Abstract
The importance of proper consumption of dietary folate for human health has been highlighted by an extensive number of publications over several decades. Fortification of grain products with folic acid was initiated with the specific intent to prevent neural tube defects, and the scope of this endeavor is unique in that its target population (women of the periconceptional period) is many times smaller than the population it affects (everyone who ingests fortified grain products). Folate fortification has been wildly successful in terms of its goal; since its inception, the incidence of neural tube defects has markedly decreased. In the wake of this public health triumph, it is important to catalogue both the serendipitous benefits and potential side effects of folic acid supplementation. The vitamin is generally regarded as a harmless nutrient based on studies evaluating the safe upper limits of folate intake. In recent years, however, a concern has been raised with respect to a potential downside to folate supplementation; namely, its proposed ability to enhance proliferation of malignant tumors. The current review summarizes the available literature on the effects of folate supplementation and the molecular mechanisms by which high doses of folate may have negative consequences on human health, especially with regard to cancer.
Keywords: cancer, folate, folic acid, folate enzymes, metastasis, molecular mechanisms, dietary supplementation
Introduction
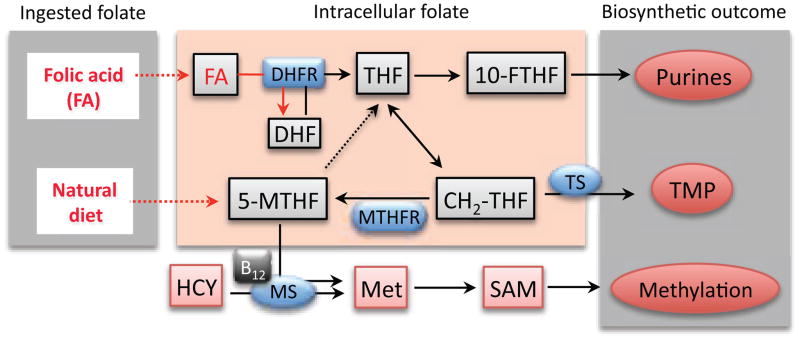
Folates are a group of coenzymes that function to carry single-carbon groups used in the biosynthesis of nucleotides and the metabolism of amino acids (schematically depicted in Fig. 1). The importance of folate first became evident after it was demonstrated as the active compound for correcting certain macrocytic anemias (reviewed in (1)). Since that time, folate deficiency has been implicated in other pathologies including neural tube defects (NTDs), homocysteinemia, cardiovascular disease, and cancer (1). Humans rely upon the presence of folate in their diet because they are unable to synthesize the molecule de novo. Natural foods rich in folate include a variety of vegetables, beans and fruits, as well as beef liver. The fact that folate is necessary for cellular functions has been exploited by medical science, and antifolates are routinely prescribed for the treatment of cancers and rheumatologic disease (2). In contrast to humans, bacteria do not acquire folate from their environment but directly synthesize it from pteridine and p-aminobenzoic acid (PABA). Of note, this pathway is selectively targeted by sulfonamide antibiotics, which are structurally similar to PABA and competitively inhibit the enzyme, dihydropteroate synthetase.
Figure 1. Folate metabolism.
Folate is taken up by the cell in forms of folic acid (FA, supplements or fortified foods) or 5-MTHF (natural diet). In the cell, FA is sequentially converted to dihydrofolate (DHF) and then to the active form of the coenzyme, tetrahydrofolate (THF). Both reactions are catalyzed by DHFR (dihydrofolate reductase). Upon accepting a one-carbon group (comes either from serine, glycine, histidine or formate), THF is converted to other forms of folate. For simplicity, only folate coenzymes directly participating in the biosynthesis of nucleotides and methionine are shown (10-FTHF, 10-formyl-THF; CH2-THF, 5,10-methylene-THF). HCY, homocysteine; SAM, S-adenosylmethionine; MS, methionine synthase; TS, thymidylate synthase; MTHFR, methylenetetrahydrofolate reductase. Reaction catalyzed by MS converts 5-MTHF to THF (indicated by dotted arrow) and requires vitamin B12.
