Abstract
Estradiol effects on memory depend on hormone levels and the interaction of different estrogen receptors within neural circuits. Estradiol induces gene transcription and rapid membrane signaling mediated by estrogen receptor-alpha (ERα), estrogen receptor-beta (ERβ), and a recently characterized G-protein coupled estrogen receptor, each with distinct distributions and ability to influence estradiol-dependent signaling. Investigations using receptor specific agonists suggest that all three receptors rapidly activate kinase-signaling and have complex dose-dependent influences on memory. Research employing receptor knockout mice demonstrate that ERα maintains transcription and memory as estradiol levels decline. This work indicates a regulatory role of ERβ in transcription and cognition, which depends on estradiol levels and the function of ERα. The regulatory role of ERβ is due in part to ERβ acting as a negative regulator of ERα-mediated transcription. Vector-mediated expression of estrogen receptors in the hippocampus provides an innovative research approach and suggests that memory depends on the relative expression of ERα and ERβ interacting with estradiol levels. Notably, the ability of estradiol to improve cognition declines with advanced age along with decreased expression of estrogen receptors. Thus, it will be important for future research to determine the mechanisms that regulate estrogen receptor expression during aging.
Keywords: estrogen, aging, memory, hippocampus, estrogen receptor-alpha (ERα)
Introduction
Estrogens are steroid hormones that are synthesized in the gonads as well as in various tissues throughout the body, including the brain. 17β-Estradiol (E2), the most potent and predominant form of estrogen, has a number of effects on cognition and brain function. Several cognitive processes appear to be dependent on the level of E2 such that cognitive impairment occurs when E2 concentrations are above or below an optimal level (Fig. 1) (Foster 2005; 2012). Numerous clinical research studies have examined memory and cognitive function in women whose levels of E2 have been altered either through ovariectomy or menopause. In general, the results of these studies show a decrease of cognitive function in women following surgical removal of their ovaries or menopause, and that these deficits can be reversed by estrogen replacement therapy (ERT) if the therapy is initiated immediately following ovarian hormone loss (Sherwin 2009). The observation that the effectiveness of ERT is tightly linked to the woman's physiological status at the time of initiation of treatment is echoed by the results of more recent studies that show that the cognitive benefits of ERT are minimal in women older than 65 years (Hogervorst and Bandelow 2010; Maki and Henderson 2012). Indeed, the effectiveness of ERT to reduce risk of Alzheimer's disease in menopausal women is lost when treatments are initiated in women of advanced age (Brinton 2001; Resnick and others 1997; Zandi and others 2002).
Figure 1.
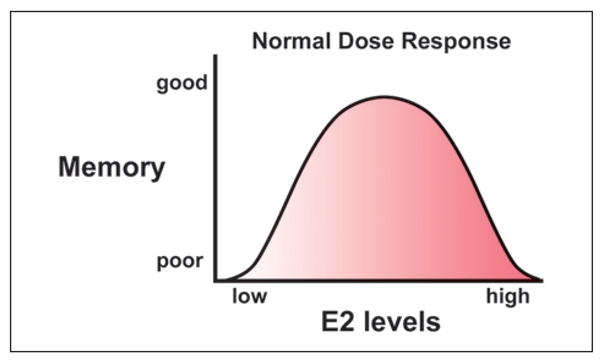
Dose–response function for 17β-estradiol (E2) effects on cognition. The relationship between the E2 dose and memory can be described by an inverted U function, such that ovariectomy and a low level of E2 result in poor memory. Memory is enhanced by increasing E2 to within the physiological range. Memory improvement is supplanted by impaired memory as E2 levels increase to supraphysiological levels.
Much of what we currently know about the mechanisms for estrogen's effects on cognitive function comes from studies of learning and memory in rodent models, the results of which parallel those observed in women. For example, the ability of E2 to improve performance on spatial memory learning tasks in ovariectomized rodents is highly dependent on the age of the animal, the dose of E2, and the duration of estrogen decline prior to initiation of E2 treatments. In these studies, E2 treatment was observed to improve spatial memory in young (3 months) ovariectomized rats when the treatments were initiated at middle-age but not when begun at later ages (Foster and others 2003; Gibbs 2000; Markham and others 2002; Markowska and Savonenko 2002). However, when animals were ovariectomized in middle-age (12-13 months), treatments initiated at 17 to 21 months did not improve spatial working memory (Daniel 2006; Gibbs 2000; Markowska and Savonenko 2002). The reduced ability of E2 to improve memory in these rat studies mirrors ERT clinical observations and indicates that the underlying mechanism(s) that account for the effectiveness of ERT to improve memory performance in women are likely to be multifaceted and influenced by age and the duration of hormone deprivation in each individual.
E2 influences brain function through gene transcription and rapid membrane signaling. Furthermore, several transcriptional and post-translational feedback mechanisms control E2 activity, which likely contributes to the complex dose–response pattern. While the mechanism(s) underlying the reduced ability of ERT to provide cognitive protection with advancing age is unknown, it is known that E2 effects on several biological processes decline with age, including the ability to preserve the blood-brain barrier (Bake and Sohrabji 2004), protect the brain from stroke (Suzuki and others 2009) and neuroinflammation (Benedusi and others 2012), promote growth of glutamatergic synapses (Adams and others 2001; Aenlle and Foster 2010), influence mitochondrial function (Aenlle and Foster 2010; Jones and Brewer 2009), and activate rapid signaling cascades (Fan and others 2010; Foster 2005; Wu and others 2013).
The purpose of this review is to highlight our current understanding of how E2 signaling may affect hippocampaldependent cognitive function. We begin with a brief overview of rapid E2 signaling mechanisms and the classical nuclear E2 pathways, with an emphasis on the role of the different estrogen receptors. The second section explores the role of estrogen receptors in the regulation of cognition by examining studies of spatial memory that employ receptor-specific agonists, receptor knockout mice, and viral vectors to overexpress receptors in specific hippocampal cells. Finally, we examine how changes in estrogen receptor expression interact with the level of E2 to determine cognitive decline during aging and discuss the mechanisms for regulation of estrogen receptor expression. The data indicate that the various receptors can have similar or antagonistic effects and the influences on memory are because of the interaction of estrogen receptors, which in turn depend on their level of expression and the level of agonist.
Estrogen Receptor Signaling Mechanisms
E2 is thought to exert the majority of its biological effects through the interaction of two primary estrogen receptors: estrogen receptor-alpha (ERα) and estrogen receptor-beta (ERβ). In addition, a G-protein coupled estrogen receptor (GPR30 or GPER1) has recently been characterized as a modulator of second messenger pathways. The relative levels and subcellular distributions of ERα, ERβ, and GPER1, which are found throughout the nervous system, vary across brain regions (Brailoiu and others 2007; Milner and others 2001; Mitra and others 2003; Mitterling and others 2010). In human and rat hippocampus, ERβ levels are greater than ERα levels (Foster 2012) and ERα is more likely to be localized to the nucleus (Mitra and others 2003; Mitterling and others 2010). Because of differences in structure and subcellular distribution, it has been argued that estrogen receptors may have different biological actions and differential effects on cognition.
Rapid Signaling Mechanisms
Rapid signaling is mediated through membrane receptors, including the GPER1 G-protein coupled receptor, and the interaction of ERα and ERβ with metabotropic receptors (Fig. 2A). These membrane receptors initiate Ca2+, phospholipase C, and adenylyl cyclase signaling with subsequent activation of a host of kinases (B-RAF, IP3K, Src, ERK, AKT, PKA, PKC), which in turn can rapidly influence neuronal physiology or phosphorylate transcription factors such as CREB or ERα to induce gene transcription (Foster 2005). As such, E2 signaling interacts with molecular pathways associate with growth factor signaling as well as learning and memory. Time course and dose–response studies indicate that kinase activity rapidly rises and declines within minutes and activation exhibits an inverted U function, with inhibition at higher doses (Kuroki and others 2000).
Figure 2.
