Abstract
Background
We sought to disentangle the contributions of hyperthyrotropinemia (an indicator of thyroid dysfunction) (HTT) and intermittent or sustained systemic inflammation (ISSI) to structural and functional indicators of brain damage.
Methods
We measured the concentrations of TSH on day 14, and of 25 inflammation-related proteins in blood collected during the first 2 postnatal weeks from 786 infants born before the 28th week of gestation who were not considered to have hypothyroidism. We defined hyperthyrotropinemia (HTT) as a TSH concentration in the highest quartile for gestational age on postnatal day 14 and ISSI was defined as a concentration in the top quartile for gestational age of a specific inflammation-related protein on two separate days a week apart during the first two postnatal weeks. We first assessed the risk of brain damage indicators comparing 1) neonates who had HTT to those without (regardless of ISSI), and 2) neonates with HTT only, ISSI only, or HTT+ ISSI, to those who were exposed to neither HTT nor ISSI. HTT was defined as a TSH concentration in the highest quartile for gestational age on postnatal day 14.
Results
In univariable models that compared those with HTT to those without, HTT was not significantly associated with any indicator of brain damage. In models that compared HTT only, ISSI only, and HTT+ISSI, to those with neither, children with ISSI only or with HTT+ISSI were at significantly higher risk of ventriculomegaly [odds ratios (OR) ranged from 2–6], while those with HTT only were at significantly reduced risk of a hypoechoic lesion [ORs ranged from 0.2–0.4]. Children with HTT only had a higher risk of quadriparesis and those with ISSI alone had a higher risk of hemiparesis [ORs ranged from 1.6–2.4]. Elevated risk of a very low mental development score was associated with both ISSI only and with HTT+ISSI while a very low motor development score and microcephaly were associated with HTT+ISSI.
Conclusions
The association of HTT with increased or decreased risk of indicators of brain damage depends upon the presence or absence of ISSI.
Keywords: thyroid stimulating hormone, inflammation, cerebral palsy, microcephaly
Introduction
Thyroid hormones play important roles in brain development[1], and early deficiency can result in brain damage [2]. It is unclear to what extent atypical neonatal thyroid hormone patterns that do not meet clinical criteria for the diagnosis of congenital hypothyroidism contribute to an elevated risk of brain injury.
Preterm newborns with elevated TSH levels (hyperthyrotropinemia) have at times appeared to be at increased risk of brain damage [3–5], but not always [6–12]. These inconsistent findings, the apparent failure, so far, of thyroid hormone treatment to reduce the risk of brain damage [13], and the potential confounding of thyroid hormone levels by inflammation [14–16] raise the possibility that indicators of thyroid dysfunction may be an epiphenomenon [17].
In the ELGAN study, elevated concentrations of TSH were associated with markers of systemic inflammation [Soto-Rivera et al., under review], while intermittent or sustained systemic inflammation (ISSI) was associated with findings of abnormal brain structure and function [18–20]. We reasoned that if hyperthyrotropinemia (HTT) (defined as a TSH concentration in the highest quartile for gestational age on postnatal day 14) conveyed information about increased risks of brain damage only when we knew about the presence or absence of ISSI (defined as a concentration in the top quartile for gestational age and postnatal day of a specific inflammation-related protein on two separate days a week apart during the first two postnatal weeks), then HTT and ISSI were probably both important and influenced each other’s capacity to convey risk information or to damage the brain. In this report, we describe what we found when we assessed the association between hyperthyrotropinemia and multiple indicators of brain damage, in light of information about the presence or absence of ISSI.
Methods
The ELGAN Study
The ELGAN study was designed to identify characteristics and exposures that increase the risk of structural and functional neurologic disorders in ELGANs (the acronym for Extremely Low Gestational Age Newborns)[21]. During the years 2002–2004, women delivering before 28 weeks gestation at one of 14 participating institutions were asked to enroll in the study. The enrollment and consent processes were approved by the individual institutional review boards. Financial limitations constrained neonatal blood protein measurement to the 939 survivors who had a developmental evaluation at 24 months adjusted age. The sample for this report consists of the 786 newborns who had protein measurements on protocol day 14.
Newborn variables
Most (62%) estimates of gestational age at birth were based on the dates of embryo retrieval, intrauterine insemination or fetal ultrasound before the 14th week of gestation. Otherwise, reliance was placed sequentially on a fetal ultrasound at 14 or more weeks (29%), LMP without fetal ultrasound (7%), and gestational age recorded in the neonatal intensive care unit log (1%).
Blood spot collection
Drops of blood remaining from clinically obtained specimens were collected on filter paper on the first (range: 1–3 days), the 7th (range: 5–8 days), and the 14th postnatal day (range: 12–15 days) and stored at −70°C in sealed bags with desiccant until processed.
Protein measurement
Details about elution of the 25 inflammation-related proteins from blood spots and measurement of the proteins are provided elsewhere [22]. Validated by comparisons with traditional ELISA this system has high content validity and inter-assay variations that are invariably less than 20%.
The Laboratory of Genital Tract Biology of the Department of Obstetrics, Gynecology and Reproductive Biology at Brigham and Women's Hospital, Boston, measured the following 25 proteins using the Meso Scale Discovery (MSD) electrochemiluminescence system: (MSD) electrochemiluminescence system: IL-1β (Interleukin-1beta), IL-6 (Interleukin-6), IL-6R (interleukin-6 receptor), TNF-α (tumor necrosis factor-alpha), TNF-R1 (tumor necrosis factor-alpha-receptor1), TNF-R2 (tumor necrosis factor-alpha-receptor2), IL-8 (CXCL8) (interleukin-8), MCP-1 (CCL2) (monocyte chemotactic protein-1), MCP-4 (CCL13) (monocyte chemoattractant protein-4) (CCL13), MIP-1β (CCL4) (Macrophage Inflammatory Protein-1beta) (CCL4), RANTES (CCL5) (regulated upon activation, normal T-cell expressed, and [presumably] secreted), I-TAC (CXCL11) (Interferon-inducible T cell Alpha-Chemoattractant), ICAM-1 (CD54) (intercellular adhesion molecule-1), ICAM-3 (CD50) (intercellular adhesion molecule-3), VCAM-1 (CD106) (vascular cell adhesion molecule-1), E-SEL (CD62E) (E-selectin) (CD62E), MMP-1 (matrix metalloproteinase-1), MMP-9 (matrix metalloproteinase-9), CRP (C-Reactive Protein), SAA (serum amyloid A), MPO (myeloperoxidase). VEGF (vascular endothelial growth factor), VEGF-R1 (vascular endothelial growth factor-receptor1), VEGF-R2 (vascular endothelial growth factor-receptor2), and IGFBP-1 (insulin-like growth factor binding protein-1). Measurements were made in duplicate and the mean served as the basis for all tables and analyses. Measurements of each protein were normalized to mg total protein. The 25 proteins were selected to represent as broad a representation of cytokines, chemokines, adhesion molecules, metalloproteinases, and growth factors with accuracy and at reasonable cost using the platforms.
