United States due to a ban or restriction on lead additives in the mid 1990s (1, 2).
Lead exposure has been associated with adverse health effects in numerous human body systems. While the neural-, renal-, and blood forming- toxic properties of lead are well documented in both occupational and non-occupational settings, its carcinogenic activity has only recently been carefully scrutinized (3). In 2004, the International Agency for Research on Cancer Working Group reviewed six occupational cohort studies to determine the cancer hazards from lead exposure. They reported evidence for carcinogenicity particularly for stomach, brain, and kidney cancer to upgrade inorganic lead exposure from a “possible” human carcinogen (defined as such in 1984) to a “probable” human carcinogen (4). Silbergeld suggests that lead may be a facilitative or permissive carcinogen which means that lead may permit or augment the genotoxic effects of other exposures (5). In other words, lead exposure may increase the likelihood of permanent damage to DNA, either by inhibiting DNA repair or by interacting with proteins involved in DNA repair (5).
For non-occupationally exposed adult women, food and air (breathing aerosol/dust), in approximate equal proportions, are considered the largest vectors of lead intake worldwide (6); however, lead exposure from air may be a less significant contributor in countries where leaded gasoline has been banned (7). Regardless of source, the toxic effects are the same (3).
In this study, we evaluated the association between urinary lead levels (a surrogate for lead body burden and therefore exposure) and breast cancer risk in a population-based case-control study of Wisconsin women.
Materials and Methods
Identification of case participants
Only participants who had been a first diagnosis of breast cancer within 24 months of the time of the interview, whose cancers had been reported to Wisconsin's mandatory statewide cancer reporting system (the Wisconsin Cancer Reporting System) and had a listed telephone number were eligible for enrollment in the study. Information on stage of disease and reported treatment(s) for all participating case participants was obtained from the Wisconsin Cancer Reporting System.
Identification of control participants
Community participants (controls) were randomly selected, in an ongoing manner, from a list of licensed drivers (approximately 80% of the eligible population (8)) within 5-year age strata to yield an age distribution similar to the breast cancer cases. Controls were required to have no personal history of breast cancer and a listed telephone number.
The participation proportions—i.e., the percentage of participants eligible to participate in the population-based breast cancer parent study, who completed the interview—was 75% for case participants and 71% for control participants. Of those who completed the interview and agreed to participate in the metal sub-study, a participation proportion of 90% was achieved for urine specimen returns for both case and control participants. University of Wisconsin, Madison Health Sciences Human Subjects Committee approved this study. Oral consent was obtained for the interview, and written informed consent was obtained for the urine specimen collection.
Data Collection
Survey questions
Study participants were sent letters briefly describing the breast cancer study before they were contacted by telephone by trained interviewers. In a 35-minute questionnaire, women were asked to report menstrual and reproductive histories, usual alcohol consumption, height and weight, use of exogenous hormones, relevant personal and family medical history, tobacco use history, and demographic factors. Interview data were collected from September 2004 to February 2005.
Urine specimen
At the conclusion of the telephone interview, women were invited to participate in the metal sub-study. Specially-prepared urine home collection kits and detailed photo-essay instructions were mailed to the recruited participants. This urine home collection kit was carefully designed to minimize trace element contamination during specimen collection and handling. Specifically, urine samples contacted only Teflon or polyethylene materials which were exhaustively pre-cleaned in multi-step acid leachings. Study participants were instructed to clean hands with water and wear supplied clean gloves while collecting samples. Sample bottles were protected with zip-lock plastic bags. Tared urine collection bottles contained sufficient high purity nitric acid (1.0 mL of 50% v/v 16N) to stabilize the trace metals in the urine. Samples, collected from September 2004 to February 2005 were reweighed (to determine volume), and held at 4° C until inductively-coupled plasma mass spectrometry (ICP-MS) analysis. Samples were processed at the Wisconsin State Laboratory of Hygiene in a laboratory supplied with HEPA-filtered air (high-efficiency particulate air) that was specially designed for, and dedicated to, trace element analysis. Critical sample and equipment handling (bottle/vial drying, sub-sampling, and dilutions) were performed under polypropylene/acrylic HEPA-laminar-flow benches. Trace metal analyses (ICP-MS; refer to section on Lead Analyses) were performed from November 2004 to August 2005.
