Abstract
Mangled upper extremity injuries usually involve high-impact trauma with crushing and tearing of the limb and its associated soft tissue structures. Such trauma is particularly mutilating because of the nature of the injury and the involvement of structures vital for proper function. Although advancements in flap technique and improvements in bone fixation methods have enabled good functional and clinical outcomes in limb salvage reconstruction, this remains a challenging area. Attempts at limb preservation should be fully exhausted before consideration is given for amputation, which results in significantly decreased function. Here the authors focus on the various modalities of soft tissue coverage available including allogenic substitutes, the adjunctive use of negative pressure wound therapy, and the design and utilization of flaps to address various defect configurations for the goals of wound healing, aesthetics, and functional restoration in the mangled upper extremity.
Keywords: upper extremity, hand, injury, soft tissue flap
Major trauma to the extremities often incorporates significant injuries to the various soft tissue components of the involved limb including the integumentary, neurovascular, and osseotendinous structures.1 The resulting damage from such traumatic injuries often leads to a mangled limb that is cut, torn, or crushed beyond recognition. As much as it is devastating for the patient, a primary amputation may sometimes be the only option for the control of infection or hemorrhage—“life over limb” in accordance with Advanced Trauma Life Support (ATLS) principles compared with futile attempts at salvaging a severely mutilated limb with no realistic prospect of functional reconstitution.
The decision to amputate or salvage a mangled limb has largely been dependent on the judgment and skill of the surgeon despite the development of scoring systems such as the Mangled Extremity Severity Score (MESS), which has only been validated in severe lower limb injuries,2 while its applicability in upper extremities has been challenged.3 Moreover, amputation is associated with persistent pain in up to 80% of patients due to neuroma formation and complex regional pain syndrome. In addition, clinical outcomes after prosthesis fitting have also been less than ideal with almost a third of such patients abandoning their usage completely.4 However, as much as a mangled but successfully replanted upper limb may never achieve full functional recovery, the residual sensation and function afforded is still preferable compared with a prosthesis.5 Overall, the functional results that can be achieved with replantation is superior to that of amputation with prosthesis fitting, regardless of the level of upper limb loss.6
The fundamental reconstructive principle of restoring both form and function therefore guides the management of patients with mangled upper extremities because they will require the provision of soft tissue coverage that is both aesthetically acceptable and durable to enable early and sustained functional rehabilitation.
Requirements and Aims
Adequate wound debridement is the absolute prerequisite prior to any plans for reconstruction. This may be achieved either in the early or delayed setting, depending on the circumstances of the patient, the nature of the wound, or the availability of reconstructive expertise.7 Either way, debridement is necessary not only to reduce the bacterial load by converting a traumatized and contaminated wound bed into a fresh surgical one ready to accept soft tissue coverage; it also attempts to even out, as much as possible, the contours of the wound bed for easier resurfacing.
Additionally, the full extent of reconstruction required will only be evident after thorough wound debridement. This may include bone defects that may require vascularized bone grafts from the fibula, iliac crest, radial shaft, or scapula; vascular injuries needing vein grafts for re-establishment of the circulation and thus perfusion; peripheral nerve damage that can be addressed either with synthetic conduits or autologous nerve grafts to restore protective sensation and/or motor function; tendon transfers or grafts for defects not amenable to direct repair to achieve proper excursion and functioning of muscle units; and muscle flaps to reduce the risk of infection by dead-space obliteration and free functioning muscle transfers for the restoration of function.
The aims of soft tissue coverage in the mangled upper extremity are thus (1) to salvage the limb by allowing the underlying wound defect to heal; (2) to reconstruct, if necessary, composite defects involving associated soft tissue structures simultaneously; and (3) to enable early and graduated mobilization efforts to restore meaningful function as much as possible.