TSH was measured with an MSD prototype platform that had documented desirable levels of validity against a Scripps Laboratories standard, and inter-assay variations below 20%.
Primary exposures
ISSI was defined separately using each inflammation-related protein as a concentration in the highest quartile for gestational age on two separate days a week apart during the first two postnatal weeks.
HTT was defined as a TSH concentration in the highest quartile on postnatal day 14 according to the interval of gestational age at delivery (> 25 nano (International) Units/mg total protein among infants born at 23–24 weeks, and above 33–34 nanoUnits/mg protein for infants > 25 weeks).
These two exposures were combined to form four mutually exclusive groups: 1) HTT only; 2) ISSI only; 3) HTT +ISSI; and 4) neither HTT nor ISSI.
Protocol ultrasound scans
Procedures for obtaining and reading ultrasound scans are described elsewhere [23]. Two independent readers had to agree on the presence of every lesion.
24-month developmental assessment
Fully, 91% of surviving children returned for a developmental assessment at about 24-months corrected age; 77% had their exam within the range of 23.5–27.9 months, which included both the Mental and Psychomotor Indices (MDI and PDI) of the Bayley Scales of Infant Development – Second Edition [24] and a neurologic assessment by examiners who demonstrated acceptably low inter-examiner variability[25]. Very low developmental indices were defined as a score below 55, which is three standard deviations below the expected mean. The topographic diagnosis of cerebral palsy (CP) (quadriparesis, diparesis, or hemiparesis) was based on an established algorithm [26]. All head circumferences were converted to Z-scores based on standards provided by the CDC [27]. The largest occipital-frontal circumference was measured to the nearest 0.1 centimeter. Microcephaly was defined as a head circumference Z-score <−2, which is two standard deviations below the expected mean.
Data analysis
Logistic regression models, which adjusted for gestational age category (i.e., 23–24, 25–26, and 27 weeks), were fit to estimate magnitudes of association (odds ratios (OR) with 95% confidence intervals (CI)) between HTT and each of eight indicators of brain damage, including ultrasound scan diagnoses of ventriculomegaly and a hypoechoic lesion,, and at age 2 years, quadriparetic, diparetic and hemiparetic cerebral palsy; very low mental and motor developmental indices; and microcephaly. When the 95% confidence intervals do not include 1.0, the odds ratios are statistically significant.
Twenty-five additional logistic regression models (one for each inflammation-associated protein) were fit for each of the eight brain damage indicators to evaluate risks associated with HTT only, ISSI only, and the combination of HTT+ISSI. These exposures were compared in each model to the absence of both HTT and ISSI (i.e., children who did not have any of the three exposures included in each model) and included variables for the gestational age category.
Magnitudes of association between selected exposures and each cerebral palsy subtype were analogously fit using a multinomial logistic model. These models included the same exposures as those fit for dichotomous outcomes, but the outcome was a four-level categorical variable representing the cerebral palsy subtype (events: 1. quadriparetic, 2. hemiparetic or 3. diparetic), or the absence of this disorder (reference).
Results
Newborns who had HTT were more likely than others to have a low birth weight and a low birth weight Z-score, but not a lower gestational age (reflecting the classification of quartiles of TSH concentrations within gestational age groups) (Table 1). In univariable analyses, HTT was not significantly associated with any of the eight brain damage indicators. None of the study participants was diagnosed with congenital hypothyroidism.
Table 1.
Percent of children classified by their TSH concentration for gestational age on postnatal day 14 who also had the disorders and dysfunctions listed on the left and magnitudes of association
| Descriptive Characteristics and Neurodevelopmental Outcomes |
TSH quartile, day 14 | Odds Ratio* for highest Quartile vs. lowest three quartiles |
Row N | |||
|---|---|---|---|---|---|---|
| Lowest | Middle two | Highest | ||||
| Gestational age at delivery (weeks) | 23–24 | 21 | 21 | 21 | 163 | |
| 25–26 | 46 | 46 | 46 | 365 | ||
| Birth weight (g) | ≤ 750 | 34 | 36 | 46 | 2.0 (1.3, 2.9) | 299 |
| Birth weight Z-score | < −2 | 6 | 4 | 10 | 2.2 (1.2, 4.1) | 46 |
| Ultrasound lesion | Ventriculomegaly | 12 | 8 | 12 | 1.3 (0.9, 2.2) | 79 |
| Hypoechoic lesion | 10 | 8 | 4 | 0.4 (0.2, 0.9) | 58 | |
| Cerebral palsy type^ | Quadriparesis | 6 | 5 | 8 | 1.6 (0.8, 3.0) | 48 |
| Diparesis | 5 | 3 | 4 | 0.9 (0.4, 2.2) | 30 | |
| Hemiparesis | 2 | 2 | 3 | 1.5 (0.5, 4.6) | 15 | |
| Bayley Scale <55 | Mental | 12 | 16 | 17 | 1.2 (0.8, 1.9) | 120 |
| Motor | 11 | 16 | 18 | 1.3 (0.9, 2.0) | 120 | |
| Microcephaly | HC Z-score < −2 | 13 | 8 | 13 | 1.4 (0.8, 2.3) | 84 |
| Column N | 198 | 390 | 198 | 786 | ||
Note: column percentages are shown;
odds Ratios are adjusted for gestational age category and shown with 95% confidence intervals; and
cerebral palsy subtype odds ratios were modeled using a multinomial logistic model.