Of particular importance, the connection between folate deficiency in early pregnancy and the fetal development of NTDs led to a public health initiative to fortify foods with folic acid. As a result, the United States Food and Drug Administration has mandated the addition of folic acid to cereals and grain products since 1998. This practice still remains a strong recommendation by the United States Preventive Services Task Force and also has been implemented in more than fifty countries worldwide (3, 4). This endeavor is unique in that the target population (women of the periconceptional period) is many times smaller than the population affected. The fortification resulted in a significant (36%) reduction in the incidence of NTDs as of 2006 (5), thus achieving its goal. Folate status can potentially benefit human health in many ways, and, since the inception of the mandatory folate fortification, there have been many studies that suggest a variety of coincidental health benefits for the general population. For example, in addition to preventing NTDs, preconceptional intake of folate has been associated with a significant reduction in the incidence of early spontaneous preterm births (6). As well, studies have underscored an inverse correlation between folate intake and diseases such as venous thrombosis, atherosclerosis, stroke, and even mood disorders (reviewed in (7)).
Folate controversies: is there an adverse effect of folate supplementation?
FA (pteroylmonoglutamate, vitamin B9) is not a natural form of folate; it is a synthetic vitamer not found in significant amounts in fresh foods and non-fortified food products. There are distinct chemical differences between the reduced folates naturally present in the human diet and FA used in the fortification of food. A folate molecule consists of a pteridine ring conjugated to p-aminobenzoic acid that is modified by one or more glutamic acid residues. There are a variety of folate species, and each is distinguished by the presence and oxidation state of carbon attached to the N5 and/or N10 positions of a tetrahydrofolate backbone. FA differs from natural folates in that (i) it contains a single glutamate residue and (ii) it is an oxidized and inactive form of the coenzyme. To become an active coenzyme, FA must be reduced twice: first to dihydrofolate and then to tetrahydrofolate (Fig. 1). Dihydrofolate reductase (DHFR) is the enzyme responsible for catalyzing both steps; however, it is a relatively slow enzyme in humans and appears incapable of completely converting large amount of FA to tetrahydrofolate (8). In contrast to natural folates, which are unstable and can readily degrade in food preparation and storage, FA is a stable compound (9). FA, being only a monoglutamate, also has excellent bioavailability (10). It should be noted that the combination of fortified foods and multivitamin supplements can result in a substantial accumulation of unmetabolized FA in cells (8). Moreover, this accumulation may be highly variable in individuals since up to five-fold differences in DHFR activity have been reported in humans (8). The accumulation of intracellular FA is likely driven by increased levels of unmetabolized FA in blood, a phenomenon observed in numerous studies assessing the effects of FA intake (4, 11–15). Whether increased circulating FA is a risk factor for certain pathologies or whether it might have a beneficial effect is not clear at present. Even though the majority of the population in many countries is affected by the mandatory supplementation, there has been a lack of targeted efforts to investigate the broad effects of FA on human health to become aware about its possible side effects. Numerous reviews have discussed the possibilities of adverse effects of supplementation with FA, and each has been careful to advocate further research on the effect of supra-physiologic levels of FA on human health (4, 16–19).
Exemplifying this concern, there is a clinically important relationship between folate and vitamin B12 deficiency. Vitamin B12 (cobalamin) is a cofactor of methionine synthase, the enzyme catalyzing the regeneration of methionine from homocysteine and 5-methyltetrahydrofolate (5-MTHF). In instances of B12 deficiency, this enzyme is inactive leading to elevated homocysteine levels and the symptoms of megaloblastic anemia. Since this is the only reaction utilizing 5-MTHF in the cell, the absence of B12 causes the accumulation of 5-MTHF at the expense of other forms of folate. Direct experimental evidence for this phenomenon, commonly known as the “methylfolate trap,” has been obtained in a mouse model where disruption of the methionine synthase reductase gene (essential for methionine synthase activity) led to decreased plasma methionine and increased plasma homocysteine and tissue 5-MTHF (20). FA supplementation can correct the megaloblastic anemia caused by B12 deficiency, and this fact has raised the concern that folate may “mask” this easily detectable symptom and thus exacerbate the more toxic neurological sequelae of vitamin B12 deficiency, such as sub-acute degeneration of the spinal cord (1).