Analysis
Survey data
The interviewed community control subjects were assigned a reference date that corresponded to the average diagnosis date for breast cancer subjects of a similar age within 5-year strata. Reference age was defined as the woman's age at the reference date (4 categories; < 40, 40-49, 50-59, and 60-69 years old). Recent alcohol consumption was computed as the total number of drinks of beer, wine and/or hard liquor typically consumed per week approximately two years before interview (4 categories: 0, up to 7, >7 drinks/week). Parity was defined as the number of full term pregnancies (greater than 6 months gestation resulting in a live birth or stillbirth) (4 categories: nulliparous, 1-2, 3, >3 children). We defined 3 categories of menopausal status (premenopausal, postmenopausal, unknown). Family history of breast cancer was defined as absent, present, or unknown. A woman was classified as premenopausal if she reported still having menstrual periods and was not using hormone replacement therapy and postmenopausal if she reported an oophorectormy or natural menopause (no menstrual periods for at least six months) before the reference date. Women who reported that they were currently taking postmenopausal hormones and still having periods, and women who reported hysterectomy alone were classified as premenopausal if their reference ages were in the first decile of age at natural menopause among the controls (<41 years of age for current smokers and < 43 years of age for non-smokers), and postmenopausal if their reference ages were in the highest decile for age at natural menopause in the control group (≥ 54 years of age for current smokers and ≥ 56 years of age for non-smokers ). For women in the intermediate ages (second to ninth decile), menopausal status was considered unknown. Other variables in this analysis were body mass index (calculated as weight (in kilogram) divided by height (in meters) squared); 4 categories in quartiles and unknown), age at menopause (5 categories: <45, 45-59, 50-54, ≥55 years old), age at menarche (3 categories: <12, 12, >12 years old), smoking status (3 categories: never, former, current), age of first full term pregnancy in years (4 categories: <20, 20-24, 25- 29, ≥30), marital status (3 categories: never married, married or living as married, separated/widowed/divorced), lifetime supplemental multivitamin or mineral use in years (5 categories: never, 1-3, 4-11, ≥12, unknown), education status (5 categories: less than high school diploma, high school graduate, some college, college degree, unknown), physical activity level (6 categories based on hours per week of moderate (5 Metabolic Equivolent, METS) or vigorous (9 METS): none, <10, 10.0-17.4, 17.5-34.1, ≥34.2 METS, unknown), and type of postmenopausal hormone therapy use for postmenopausal women only (5 categories: never, estrogen only, estrogen and progestin only, other combinations, unknown).
Lead Analysis
Lead in urine was quantified by using inductively coupled plasma mass spectrometry (ICP-MS). A Perkin Elmer DRC II ICP-MS was used in standard mode following a method based upon the Centers for Disease Control and Prevention, National Health and Nutrition Examination Survey (NHANES) ICP-MS protocol (9).
Immediately before analysis, urine samples were diluted 1+9 with a dilute nitric acid solution (containing internal standards) in trace metal clean polypropylene autosampler tubes. Lead quantification was performed using external standards with internal normalization. Isotopes were acquired in peak jumping mode with a minimum of three replicate analyses performed on each sample after a 60 second uptake and 60 second stabilization period. A 90 second rinse (5%v/v nitric acid + v0.01% v/v triton) between samples virtually eliminated carry-over, and improved quantification limits (10). To account for minor variations in the natural isotopic distribution of lead, quantification was performed by summing the three primary lead isotopes (m/z 206, 207, 208). Analytical batches consisted of 20 urine samples + 16 quality control samples. Sample concentrations were blank-baseline corrected using the mean of three batch specific matrix blanks. Field blanks were insignificant; therefore, no additional method blank was applied.
A comprehensive quality-control program, described by Shafer and Overdie (10), incorporating numerous method/bottle blanks, monitoring of multiple lead isotopes (m/z 206, 207, 208), internal and external controls, sample spikes and duplicates, and routine inclusion of National Institute of Standards and Technology (NIST 2670a Trace Elements in Urine) standard reference materials ensured reliable and robust data. A 3-sigma method reporting limit of 0.19 μg/L was determined from the variation in lead levels in field method blanks. We measured creatinine (11) and specific gravity on each urine sample. A strong correlation (r=0.83) was observed between the two metrics, as has been noted in other published studies.Miller, 2004 3405 /id}(12) Both normalization approaches have been used in the literature, with no clear performance edge of any one approach, though some studies suggest that specific gravity may be superior.(13, 14) In the context of this study, specific gravity normalization was judged to be equivalent to creatinine, and therefore specific gravity-normalized data were used in the statistical analysis. Specific gravity was determined with a Schuco clinical refractometer (Model 511-2020).
Statistical analysis
Multivariate logistic regression models were used to assess the effects of urinary lead levels on breast cancer risk, controlling for age and for suspected or established risk factors (age, parity, age at first birth, family history of breast cancer, recent alcohol consumption, body mass index, age at menarche, menopausal status, age at menopause, type of postmenopausal hormone use, education, physical activity, and marital status). Established risk factors were defined categorically (Table 1). Models were fit using lead concentrations as continuous (linear) and categorical (quartiles) variables. Relative risk estimates, which assess the strength of association between urinary lead levels and breast cancer, for the continuous (linear) term are presented for the interquartile range of lead levels in the controls (0.68 μg/L). Urine samples that were below the limit of detection were assigned one-half of the value (0.096 μg/L). Additional multivariate logistic regression models that exluded women who were currently using non-steroidal aromatase inhibitors, adjusting for suspected or established risk factors were also used.
Table 1.