Soft Tissue Coverage Options
In general, the choice for soft tissue coverage can be approached using the reconstructive ladder, especially with increasing recognition of the utility of adjuncts such as negative-pressure wound therapy (NPWT) and the various skin substitutes including acellular dermal matrices and collagen wound matrix (Integra Life Sciences, Plainsboro, NJ), which are dermal regeneration templates. Special consideration is also warranted for the use of “spare parts” in cases where the upper limb (or part thereof) cannot be replanted or salvaged because it provides a ready supply of optimally matched tissues for coverage of both the wound and amputation stump as well as having the potential to both preserve limb length and improve remaining function without the need for further donor site morbidity.8
Skin-only defects may be best addressed by the mobilization of a local flap or in combination with a split-thickness skin graft (STSG). The use of NPWT, most commonly in the form of the vacuum-assisted closure (VAC; Kinetic Concepts, Inc., San Antonio, TX) device can also promote success of the STSG.9 Similarly, synthetic skin substitutes may be used together with STSG and NPWT.10 Acellular dermal matrix can be used on its own or simultaneously with a very thin STSG. It has most commonly been used for the coverage of acute hand burns and donor-site reconstruction after radial forearm flap harvest,11 but has recently been reported by Bastidas et al for use as a temporary dressing in extremity wounds to prevent desiccation of exposed neurovascular structures while promoting granulation tissue formation at the same time.12 By comparison, collagen wound matrix usually requires 3 to 4 weeks to incorporate fully before it is ready to accept a STSG during a second procedure. Nevertheless, its utility has been demonstrated by Weigert et al for successful coverage of traumatic hand wounds with exposure of bone, joints, and tendons.13 Fig. 1 illustrates the use of collagen wound matrix with subsequent STSG for dorsal hand coverage. The main downsides to the use of these dermal regeneration templates are (1) the need for a period of immobilization to allow for full incorporation, and (2) the financial costs involved. When the dorsum of the hand and wrist is involved, the choice of reconstruction is important as it is an aesthetically noticeable area with thin and highly mobile skin.14 Local flaps will be optimal, but this may be limited by the zone of injury; more distant options such as the reversed radial or ulnar forearm flaps may require the sacrifice of a major source artery to the hand in an already injured upper limb and usually need skin grafting of the donor site after flap harvest. Free fascial flaps and venous flaps have since been reported by Parrett et al to be the flaps of choice for coverage of dorsal hand wounds with only 6% (1/17 flaps) of the former and none in the latter requiring further debulking15 venous flaps were also judged to provide the best aesthetic results with fasciocutaneous flaps performing poorly.
Fig. 1.
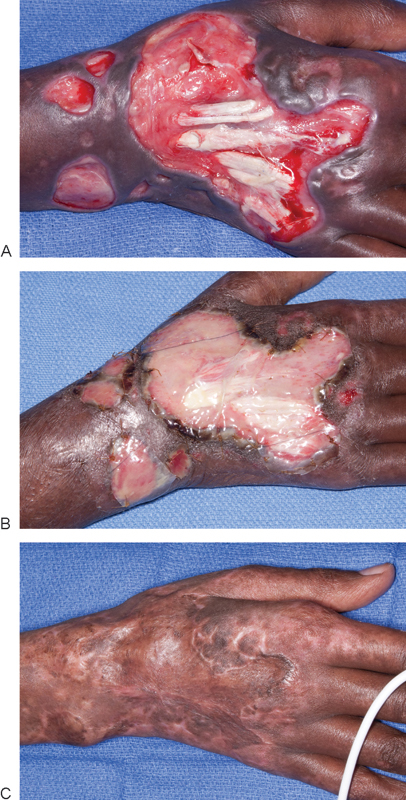
A 52-year-old woman presented with a dorsal hand wound with exposed extensor tendons (A). The wound was covered with collagen wound matrix (Integra, Integra Life Sciences, Plainsboro, NJ) and skin grafted 1 month later, (B) Appearance of wound prior to skin grafting. (C) 3-month postoperative result.