Other health concerns about folate supplementation have been raised in the literature. For example, some studies have suggested that high intake of FA may lead to cognitive dysfunction (21, 22). Also, a link between autism and folate has been suggested based upon the temporal correlation of the rise in autism diagnoses and FA fortification (23). While these potential negative effects are questionable, it would still be prudent to account for the possibility that FA supplementation may have no clear positive effect on diseases other than NTDs. For instance, recent meta-analyses failed to find a link between folate supplementation and the incidence of cardiovascular disease (24, 25). It is also important to consider that folate supplementation alone may not be a panacea for NTDs, and it is possible that the ability of folate to reduce the development of NTDs may be limited to certain genetic sub-populations (26). Moreover, a recent study in mice has demonstrated that depending on the genetic background, FA supplementation may even increase the incidence of NTDs (27).
Molecular mechanism underlying adverse cellular response to folate increase
Oral FA is considered non-toxic to humans: being a water-soluble vitamin, it is readily excreted in both sweat and urine (28). The upper limit for its ingestion has been set by the Institute of Medicine Food and Nutrition Board as 1 mg/day, but this restriction seems arbitrary considering there are no definitive toxic effects of FA at a much higher daily dose (up to 15 mg/day) in healthy individuals (28). As mentioned above, FA is a stable molecule while most reduced folate coenzymes are unstable and can be chemically degraded through oxidation and breakage of the bond between pterine ring and glutamylated PABA moiety (reviewed in (9)). It should be taken into consideration that the majority of folate coenzymes are protein-bound since the concentration of folate-utilizing enzymes in the cell is higher than the total concentration of cellular folate, and such binding to proteins protects the cofactors from degradation (9). Interestingly, studies from our laboratory have demonstrated that excessive supplementation of cultured cells with 5-formyltetrahydrofolate (also known as folinic acid or leucovorin) dramatically (three-fold) increases the total intracellular level of reduced folates (29). Such a high total concentration could result in increased levels of free (non-bound) folate, which may then increase their degradation rate. However, even if folate degradation takes place at a significant rate, the products of this degradation are not toxic and can either be re-utilized in other biochemical reactions or excreted.
Other mechanisms of adverse effects of the vitamin have been suggested in the literature. Theoretically, increased blood levels of FA may interfere with cellular folate transport and metabolism, or regulatory functions of the coenzyme through competitive inhibition of binding of natural folates to enzymes and/or carrier proteins (30). High intake of FA may exert an antagonistic effect towards natural folates due to accumulation of dihydrofolate, which is known to inhibit thymidylate synthase (TS) and methylenetetrahydrofolate reductase (MTHFR), leading to decreased levels of thymidylate and 5-MTHF (30, 31). Importantly, the shortage of thymidylate impairs DNA integrity and cellular division while the shortage of 5-MTHF decreases methionine biosynthesis thus affecting protein production and DNA methylation. As well, high doses of FA may simply saturate DHFR and potentially inhibit the entire folate metabolism (8). Thus, paradoxically, high doses of FA may induce effects similar to those produced by deficiency of the bioactive forms of folate. It has been also shown that folate-enriched diets are associated with reduced cytotoxicity of natural killer cells; immune cells thought to be a major line of defense against arising neoplasia (32). However, a recent study involving healthy individuals did not confirm an association between serum folate levels and the cytotoxic activity of natural killer cells (33), leaving the above mechanism an open question.
Folate and cancer
In addition to the mechanisms discussed above, FA could also produce an effect through the elevation of the reduced folate pool thus boosting folate metabolism and promoting proliferation, a phenomenon especially relevant to the pathogenesis of cancer. The first observation that FA promotes cancer proliferation dates back to the 1940s when studies by Sidney Farber and colleagues demonstrated that the administration of FA accelerated the progression of leukemia in children (34). The relationship between folate supplementation and cancer, however, is perhaps one of the most controversial subjects in the field. Early epidemiological studies have indicated that a low folate status or low folate intake increases, while high folate intake decreases, the risk of certain types of cancer in humans (35, 36). A list of potential mechanisms by which folate deficiency can promote carcinogenesis includes: induction of DNA hypomethylation, secondary deficiency of choline, diminution in natural killer cell surveillance, increased chromosomal aberrations and fragility, uracil misincorporation in DNA, and facilitation of tumorigenic virus metabolism (37). While folate supplementation of normal cells appears to have a protective effect, it may also promote the progression of neoplastic lesions because rapidly dividing cells are critically dependent on an abundant supply of reduced folates to support de novo nucleotide biosynthesis and the methylation reactions necessary for cell division. This serves as the basis for treatment of cancer patients with antifolates, drugs that inhibit folate enzymes and thus prevent the de novo biosynthesis of nucleotides (38). Of note, later epidemiological studies have failed to provide a definite conclusion on the role of folate intake in mediating cancer risk (reviewed in (3, 16, 18, 19)). Part of the problem, however, lies in discriminating between the effect of folate on tumorigenesis or on undetected pre-neoplastic lesions, which are expected to be quite opposite. Interestingly, animal studies have demonstrated that the exposure to high levels of FA in utero may increase the risk of mammary tumors in the offspring (39) while folate depletion post-weaning may be protective against intestinal neoplasia (40), and these findings argue in favor of a direct effect of excess FA in promoting tumorigenesis. Though mechanisms underlying the observed phenomena have yet to be pinpointed, the effect in the former study was attributed to altered DNA methylation as a function of folate status.