In contrast, Figures 1–4 show that HTT was significantly associated with several indicators of brain damage when we considered the co-occurrence or absence of ISSI. (Figures 1–4). Rather than discuss the many details of these figures, we focus on identifying which of the three patterns applies to each indicator of brain damage (summarized and presented in color in Table 2).
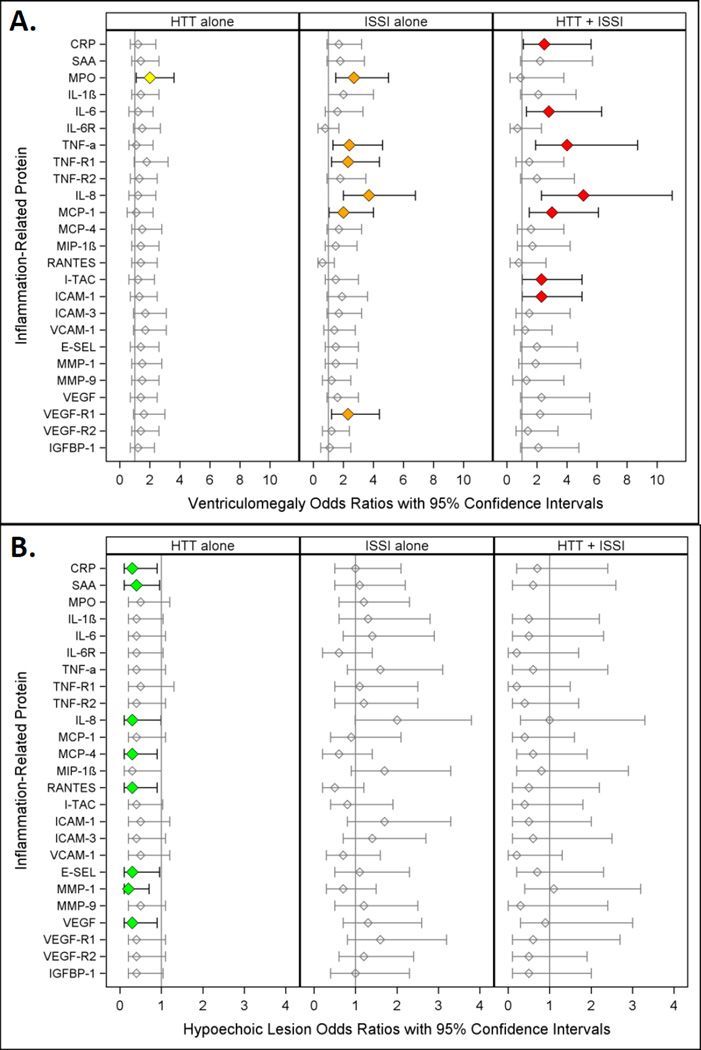
Figure 1.
Odds ratios (and 95% confidence intervals) for ventriculomegaly (A.) and hypoechoic lesion (B.) calculated with logistic regression models. The three risk groups ISSI (intermittent or sustained systemic inflammation) without HTT, HTT (relative hyperthyrotropinemia) without ISSI, and both ISSI and HTT are each compared to the referent group that consists of newborns with neither ISSI nor HTT. All logistic regression equations also included a variable for gestational age.
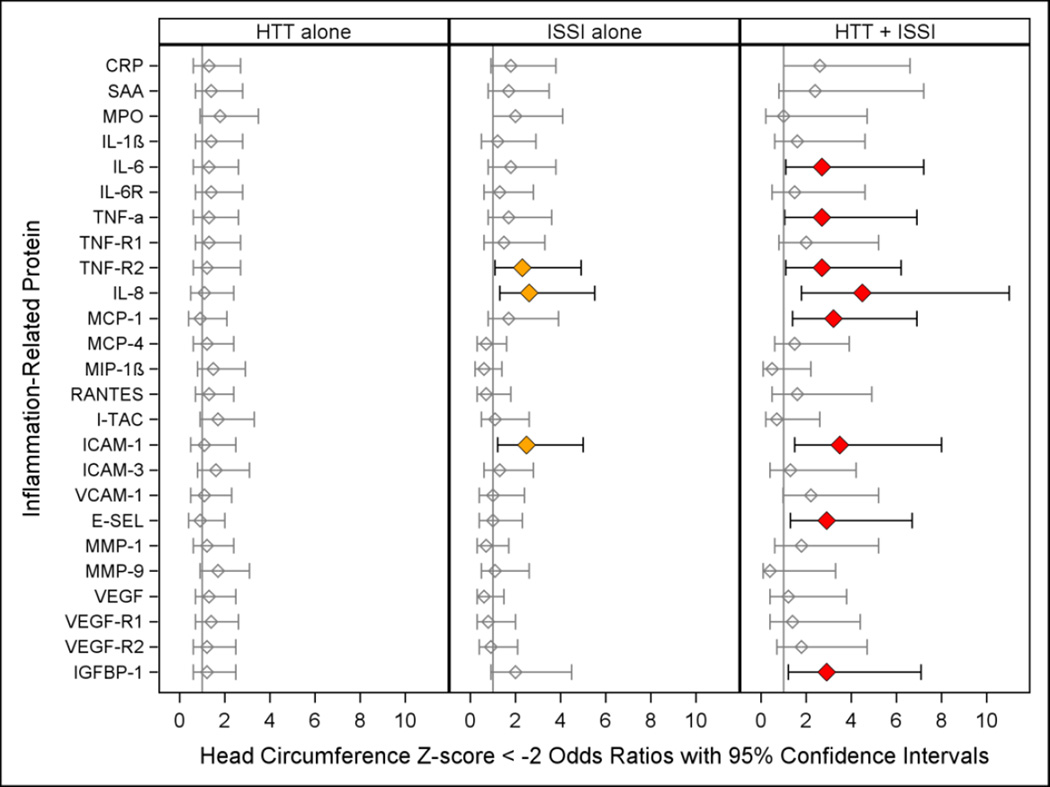
Figure 4.