More extensive defects with greater exposure of underlying structures may require resurfacing and dead-space obliteration with pedicled or free flap options in the form of fasciocutaneous, muscle-only, or myocutaneous flaps. Despite the well-reported advantages of free tissue transfers, pedicled options remain an invaluable tool in modern reconstructive surgery, especially when recipient vessels or microsurgical facilities are inadequate.16 The choice of pedicled flap for upper extremity reconstruction is guided by the extent and location of injury. Commonly used options include the lateral arm flap, radial forearm flap, latissimus dorsi flap, and groin flap. The forearm-based flaps may be harvested as fasciocutaneous or fascia-only flaps to allow pliable coverage of exposed and/or repaired tendons and enable smooth gliding to minimize adhesions, but this leads to further morbidity in the same, already injured limb. Moreover, the use of forearm-based flaps may not be possible due to the proximity to, or location within the zone of injury depending on the extent of the defect. Risk of flap compromise can be circumvented using more distant, pedicled flap options from the patient's back or abdomen, but a second-stage procedure for division of the pedicle becomes necessary in such cases. A common complication arising from the use of such pedicled flaps is the risk of distal flap necrosis.17 Free flap options on the other hand are versatile, and well described in the literature (Table 1). Such flaps can be fashioned at different levels of harvest as described previously, or designed in chimeric fashion, most commonly based on the anterolateral thigh (ALT) flap18 or scapular system,19 to simultaneously address composite defects involving the different soft tissue components.
Table 1. Comparison of commonly used free flaps for mangled upper extremities.
| Free flap | Pedicle | Flap size | FFMT | Intraop position change | Can be part of chimeric flap? | Primary debulking |
|---|---|---|---|---|---|---|
| Muscle-only/Myocutaneous | ||||||
| Rectus abdominis | DIEA | + + | No | No | No | No; atrophies later from denervation |
| Latissimus dorsi | TDA | + + + | Yes | Yes | Yes | |
| Gracilis | MCFA | + + | Yes | No | Yes | |
| Serratus anterior | Branch of TDA | + | Yes | Yes | Yes | |
| Fasciocutaneous | ||||||
| ALT | LCFA | + + | N/A | No | Yes | Yes; often requires further revision |
| SIEA/DIEP | SIEA/DIEP | + + | N/A | No | No | |
| TAP | TDA | + + + | N/A | Yes | Yes | |
| Radial forearm | Radial artery | + | N/A | No | Yes | |
| Lateral arm | PRCA | + | N/A | No | Yes | |
| Fascial | ||||||
| ALT | LCFA | + + | N/A | No | Yes | N/A |
| Dorsal thoracic | CSA | + + + | N/A | Yes | Yes | N/A |
| Temporoparietal | STA | + | N/A | No | No | N/A |
| Lateral arm | PRCA | + | N/A | No | Yes | N/A |
Abbreviations: ALT, anterolateral thigh; CSA, circumflex scapular artery; DIEA, deep inferior epigastric artery; FFMT, free functioning muscle transfer; LCFA, lateral circumflex femoral artery; MCFA, medial circumflex femoral artery; PRCA, posterior radial collateral artery; SIEA, superficial inferior epigastric artery; STA, superficial temporal artery; TAP, thoracodorsal artery perforator; TDA, thoracodorsal artery.
Muscle-only and myocutaneous flaps are usually indicated for sizable defects to eliminate dead space or prior infections. Wound healing is also promoted by re-establishment of the venous and lymphatic circulations.20 A disadvantage of muscle flaps is the formation of adhesions between the flap and underlying tissue. Hence when tendon gliding is a concern or flap re-elevation is anticipated, a fasciocutaneous flap may be a better option. Though the superiority of soft tissue coverage between muscle-only and myocutaneous flaps remains equivocal,21 skin-grafting of the former has been reported to provide favorable color match at the recipient site.22 In addition, such muscle flaps also contour well as they undergo subsequent denervation atrophy and require less secondary debulking procedures. Improved knowledge of flap anatomy has also enabled reconstructive surgeons to harvest partial muscle flaps such as partial latissimus dorsi and medial rectus abdominis flaps.23 Such flaps allow for improved match between the size and contour of the defect and the flap while minimizing donor site morbidity.24 Finally, there is growing recognition for the utility of free functioning muscle transfers, such as gracilis and latissimus dorsi muscles in restoring hand opposition and elbow flexion, respectively,25 in achieving restoration of purposeful function in severe upper extremity injuries.
