Abstract
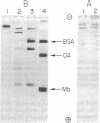
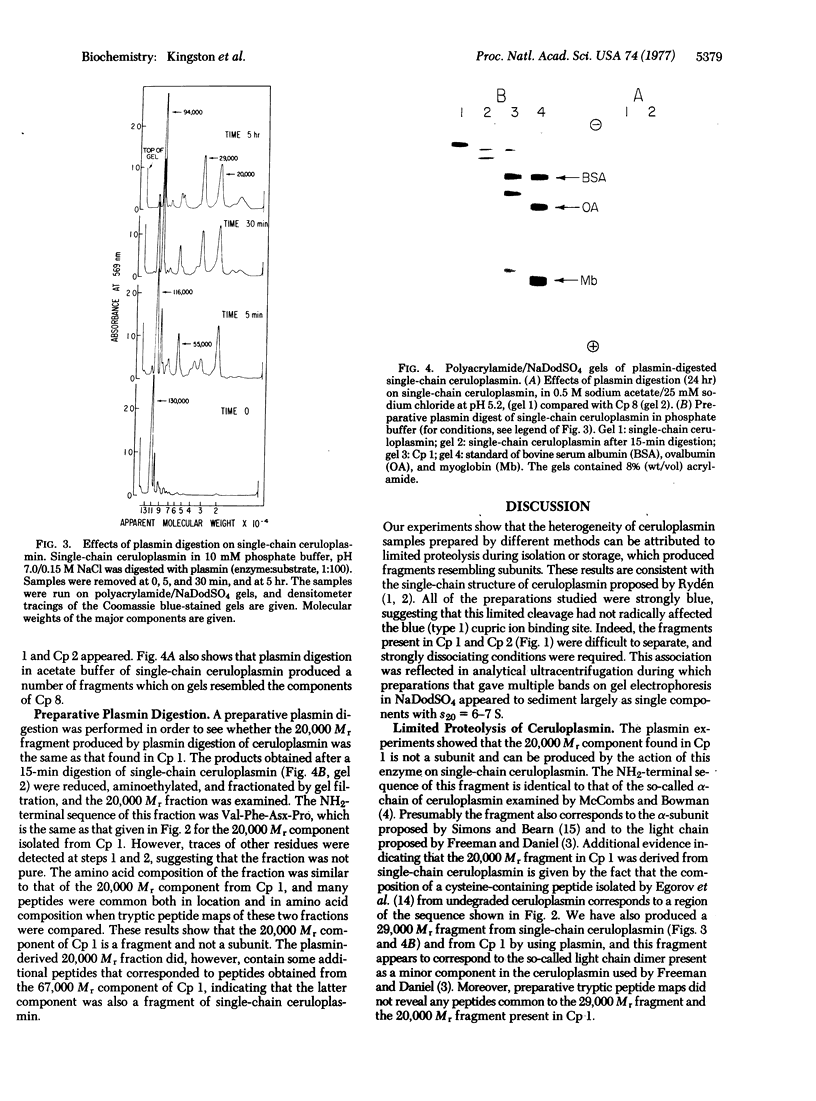
Nine samples of human ceruloplasmin [iron(II):oxygen oxidoreductase; EC 1.16.3.1] prepared by different procedures have been examined for heterogeneity; gel electrophoresis showed that seven contained a number of components with molecular weights ranging from 20,000 to 130,000, and two contained largely a single component of molecular weight 130,000. Digestion of a single-component preparation with plasmin produced fragments with molecular weights similar to those found in the multicomponent preparations. Amino-terminal analysis, peptide mapping, and amino acid analysis showed that plasmin digestion generated a fragment of 20,000 molecular weight, which corresponded to a component present in a multicomponent ceruloplasmin preparation. The 20,000 molecular weight fragment appears to correspond to the so-called alpha-subunit or L-chain of human ceruloplasmin. Chemical evidence is thus provided that ceruloplasmin is a single-chain protein and that the so-called subunits are fragments. The 20,000 molecular weight fragment contains a single cysteine; amino acid sequence studies have shown that the sequence in the vicinity of this residue is similar to that around the single cysteine residue in plant plastocyanins and bacterial azurins, which are small, blue, copper-containing proteins.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aitken A. Prokaryote-eukaryote relationship and the amino acid sequence of plastocyanin from Anabaena variabilis. Biochem J. 1975 Sep;149(3):675–683. doi: 10.1042/bj1490675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambler R. P., Brown L. H. The amino acid sequence of Pseudomonas fluorescens azurin. Biochem J. 1967 Sep;104(3):784–825. doi: 10.1042/bj1040784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames G. F. Resolution of bacterial proteins by polyacrylamide gel electrophoresis on slabs. Membrane, soluble, and periplasmic fractions. J Biol Chem. 1974 Jan 25;249(2):634–644. [PubMed] [Google Scholar]
- DEUTSCH H. F., KASPER C. B., WALSH D. A. Rapid method for preparation of crystalline human ceruloplasmin from Cohn fraction IV-1. Arch Biochem Biophys. 1962 Oct;99:132–135. doi: 10.1016/0003-9861(62)90255-2. [DOI] [PubMed] [Google Scholar]
- Dockal E. R., Jones T. E., Sokol W. F., Engerer R. J., Rorabacker D. B., Ochrymowycz L. A. Letter: Redox properties of copper-thiaether complexes. Comparison to blue copper protein behavior. J Am Chem Soc. 1976 Jul 7;98(14):4322–4324. doi: 10.1021/ja00430a058. [DOI] [PubMed] [Google Scholar]
- Egorov T. A., Svenson A., Rydén L., Carlsson J. A rapid and specific method for isolation of thiol-containing peptides from large proteins by thiol-disulfide exchange on a solid support. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3029–3033. doi: 10.1073/pnas.72.8.3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Freeman S., Daniel E. Dissociation and reconstitution of human ceruloplasmin. Biochemistry. 1973 Nov 6;12(23):4806–4810. doi: 10.1021/bi00747a038. [DOI] [PubMed] [Google Scholar]
- Kelly J., Ambler R. P. The amino acid sequence of plastocyanin from Chlorella fusca. Biochem J. 1974 Dec;143(3):681–690. doi: 10.1042/bj1430681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liu T. Y., Chang Y. H. Hydrolysis of proteins with p-toluenesulfonic acid. Determination of tryptophan. J Biol Chem. 1971 May 10;246(9):2842–2848. [PubMed] [Google Scholar]