Odds ratio (and 95% confidence interval) of 24 month head circumference Z-score < −2 (when those with a birth head circumference Z-score < −2 are excluded) calculated with logistic regression models. The three risk groups ISSI (intermittent or sustained systemic inflammation) without HTT, HTT (relative hyperthyrotropinemia) without ISSI, and both ISSI and HTT are each compared to the referent group that consists of newborns with neither ISSI nor HTT. All logistic regression equations also included a variable for gestational age. All logistic regression equations also included a variable for gestational age.
Table 2.
We identify three patterns of increased risk of indicators of brain damage associated with HTT and ISSI.
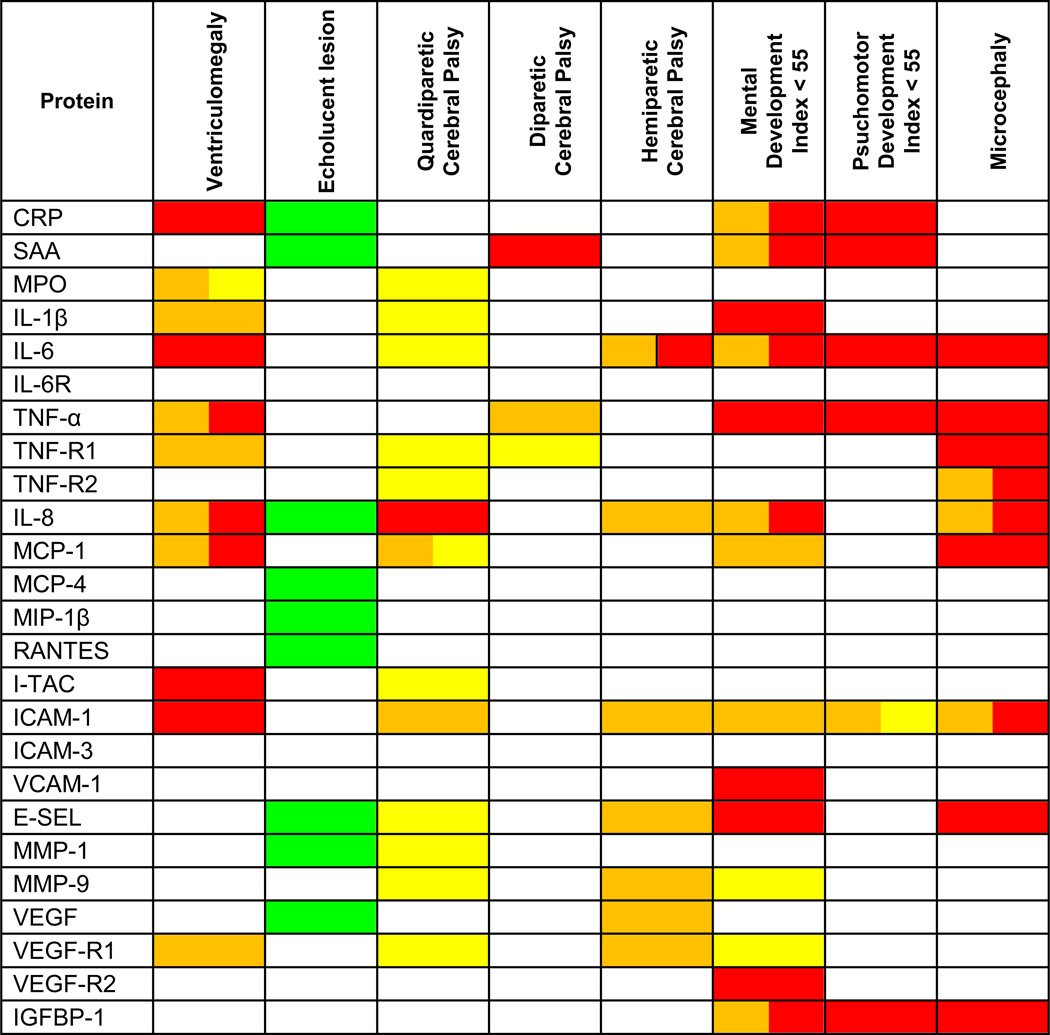 |
HTT in the absence of ISSI is identified with yellow, ISSI in the absence of HTT is identified with orange, and the combination of ISSI + HTT is identified with red. Reduced risk of an echolucent lesion associated with HTT alone is identified with green. Boxes with 2 separate colors indicate that 2 patterns were identified.
Ventriculomegaly (Figure 1A.)
Children with HTT alone were not at increased risk of ventriculomegaly. On the other hand, those with ISSI alone (e.g. IL-1β, TNF-R1, VEGF-R1)(orange) and those with HTT+ISSI (e.g., CRP, IL-6, I-TAC, ICAM-1)(red) were. For TNF-α, IL-8, and MCP-1, two groups of children--those with ISSI alone and those with HTT+ISSI (orange and red)--were at increased risk of ventriculomegaly.
A hypoechoic lesion (Figure 1B.)
Children with ISSI alone or with HTT+ISSI were not at risk of a hypoechoic lesion. Children with HTT alone (green) in nine models with non-significant ISSI and non-significant HTT+ISSI were at significantly reduced risk of this lesion.
Quadriparetic cerebral palsy (Figure 2A.)