Although the utility of muscle flaps in wound coverage and limb salvage is undoubted, the success in the management of the mangled upper extremity is more accurately assessed by the degree of restoration of preinjury function and appearance. This translates to a need for secondary procedures after successful limb salvage, which may involve bone grafting for non-unions or malunions, capsulotomies or arthrodeses for persistent joint stiffness, tenolysis or tendon transfers and secondary tendon reconstructions, neuroma excision and nerve grafting or neurovascular island flap transfers for residual sensory and motor loss, as well as release of scar contractures and further flap debulking.26 For such cases, re-elevation of flaps would be necessary and can prove difficult in case of muscle flaps for coverage. Compared with muscle flaps, free fasciocutaneous flaps are easier to harvest, allow safer primary debulking for better contouring, and easier re-elevation due to decreased adhesions to underlying tissue. Fig. 2 illustrates the use of an anterolateral thigh flap for volar hand coverage. Furthermore, based on the principle of peripheral neovascularization as seen in pedicled fasciocutaneous flaps,27 aggressive debulking of fasciocutaneous free flaps can be performed safely at approximately 3 to 6 months after the initial surgery. The advent and popularization of perforator flaps have made fasciocutaneous flaps even more popular as an option for upper extremity coverage as perforator-based flaps can be harvested very thin, providing a better contour match for hand coverage. Fig. 3 shows the use of a superficial circumflex iliac artery perforator (SCIP) flap for dorsal hand coverage. The disadvantages associated with the use of fasciocutaneous flaps include the need for further debulking, potential excess in flap thickness in overweight patients,28 and the risk of vessel caliber mismatch during anastomosis.
Fig. 2.
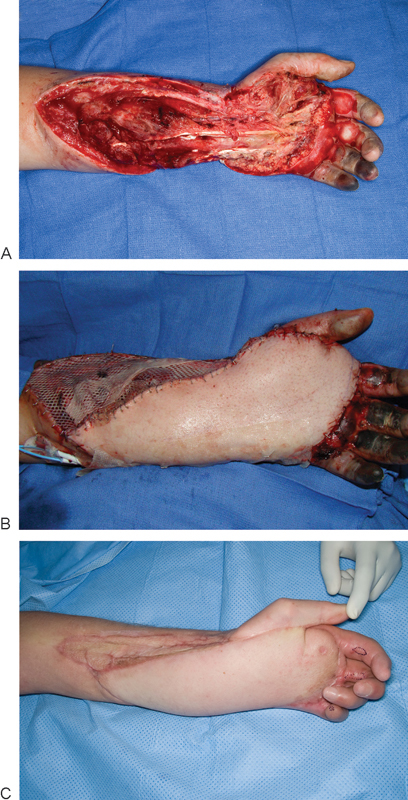
A 43-year-old man sustained a degloving injury of the volar aspect of his left hand and forearm from a cornpicker machine (A). (B) An anterolateral thigh flap was used for coverage. (C) 4-month postoperative result.
Fig. 3.
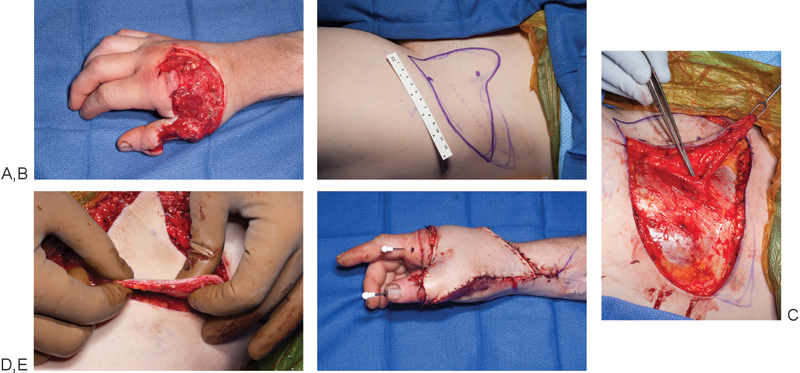
A 25-year-old man sustained a saw injury to his right hand causing dorsal skin loss (A). (B) A superficial circumflex iliac artery perforator (SCIP) flap was designed and elevated. (C) Perforator seen entering the flap. (D) The flap has been harvested very thin to approximate the contour of the dorsal skin of the hand. (E) Immediate postoperative result.