Characteristics of Community Controls and Breast Cancer Cases Aged 20-69 Years
| Cases (N=246) | Controls (N=254) | |||
|---|---|---|---|---|
| N | % | N | % | |
| Family history of breast cancer | ||||
| Absent | 187 | 76% | 218 | 86% |
| Present | 52 | 21% | 30 | 12% |
| Unknown | 7 | 3% | 6 | 2% |
| Recent alcohol consumption | ||||
| None | 51 | 21% | 51 | 20% |
| 1-6 drinks/week | 168 | 68% | 177 | 70% |
| ≥ 7 drinks/week | 27 | 11% | 25 | 10% |
| Parity | ||||
| 0-1 | 69 | 28% | 45 | 18% |
| 2 | 95 | 39% | 100 | 39% |
| ≥3 | 81 | 33% | 107 | 42% |
| Age at first full term pregnancy (years) | ||||
| < 20 | 36 | 15% | 43 | 17% |
| 20-24 | 80 | 33% | 105 | 41% |
| 25-29 | 55 | 22% | 54 | 21% |
| ≥30 | 36 | 15% | 21 | 8% |
| Nulliparous | 39 | 16% | 31 | 12% |
| Menopausal status | ||||
| Post-menopausal | 147 | 60% | 152 | 60% |
| Pre-menopausal | 67 | 27% | 75 | 29% |
| Unknown | 32 | 13% | 27 | 11% |
| Age at menopause (years)† | ||||
| <45 | 25 | 17% | 43 | 28% |
| 45-49 | 38 | 26% | 37 | 24% |
| 50-54 | 50 | 34% | 33 | 22% |
| ≥55 | 22 | 15% | 22 | 15% |
| Unknown | 12 | 8% | 17 | 11% |
| Body Mass Index (kg/m2)† | ||||
| < 22.5 | 34 | 23% | 27 | 18% |
| 22.5-25.0 | 30 | 20% | 30 | 20% |
| 25.1-28.8 | 35 | 24% | 41 | 27% |
| ≥28.9 | 48 | 33% | 52 | 34% |
| Age at menarche (years) | ||||
| <12 | 59 | 24% | 51 | 20% |
| 12 | 51 | 21% | 58 | 23% |
| 13 | 60 | 24% | 72 | 28% |
| ≥14 | 67 | 27% | 65 | 26% |
| Unknown | 9 | 4% | 8 | 3% |
| Education | ||||
| < High School diploma | 4 | 2% | 13 | 5% |
| High School diploma | 103 | 42% | 97 | 38% |
| Some College | 60 | 24% | 69 | 27% |
| College degree | 77 | 31% | 72 | 28% |
| Smoking | ||||
| Never | 135 | 55% | 155 | 61% |
| Former | 77 | 31% | 65 | 26% |
| Current | 33 | 13% | 31 | 12% |
| Postmenopausal hormone use† | ||||
| Never | 52 | 35% | 61 | 40% |
| Estrogen only | 32 | 22% | 37 | 24% |
| Estrogen and progestin only | 47 | 32% | 37 | 24% |
| Other combinations | 9 | 6% | 10 | 7% |
| Unknown | 7 | 5% | 7 | 5% |
| Marital Status | ||||
| Never married | 17 | 7% | 7 | 3% |
| Married/living as married | 199 | 81% | 201 | 79% |
| Separated/widowed | 28 | 11% | 43 | 17% |
| Physical Activty (MET-hrs/wk) | ||||
| None | 43 | 18% | 40 | 16% |
| <10.0 | 46 | 19% | 49 | 19% |
| 10.0-17.4 | 58 | 24% | 51 | 29% |
| 17.5-34.1 | 54 | 22% | 53 | 21% |
| ≥34.2 | 45 | 18% | 56 | 22% |
| Unknown | 0 | 0% | 5 | 2% |
*Logistic regression models adjusted for age.
Among postmenopausal women only.
Results
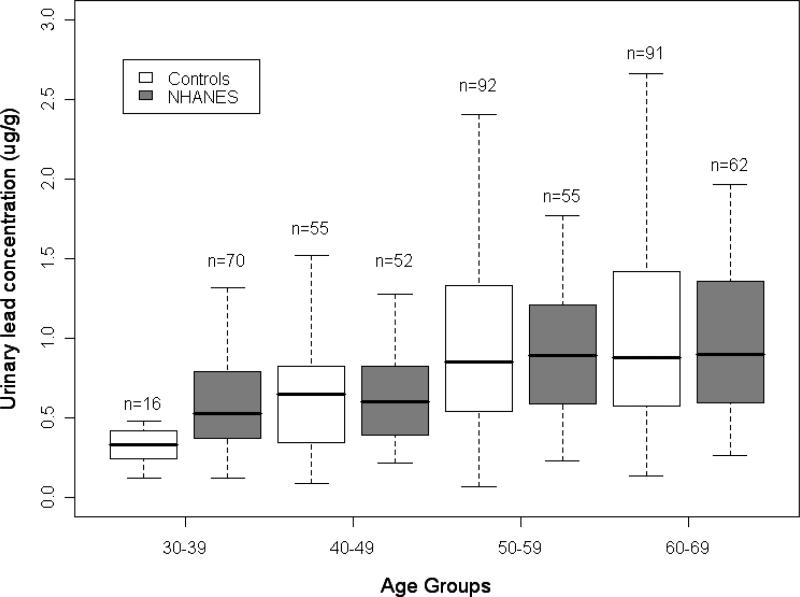
Case participants, as compared with similarly aged control subjects, were older at first full-term pregnancy, were more likely to have a family history of breast cancer, had fewer children, reported higher income, exercised less, and were more likely to have never married (Table 1). Specific gravity-adjusted lead levels ranged from 0.07 μg/L to 17.78 μg/L (median = 0.86μg/L) for case participants and from 0.05 μg/g to 3.90 μg/L (median = 0.64 μg/L) for control subjects. Of the 45 urine samples below the level of detection, 18 were from cases and 27 were from controls. The creatinine-adjusted lead values for each 10-year age group in the control subjects were similar to those reported from a national age-stratified sampling of the general population of Caucasian women in the NHANES III (2003-2004 National Health and Nutrition Examination Survey) (9) (Figure 1).