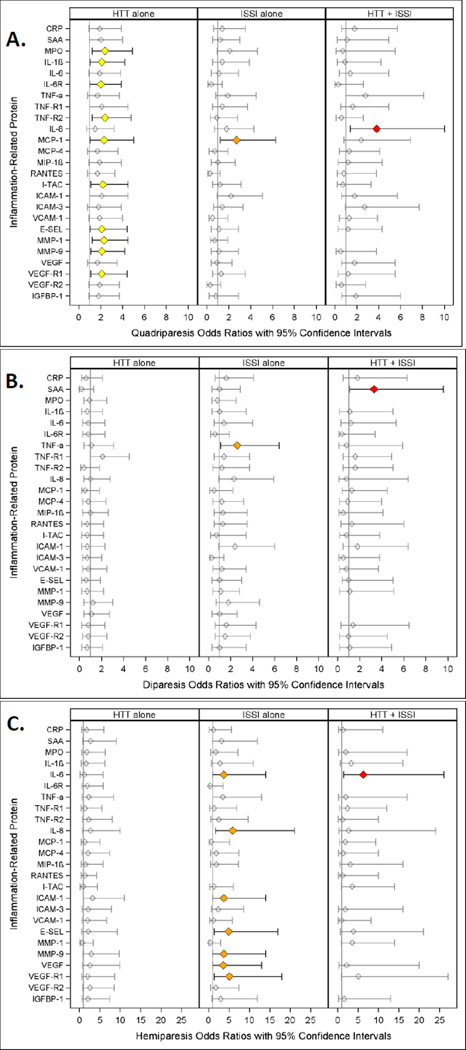
Figure 2.
Odds ratio (and 95% confidence interval) of quadriparesis (A.), (B.) diparesis and (C.) hemiparesis calculated with logistic regression models. The three risk groups ISSI (intermittent or sustained systemic inflammation) without HTT, HTT (relative hyperthyrotropinemia) without ISSI, and both ISSI and HTT are each compared to the referent group that consists of newborns with neither ISSI nor HTT. All logistic regression equations also included a variable for gestational age. Missing values indicate an inability to estimate odds due to complete separation of outcomes among exposed and unexposed in light of the small sample size.
Those with HTT only were at increased risk of quadriparesis in ten separate models with different inflammation-associated proteins (MPO, IL-1 β, IL6-R, TNF-R1, TNF-R2, I-TAC, E-SEL, MMP-1, MMP-9, VEGF-R1) that did not contribute risk information (yellow). Children with HTT+ISSI (ISSI with IL-8)(red) were at increased risk of quadriparesis, as were two groups of children in the MCP-4 model-- − those with HTT only and those with ISSI only (yellow and orange).
Hemiparetic cerebral palsy (Figure 2B.)
Children who had ISSI with inflammation-associated proteins IL-8, MMP-9, ICAM-1, E-SEL, VEGF, and VEGF-R1 were at increased risk of hemiparesis (orange). Children with IL-6 ISSI (orange) and those with IL-6 HTT+ISSI (IL-6)(orange and red) were also at increased risk.
Diparetic cerebral palsy (Figure 2C.)
By and large, children with HTT only, ISSI only or HTT+ISSI were not at increased risk of diparetic cerebral palsy.
Very low mental developmental index (Figure 3A.)
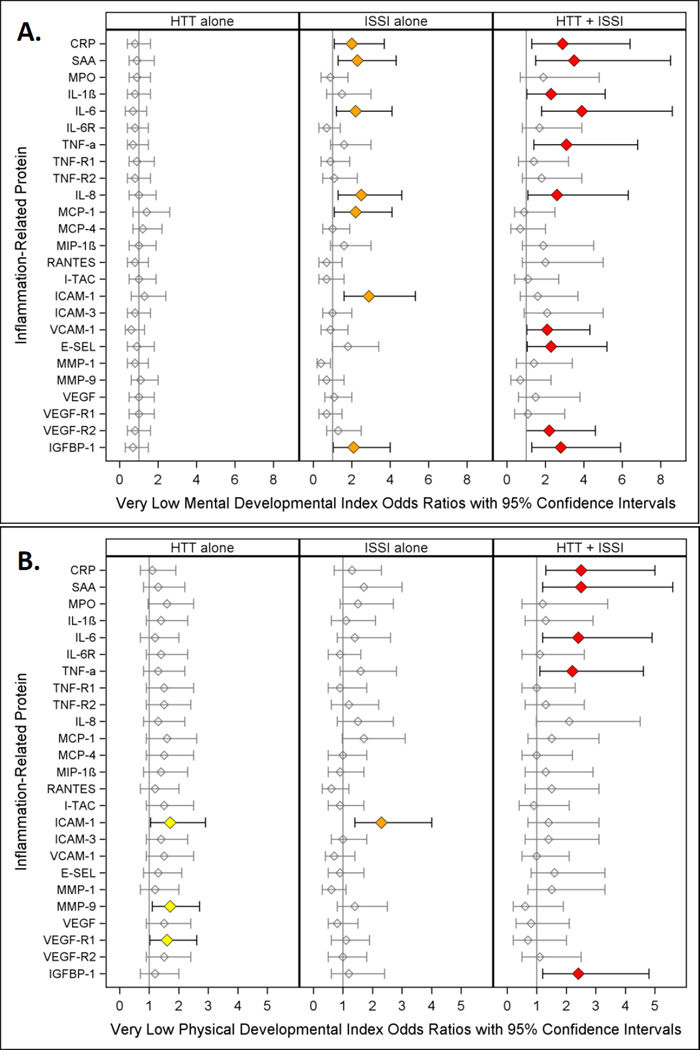
Figure 3.
Odds ratio (and 95% confidence interval) of Very Low Mental Developmental Index (A.) and Very Low Physical Developmental Index (B.) calculated with logistic regression models. The three risk groups ISSI (intermittent or sustained systemic inflammation) without HTT, HTT (relative hyperthyrotropinemia) without ISSI, and both ISSI and HTT are each compared to the referent group that consists of newborns with neither ISSI nor HTT. All logistic regression equations also included a variable for gestational age. Note: Children who had quadriparetic cerebral palsy were not included in these analyses
Two patterns were most associated with increased risk of a Mental Developmental Index < 55. Children who had HTT+ISSI with five inflammation-associated proteins, IL-1β, TNF-α, VCAM-1, E-SEL, and VEGF-R2, were at increased risk of a very low index (red). For five other proteins (CRP, SAA, IL-6, IL-8, and IGFBP-1) children with ISSI and, separately, children with HTT+ISSI were also at increased risk of a very low mental developmental index (orange and red).
Motor developmental index (Figure 3B.)
Children with HTT+ISSI (in models with CRP, SAA, IL-6, TNF-α, and IGFBP-1) were at increased risk of a very low Psychomotor Development Index (red).