As mentioned previously, fascial flaps are better suited for dorsal hand wounds or wounds with exposed tendons as the skin required for such coverage is usually thin. Use of fascial flaps allows for the overlying skin from the donor site to be retained for primary closure while STSG is required at the recipient site for flap coverage and thus a second donor site is created. Fig. 4 illustrates the use of a lateral arm fascial flap for dorsal hand coverage. Venous flaps are usually harvested from the volar forearm in the suprafascial plane, which provides better cosmesis and pliability. This flap maintains flow via the venous plexus with eventual arterialization of the inflow veins. However, the size of venous flaps that can be harvested is limited and are thus indicated mainly for smaller defects involving the hand and fingers.29
Fig. 4.
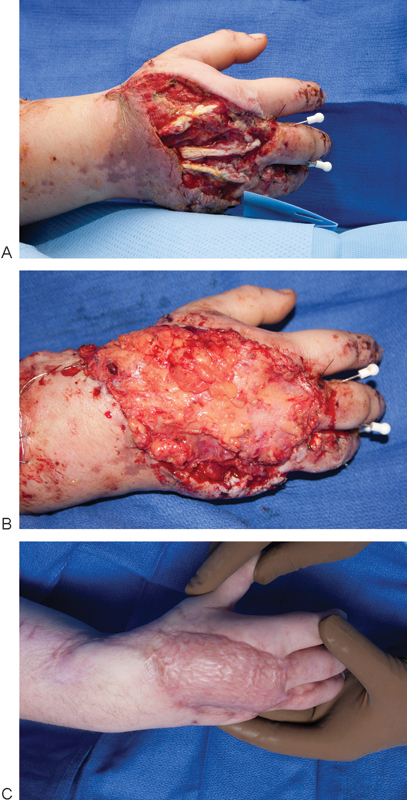
A 32-year-old woman sustained a right hand degloving injury following a motor vehicle accident with dorsal skin loss (A). (B) The wound was covered with a free lateral arm fascia only flap and later with a split thickness skin graft. (C) 5-month postoperative result.
The decision to raise different types of free flaps is based on several considerations, including the characteristics of the defect such as the shape, the etiology of the wound, the extent of exposure of underlying structures, and patient-related factors such as increased body fat that can affect the bulkiness of a flap (e.g., ALT) or previous surgeries in certain donor sites of the body that may affect the integrity of the vascular pedicle as well as surgery-related factors such as the operating surgeon's experience with particular flaps and the need for an intraoperative change in patient positioning. With increasing success in free flap reconstructions, an often overlooked consideration is the ease of flap re-elevation in situations where secondary reconstruction or revision is anticipated as part of the goal of soft tissue reconstruction of the mangled upper extremity. This final point should always be incorporated into the initial evaluation and planning prior to execution of the reconstructive plan.
Postoperative Care and Rehabilitation
Depending on the type of soft tissue coverage selected, postoperative care may vary from VAC dressing changes in cases with delayed reconstruction, to bedside monitoring protocols of free flaps. The primary goal of immediate postoperative care is to detect any possible complications that may threaten the viability of soft tissue coverage early and to rectify them early before it results in complete or partial loss of the soft tissue coverage. Once the wounds are noted to be stable, individualized rehabilitation protocols are initiated depending on the extent of reconstruction (i.e., flexor or extensor tendons, functional muscle transfers). The traumatized upper limb is especially intolerant of immobility with the resulting edema and joint stiffness further compounding the problem. Extra precaution is required as the patient initiates rehabilitation exercises to prevent the shearing of STSG before they are fully adherent or undue pressure points on flaps, which in turn may compromise the circulation or even the pedicle itself.