Figure 1.
Comparison of NHANES 2003-2004 creatinine-adjusted urinary lead data to our study.
After adjustment for age only, women in the highest quartile had twice the risk of breast cancer as those in the lowest quartile (OR = 1.93, 95% CI = 1.1, 3.3; Pvalue = 0.001) (Table 2). In the multivariate adjustment with known and suspected risk factors, the OR was essentially unchanged, 1.91 (95% CI = 1.0 to 3.5; Pvalue = 0.006). The odds ratio in the multivariate model, which excluded 52 women who were current users of nonsteroidal aromatase inhibitors as adjuvant therapy was 1.01 (95% CI = 0.5 to 2.0; Pvalue = 0.31) (Table 2). Similar results were observed for creatinine-adjusted urinary lead levels in all models (data not shown).
Table 2.
Multivariate Odds Ratio of Breast Cancer Associated with Specific Gravity-adjusted Urinary Lead
| Cases | Controls | Odds | Odds | |||||
|---|---|---|---|---|---|---|---|---|
| Lead concentration levels‡ | N | (%) | N | (%) | Ratio* | 95% CI* | Ratio† | 95% CI† |
| All participants | ||||||||
| 1 | 48 | 20% | 64 | 25% | 1.00 | 1.00 | ||
| 2 | 43 | 17% | 63 | 25% | 0.91 | 0.5-1.6 | 0.90 | 0.5-1.7 |
| 3 | 68 | 28% | 64 | 25% | 1.46 | 0.9-2.5 | 1.73 | 1.0-3.2 |
| 4 | 87 | 35% | 63 | 25% | 1.93 | 1.1-3.3 | 1.91 | 1.0-3.5 |
| continuous | 1.36 | 1.1-1.6 | 1.34 | 1.1-1.7 | ||||
| p-value | 0.001 | 0.006 | ||||||
| Excluding users of non-steroidal aromatase inhibitors not taking bisphosphonates | ||||||||
| 1 | 46 | 24% | 63 | 25% | 1.00 | 1.00 | ||
| 2 | 37 | 19% | 64 | 25% | 0.83 | 0.5-1.5 | 0.82 | 0.4-1.6 |
| 3 | 63 | 32% | 64 | 25% | 1.48 | 0.9-2.5 | 1.75 | 0.9-3.2 |
| 4 | 48 | 25% | 63 | 25% | 1.17 | 0.7-2.0 | 1.01 | 0.5-2.0 |
| continuous | 1.12 | 0.9-1.3 | 1.10 | 0.9-1.3 | ||||
| p-value | 0.20 | 0.31 | ||||||
Adjusted for age.
Adjusted for age, parity, age at first birth, family history of breast cancer, recent alcohol consumption, body mass index, age at menarche, menopausal status, age at menopause, type of postmenopausal hormone use, education, marital status and physical activity.
Lead concentration levels (in μg/L): 1 = < 0.42; 2 = 0.42-0.64; 3 = 0.0.65-1.10; 4 = ≥1.10.
Finally, we attempted to examine whether the disease process per se or treatment may have altered lead levels in analyses stratified by disease stage (localized versus regional/distant) and by treatment (surgery and/or radiation only versus chemotherapy and/or hormonal therapy). Similar associations between lead levels and breast cancer risk were observed among participants with localized breast cancer (OR=1.32; 95% CI: 1.0-1.7) and participants with regional/distant breast cancer (OR=1.59; 95% CI: 1.2-2.1). The association of breast cancer risk with lead levels did not differ between participants treated by surgery and/or radiation only (OR=1.36; 95% CI: 1.1-1.7) and those treated with chemotherapy (OR=1.36; 95% CI: 1.0-1.8).
Discussion
Our finding indicates that exposure to lead is not associated with an increased breast cancer risk. However, the use of adjuvant therapy in the form of nonsteroidal aromatase inhibitors may influence the amount of biologically active lead levels in women.
Almost all the epidemiologic cancer research associated with lead exposure has been derived from occupationally exposed individuals (see (2) for review). Most of these studies have evaluated only men and, not unexpectedly, none of these studies noted a breast cancer risk associated with lead exposure (3, 4). In addition, studies have found little difference in lead concentration levels between corresponding and neoplastic human breast tissue in paired specimens (15-19). These studies involved a small number of patients (5 – 25 patients) in which the tissue was obtained from surgical removal of breast tissue (usually mastectomy) due to cancer (15-19). Samples for analysis were carefully selected as neoplastic and corresponding non cancerous tissue (15-19).