Microcephaly (Figure 4)
The risk of microcephaly was increased in children with HTT+ISSI with IL-6, TNF-α, TNF-R1, MCP-1, E-SEL, and IGFBP-1 (red). For three inflammation-associated proteins (TNF-R2, IL-8, ICAM-1) children with ISSI and, separately, children with HTT+ISSI were also at increased risk of microcephaly (orange and red).
Discussion
Our two most important findings can be characterized as follows. First, HTT when considered alone did not convey information about increased risk of any of the structural and functional indicators of brain damage. Second, HTT was associated with elevated risk of multiple indicators of brain damage, but only when classified according to whether ISSI was present or absent. These two findings alone allow us to infer that risk of brain damage associated with HTT in very preterm newborns needs to be assessed in light of information about ISSI. They also lead to the inference that HTT and ISSI are probably influenced by each other’s capacity to convey risk information or to damage the brain. They also raise the possibility that the failure to identify an association between indicators of thyroid dysfunction and neurodevelopmental outcomes in some studies of very preterm neonates [6–12] is attributable, at least in part, to the lack of consideration for ISSI.
The association of HTT alone with a lower frequency of hypoechoic lesions was unexpected. Taken together with the observation that isolated HTT was also associated with a non-significant reduction in risk of spastic diplegia in nearly 80% of protein-specific models, we consider the possibility that HTT might protect against some forms of brain damage under some circumstances.
Synthesis
We are not sure how HTT conveys information about brain damage risk or actually contributes to brain damage. One possibility is that HTT is a measure of low thyroid availability. Thyroid hormone advances the maturation of oligodendrocytes passed past a period of vulnerability, perhaps thereby reducing the risk of brain damage[28]. In this scenario, HTT is a surrogate for thyroid deficiency. Since HTT has been linked to inflammatory phenomena both in adults [29, 30]and in neonates [Soto-Rivera et al., under review], it is possible that HTT is also marker of inflammation severity.
Thyroid dysfunction is common in the setting of critical illness among newborns, children and adults, a condition now referred to as nonthyroidal illness syndrome [15, 31]. Newborns with severe illness typically have reduced T4 and T3 concentrations, whereas TSH levels are largely unaffected [14, 32].
Most studies of the relation between illness severity and thyroid hormone levels in children born preterm have focused on respiratory distress syndrome (RDS) and sepsis [32–35]. Blood levels of T4 are also lower on average in earlier compared to later preterm births and in newborns who had bacteremia, endotracheal bacterial cultures, persistent ductus arteriosus, or necrotizing enterocolitis [36]. Thyroid hormone levels in preterm newborns are especially low in those who have such markers of systemic inflammation, such as elevated serum concentrations of IL-6 and CRP [16].
Another possibility is that HTT is an expression of immaturity/vulnerability. TSH concentrations tend to be higher in the lowest gestational age newborns. But not all babies born at 23–24 weeks are of similar maturation. In this situation, HTT adds information beyond gestational age about the immaturity/vulnerability of the newborn. This might reflect brain immaturity or the immaturity of systems that have the capability to protect the brain[37].
We are unable to select one of these three possibilities as more plausible than the others. Indeed, we consider it possible that each is likely.
Strengths and limitations
Our study has several strengths. First, we included a large number of infants providing power to perceive a doubling or halving of risk. Second, we enrolled infants based on gestational age and not birth weight [38]. Third, we minimized observer variability as best we could. Fourth, our protein data are of high quality.
Our major limitation is the lack of lack information about thyroxine levels at the time TSH levels were determined. Consequently, we cannot separate the possibility of thyroxine deficiency from the less likely possibility that HHT has adverse effects. Four other potential limitations deserve identification. First, children had to survive until age 2 years and return for a developmental assessment to be included in this study. Consequently, children who died with brain damage are not included. To the extent that these children differed from survivors, the survivor requirement might have introduced bias. Second, this is an observational study, therefore limiting our ability to infer causation. Third, the measured proteins probably represent only a subset of those involved in the pathogenesis of perinatal brain damage. Fourth, in our desire to avoid the error of inappropriately drawing the inference that HTT has no effect, we did not adjust for multiple comparisons. This might have inappropriately increased the probability of identifying an HTT effect when none exists.
Conclusion
HTT in very preterm newborns is associated with increased risks of brain damage when modeled according to the absence or especially the presence of ISSI. HTT was also associated with lower risk of hypoechoic lesion in some models, but only in the absence of ISSI. Without information about thyroid function, we do not know how much of what we see represents unduly low thyroid hormone availability. We cannot identify to what extent the HTT represents inflammation, immaturity of neuroprotective systems, immaturity/vulnerability of the brain, or other reasons for these associations.
Acknowledgements
The authors gratefully acknowledge the contributions of their subjects, and their subjects’ families, as well as those of their colleagues.
Financial support:
This study was supported by The National Institute of Neurological Disorders and Stroke (5U01NS040069-05; 2R01NS040069-06A2), The National Eye Institute (1-R01-EY021820-01), and the National Institute of Child Health and Human Development (5P30HD018655-28).