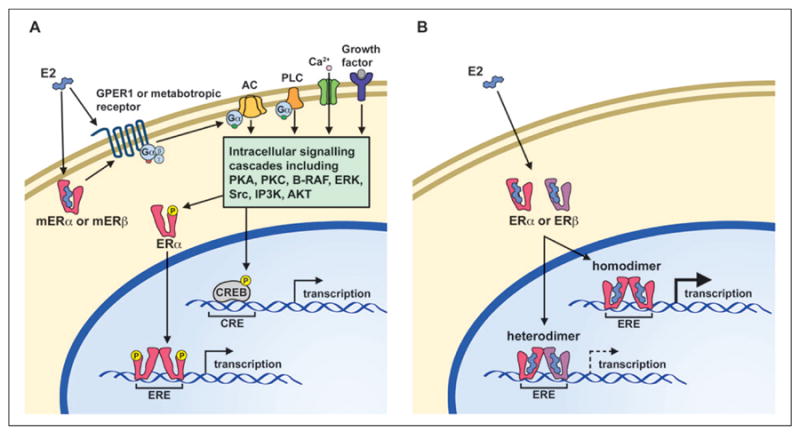
Simplified models depicting rapid estrogen receptor signaling (left) and classical nuclear pathway (right). (A) In the rapid estrogen receptor signaling pathway, estrogen activates membrane associated estrogen receptors and associated G-protein coupled receptors to induce intracellular signaling cascades, which rapidly influence neuronal physiology or lead to the phosphorylation of the estrogen receptor-alpha (ERα) or CREB proteins. (B) In the classical pathway, estrogen binds to an inactive ERα or ERβ within the cytoplasm or the nucleus of the cell. The activated estrogen receptor monomer forms a dimer with another activated estrogen receptor monomer to create either a homodimer or a heterodimer, which then binds to an estrogen response element (ERE) within the DNA, along with other co-regulator proteins (not shown) to modify transcription of target genes. Note that ERα homodimers exhibit increased transcription relative to estrogen receptor heterodimers or ERβ homodimers because of ERβ acting as a negative regulator of ERα-mediated transcription.
Research using receptor-specific agonists indicates that membrane ERα and GPER1 initiate AKT cell survival signaling and all three receptors can initiate Ca2+ to ERK signaling (Boulware and others 2013; Gingerich and others 2010; Maruyama and others 2013; Wu and others 2011; Zhang and others 2009), which is thought to be involved in cell survival and modulation of memory. Similarly, work with estrogen receptor knockout (ERKO) mice indicates that multiple estrogen receptors contribute to rapid effects of E2 on synaptic transmission (Foster and others 2008; Fugger and others 2001). Finally, in an elegant series of studies, it was shown that the rapid kinase signaling cascades enhanced ERα-mediated transcription through the phosphorylation of ERα (Clark and others 2013). Thus, for hippocampal cells that express both genomic and membrane receptors, the specificity of action of ERα and ERβ on rapid signaling cascades may be mute if all receptors activate the same rapid signaling systems and ultimately contribute to ERα-dependent transcription (Fig. 2A).
Classical Nuclear Pathways for Regulating Transcription
Considering the diverse assortment of hippocampal molecular and biological processes influenced by E2, it is not unanticipated that E2 treatment regulates the expression of hundreds of hippocampal genes (Aenlle and Foster 2010; Aenlle and others 2009; Han and others 2013). Receptor specific agonists have been employed to examine the possible role of ERα and ERβ on biological processes that depend on transcription and likely contribute to cognitive function during aging. Activation of ERα or ERβ differentially influences the expression of neuroprotective genes and neuroprotection to ischemia and β-amyloid protein (Carroll and Pike 2008; Farr and others 2007; Gingerich and others 2010). E2 increases the expression of genes linked to the cellular components of the synapse (Aenlle and Foster 2010). Both receptors appear to influence synaptogenesis, but most studies using receptor-specific agonists find that synaptic markers are increased more by ERα activation, relative to the ERβ agonists, suggesting that ERα is the predominate receptor for synaptogenesis (Foster 2012). Interestingly, the extent of synaptogenesis may depend on the relative expression of ERα/ERβ, with a decrease in synaptogenesis observed as ERβ levels increase (Szymczak and others 2006).
Despite the fact that ERβ is the predominately expressed receptor in the hippocampus, ERα is transcriptionally more active than ERβ (Fig. 2B). Although both receptors exhibit similar homology for the DNA binding domain, the difference in transcriptional activity is, in part, beause of the structural differences that influence the affinity of the receptor for E2 and interactions with transcription activators/repressors (Barkhem and others 1998) (Fig. 3). The consequence of these structural differences is that low levels of E2 will preferentially activate ERα-dependent transcription (Foster 2012). However, for low E2 levels, ERβ can heterodimerize with ERα to act as a negative regulator of ERα-mediated transcription (Pettersson and others 2000). Co-expression of both receptors in cell cultures results in an ERβ gene-dose inhibition of transcription (Hall and McDonnell 1999; Pettersson and others 2000). Thus, the higher level of ERβ in the hippocampus may act to inhibit ERα-dependent transcription (Fig. 2B). Indeed, some ERβ isoforms found in the hippocampus act as dominant negative regulators of ERα-dependent transcription (Chung and others 2007). Thus, in many instances, ERβ has a role in regulating ERα activity.
Figure 3.
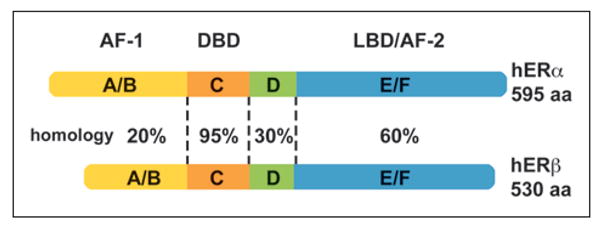
Non-homologous domains/regions within human nuclear estrogen receptors (hERs) contribute to dissimilarities in transcription. Although ERα and ERβ are highly homologous (95%) within the DNA binding domain (DBD), ERα has greater transcriptional activity. Differences in the ligand binding domain (LBD) contribute to increased affinity of 17β-estradiol (E2) for ERα. Furthermore, differences in the structure of the activation function regions (AF-1 and AF-2) influence the ability of transcriptional co-regulators to interact with the receptors.
ERβ regulation of ERα-mediated transcription is particularly evident in mice with a functional knockout of ERα or ERβ. Relative to wildtype controls, ERα-mediated transcription is increased in tissues from ERβKO mice (Foster 2012). Recent work (Han and others 2013) employing mutant mice revealed that, in the hippocampus, E2-related transcription was observed in ovariectomized ERβKO mice in the absence of E2 treatment (Box 1). The results indicate that ERα-mediated transcription can occur under conditions of low E2 and confirms an inhibitory role for ERβ (Fig. 4A). Interestingly, a single E2 treatment decreased expression of E2-sensitive genes in ERβKO mice suggesting that feedback mechanisms may attempt to limit over activation of ERα following E2 treatment. Finally, a single injection of E2 resulted in E2-dependent transcription in ERαKO mice, suggesting that other E2 sensitive mechanisms, possibly involving a transcriptionally weaker ERβ, can induce transcription and maintain hippocampal function provided E2 levels are increased (Fig. 4B).
Box 1.
How can ERα induce transcription in ovariectomized animals in the absence of E2 treatment? E2 is synthesized in the hippocampus and the level of synthesized E2 is within the range that preferentially binds and activates ERα (Hojo and others 2004; Prange-Kiel and others 2003). However, an extended period of hormone deprivation results in the loss of locally synthesized E2 (Barker and Galea 2009). In turn, a decrease in local E2 synthesis results in a decrease in ERα expression. Similarly, a loss of E2 synthesis and ERα is observed in Alzheimer's disease (Ishunina and Swaab 2007). The results emphasize the interactions of feed forward and feedback mechanisms such that the local level of E2 interacts with ERα and ERβ expression to determine transcriptional activity, including the transcription of ERα. The interaction of E2, ERα, and ERβ may provide a mechanism to insure proper transcription and maintenance of hippocampal function as E2 levels fluctuate across the estrus cycle in adults (Foster 2012; Han and others 2013). However, this same interaction may also contribute to impaired cognition following the loss of ovarian hormone and a decline in ERα expression during menopause.