Of the four biospecimens that could potentially be used in a large population based study-- bone, plasma, whole blood, and urine--none are without limitations. Skeletal levels of lead are considered the best indicator of lifetime exposure (4) (half-life >25 years) and lead stored in bone may be an important endogenous source of bioactive lead (20-23). Although the method used to determine in vivo bone lead levels, XRF (X-ray fluorescence method) is noninvasive, it demands substantial time burden on participant (approximately 30 minutes to obtain a measurement and the participants must travel to a testing center); to date 7- 12 research institutions in North America have this tool (24, 25). More importantly, the K-XRF approach is subject to relatively high measurement uncertainties arising from fundamental and practical constraints of the x-ray technique itself (variations range from 3 to 30 μg/g-levels encompassing a significant fraction of the observed range of lead levels in bone) (25), and also native variation in the deposition of lead within various bone structures and compartments (26).
Plasma lead may be the most biologically active matrix since a large fraction of lead in plasma is unbound (3). The extremely low concentrations of lead in plasma (especially at whole blood level of <20 μg/dL), necessitating both extreme measures to prepare collection materials and the use of ICP-MS instrumentation, makes this matrix problematic (22, 27). The rapid cycling of lead through the plasma compounds the difficulty in inferring body burden from plasma measurements.
Whole blood has historically been the benchmark matrix for assessment of lead exposure (more than 99 percent of total blood lead is associated with the erthyrocytes (28)) (4). The half life of lead in blood is 4-5 weeks reflecting recent exposure as well as mobilization of lead from other tissues including bone (21). Therefore, a single blood lead measurement does not differentiate between recent exposure and historic exposure that may be represented in the blood from cycling among blood and bone/soft tissue lead storage (24, 29). Several studies have reported good correlation (0.4-0.8) between urine and blood lead levels, especially above 10 μg/L —where the lack of a better correlation is in part attributed to the shorter residence time of lead in blood (30-32).
The validity of urine to assess the body burden (a surrogate for exposure history) of lead must be carefully considered. Urinary lead, as a measure of body burden, has support in the literature (30, 33), but a consensus has not been reached in the research community as to the appropriate matrix for body burden estimation in cancer studies (3, 24). One limitation of its appropriateness may be that urinary lead values do not adequately reflect the participant's lead exposure at clinically significant time periods. However, the same argument/constraint is likely present for other biological matrices. Evidence for the exchange of bone lead, where approximately 94% of the total body burden of lead resides (34), with soft tissue stores indicates that a significant portion of urinary lead may reflect this historic accumulation (35). Smith et al's study demonstrated that bone lead contributed 40-70% of the lead in blood (23) of which a large portion is then excreted via the kidneys (24). Urinary lead may be indicative of the degree of lead that is biologically active by reflecting lead from plasma, excreted through the kidneys (30).
Lead exposure begins in utero (36) and continues throughout life. Increases in bone lead plateaus at middle age and decreases at higher ages with older adults having among the highest blood lead levels (37); this trend is more pronounced in females. Lead interacts with essential elements, particularly calcium, zinc, and iron (for review see (38)). Menopausal status, pregnancy and lactation, bone mineral density decline in women with age (39-43) have been linked with increased endogenous exposure of lead release from resorbed bone to blood. In our study, we adjusted for menopausal status and parity. Urinary lead levels may be influenced by kidney function. Although we adjusted for dilution of spot-urine sample using specific gravity normalization, we were unable to determine the kidney function status of our participations.
We also considered whether treatment for breast cancer (chemotherapy/hormonal vs radiation/surgery) may have altered lead levels. We obtained the treatment data from the cancer registry records and not from medical record abstraction. In our analyses, we did not find any statistical differences in the main effect's odds ratios when stratifying by treatment. However, the quality and completeness of the treatment data is less than ideal.
We were able to evaluate the influence of some types of adjuvant therapy on lead levels most likely through bone turnover. Specifically, nonsteroidal aromatase inhibitors are commonly prescribed for postmenopausal estrogen sensitive breast cancer patients though a known side effect, particularly for non-steroidal aromatase inhibitors (e.g., letrozole) is bone loss (44-47). Studies have shown that aromatase inhibitors deplete estrogen levels in postmenopausal women to near zero which could and, thus, are exacerbate bone loss (48). In contrast, bisphosphonates, such as zolendronic acid, is known to prevent bone loss in premenopausal women who receive this adjuvant estrogen suppression therapy (49). In our study, we did not have the estrogen status of the tumor cells (estrogen positive or negative). However, we did collect data on adjuvant therapy, including use of both types of aromatase inhibitors (steroidal and nonsteroidal), bisphosphates, tamoxifen and raloxifene. Of the 246 women diagnosed with breast cancer, 21 percent (n=52) were using non-steroidal aromatase inhibitors without any bisphosphonate therapy subsequent to their diagnosis. Excluding these women from the analyses nullified our finding of a significant increased breast cancer risk with lead exposure. This finding suggests that the elevated lead levels in women diagnosed with breast cancer compared to controls were strongly associated with non-steroidal aromatase inhibitor use and not an indication of breast cancer risk.