- Malkin R., Malmström B. G. The state and function of copper in biological systems. Adv Enzymol Relat Areas Mol Biol. 1970;33:177–244. doi: 10.1002/9780470122785.ch4. [DOI] [PubMed] [Google Scholar]
- McCombs M. L., Bowman B. H. Biochemical studies on human ceruloplasmin. Biochim Biophys Acta. 1976 Jun 15;434(2):452–461. doi: 10.1016/0005-2795(76)90235-x. [DOI] [PubMed] [Google Scholar]
- Miskowski V., Tang S. P., Spiro T. G., Shapiro E., Moss T. H. The copper coordination group in "blue" copper proteins: evidence from resonance Raman spectra. Biochemistry. 1975 Mar 25;14(6):1244–1250. doi: 10.1021/bi00677a024. [DOI] [PubMed] [Google Scholar]
- Morpurgo L., Finazzi-Agrò A., Rotilio G., Mondovì B. Studies of the metal sites of copper proteins. IV. Stellacyanin: preparation of apoprotein and involvement of sulfhydryl and tryptophan in the copper chromophore. Biochim Biophys Acta. 1972 Jul 21;271(2):292–299. doi: 10.1016/0005-2795(72)90203-6. [DOI] [PubMed] [Google Scholar]
- Ryden L., Lundgren J. Homology relationships among the small blue proteins. Nature. 1976 May 27;261(5558):344–346. doi: 10.1038/261344a0. [DOI] [PubMed] [Google Scholar]
- Rydén L., Björk I. Reinvestigation of some physicochemical and chemical properties of human ceruloplasmin (ferroxidase). Biochemistry. 1976 Aug 10;15(16):3411–3417. doi: 10.1021/bi00661a003. [DOI] [PubMed] [Google Scholar]
- Rydén L., Eaker D. Reactivities fo the cysteinyl residues of human ceruloplasmin (ferroxidase). FEBS Lett. 1975 May 15;53(3):279–281. doi: 10.1016/0014-5793(75)80036-6. [DOI] [PubMed] [Google Scholar]
- Rydén L. Evidence for proteolytic fragments in commercial samples of human ceruloplasmin. FEBS Lett. 1971 Nov 1;18(2):321–325. doi: 10.1016/0014-5793(71)80477-5. [DOI] [PubMed] [Google Scholar]
- Rydén L. Single-chain structure of human ceruloplasmin. Eur J Biochem. 1972 Apr 11;26(3):380–386. doi: 10.1111/j.1432-1033.1972.tb01777.x. [DOI] [PubMed] [Google Scholar]
- SGOURIS J. T., CORYELL F. C., GALLICK H., STOREY R. W., McCALL K. B., ANDERSON H. D. A large scale method for the preparation and sterilization of ceruloplasmin and apoceruloplasmin from human plasma. Vox Sang. 1962 Jul-Aug;7:394–405. doi: 10.1111/j.1423-0410.1962.tb03272.x. [DOI] [PubMed] [Google Scholar]
- Simons K., Bearn A. G. Isolation and partial characterization of the polypeptide chains in human ceruloplasmin. Biochim Biophys Acta. 1969 Mar;175(2):260–270. doi: 10.1016/0005-2795(69)90004-x. [DOI] [PubMed] [Google Scholar]
- Solomon E. I., Hare J. W., Gray H. B. Spectroscopic studies and a structural model for blue copper centers in proteins. Proc Natl Acad Sci U S A. 1976 May;73(5):1389–1393. doi: 10.1073/pnas.73.5.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toraño A., Tsuzukida Y., Liu Y. S., Putnam F. W. Location and structural significance of the oligosaccharides in human Ig-A1 and IgA2 immunoglobulins. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2301–2305. doi: 10.1073/pnas.74.6.2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner A. M., Platt T., Weber K. Amino-terminal sequence analysis of proteins purified on a nanomole scale by gel electrophoresis. J Biol Chem. 1972 May 25;247(10):3242–3251. [PubMed] [Google Scholar]