Figure 4.
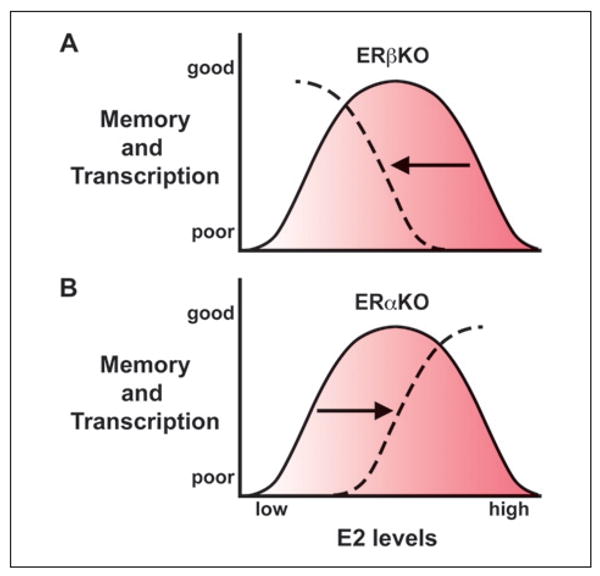
Role of estrogen receptor-alpha (ERα) and ERβ in transcription and cognition: studies using ERKO mice. (A) For ERβKO mice, the absence of ERβ results in a leftward shift in the descending arm of the dose–response curve. Ovariectomized ERβKO mice exhibit increased transcription of E2-sensitive genes and better cognition. Acute E2 treatment decreases transcription and fails to improve memory and chronic treatment impairs memory. (B) For ERαKO mice, the ascending arm of the dose response function for memory and 17β-estradiol (E2)- mediated transcription is shifted to the right. In this case, memory impairment is observed in low estrogen condition such as following ovariectomy and E2 treatment improves cognition and increases E2-sensitive transcription.
Estrogen Receptors in Cognition
The various estrogen receptors may differentially contribute to time-dependent and dose-dependent effects of E2 treatments on cognition. For example, E2 is thought to influence memory through estrogen receptors in the membrane that rapidly activate signaling cascades involved in synaptic plasticity processes that are required for learning and memory (Fig. 2A). In this case, E2-mediated enhancement of memory is observed when treatment is delivered around the time of training, but not when it is delayed by 2 hours (Packard 1998). Long-term effects are thought to result from transcription of genes that maintain cognitive function such that memory facilitation is observed several days after an E2 treatment. In this way, cyclic E2 treatment improved memory in middle-age mice 48 to 72 hours after E2 treatment (Aenlle and others 2009; Han and others 2013). Finally, difference may be observed for cyclic and chronic treatments, possibly because of feedback mechanisms (Gibbs 2000; Markowska and Savonenko 2002).
The relationship between the E2 dose and memory can be described by an inverted U function, such that memory improvement is supplanted by impaired memory as E2 levels increase (Foster 2012) (Fig. 1). E2 induces a similar biphasic response in a wide range of tissues and biological processes (Calabrese 2001). In other receptor systems, receptor subtypes contribute to a biphasic dose response in rapid signaling due to differences in receptor affinity and the effectiveness of signal transduction for receptor subtypes with antagonistic actions. As noted above, E2-mediated transcriptional activity is a function of differences in ligand affinity and the efficacy of transcription driven by homodimers and heterodimers suggesting one possible mechanism for the inverted U dose–response.
Receptor Specific Agonists
In an attempt to examine the role of each receptor subtype in memory, recent work has employed receptor specific agonists, mutant mice, and viral vector delivery of different estrogen receptors. The results suggest a framework for thinking about how the various estrogen receptors interact with the level of E2 to influence memory. For studies on the role of each receptor in rapid effects in memory modulation, receptor specific agonists are delivered within a relatively short time prior to or just after a learning episode. For example, post-training treatment with the ERα-specific agonist, propyl pyrazole triol (PPT, 0.9 mg/kg), but not the ERβ-specific agonist, diarylpropionitrile (DPN, 0.9 mg/kg) improved retention on the object placement task in 2- to 4-month ovariectomized rats (Frye and others 2007). Interestingly, the opposite effect was observed by another group (Jacome and others 2010), which used a higher dose (3-5 mg/kg) and found that DPN, but not PPT, improved memory in 2-month ovariectomized rats. One study has specifically examined the dose–response function for ERα- and ERβ-specific agonists on the object placement task (Phan and others 2011). The results indicated that in 2-month ovariectomized mice, PPT treatment prior to training resulted in an inverted U function for memory and hippocampal dendritic spine number. In contrast, DPN had only a modest effect on spatial memory and no effect on spine number. The differences emphasize the complex dose–response relationship for estrogen receptor agonists and the need to examine multiple doses.
Long-term treatment with PPT, DPN, or the GPER1 agonist G-1 improved delayed matching-to-position on a T-maze in young rats (Hammond and others 2009), suggesting a lack of receptor specificity for long-term effects on this task. Because the activation of membrane ERα, ERβ, or GPER1 receptors lead to activation of similar kinase pathways, ERα phosphorylation, and ERα-mediated transcription (Fig. 2A), it will be important for futures studies to determine the mechanism for improved cognition. Several studies have examined the effects of chronic ERα- or ERβ-specific agonists on spatial memory in middle-age animals. In one study, rats were ovariectomized at 3 months of age and treatment with the ERα agonist PPT (1 mg/kg) was initiated at ∼5 months. Animals were tested after 2 months of treatment and exhibited improved spatial learning and memory on the water maze, which was associated with neuroprotection and preservation of synaptic function (Qu and others 2013). The dose–response (0.02-0.2 mg/kg) influence of PPT and DPN was examined in middle-age (12 months old) ovariectomized rats on a delayed spatial alternation task (Neese and others 2010). This study reported that the lowest dose of DPN impaired performance and intact performance was observed for higher doses. In contrast, PPT had only subtle effects at the highest dose. Together, the studies emphasize that, similar to acute studies, chronic studies of receptor specific agonists should employ multiple doses to determine the dose–response function. Furthermore, for chronic studies, it will be important to determine which mechanisms, rapid activation of kinases and/or ERα-mediated transcription, mediate the preservation of cognitive function during aging.
Estrogen Receptor Knockout Mice
Other studies have attempted to differentiate cognitive effects of estrogen receptor subtypes through receptor knockout mice. In many ways, these studies mirror the results of hippocampal transcription in receptor knockout mice, indicating that ERα maintains hippocampal function, particularly when E2 levels are low, and ERβ can have an inhibitory effect on ERα-mediated effects. In the absence of E2 treatment, ovariectomized young and middle-age ERαKO mice exhibit impaired memory (Foster and others 2008; Fugger and others 2000; Han and others 2013), supporting a role for ERα in maintaining cognitive function. The results are consistent with a growing literature that indicates that memory function is positively correlated with hippocampal ERα levels and, under conditions of low E2, cognitive deficits are observed when ERα function is impaired (Foster 2012). In women, some ERα polymorphisms are associated with an increased susceptibility to Alzheimer's disease (Corbo and others 2006; Ji and others 2000). Interestingly, ERα polymorphisms are also associated with an increased propensity for cognitive impairments, which emerge as E2 levels decline during middle-age (Olsen and others 2006; Yaffe and others 2002). The results suggest that prior to menopause; elevated levels of E2 may be able to compensate for impaired ERα function. This idea was confirmed in ERαKO mice in which E2 treatment improved memory, suggesting that other estrogen sensitive mechanisms, possibly involving ERβ, may be engaged by elevation of E2 to compensate for a decrease in ERα function (Fugger and others 2000; Han and others 2013; Liu and others 2008).