Although considerable evidence of bone loss during chemotherapy has been reported (50, 51), our study did not find an attenuation of the results for the full dataset (i.e., included in the dataset were those who are non-steroidal aromatase inhibitors users) when stratified by breast cancer treatment type. Since the nonsteroidal aromatase inhibitors were divided between treatment types (23 women who received chemotherapy/hormonal therapy and 28 women who received surgery/radiation), these data support our understanding that the lead levels reflect bone exchange from the adjuvant therapy.
In conclusion, more research is needed to examine the relation of endogenous lead exposure from bone turnover and loss due to adjuvant hormonal therapy. The results of our population-based case–control study indicated little influence of lead exposure on breast cancer risk, although we did find nonsteroidal aromatase inhibitor use strongly influenced potentially biologically active lead levels. Given the ubiquitous exposure of the general population to lead and the potential accessibility of endogenous exposure from bone cycling, the mode of the association between lead exposure from bone cycling and adjuvant therapy use for breast cancer warrants further study.
Acknowledgment
We thank Dr. Henry Anderson and Laura Stephenson at the Wisconsin State Department of Health and Vital Statistics Section for support and assistance with the cancer data. The authors are appreciative of the staff of the Wisconsin Women's Health Study including Dr. Amy Trentham-Dietz and John Hampton, and the State Laboratory of Hygiene for data collection and laboratory support on this project. We are especially grateful to the study participants, whose generosity made this research possible.
Funding for this study was provided by the National Institutes of Health grants (R03 CA110796 and R01 CA47147).
References
- 1.Thomas VM, Socolow RH, Fanelli JJ, Spiro TG. Effects of reducing lead in gasoline: an analysis of the international experience. Environ Sci Technol. 1999;33:3942–8. [Google Scholar]
- 2.Landrigan PJ, Boffetta P, Apostoli P. The reproductive toxicity and carcinogenicity of lead: a critical review. Am J Ind Med. 2000;38:231–43. doi: 10.1002/1097-0274(200009)38:3<231::aid-ajim2>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 3.Agency for Toxic Substances and Disease Registry . Toxicological profile for lead. U.S. Department of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry; Atlanta, Ga.: 2007. [Google Scholar]
- 4.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans Inorganic and organic lead compounds. IARC Monogr Eval Carcinog Risks Hum. 2006;87:1–471. [PMC free article] [PubMed] [Google Scholar]
- 5.Silbergeld EK. Facilitative mechanisms of lead as a carcinogen. Mutat Res. 2003;533:121–33. doi: 10.1016/j.mrfmmm.2003.07.010. [DOI] [PubMed] [Google Scholar]
- 6.Jarup L. Hazards of heavy metal contamination. Br Med Bull. 2003;68:167–82. doi: 10.1093/bmb/ldg032. [DOI] [PubMed] [Google Scholar]
- 7.Paschal DC, Ting BG, Morrow JC, et al. Trace metals in urine of United States residents: reference range concentrations. Environ Res. 1998;76:53–9. doi: 10.1006/enrs.1997.3793. [DOI] [PubMed] [Google Scholar]
- 8.Pawasarat J. The driver license status of the voting age population in Wisconsin. University of Wisconsin-Milwaukee Employment and Training Institute; Milwaukee, WI: 2005. [2008 January 3]. Available from: http://www.uwm.edu/Dept/ETI/barriers/DriversLicense.pdf. [Google Scholar]
- 9.Centers for Disease control and Prevention (CDC), National Center for Health Statistics (NCHS) National Health and Nutrition Examination Survey Data (NHANES), 2003-2004. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; Hyattsville, MD: [2008 June 3]. Available from: http://www.cdc.gov/nchs/about/major/nhanes/nhanes2003-2004/nhanes03_04.htm. [Google Scholar]
- 10.Shafer M, Overdier J. Analysis of surface waters for trace elements by inductively-coupled plasma mass spectrometry. Lake Michigan Mass Balance methods compendium. Volume 3. Metals, conventionals, radiochemistry, and biomonitoring sample analysis techniques. U.S. Environmental Protection Agency, Great Lakes National Program Office; Chicago, IL: 1997. pp. 3–85-3-129. [Google Scholar]
- 11.Elinder CG. Normal values for cadmium in human tissues, blood, and urine in different countries. In: Friberg L, Elinder CG, Kjellstrom T, Nordberg GF, editors. Cadmium and health: a toxicological and epidemiological appraisal, volume I exposure, dose, and metabolism. CRC Press; Boca Raton, FL: 1985. pp. 81–102. [Google Scholar]