Reconstructive and Functional Outcomes
Previously, emergency free flaps for reconstruction of extensive open fractures in the extremity was advocated based on the landmark work by Godina.30 Although this did not differentiate between upper and lower extremity injuries, subsequent reports have demonstrated the utility of this approach in mangled upper limbs. Lister and Scheker were able to achieve good functional recovery with 51% and 87% of patients returning to their original employment or some form of work, respectively,31 whereas Saint-Cyr and Daigle showed an overall statistically significant reduced length of hospital stay and number of surgeries required for definitive wound closure as compared with staged reconstruction.32
The advent of NPWT, allogenic skin substitutes, and cadaveric skin grafts among many other options has influenced the perioperative care of such injuries. A systemic review by Harrison et al33 has also shown that delayed reconstruction with the adjunctive use of NPWT does not impact the reconstructive outcomes, whereas Parrett et al34 have demonstrated its potential to simplify the reconstructive procedure required with no difference in clinical outcomes at one year follow-up. Additionally, Parrett at al have also shown that an individualized approach in which all aspects of the reconstruction including the defect, the patient, and flap options are considered, will allow the best aesthetic outcomes.15 They recommended the use of free fascial flaps or venous flaps for coverage of dorsal hand wounds to achieve such outcomes. Similarly, Fox and colleagues have recently shown that under the right circumstances, the fascia-only ALT flap could provide adequate soft tissue cover without the need for further debulking as compared with fasciocutaneous or muscle flaps.35 Further randomized studies comparing long-term functional outcomes between different types of soft tissue cover are warranted to determine the best reconstructive option for the different types of mangled upper extremity wounds.
Summary
The goal of soft tissue coverage in mangled upper extremity injuries is to close the wound bed, reduce the risks of associated sequela such as infection, allow early mobilization and rehabilitation of the injured limb, and ultimately to enable limb preservation with functional restoration so that the patient can successfully return to his or her preinjury status in society. This is achieved through the selection of the reconstructive option that provides for durable coverage and addresses all missing tissue in the wound. Although an ever-increasing number of flaps and their many permutations are being described for various indications due to improved knowledge of microsurgical anatomy, various adjuncts such as allogenic skin substitutes and NPWT have proved invaluable as temporizing measures and are able to render the subsequent, final reconstruction less complex. Ultimately, the choice of soft tissue coverage depends on a complex interplay of patient-, defect-, and surgeon-specific factors.
References
- 1.Gregory R T, Gould R J, Peclet M. et al. The mangled extremity syndrome (M.E.S.): a severity grading system for multisystem injury of the extremity. J Trauma. 1985;25(12):1147–1150. [PubMed] [Google Scholar]
- 2.Helfet D L, Howey T, Sanders R, Johansen K. Limb salvage versus amputation. Preliminary results of the Mangled Extremity Severity Score. Clin Orthop Relat Res. 1990;(256):80–86. [PubMed] [Google Scholar]
- 3.Togawa S, Yamami N, Nakayama H, Mano Y, Ikegami K, Ozeki S. The validity of the mangled extremity severity score in the assessment of upper limb injuries. J Bone Joint Surg Br. 2005;87(11):1516–1519. doi: 10.1302/0301-620X.87B11.16512. [DOI] [PubMed] [Google Scholar]