Research using ERβKO mice indicates that the role of ERβ in cognition depends not only on the level of E2 but also on the function of ERα. Unlike ERαKO mice, ovariectomized ERβKO mice do not exhibit an initial impairment in memory (Fugger and others 2000; Han and others 2013). In fact, in middle-age mice and young mice, 6 weeks following ovariectomy, cognition of ERβKO mice is enhanced relative to both ERαKO and wild-type mice (Han and others 2013). The contrast between wild-type and ERβKO mice suggests that, when E2 levels are low, ERβ expression has an antagonistic effect on cognition. This effect of ERβ is thought to result in part from ERβ acting as a negative regulator of ERα-mediated transcription. The antagonistic action of ERβ may also protect animals from cognitive impairment because of overactivation of ERα. In ERβKO, but not wild-type mice, chronic E2 treatment resulted in a dose-dependent impairment of cognition (Fig. 4A) and a decrease in ERα expression (Rissman and others 2002), suggesting overactivation of ERα and subsequent feedback regulation. Finally, ERβKO mice do not exhibit the memory-enhancing effects of acute E2 treatment normally observed in wildtype and ERαKO mice (Fugger and others 2000; Han and others 2013; Walf and others 2008). Together, the results demonstrate that ERα maintains cognitive function in the face of declining E2 levels and that ERβ can be antagonistic to memory for low E2 levels (Fig. 4), suggesting that dose-dependent effects of E2 on cognition may depend on the relative expression of ERα and ERβ.
Viral Vector Delivery of ERα and ERβ
Viral-mediated delivery of estrogen receptors provides a novel approach to determine the role of estrogen receptor expression in cognitive function. This technique has the advantage of enhancing receptor function in specific brain regions and cell types, and at specific ages. Vector delivery of ERα to neurons of the hippocampus rescues memory function in adult ovariectomized ERαKO mice (Foster and others 2008). The results indicate that memory impairments of ERαKO mice are not simply because of the absence of ERα during development; rather, ERα is important in maintaining cognitive function in adults. This idea was confirmed for middle-age rats in a study that showed that, in the absence of exogenous E2, viral vector–mediated expression of ERα in pyramidal cells of the hippocampus improved spatial working memory (Witty and others 2012). It is important to note that in all cases, memory enhancement was observed in the absence of E2 treatment suggesting that an increase in the ERα expression can preserve cognitive function in adults, at least up through middle-age. In contrast, delivery of a viral vector to increase expression of ERβ in neurons of the hippocampus reverses the protective effect on cognition observed in middle-age ERβKO mice (Han and others 2013). It is unclear if the cognitive impairment associated with delivery of ERβ reestablishes the ability of E2 to improve cognition. Regardless, the results confirm that ERβ expression can contribute to impaired cognition associated with E2 deprivation.
Together, the results suggest that the dose-dependent effects of E2 on cognition are linked to the relative expression of ERα and ERβ (Foster 2012). A decrease in the expression of ERβ (e.g., ERβKO mice) results in a leftward shift in the descending arm of the dose–response curve observed as improved cognition and increased ERα-mediated transcription for ovariectomized animals (i.e., low E2 conditions). Furthermore, with a loss of ERβ, acute E2 treatments fail to improve memory and may activate feedback systems to decrease transcription. Moreover, chronic E2 treatment impairs memory, possibly because of a loss of ERβ-mediated regulation of ERα (Fig. 4A). In the case of impaired ERα function (e.g., ERαKO mice), the ascending arm of the dose response function is shifted to the right. Impairments are more profound for ovariectomized animals and an increase in E2 level can promote transcription and cognitive function (Fig. 4B).
Estrogen Receptors and Age-Related Cognitive Decline
While the exact reason for the loss of the cognitive enhancing effects of E2 with advanced age is unknown, evidence suggests it is linked to age-related changes in expression and signaling of the estrogen receptors (see Foster 2012 for review). Gene profiling studies indicate that aged animals are less responsive than young and middle-age animals, exhibiting reduced E2-induced hippocampal transcription (Aenlle and Foster 2010). Interestingly, the decreased responsiveness was observed for genes that decline with advancing age, including genes linked to transcriptional regulation, growth, synaptic activity, and neuroprotection (Box 2). Similarly, loss of ERα due to extended E2 deprivation (Zhang and others 2009) or in ERαKO mice (Suzuki and others 2009) is linked to a loss of E2-mediated neuroprotection and synaptogenesis. Together, the results suggest that a decline in ERα expression may contribute to decreased E2 responsiveness.
Box 2.
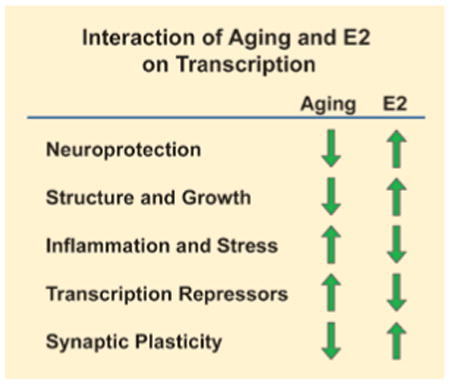
The effect of aging and E2 treatment on hippocampal genes associated with specific biological responses. Aging is associated with an increase in expression of genes associated with transcription repression, inflammation and stress, and a decrease in expression of genes associated with neuroprotection, structure and growth, and synaptic plasticity. E2 treatments can reverse the age-related expression profile.
There is a significant decrease in the number of estrogen receptor positive neurons in the hippocampus during aging. For example, there is a 56% and 41% decrease of ERα and ERβ respectfully in the CA1 region of aged rats compared to young rats (Mehra and others 2005). Interestingly, this decrease in hippocampal estrogen receptors is not reflected in the uterus, which remains responsive to estrogen treatment. There is also a 50% reduction in the number of spines/synapses that contain ERα in the rat CA1 region of the hippocampus and the ability of estrogen treatment to induce spine formation declines with age and is associated with this loss of ERα (Adams and others 2002). Like ERα, the level of ERβ at the synapse decreases with age, but the level of synaptic ERβ increases following E2 treatment (Waters and others 2011). These observations suggest that changes in mechanisms for regulation of estrogen receptor expression contribute to the shift in the relative expression of ERα and ERβ. Thus, in older animals a decline in ERα and an E2-mediated increase in ERβ would result in a decrease in the ratio of ERα/ERβ. As noted above, a decrease in the relative expression of ERα/ERβ due to loss of ERα or an increase in ERβ is associated with a decrease in transcription of neuroprotection and synaptic plasticity genes (Aenlle and Foster 2010; Aenlle and others 2009; Han and others 2013) and reduced synaptogenesis (Szymczak and others 2006). The results suggest that the expression of ERα and ERβ is important for maintaining hippocampal function with advancing age.
Regulation of Estrogen Receptor Expression
While the expression profile for estrogen receptors is well characterized by age and brain region, the molecular mechanisms that regulate estrogen receptor expression in the hippocampus are not well understood. Experiments to examine regulation of estrogen receptor expression have mainly been performed in breast cancer cell cultures. Because of their importance in regulating numerous genes and physiological processes, several transcriptional and post-translational feedback mechanisms control estrogen receptor expression. Post-translational mechanisms include protein down-regulation via the ubiquitin–proteasome pathway (Fig. 5). ERβ binds to the heat shock cognate protein 70 (Hsc70)-interacting protein (CHIP) and induces degradation via ubiquitination and proteolysis. The degradation of ERβ results in a decrease in ERβ-mediated transcription (Tateishi and others 2006). In addition, degradation of ERα through the ubiquitin–proteasome pathway has been reported in the CA1 region of the rat hippocampus (Zhang and others 2011). In this study, an increase in ERα–CHIP interaction was observed in ovariectomized young rats and intact aged female rats. However, it is important to note that unlike ERβ, cycling of ligandbound ERα through the ubiquitin–proteasome degradation pathway is required for ERα-mediated transcription (Lonard and others 2000) (Fig. 5). Interruption of ERα ubiquitination and proteolysis will stabilize the receptor in the nuclear matrix, resulting in inhibition of transcription. Thus, it will be important for future studies to determine if the increase in ERα–CHIP interaction indicates increased or decreased ERα-mediated transcription.