Participating institutions and ELGAN Study collaborators who made this report possible
Children's Hospital, Boston, MA
Kathleen Lee, Anne McGovern, Jill Gambardella, Susan Ursprung, Ruth Blomquist Kristen Ecklund, Haim Bassan, Samantha Butler, Adré Duplessis, Cecil Hahn, Catherine Limperopoulos, Omar Khwaja, Janet S. Soul
Baystate Medical Center, Springfield, MA
Karen Christianson, Frederick Hampf, Herbert Gilmore, Susan McQuiston
Beth Israel Deaconess Medical Center, Boston, MA
Camilia R. Martin, Colleen Hallisey, Caitlin Hurley, Miren Creixell, Jane Share,
Brigham & Women's Hospital, Boston, MA
Linda J. Van Marter, Sara Durfee
Massachusetts General Hospital, Boston, MA
Robert M. Insoft, Jennifer G. Wilson, Maureen Pimental, Sjirk J. Westra, Kalpathy Krishnamoorthy
Floating Hospital for Children at Tufts Medical Center, Boston, MA
Cynthia Cole, John M. Fiascone, Janet Madden, Ellen Nylen, Anne Furey Roy McCauley, Paige T. Church, Cecelia Keller, Karen J. Miller
U Mass Memorial Health Care, Worcester, MA
Francis Bednarek, Mary Naples, Beth Powers, Jacqueline Wellman, Robin
Adair, Richard Bream, Alice Miller, Albert Scheiner, Christy Stine
Yale University School of Medicine, New Haven, CT
Richard Ehrenkranz, Joanne Williams, Elaine Romano
Wake Forest University Baptist Medical Center and Forsyth Medical Center, Winston-Salem, NC
T. Michael O’Shea, Debbie Gordon, Teresa Harold, Barbara Specter, Deborah Allred, Robert Dillard, Don Goldstein, Deborah Hiatt (deceased), Gail Hounshell, Ellen Waldrep, Lisa Washburn, Cherrie D. Welch
University Health Systems of Eastern Carolina, Greenville, NC
Stephen C. Engelke, Sherry Moseley, Linda Pare, Donna Smart, Joan Wilson, Ira Adler, Sharon Buckwald, Rebecca Helms, Kathyrn Kerkering, Scott S. MacGilvray, Peter Resnik
North Carolina Children's Hospital, Chapel Hill, NC
Carl Bose, Gennie Bose, Lynn A. Fordham, Lisa Bostic, Diane Marshall, Kristi Milowic, Janice Wereszczak
Helen DeVos Children's Hospital, Grand Rapids, MI
Mariel Poortenga, Dinah Sutton, Bradford W. Betz, Steven L. Bezinque, Joseph Junewick, Wendy Burdo-Hartman, Lynn Fagerman, Kim Lohr, Steve Pastyrnak,
Sparrow Hospital, Lansing, MI
Carolyn Solomon, Ellen Cavenagh, Victoria J. Caine, Nicholas Olomu, Joan Price
Michigan State University, East Lansing, MI
Nigel Paneth, Padmani Karna, Madeleine Lenski
University of Chicago Medical Center, Chicago, IL
Michael D. Schreiber, Grace Yoon, Kate Feinstein, Leslie Caldarelli, Sunila E. O’Connor, Michael Msall, Susan Plesha-Troyke
William Beaumont Hospital, Royal Oak, MI
Daniel Batton, Beth Kring, Karen Brooklier, Beth Kring, Melisa J. Oca, Katherine M. Solomon
Arkansas Children's Hospital
Joanna J Seibert
Children’s Hospital of Atlanta
Robert Lorenzo
Footnotes
None of the authors has any financial issue or conflict of interest to disclose
References
- 1.Horn S, Heuer H. Thyroid hormone action during brain development: More questions than answers. Molecular and Cellular Endocrinology. 2010;315(1–2):19–26. doi: 10.1016/j.mce.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 2.Fisher DA. Thyroid System Immaturities in Very Low Birth Weight Premature Infants. Seminars in Perinatology. 2008;32(6):387–397. doi: 10.1053/j.semperi.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 3.Belcari F, et al. Thyroid-stimulating hormone levels in the first days of life and perinatal factors associated with sub-optimal neuromotor outcome in pre-term infants. Journal of Endocrinological Investigation. 2011;34(10):E308–E313. doi: 10.3275/7795. [DOI] [PubMed] [Google Scholar]
- 4.Azizi F, et al. Effects of transient neonatal hyperthyrotropinemia on intellectual quotient and psychomotor performance. International Journal for Vitamin and Nutrition Research. 2001;71(1):70–73. doi: 10.1024/0300-9831.71.1.70. [DOI] [PubMed] [Google Scholar]
- 5.Alm J, et al. Incidence of Congenital Hypothyroidism - Retrospective Study of Neonatal Laboratory Screening Versus Clinical Symptoms as Indicators Leading to Diagnosis. British Medical Journal. 1984;289(6453):1171–1175. doi: 10.1136/bmj.289.6453.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tyfield LA, et al. Persistent hyperthyrotropinaemia since the neonatal period in clinically euthyroid children. European Journal of Pediatrics. 1991;150(5):308–309. doi: 10.1007/BF01955927. [DOI] [PubMed] [Google Scholar]
- 7.Miki K, et al. Transient infantile hyperthyrotrophinaemia. Archives of Disease in Childhood. 1989;64(8):1177–1182. doi: 10.1136/adc.64.8.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cody D, et al. The differing outcomes of hyperthyrotropinaemia. Journal of Pediatric Endocrinology & Metabolism. 2003;16(3):375–378. doi: 10.1515/jpem.2003.16.3.375. [DOI] [PubMed] [Google Scholar]
- 9.Kohler B, et al. Transient congenital hypothyroidism and hyperthyrotropinemia: Normal thyroid function and physical development at the ages of 6–14 years. Journal of Clinical Endocrinology and Metabolism. 1996;81(4):1563–1567. doi: 10.1210/jcem.81.4.8636368. [DOI] [PubMed] [Google Scholar]
- 10.Oken E, et al. Neonatal Thyroxine, Maternal Thyroid Function, and Child Cognition. Journal of Clinical Endocrinology & Metabolism. 2009;94(2):497–503. doi: 10.1210/jc.2008-0936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dilli D, et al. Neurodevelopmental evaluation of very low birth weight infants with transient hypothyroxinemia at corrected age of 18–24 months. Indian Pediatrics. 2012;49(9):711–715. doi: 10.1007/s13312-012-0162-x. [DOI] [PubMed] [Google Scholar]