- 12.Barr DB, Wilder LC, Caudill SP, Gonzalez AJ, Needham LL, Pirkle JL. Urinary creatinine concentrations in the U.S. population: implications for urinary biologic monitoring measurements. Environ Health Perspect. 2005;113:192–200. doi: 10.1289/ehp.7337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mason HJ, Williams NR, Morgan MG, Stevenson AJ, Armitage S. Influence of biological and analytical variation on urine measurements for monitoring exposure to cadmium. Occup Environ Med. 1998;55:132–7. doi: 10.1136/oem.55.2.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haddow JE, Knight GJ, Palomaki GE, Neveux LM, Chilmonczyk BA. Replacing creatinine measurements with specific gravity values to adjust urine cotinine concentrations. Clin Chem. 1994;40:562–4. [PubMed] [Google Scholar]
- 15.Rizk SL, Sky-Peck HH. Comparison between concentrations of trace elements in normal and neoplastic human breast tissue. Cancer Res. 1984;44:5390–4. [PubMed] [Google Scholar]
- 16.Mulay IL, Roy R, Knox BE, Suhr NH, Delaney WE. Trace-metal analysis of cancerous and noncancerous human tissues. J Natl Cancer Inst. 1971;47:1–13. [PubMed] [Google Scholar]
- 17.Tariq MA, Qamar un N, Fatima A. Concentrations of Cu, Cd, Ni, and Pb in the blood and tissues of cancerous persons in a Pakistani population. Sci Total Environ. 1995;175:43–8. doi: 10.1016/0048-9697(95)04823-5. [DOI] [PubMed] [Google Scholar]
- 18.Santoliquido PM, Southwick HW, Olwin JH. Trace metal levels in cancer of the breast. Surg Gynecol Obstet. 1976;142:65–70. [PubMed] [Google Scholar]
- 19.Schwartz AE, Leddicotte GW, Fink RW, Friedman EW. Trace elements in normal and malignant human breast tissue. Surgery. 1974;76:325–9. [PubMed] [Google Scholar]
- 20.Cake KM, Bowins RJ, Vaillancourt C, et al. Partition of circulating lead between serum and red cells is different for internal and external sources of lead. Am J Ind Med. 1996;29:440–5. doi: 10.1002/(SICI)1097-0274(199605)29:5<440::AID-AJIM2>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 21.Gulson BL, Mahaffey KR, Mizon KJ, Korsch MJ, Cameron MA, Vimpani G. Contribution of tissue lead to blood lead in adult female subjects based on stable lead isotope methods. J Lab Clin Med. 1995;125:703–12. [PubMed] [Google Scholar]
- 22.Hernandez-Avila M, Smith D, Meneses F, Sanin LH, Hu H. The influence of bone and blood lead on plasma lead levels in environmentally exposed adults. Environ Health Perspect. 1998;106:473–7. doi: 10.1289/ehp.106-1533211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith DR, Osterloh JD, Flegal AR. Use of endogenous, stable lead isotopes to determine release of lead from the skeleton. Environ Health Perspect. 1996;104:60–6. doi: 10.1289/ehp.9610460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu H, Shih R, Rothenberg S, Schwartz BS. The epidemiology of lead toxicity in adults: measuring dose and consideration of other methodologic issues. Environ Health Perspect. 2007;115:455–62. doi: 10.1289/ehp.9783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ambrose TM, Al Lozi M, Scott MG. Bone lead concentrations assessed by in vivo X-ray fluorescence. Clin Chem. 2000;46:1171–8. [PubMed] [Google Scholar]
- 26.Rabinowitz MB. Toxicokinetics of bone lead. Environ Health Perspect. 1991;91:33–7. doi: 10.1289/ehp.919133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith D, Hernandez-Avila M, Tellez-Rojo MM, Mercado A, Hu H. The relationship between lead in plasma and whole blood in women. Environ Health Perspect. 2002;110:263–8. doi: 10.1289/ehp.02110263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ong CN, Lee WR. Distribution of lead-203 in human peripheral blood in vitro. Br J Ind Med. 1980;37:78–84. doi: 10.1136/oem.37.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barbosa F, Jr., Tanus-Santos JE, Gerlach RF, Parsons PJ. A critical review of biomarkers used for monitoring human exposure to lead: advantages, limitations, and future needs. Environ Health Perspect. 2005;113:1669–74. doi: 10.1289/ehp.7917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bergdahl IA, Schutz A, Gerhardsson L, Jensen A, Skerfving S. Lead concentrations in human plasma, urine and whole blood. Scand J Work Environ Health. 1997;23:359–63. doi: 10.5271/sjweh.232. [DOI] [PubMed] [Google Scholar]
- 31.Fukui Y, Miki M, Ukai H, et al. Urinary lead as a possible surrogate of blood lead among workers occupationally exposed to lead. Int Arch Occup Environ Health. 1999;72:516–20. doi: 10.1007/s004200050409. [DOI] [PubMed] [Google Scholar]
- 32.Gulson BL, Cameron MA, Smith AJ, et al. Blood lead-urine lead relationships in adults and children. Environ Res. 1998;78:152–60. doi: 10.1006/enrs.1997.3810. [DOI] [PubMed] [Google Scholar]