Figure 5.
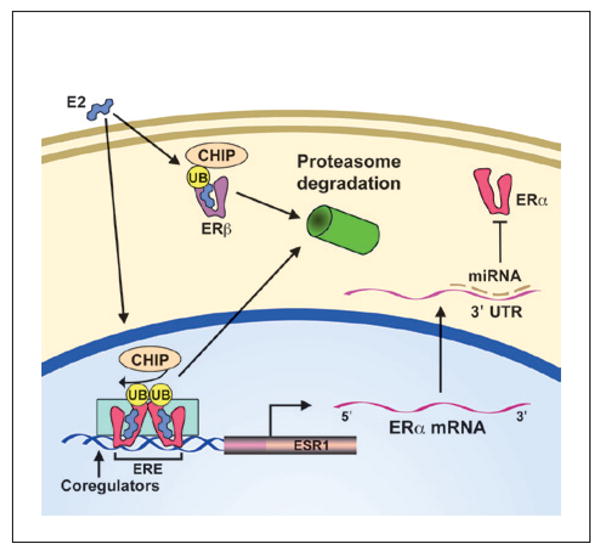
Post-translational regulation of estrogen receptor expression. Ligand-bound estrogen receptor-alpha (ERα)- mediated transcription is coupled with the Hsc70-interacting protein (CHIP) and ubiquination–proteolysis process, such that ligand-bound ERα is degraded following successful transcription of the target gene. In contrast, ERβ interacts with CHIP and ubiquination and subsequent ligand binding can induce transcription or transport to the proteasome for degradation. Thus, ligand-bound receptor degradation may indicate ongoing transcription for ERα or act to down-regulate ERβ transcriptional activity. Other posttranscriptional modifications include ERα miRNAs that bind to the 3′ untranslated region and prevent mRNA stability or translation.
The mRNA for ERα exhibits a relatively long 3′-untranslated region (3′UTR), which makes it subject to post-transcriptional regulation by binding of proteins and small noncoding microRNA (miRNA) (Fig. 5). Expressions of miR-18a, miR-22, miR-206, miR-221, and miR-222 are associated with decreased ERa expression in human cancer cell lines. Interestingly, miR-206 expression was inhibited by PPT, but not DPN, suggesting a reciprocal inhibitory feedback loop. However, it appears that the levels for known miRNAs that regulate ERα expression do not change in the hippocampus with E2 treatment or with age (Rao and others 2013).
A number of epigenetic mechanisms can produce stable changes in transcription and several epigenetic mechanisms for regulating estrogen receptor transcription have been described (Fig. 6). Histones are proteins involved in the packaging and unwinding of DNA regions through chemical modifications, including histone acetylation. Histone deacetylation is associated with silent chromatin and in breast cancer cells, a loss of ERα expression can be reversed by inhibition of histone deacetylase. Again, a mutual inhibitory feedback system could be in play since E2 treatment decreases hippocampal expression of genes (HDAC2, Sap18) involved in histone deacetylation in young and middle-age mice, but not aged mice (Aenlle and Foster 2010). Repression of gene expression can also occur through methylation of cytosines in guanine-cytosine-rich areas of the gene promoter region, termed CpG islands (Fig. 6). In several systems, ERα gene promoter methylation rises with age and is associated with decreased ERα expression and increased incidence of disease, particularly in cancer where it has been studied in depth (Li and others 2004; Post and others 1999). Similarly, methylation of the ERβ promoter was found in atherosclerosis, a common condition in vascular aging, and in senescence of smooth muscle and endothelial cell lines. The epigenetic mechanism was confirmed in the cell lines by the exposure of a DNA demethylating agent and histone deacetylase, which caused the recovery of ERβ expression (Kim and others 2007). In the brain, methylation of the ERα promoter during development results in a decrease in ERα expression and contributes to lifelong physiological and behavioral differences (Gore and others 2011; Schwarz and others 2010). Little is known concerning estrogen receptor promoter methylation in the hippocampus during aging. However, modifications in methylation of ERα and ERβ promoters have been reported in the cortex of middle-age rats and following brain injury (Westberry and others 2011). The results point to promoter methylation as an important, but relatively unexplored, regulator of estrogen receptor expression in the brain across the lifespan.
Figure 6.
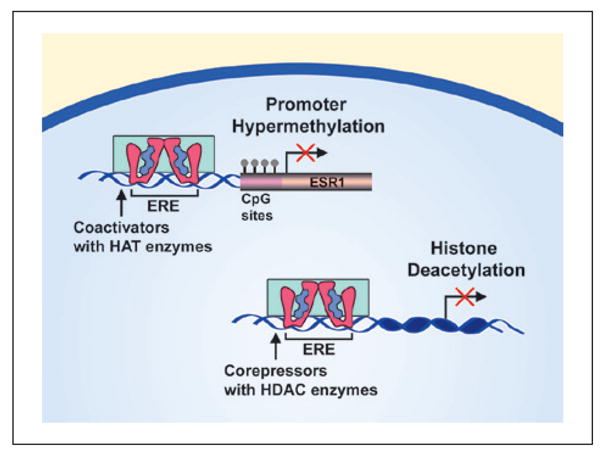
Transcriptional regulation of estrogen receptor expression. The promoter region of the estrogen receptoralpha (ERα) gene (ESR1) contains CpG sites and methylation within this region is associated with reduced transcription of ESR1. Successful transcription requires histone acetyltransferase (HAT) enzyme activity to increase access to DNA for transcription. Histone deacetylases (HDACs) prevent the unwinding of the DNA from the histone thereby preventing transcription.
Finally, estrogen receptor expression is controlled by ERα promoter activity, which can be regulated by mitogen and growth factor signaling pathways, as well as through other nuclear receptors, including auto-regulation through ERα (Pinzone and others 2004). Interestingly, E2 treatment within the physiological range, promotes expression of ERα in the hippocampus, while supraphysiological levels result in a decrease in ERα expression indicating tightly controlled feedback mechanisms (Foster 2012). However, long-term E2 deprivation or the loss of locally synthesized E2 results in a decrease in ERα expression and impairment in the ability of E2 treatment to increase ERα expression (Bohacek and Daniel 2009; Prange-Kiel and others 2003). In one study, 40 days of E2 treatment, initiated in middle-age rats, provided long-term enhancement of memory into old age that coincided with increased ERα levels for up to 8 months following termination of treatment (Rodgers and others 2010). Another experiment saw a significant increase in ERα levels in the hippocampus of rats ovariectomized at 15 months of age and treated with E2 for 10 days; however, there was no significant increase in ERα levels when animals were ovariectomized at 10 months of age and the E2 treatment was delayed for 5 months, suggesting that a prolonged delay in E2 therapy triggers mechanisms to render ERα expression insensitive to E2 treatment (Bohacek and Daniel 2009). It is important to note that in the above experiments there was no change in ERβ levels in the rat hippocampus. Together, these results imply that reduced expression and/or function of ERα with age or ovariectomy may be a critical factor in mediating the decrease ability of E2 to promote memory with advancing age.
Conclusion
In summary, work using receptor specific agonists, mutant mice, and viral vector delivery of different estrogen receptors has revealed a framework for thinking about the role of estrogen receptors in memory. This work indicates that ERα, ERβ, and GPER1 interact to promote or inhibit signaling. Membrane ERα, ERβ, and GPER1 receptors initiate similar signaling cascades that activate kinases involved in modulating memory, including initiating ERα-mediated transcription through phosphorylation of ERα. Genomic estrogen receptor activity induces the transcription of genes involved in memory and maintenance of hippocampal function, including genes for rapid signaling cascades, neuroprotection, and neuronal plasticity. When E2 levels are low, ERβ can act as a negative regulator of ERα-mediated transcription, such that an elevation of E2 (i.e., ERT) may be required to obtain optimal cognitive function. Recent work using gene delivery indicates that shifting the relative expression of ERα/ERβ, to favor ERα, preserves cognition in the face of declining E2 levels during menopause. With advancing aging, a decrease in the relative expression of ERα/ERβ, mainly due to a loss of ERα, is associated with a loss of E2 responsiveness. It is clear that ERα expression decreases in the hippocampus with advanced age; however, the molecular mechanisms that mediate the decrease in ERα remain to be elucidated.