- 4.Tintle S M, Baechler M F, Nanos G P III, Forsberg J A, Potter B K. Traumatic and trauma-related amputations: Part II: Upper extremity and future directions. J Bone Joint Surg Am. 2010;92(18):2934–2945. doi: 10.2106/JBJS.J.00258. [DOI] [PubMed] [Google Scholar]
- 5.Peacock K, Tsai T M. Comparison of functional results of replantation versus prosthesis in a patient with bilateral arm amputation. Clin Orthop Relat Res. 1987;(214):153–159. [PubMed] [Google Scholar]
- 6.Graham B, Adkins P, Tsai T M, Firrell J, Breidenbach W C. Major replantation versus revision amputation and prosthetic fitting in the upper extremity: a late functional outcomes study. J Hand Surg Am. 1998;23(5):783–791. doi: 10.1016/s0363-5023(98)80151-2. [DOI] [PubMed] [Google Scholar]
- 7.Saint-Cyr M, Gupta A. Indications and selection of free flaps for soft tissue coverage of the upper extremity. Hand Clin. 2007;23(1):37–48. doi: 10.1016/j.hcl.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 8.Russell R C Neumeister M W Ostric S A Engineer N J Extremity reconstruction using nonreplantable tissue (“spare parts”) Clin Plast Surg 2007342211–222., viii viii [DOI] [PubMed] [Google Scholar]
- 9.Blackburn J H II, Boemi L, Hall W W. et al. Negative-pressure dressings as a bolster for skin grafts. Ann Plast Surg. 1998;40(5):453–457. doi: 10.1097/00000637-199805000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Kim E K, Hong J P. Efficacy of negative pressure therapy to enhance take of 1-stage allodermis and a split-thickness graft. Ann Plast Surg. 2007;58(5):536–540. doi: 10.1097/01.sap.0000245121.32831.47. [DOI] [PubMed] [Google Scholar]
- 11.Ellis C V, Kulber D A. Acellular dermal matrices in hand reconstruction. Plast Reconstr Surg. 2012;130(5) 02:256S–269S. doi: 10.1097/PRS.0b013e318265a5cf. [DOI] [PubMed] [Google Scholar]
- 12.Bastidas N, Ashjian P J, Sharma S. Acellular dermal matrix for temporary coverage of exposed critical neurovascular structures in extremity wounds. Ann Plast Surg. 2009;62(4):410–413. doi: 10.1097/SAP.0b013e318184ab2f. [DOI] [PubMed] [Google Scholar]
- 13.Weigert R, Choughri H, Casoli V. Management of severe hand wounds with Integra® dermal regeneration template. J Hand Surg Eur Vol. 2011;36(3):185–193. doi: 10.1177/1753193410387329. [DOI] [PubMed] [Google Scholar]
- 14.Friedrich J B, Katolik L I, Vedder N B. Soft tissue reconstruction of the hand. J Hand Surg Am. 2009;34(6):1148–1155. doi: 10.1016/j.jhsa.2009.04.035. [DOI] [PubMed] [Google Scholar]
- 15.Parrett B M, Bou-Merhi J S, Buntic R F, Safa B, Buncke G M, Brooks D. Refining outcomes in dorsal hand coverage: consideration of aesthetics and donor-site morbidity. Plast Reconstr Surg. 2010;126(5):1630–1638. doi: 10.1097/PRS.0b013e3181ef8ea3. [DOI] [PubMed] [Google Scholar]
- 16.Mih A D. Pedicle flaps for coverage of the wrist and hand. Hand Clin. 1997;13(2):217–229. [PubMed] [Google Scholar]
- 17.Sabapathy S R Bajantri B Indications, selection, and use of distant pedicled flap for upper limb reconstruction Hand Clin 2014302185–199., vi [DOI] [PubMed] [Google Scholar]
- 18.Peng F, Chen L, Han D, Xiao C, Bao Q, Wang T. Reconstruction of two separate defects in the upper extremity using anterolateral thigh chimeric flap. Microsurgery. 2013;33:631–637. doi: 10.1002/micr.22170. [DOI] [PubMed] [Google Scholar]
- 19.Sabino J, Franklin B, Patel K, Bonawitz S, Valerio I L. Revisiting the scapular flap: applications in extremity coverage for our U.S. combat casualties. Plast Reconstr Surg. 2013;132(4):577e–585e. doi: 10.1097/PRS.0b013e31829f4a08. [DOI] [PubMed] [Google Scholar]