- 33.Tsaih SW, Schwartz J, Lee ML, et al. The independent contribution of bone and erythrocyte lead to urinary lead among middle-aged and elderly men: the normative aging study. Environ Health Perspect. 1999;107:391–6. doi: 10.1289/ehp.99107391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barry PS. A comparison of concentrations of lead in human tissues. Br J Ind Med. 1975;32:119–39. doi: 10.1136/oem.32.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Manton WI. Significance of lead isotope composition in blood. Nature. 1973;244:165–7. doi: 10.1038/244165a0. [DOI] [PubMed] [Google Scholar]
- 36.Shen XM, Yan CH, Guo D, et al. Low-level prenatal lead exposure and neurobehavioral development of children in the first year of life: a prospective study in Shanghai. Environ Res. 1998;79:1–8. doi: 10.1006/enrs.1998.3851. [DOI] [PubMed] [Google Scholar]
- 37.Paschal DC, Burt V, Caudill SP, et al. Exposure of the U.S. population aged 6 years and older to cadmium: 1988-1994. Arch Environ Contam Toxicol. 2000;38:377–83. doi: 10.1007/s002449910050. [DOI] [PubMed] [Google Scholar]
- 38.Magos L. Epidemiological and experimental aspects of metal carcinogenesis: physicochemical properties, kinetics, and the active species. Environ Health Perspect. 1991;95:157–89. doi: 10.1289/ehp.9195157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Webber CE, Chettle DR, Bowins RJ, et al. Hormone replacement therapy may reduce the return of endogenous lead from bone to the circulation. Environ Health Perspect. 1995;103:1150–3. doi: 10.1289/ehp.951031150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gulson BL, Mizon KJ, Korsch MJ, Palmer JM, Donnelly JB. Mobilization of lead from human bone tissue during pregnancy and lactation--a summary of long-term research. Sci Total Environ. 2003;303:79–104. doi: 10.1016/s0048-9697(02)00355-8. [DOI] [PubMed] [Google Scholar]
- 41.Hernandez-Avila M, Gonzalez-Cossio T, Palazuelos E, et al. Dietary and environmental determinants of blood and bone lead levels in lactating postpartum women living in Mexico City. Environ Health Perspect. 1996;104:1076–82. doi: 10.1289/ehp.961041076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Korrick SA, Schwartz J, Tsaih SW, et al. Correlates of bone and blood lead levels among middle-aged and elderly women. Am J Epidemiol. 2002;156:335–43. doi: 10.1093/aje/kwf042. [DOI] [PubMed] [Google Scholar]
- 43.Nash D, Magder LS, Sherwin R, Rubin RJ, Silbergeld EK. Bone density-related predictors of blood lead level among peri- and postmenopausal women in the United States: The Third National Health and Nutrition Examination Survey, 1988-1994. Am J Epidemiol. 2004;160:901–11. doi: 10.1093/aje/kwh296. [DOI] [PubMed] [Google Scholar]
- 44.Miki Y, Suzuki T, Sasano H. Aromatase inhibitor and bone. Biomed Pharmacother. 2007;61:540–2. doi: 10.1016/j.biopha.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 45.Coombes RC, Hall E, Gibson LJ, et al. A randomized trial of exemestane after two to three years of tamoxifen therapy in postmenopausal women with primary breast cancer. N Engl J Med. 2004;350:1081–92. doi: 10.1056/NEJMoa040331. [DOI] [PubMed] [Google Scholar]
- 46.Howell A, Cuzick J, Baum M, et al. Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years' adjuvant treatment for breast cancer. Lancet. 2005;365:60–2. doi: 10.1016/S0140-6736(04)17666-6. [DOI] [PubMed] [Google Scholar]
- 47.Thurlimann B, Keshaviah A, Coates AS, et al. A comparison of letrozole and tamoxifen in postmenopausal women with early breast cancer. N Engl J Med. 2005;353:2747–57. doi: 10.1056/NEJMoa052258. [DOI] [PubMed] [Google Scholar]
- 48.Winer EP, Hudis C, Burstein HJ, et al. American Society of Clinical Oncology technology assessment on the use of aromatase inhibitors as adjuvant therapy for postmenopausal women with hormone receptor-positive breast cancer: status report 2004. J Clin Oncol. 2005;23:619–29. doi: 10.1200/JCO.2005.09.121. [DOI] [PubMed] [Google Scholar]
- 49.Coleman RE, Body JJ, Gralow JR, Lipton A. Bone loss in patients with breast cancer receiving aromatase inhibitors and associated treatment strategies. Cancer Treat Rev. 2008 doi: 10.1016/j.ctrv.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 50.Waltman NL, Ott CD, Twiss JJ, Gross GJ, Lindsey AM, Moore TE. Bone mineral density and bone turnover in postmenopausal women treated for breast cancer. Cancer Nurs. 2008;31:182–90. doi: 10.1097/01.NCC.0000305722.75647.26. [DOI] [PubMed] [Google Scholar]
- 51.Ramaswamy B, Shapiro CL. Osteopenia and osteoporosis in women with breast cancer. Seminars in Oncology. 2003;30:763–75. doi: 10.1053/j.seminoncol.2003.08.028. [DOI] [PubMed] [Google Scholar]