Acknowledgments
Special thanks to Sue Semple-Rowland for her editorial advice.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Institute of Aging Grants, RO1AG037984 and R37AG036800, and the Evelyn F. McKnight Brain Research Foundation.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Adams MM, Fink SE, Shah RA, Janssen WG, Hayashi S, Milner TA, et al. Estrogen and aging affect the subcellular distribution of estrogen receptor-alpha in the hippocampus of female rats. J Neurosci. 2002;22(9):3608–14. doi: 10.1523/JNEUROSCI.22-09-03608.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams MM, Shah RA, Janssen WG, Morrison JH. Different modes of hippocampal plasticity in response to estrogen in young and aged female rats. Proc Natl Acad Sci U S A. 2001;98(14):8071–6. doi: 10.1073/pnas.141215898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aenlle KK, Foster TC. Aging alters the expression of genes for neuroprotection and synaptic function following acute estradiol treatment. Hippocampus. 2010;20(9):1047–60. doi: 10.1002/hipo.20703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aenlle KK, Kumar A, Cui L, Jackson TC, Foster TC. Estrogen effects on cognition and hippocampal transcription in middle-aged mice. Neurobiol Aging. 2009;30(6):932–45. doi: 10.1016/j.neurobiolaging.2007.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bake S, Sohrabji F. 17β-Estradiol differentially regulates blood-brain barrier permeability in young and aging female rats. Endocrinology. 2004;145(12):5471–5. doi: 10.1210/en.2004-0984. [DOI] [PubMed] [Google Scholar]
- Barker JM, Galea LA. Sex and regional differences in estradiol content in the prefrontal cortex, amygdala and hippocampus of adult male and female rats. Gen Comp Endocrinol. 2009;164(1):77–84. doi: 10.1016/j.ygcen.2009.05.008. [DOI] [PubMed] [Google Scholar]
- Barkhem T, Carlsson B, Nilsson Y, Enmark E, Gustafsson J, Nilsson S. Differential response of estrogen receptor α and estrogen receptor β to partial estrogen agonists/antagonists. Mol Pharmacol. 1998;54(1):105–12. doi: 10.1124/mol.54.1.105. [DOI] [PubMed] [Google Scholar]
- Benedusi V, Meda C, Della Torre S, Monteleone G, Vegeto E, Maggi A. A lack of ovarian function increases neuroinflammation in aged mice. Endocrinology. 2012;153(6):2777–88. doi: 10.1210/en.2011-1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohacek J, Daniel JM. The beneficial effects of estradiol on attentional processes are dependent on timing of treatment initiation following ovariectomy in middle-aged rats. Psychoneuroendocrinology. 2009;35(5):694–705. doi: 10.1016/j.psyneuen.2009.10.010. [DOI] [PubMed] [Google Scholar]
- Boulware MI, Heisler JD, Frick KM. The memoryenhancing effects of hippocampal estrogen receptor activation involve metabotropic glutamate receptor signaling. J Neurosci. 2013;33(38):15184–94. doi: 10.1523/JNEUROSCI.1716-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brailoiu E, Dun SL, Brailoiu GC, Mizuo K, Sklar LA, Oprea TI, et al. Distribution and characterization of estrogen receptor G protein-coupled receptor 30 in the rat central nervous system. J Endocrinol. 2007;193(2):311–21. doi: 10.1677/JOE-07-0017. [DOI] [PubMed] [Google Scholar]
- Brinton RD. Cellular and molecular mechanisms of estrogen regulation of memory function and neuroprotection against Alzheimer's disease: recent insights and remaining challenges. Learn Mem. 2001;8(3):121–33. doi: 10.1101/lm.39601. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ. Estrogen and related compounds: biphasic dose responses. Crit Rev Toxicol. 2001;31(4-5):503–15. doi: 10.1080/20014091111785. [DOI] [PubMed] [Google Scholar]
- Carroll JC, Pike CJ. Selective estrogen receptor modulators differentially regulate Alzheimer-like changes in female 3xTg-AD mice. Endocrinology. 2008;149(5):2607–11. doi: 10.1210/en.2007-1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung WC, Pak TR, Suzuki S, Pouliot WA, Andersen ME, Handa RJ. Detection and localization of an estrogen receptor β splice variant protein (ERβ2) in the adult female rat forebrain and midbrain regions. J Comp Neurol. 2007;505(3):249–67. doi: 10.1002/cne.21490. [DOI] [PubMed] [Google Scholar]
- Clark S, Rainville J, Zhao X, Katzenellenbogen BS, Pfaff D, Vasudevan N. Estrogen receptor-mediated transcription involves the activation of multiple kinase pathways in neuroblastoma cells. J Steroid Biochem Mol Biol. 2013;139C:45–53. doi: 10.1016/j.jsbmb.2013.09.010. [DOI] [PubMed] [Google Scholar]
- Corbo RM, Gambina G, Ruggeri M, Scacchi R. Association of estrogen receptor α (ESR1) PvuII and XbaI polymorphisms with sporadic Alzheimer's disease and their effect on apolipoprotein E concentrations. Dement Geriatr Cogn Disord. 2006;22(1):67–72. doi: 10.1159/000093315. [DOI] [PubMed] [Google Scholar]
- Daniel JM. Effects of oestrogen on cognition: what have we learned from basic research? J Neuroendocrinol. 2006;18(10):787–95. doi: 10.1111/j.1365-2826.2006.01471.x. [DOI] [PubMed] [Google Scholar]
- Fan L, Zhao Z, Orr PT, Chambers CH, Lewis MC, Frick KM. Estradiol-induced object memory consolidation in middle-aged female mice requires dorsal hippocampal extracellular signal-regulated kinase and phosphatidylinositol 3-kinase activation. J Neurosci. 2010;30(12):4390–400. doi: 10.1523/JNEUROSCI.4333-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farr TD, Carswell HV, Gsell W, Macrae IM. Estrogen receptor β agonist diarylpropiolnitrile (DPN) does not mediate neuroprotection in a rat model of permanent focal ischemia. Brain Res. 2007;1185:275–82. doi: 10.1016/j.brainres.2007.09.009. [DOI] [PubMed] [Google Scholar]
- Foster TC. Interaction of rapid signal transduction cascades and gene expression in mediating estrogen effects on memory over the life span. Front Neuroendocrinol. 2005;26(2):51–64. doi: 10.1016/j.yfrne.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Foster TC. Role of estrogen receptor α and β expression and signaling on cognitive function during aging. Hippocampus. 2012;22(4):656–69. doi: 10.1002/hipo.20935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster TC, Rani A, Kumar A, Cui L, Semple-Rowland SL. Viral vector-mediated delivery of estrogen receptor-α to the hippocampus improves spatial learning in estrogen receptor-α knockout mice. Mol Ther. 2008;16(9):1587–93. doi: 10.1038/mt.2008.140. [DOI] [PubMed] [Google Scholar]
- Foster TC, Sharrow KM, Kumar A, Masse J. Interaction of age and chronic estradiol replacement on memory and markers of brain aging. Neurobiol Aging. 2003;24(6):839–52. doi: 10.1016/s0197-4580(03)00014-9. [DOI] [PubMed] [Google Scholar]
- Frye CA, Duffy CK, Walf AA. Estrogens and progestins enhance spatial learning of intact and ovariectomized rats in the object placement task. Neurobiol Learn Mem. 2007;88(2):208–16. doi: 10.1016/j.nlm.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fugger HN, Foster TC, Gustafsson J, Rissman EF. Novel effects of estradiol and estrogen receptor α and β on cognitive function. Brain Res. 2000;883(2):258–64. doi: 10.1016/s0006-8993(00)02993-0. [DOI] [PubMed] [Google Scholar]