- 20.Slavin S A Upton J Kaplan W D Van den Abbeele A D An investigation of lymphatic function following free-tissue transfer Plast Reconstr Surg 1997993730–741., discussion 742–743 [DOI] [PubMed] [Google Scholar]
- 21.Pederson W C, Lister G D. Philadelphia, PA: Churchill Livingstone; 2005. Skin flaps; pp. 1648–1703. [Google Scholar]
- 22.Chang J, Jones N. Muscle free flaps with full-thickness skin grafting: improved contour over traditional musculocutaneous free flaps. Microsurgery. 2001;21(2):70–73. doi: 10.1002/micr.1012. [DOI] [PubMed] [Google Scholar]
- 23.Buntic R F, Brooks D. Free partial medial rectus muscle flap for closure of complex extremity wounds. Plast Reconstr Surg. 2005;116(5):1434–1437. doi: 10.1097/01.prs.0000182378.42555.be. [DOI] [PubMed] [Google Scholar]
- 24.Brooks D, Buntic R F. Partial muscle harvest: our first 100 cases attempting to preserve form and function at the donor site. Microsurgery. 2008;28(8):606–611. doi: 10.1002/micr.20575. [DOI] [PubMed] [Google Scholar]
- 25.Fischer J P, Elliott R M, Kozin S H, Levin L S. Free function muscle transfers for upper extremity reconstruction: a review of indications, techniques, and outcomes. J Hand Surg Am. 2013;38(12):2485–2490. doi: 10.1016/j.jhsa.2013.03.041. [DOI] [PubMed] [Google Scholar]
- 26.Chiou G J Chang J Refinements and secondary surgery after flap reconstruction of the traumatized hand Hand Clin 2014302211–223., vi [DOI] [PubMed] [Google Scholar]
- 27.Gatti J E, LaRossa D, Brousseau D A, Silverman D G. Assessment of neovascularization and timing of flap division. Plast Reconstr Surg. 1984;73(3):396–402. doi: 10.1097/00006534-198403000-00010. [DOI] [PubMed] [Google Scholar]
- 28.Kimata Y, Uchiyama K, Ebihara S, Nakatsuka T, Harii K. Anatomic variations and technical problems of the anterolateral thigh flap: a report of 74 cases. Plast Reconstr Surg. 1998;102(5):1517–1523. doi: 10.1097/00006534-199810000-00026. [DOI] [PubMed] [Google Scholar]
- 29.Woo S H, Kim K C, Lee G J. et al. A retrospective analysis of 154 arterialized venous flaps for hand reconstruction: an 11-year experience. Plast Reconstr Surg. 2007;119(6):1823–1838. doi: 10.1097/01.prs.0000259094.68803.3d. [DOI] [PubMed] [Google Scholar]
- 30.Godina M. Early microsurgical reconstruction of complex trauma of the extremities. Plast Reconstr Surg. 1986;78(3):285–292. doi: 10.1097/00006534-198609000-00001. [DOI] [PubMed] [Google Scholar]
- 31.Lister G, Scheker L. Emergency free flaps to the upper extremity. J Hand Surg Am. 1988;13(1):22–28. doi: 10.1016/0363-5023(88)90193-1. [DOI] [PubMed] [Google Scholar]
- 32.Saint-Cyr M, Daigle J P. Early free tissue transfer for extremity reconstruction following high-voltage electrical burn injuries. J Reconstr Microsurg. 2008;24(4):259–266. doi: 10.1055/s-2008-1078697. [DOI] [PubMed] [Google Scholar]
- 33.Harrison B L, Lakhiani C, Lee M R, Saint-Cyr M. Timing of traumatic upper extremity free flap reconstruction: a systematic review and progress report. Plast Reconstr Surg. 2013;132(3):591–596. doi: 10.1097/PRS.0b013e31829ad012. [DOI] [PubMed] [Google Scholar]
- 34.Parrett B M Matros E Pribaz J J Orgill D P Lower extremity trauma: trends in the management of soft-tissue reconstruction of open tibia-fibula fractures Plast Reconstr Surg 200611741315–1322., discussion 1323–1324 [DOI] [PubMed] [Google Scholar]
- 35.Fox P, Endress R, Sen S, Chang J. Fascia-only anterolateral thigh flap for extremity reconstruction. Ann Plast Surg. 2014;72 01:S9–S13. doi: 10.1097/SAP.0000000000000146. [DOI] [PubMed] [Google Scholar]