- 12.Scratch SE, et al. Free Thyroxine Levels After Very Preterm Birth and Neurodevelopmental Outcomes at Age 7 Years. Pediatrics. 2014;133(4):e955–e963. doi: 10.1542/peds.2013-2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Osborn DA, Hunt RW. Prophylactic postnatal thyroid hormones for prevention of morbidity and mortality in preterm infants. Cochrane Database Syst Rev. 2007;(1):CD005948. doi: 10.1002/14651858.CD005948.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shih JL, Agus MSD. Thyroid function in the critically ill newborn and child. Current Opinion in Pediatrics. 2009;21(4):536–540. doi: 10.1097/MOP.0b013e32832cbc12. [DOI] [PubMed] [Google Scholar]
- 15.Chopra IJ. Clinical review 86 - Euthyroid sick syndrome: Is it a misnomer? Journal of Clinical Endocrinology and Metabolism. 1997;82(2):329–334. doi: 10.1210/jcem.82.2.3745. [DOI] [PubMed] [Google Scholar]
- 16.Dilli D, Dilmen U. The role of interleukin-6 and C-reactive protein in non-thyroidal illness in premature infants followed in neonatal intensive care unit. J Clin Res Pediatr Endocrinol. 2012;4(2):66–71. doi: 10.4274/jcrpe.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Williams FL, Visser TJ, Hume R. Transient hypothyroxinaemia in preterm infants. Early Hum Dev. 2006;82(12):797–802. doi: 10.1016/j.earlhumdev.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 18.Leviton A, et al. The Relationship between Early Concentrations of 25 Blood Proteins and Cerebral White Matter Injury in Preterm Newborns: The ELGAN Study. The Journal of Pediatrics. 2011;158(6):897.e5–903.e5. doi: 10.1016/j.jpeds.2010.11.059. [DOI] [PubMed] [Google Scholar]
- 19.Leviton A, et al. Early postnatal blood concentrations of inflammation-related proteins and microcephaly two years later in infants born before the 28th post-menstrual week. Early Human Development. 2011;87(5):325–330. doi: 10.1016/j.earlhumdev.2011.01.043. [DOI] [PubMed] [Google Scholar]
- 20.Dammann O, Leviton A. Intermittent or sustained systemic inflammation and the preterm brain. Pediatric Research. 2014 doi: 10.1038/pr.2013.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O'Shea TM, et al. The ELGAN study of the brain and related disorders in extremely low gestational age newborns. Early Hum Dev. 2009;85(11):719–725. doi: 10.1016/j.earlhumdev.2009.08.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leviton A, et al. Inflammation-related proteins in the blood of extremely low gestational age newborns. The contribution of inflammation to the appearance of developmental regulation. Cytokine. 2011;53(1):66–73. doi: 10.1016/j.cyto.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuban K, et al. Observer variability assessing US scans of the preterm brain: the ELGAN study. Pediatr Radiol. 2007;37(12):1201–1208. doi: 10.1007/s00247-007-0605-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bayley N. Bayley Scales of Infant Development-II. San Antonio, TX: Psychological Corporation; 1993. [Google Scholar]
- 25.Kuban KC, et al. Video and CD-ROM as a training tool for performing neurologic examinations of 1-year-old children in a multicenter epidemiologic study. J Child Neurol. 2005;20(10):829–831. doi: 10.1177/08830738050200101001. [DOI] [PubMed] [Google Scholar]
- 26.Kuban KCK, et al. An algorithm for diagnosing and classifying cerebral palsy in young children J. Pediat. 2008;153:466.e1–472.e1. doi: 10.1016/j.jpeds.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.CDC. CDC Growth Charts: United States. 2007 http://www.cdc.gov/nchs/data/nhanes/growthcharts/zscore/zhcageinf.xls. [PubMed]
- 28.Bernal J, Nunez J. Thyroid-Hormones and Brain-Development. European Journal of Endocrinology. 1995;133(4):390–398. doi: 10.1530/eje.0.1330390. [DOI] [PubMed] [Google Scholar]
- 29.Kamath J. Cancer-related fatigue, inflammation and thyrotropin-releasing hormone. Curr Aging Sci. 2012;5(3):195–202. doi: 10.2174/1874609811205030005. [DOI] [PubMed] [Google Scholar]
- 30.Yu YT, et al. Subclinical hypothyroidism is associated with elevated high-sensitive C-reactive protein among adult Taiwanese. Endocrine. 2013;44(3):716–722. doi: 10.1007/s12020-013-9915-0. [DOI] [PubMed] [Google Scholar]
- 31.De Groot LJ. Dangerous dogmas in medicine: The nonthyroidal illness syndrome. Journal of Clinical Endocrinology and Metabolism. 1999;84(1):151–164. doi: 10.1210/jcem.84.1.5364. [DOI] [PubMed] [Google Scholar]
- 32.Simpson J, et al. Serum thyroid hormones in preterm infants and relationships to indices of severity of intercurrent illness. Journal of Clinical Endocrinology & Metabolism. 2005;90(3):1271–1279. doi: 10.1210/jc.2004-2091. [DOI] [PubMed] [Google Scholar]
- 33.Romagnoli C, et al. Serial blood T4 and TSH determinations in low birth weight infants. Influence of gestational age, birth weight and neonatal pathology on thyroid function. Helv Paediatr Acta. 1982;37(4):331–344. [PubMed] [Google Scholar]
- 34.Paul DA, Mackley A, Yencha EM. Thyroid Function in Term and Late Preterm Infants with Respiratory Distress in Relation to Severity of Illness. Thyroid. 2010;20(2):189–194. doi: 10.1089/thy.2009.0012. [DOI] [PubMed] [Google Scholar]
- 35.Paul DA, et al. Thyroxine and illness severity in very low-birth-weight infants. Thyroid. 2001;11(9):871–875. doi: 10.1089/105072501316973136. [DOI] [PubMed] [Google Scholar]
- 36.Williams FLR, et al. Serum thyroid hormones in preterm infants: Associations with postnatal illnesses and drug usage. Journal of Clinical Endocrinology & Metabolism. 2005;90(11):5954–5963. doi: 10.1210/jc.2005-1049. [DOI] [PubMed] [Google Scholar]
- 37.Dammann O, Leviton A. Brain damage in preterm newborns: Might enhancement of developmentally regulated endogenous protection open a door for prevention? Pediatrics. 1999;104(3):541–550. doi: 10.1542/peds.104.3.541. [DOI] [PubMed] [Google Scholar]
- 38.Arnold CC, et al. Very low birth weight: a problematic cohort for epidemiologic studies of very small or immature neonates. Am J Epidemiol. 1991;134(6):604–613. doi: 10.1093/oxfordjournals.aje.a116133. [DOI] [PubMed] [Google Scholar]