- Fugger HN, Kumar A, Lubahn DB, Korach KS, Foster TC. Examination of estradiol effects on the rapid estradiol mediated increase in hippocampal synaptic transmission in estrogen receptor α knockout mice. Neurosci Lett. 2001;309(3):207–9. doi: 10.1016/s0304-3940(01)02083-3. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Long-term treatment with estrogen and progesterone enhances acquisition of a spatial memory task by ovariectomized aged rats. Neurobiol Aging. 2000;21(1):107–16. doi: 10.1016/s0197-4580(00)00103-2. [DOI] [PubMed] [Google Scholar]
- Gingerich S, Kim GL, Chalmers JA, Koletar MM, Wang X, Wang Y, et al. Estrogen receptor alpha and G-protein coupled receptor 30 mediate the neuroprotective effects of 17β-estradiol in novel murine hippocampal cell models. Neuroscience. 2010;170(1):54–66. doi: 10.1016/j.neuroscience.2010.06.076. [DOI] [PubMed] [Google Scholar]
- Gore AC, Walker DM, Zama AM, Armenti AE, Uzumcu M. Early life exposure to endocrine-disrupting chemicals causes lifelong molecular reprogramming of the hypothalamus and premature reproductive aging. Mol Endocrinol. 2011;25(12):2157–68. doi: 10.1210/me.2011-1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall JM, McDonnell DP. The estrogen receptor β-isoform (ERβ) of the human estrogen receptor modulates ERα transcriptional activity and is a key regulator of the cellular response to estrogens and antiestrogens. Endocrinology. 1999;140(12):5566–78. doi: 10.1210/endo.140.12.7179. [DOI] [PubMed] [Google Scholar]
- Hammond R, Mauk R, Ninaci D, Nelson D, Gibbs RB. Chronic treatment with estrogen receptor agonists restores acquisition of a spatial learning task in young ovariectomized rats. Horm Behav. 2009;56(3):309–14. doi: 10.1016/j.yhbeh.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Aenlle KK, Bean LA, Rani A, Semple-Rowland SL, Kumar A, et al. Role of estrogen receptor alpha and beta in preserving hippocampal function during aging. J Neurosci. 2013;33(6):2671–83. doi: 10.1523/JNEUROSCI.4937-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogervorst E, Bandelow S. Sex steroids to maintain cognitive function in women after the menopause: a meta-analyses of treatment trials. Maturitas. 2010;66(1):56–71. doi: 10.1016/j.maturitas.2010.02.005. [DOI] [PubMed] [Google Scholar]
- Hojo Y, Hattori TA, Enami T, Furukawa A, Suzuki K, Ishii HT, et al. Adult male rat hippocampus synthesizes estradiol from pregnenolone by cytochromes P45017α and P450 aromatase localized in neurons. Proc Natl Acad Sci U S A. 2004;101(3):865–70. doi: 10.1073/pnas.2630225100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishunina TA, Swaab DF. Alterations in the human brain in menopause. Maturitas. 2007;57(1):20–2. doi: 10.1016/j.maturitas.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Jacome LF, Gautreaux C, Inagaki T, Mohan G, Alves S, Lubbers LS, et al. Estradiol and ERβ agonists enhance recognition memory, and DPN, an ERβ agonist, alters brain monoamines. Neurobiol Learn Mem. 2010;94(4):488–98. doi: 10.1016/j.nlm.2010.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji Y, Urakami K, Wada-Isoe K, Adachi Y, Nakashima K. Estrogen receptor gene polymorphisms in patients with Alzheimer's disease, vascular dementia and alcohol-associated dementia. Dement Geriatr Cogn Disord. 2000;11(3):119–22. doi: 10.1159/000017224. [DOI] [PubMed] [Google Scholar]
- Jones TT, Brewer GJ. Critical age-related loss of cofactors of neuron cytochrome C oxidase reversed by estrogen. Exp Neurol. 2009;215(2):212–9. doi: 10.1016/j.expneurol.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Kim JY, Song KS, Lee YH, Seo JS, Jelinek J, et al. Epigenetic changes in estrogen receptor beta gene in atherosclerotic cardiovascular tissues and in-vitro vascular senescence. Biochim Biophys Acta. 2007;1772(1):72–80. doi: 10.1016/j.bbadis.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Kuroki Y, Fukushima K, Kanda Y, Mizuno K, Watanabe Y. Putative membrane-bound estrogen receptors possibly stimulate mitogen-activated protein kinase in the rat hippocampus. Eur J Pharmacol. 2000;400(2-3):205–9. doi: 10.1016/s0014-2999(00)00425-8. [DOI] [PubMed] [Google Scholar]
- Li LC, Shiina H, Deguchi M, Zhao H, Okino ST, Kane CJ, et al. Age-dependent methylation of ESR1 gene in prostate cancer. Biochem Biophys Res Commun. 2004;321(2):455–61. doi: 10.1016/j.bbrc.2004.06.164. [DOI] [PubMed] [Google Scholar]
- Liu F, Day M, Muniz LC, Bitran D, Arias R, Revilla-Sanchez R, et al. Activation of estrogen receptor-β regulates hippocampal synaptic plasticity and improves memory. Nat Neurosci. 2008;11(3):334–43. doi: 10.1038/nn2057. [DOI] [PubMed] [Google Scholar]
- Lonard DM, Nawaz Z, Smith CL, O'Malley BW. The 26S proteasome is required for estrogen receptor-alpha and coactivator turnover and for efficient estrogen receptor-α transactivation. Mol Cell. 2000;5(6):939–48. doi: 10.1016/s1097-2765(00)80259-2. [DOI] [PubMed] [Google Scholar]
- Maki PM, Henderson VW. Hormone therapy, dementia, and cognition: the Women's Health Initiative 10 years on. Climacteric. 2012;15(3):256–62. doi: 10.3109/13697137.2012.660613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham JA, Pych JC, Juraska JM. Ovarian hormone replacement to aged ovariectomized female rats benefits acquisition of the morris water maze. Horm Behav. 2002;42(3):284–93. doi: 10.1006/hbeh.2002.1819. [DOI] [PubMed] [Google Scholar]
- Markowska AL, Savonenko AV. Effectiveness of estrogen replacement in restoration of cognitive function after long-term estrogen withdrawal in aging rats. J Neurosci. 2002;22(24):10985–95. doi: 10.1523/JNEUROSCI.22-24-10985.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama NO, Lucas TF, Porto CS, Abdalla FM. Estrogen receptor ESR1 regulates the phospholipase C-inositol phosphate signaling in the hippocampus from rats in proestrous and estrous phases. Steroids. 2013;78(1):8–14. doi: 10.1016/j.steroids.2012.10.005. [DOI] [PubMed] [Google Scholar]
- Mehra RD, Sharma K, Nyakas C, Vij U. Estrogen receptor α and β immunoreactive neurons in normal adult and aged female rat hippocampus: a qualitative and quantitative study. Brain Res. 2005;1056(1):22–35. doi: 10.1016/j.brainres.2005.06.073. [DOI] [PubMed] [Google Scholar]
- Milner TA, McEwen BS, Hayashi S, Li CJ, Reagan LP, Alves SE. Ultrastructural evidence that hippocampal alpha estrogen receptors are located at extranuclear sites. J Comp Neurol. 2001;429(3):355–71. [PubMed] [Google Scholar]
- Mitra SW, Hoskin E, Yudkovitz J, Pear L, Wilkinson HA, Hayashi S, et al. Immunolocalization of estrogen receptor beta in the mouse brain: comparison with estrogen receptor α. Endocrinology. 2003;144(5):2055–67. doi: 10.1210/en.2002-221069. [DOI] [PubMed] [Google Scholar]
- Mitterling KL, Spencer JL, Dziedzic N, Shenoy S, McCarthy K, Waters EM, et al. Cellular and subcellular localization of estrogen and progestin receptor immunoreactivities in the mouse hippocampus. J Comp Neurol. 2010;518(14):2729–43. doi: 10.1002/cne.22361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neese SL, Korol DL, Katzenellenbogen JA, Schantz SL. Impact of estrogen receptor α and β agonists on delayed alternation in middle-aged rats. Horm Behav. 2010;58(5):878–90. doi: 10.1016/j.yhbeh.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen L, Rasmussen HB, Hansen T, Bagger YZ, Tanko LB, Qin G, et al. Estrogen receptor α and risk for cognitive impairment in postmenopausal women. Psychiatr Genet. 2006;16(2):85–8. doi: 10.1097/01.ypg.0000194445.27555.71. [DOI] [PubMed] [Google Scholar]
- Packard MG. Posttraining estrogen and memory modulation. Horm and Behav. 1998;34(2):126–39. doi: 10.1006/hbeh.1998.1464. [DOI] [PubMed] [Google Scholar]
- Pettersson K, Delaunay F, Gustafsson JA. Estrogen receptor beta acts as a dominant regulator of estrogen signaling. Oncogene. 2000;19(43):4970–8. doi: 10.1038/sj.onc.1203828. [DOI] [PubMed] [Google Scholar]
- Phan A, Lancaster KE, Armstrong JN, MacLusky NJ, Choleris E. Rapid effects of estrogen receptor α and β selective agonists on learning and dendritic spines in female mice. Endocrinology. 2011;152(4):1492–502. doi: 10.1210/en.2010-1273. [DOI] [PubMed] [Google Scholar]
- Pinzone JJ, Stevenson H, Strobl JS, Berg PE. Molecular and cellular determinants of estrogen receptor α expression. Mol Cell Biol. 2004;24(11):4605–12. doi: 10.1128/MCB.24.11.4605-4612.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post WS, Goldschmidt-Clermont PJ, Wilhide CC, Heldman AW, Sussman MS, Ouyang P, et al. Methylation of the estrogen receptor gene is associated with aging and atherosclerosis in the cardiovascular system. Cardiovasc Res. 1999;43(4):985–91. doi: 10.1016/s0008-6363(99)00153-4. [DOI] [PubMed] [Google Scholar]
- Prange-Kiel J, Wehrenberg U, Jarry H, Rune GM. Para/autocrine regulation of estrogen receptors in hippocampal neurons. Hippocampus. 2003;13(2):226–34. doi: 10.1002/hipo.10075. [DOI] [PubMed] [Google Scholar]
- Qu N, Wang L, Liu ZC, Tian Q, Zhang Q. Oestrogen receptor α agonist improved long-term ovariectomyinduced spatial cognition deficit in young rats. Int J Neuropsychopharmacol. 2013;16(5):1071–82. doi: 10.1017/S1461145712000958. [DOI] [PubMed] [Google Scholar]
- Rao YS, Mott NN, Wang Y, Chung WC, Pak TR. MicroRNAs in the aging female brain: a putative mechanism for age-specific estrogen effects. Endocrinology. 2013;154(8):2795–806. doi: 10.1210/en.2013-1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick SM, Metter EJ, Zonderman AB. Estrogen replacement therapy and longitudinal decline in visual memory. A possible protective effect? Neurology. 1997;49(6):1491–7. doi: 10.1212/wnl.49.6.1491. [DOI] [PubMed] [Google Scholar]
- Rissman EF, Heck AL, Leonard JE, Shupnik MA, Gustafsson JA. Disruption of estrogen receptor beta gene impairs spatial learning in female mice. Proc Natl Acad Sci U S A. 2002;99(6):3996–4001. doi: 10.1073/pnas.012032699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers SP, Bohacek J, Daniel JM. Transient estradiol exposure during middle age in ovariectomized rats exerts lasting effects on cognitive function and the hippocampus. Endocrinology. 2010;151(3):1194–203. doi: 10.1210/en.2009-1245. [DOI] [PubMed] [Google Scholar]
- Schwarz JM, Nugent BM, McCarthy MM. Developmental and hormone-induced epigenetic changes to estrogen and progesterone receptor genes in brain are dynamic across the life span. Endocrinology. 2010;151(10):4871–81. doi: 10.1210/en.2010-0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwin BB. Estrogen therapy: is time of initiation critical for neuroprotection? Nat Rev Endocrinol. 2009;5(11):620–7. doi: 10.1038/nrendo.2009.193. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Brown CM, Wise PM. Neuroprotective effects of estrogens following ischemic stroke. Front Neuroendocrinol. 2009;30(2):201–11. doi: 10.1016/j.yfrne.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szymczak S, Kalita K, Jaworski J, Mioduszewska B, Savonenko A, Markowska A, et al. Increased estrogen receptor β expression correlates with decreased spine formation in the rat hippocampus. Hippocampus. 2006;16(5):453–63. doi: 10.1002/hipo.20172. [DOI] [PubMed] [Google Scholar]
- Tateishi Y, Sonoo R, Sekiya Y, Sunahara N, Kawano M, Wayama M, et al. Turning off estrogen receptor β-mediated transcription requires estrogen-dependent receptor proteolysis. Mol Cell Biol. 2006;26(21):7966–76. doi: 10.1128/MCB.00713-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf AA, Koonce CJ, Frye CA. Estradiol or diarylpropionitrile administration to wild type, but not estrogen receptor β knockout, mice enhances performance in the object recognition and object placement tasks. Neurobiol Learn Mem. 2008;89(4):513–21. doi: 10.1016/j.nlm.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters EM, Yildirim M, Janssen WG, Lou WY, McEwen BS, Morrison JH, et al. Estrogen and aging affect the synaptic distribution of estrogen receptor β-immunoreactivity in the CA1 region of female rat hippocampus. Brain Res. 2011;1379:86–97. doi: 10.1016/j.brainres.2010.09.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westberry JM, Trout AL, Wilson ME. Epigenetic regulation of estrogen receptor beta expression in the rat cortex during aging. Neuroreport. 2011;22(9):428–32. doi: 10.1097/WNR.0b013e328346e1cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witty CF, Foster TC, Semple-Rowland SL, Daniel JM. Increasing hippocampal estrogen receptor α levels via viral vectors increases MAP kinase activation and enhances memory in aging rats in the absence of ovarian estrogens. PLoS One. 2012;7(12):e51385. doi: 10.1371/journal.pone.0051385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu TW, Chen S, Brinton RD. Membrane estrogen receptors mediate calcium signaling and MAP kinase activation in individual hippocampal neurons. Brain Res. 2011;1379:34–43. doi: 10.1016/j.brainres.2011.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu WW, Bryant DN, Dorsa DM, Adelman JP, Maylie J. Ovarian hormone loss impairs excitatory synaptic transmission at hippocampal CA3-CA1 synapses. J Neurosci. 2013;33(41):16158–69. doi: 10.1523/JNEUROSCI.2001-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe K, Lui LY, Grady D, Stone K, Morin P. Estrogen receptor 1 polymorphisms and risk of cognitive impairment in older women. Biol Psychiatry. 2002;51(8):677–82. doi: 10.1016/s0006-3223(01)01289-6. [DOI] [PubMed] [Google Scholar]
- Zandi PP, Carlson MC, Plassman BL, Welsh-Bohmer KA, Mayer LS, Steffens DC, et al. Hormone replacement therapy and incidence of Alzheimer disease in older women: the Cache County Study. JAMA. 2002;288(17):2123–9. doi: 10.1001/jama.288.17.2123. [DOI] [PubMed] [Google Scholar]
- Zhang QG, Han D, Wang RM, Dong Y, Yang F, Vadlamudi RK, et al. C terminus of Hsc70-interacting protein (CHIP)-mediated degradation of hippocampal estrogen receptor-α and the critical period hypothesis of estrogen neuroprotection. Proc Natl Acad Sci U S A. 2011;108(35):E617–24. doi: 10.1073/pnas.1104391108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang QG, Raz L, Wang R, Han D, De Sevilla L, Yang F, et al. Estrogen attenuates ischemic oxidative damage via an estrogen receptor α-mediated inhibition of NADPH oxidase activation. J Neurosci. 2009;29(44):13823–36. doi: 10.1523/JNEUROSCI.3574-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]