The effect of folate on cancer initiation and progression is an extremely important public health issue because the mandatory fortification of grain food with FA has resulted in increased folate intake, raising the concern that it may increase the incidence of malignancies and cancer-related death (41). End-point effects of the vitamin could depend on its ingested form, synthetic FA versus natural (reduced) folate. For example, a recent randomized clinical trial indicated that supplementation with FA doubled the risk of prostate cancer while baseline dietary (natural) folate revealed a protective effect (42). Another example of this trend is the inverse correlation between the risk of pancreatic as well as colon cancer and the dietary (natural) folate intake while no effect was demonstrated for the FA supplemented diet (43). The dose of ingested folate clearly matters as well. Of note, in the above study on prostate cancer FA was given at the dose of 1 mg per day (44), which was 2.5-fold higher than the normally recommended daily allowance of 0.4 mg and was given on top of folate obtained from natural and fortified foods. The tumorigenic response to dietary folate may also depend on the cells/organs of origin and cancer type, but results on this matter are inconsistent (3, 45). Thus, while epidemiological studies of head and neck cancer, liver cancer and neuroblastomas mostly reported protective effects of folate (46–49), results of studies on colorectal, breast, prostate and lung cancers are far less conclusive (50–53). This inconsistency prompted the idea that effects of folate in tumorigenesis depend on the timing and duration of folate administration (18, 19, 54). These effects could be further modified by other factors such as age and the status of vitamins B6 and B12 (4). Finally, it is likely that the relationship between the folate intake and cancer risk also depends on individual genotypic features including polymorphisms in folate enzymes (55–57).
In light of the controversy of the relationship between dietary folate and cancer risk, it is crucial to ascertain which molecular mechanisms are initiated/maintained by excessive folate that could promote proliferation and tumorigenesis. The negative consequences of dietary folate deficiency at the cellular level include altered protein expression (58), decreased DNA repair capability and accumulation of DNA damage (59–61), increased chromosomal aberrations and fragility (62); events that ultimately reduce growth rate and impair cell division. Conversely, the abundance of folate coenzymes would be expected to prevent these negative events and promote proliferation. Indeed, direct experimental evidence of such dependence of proliferation from folate availability has been obtained in both cell culture and animal models. For example a recent study has shown that dietary folate restriction blocks prostate cancer progression in the TRAMP mice (63), a finding that is consistent with the idea that folate promotes cellular proliferation. It has to be seen whether a similar outcome can be reproduced in cancer patients. It should be pointed out that FA supplementation may also interfere with the action of antifolates by reducing their efficacy in the treatment of cancer, rheumatologic disease, and malaria (64–67).
Effects of folate on metastasis
The direct effects of folate on metastatic disease have yet to be thoroughly evaluated, but the treatment of metastatic tumors with antifolates has shown promising results (68), suggesting the involvement of folate metabolism in the metastatic processes. In agreement with this notion, a recent case report indicated that supplementation with large amounts of folate could accelerate metastasis in patients with hormone-resistant prostate cancer (69). In addition to its potential role in enhancing proliferation, folate may promote metastatic disease via its effects on cancer cell migration. The migratory ability of malignant cells is a key feature that distinguishes metastatic disease from benign solid tumors. The presence or absence of essential nutrients is an important component of the tumor microenvironment that contributes to cancer progression and metastasis. In this regard, extracellular folate status correlates with altered expression of genes responsible for cell adhesion, migration and invasion (70, 71). The ability of the cell to move and migrate depends upon reorganization of the cytoskeleton, a complex network of actin filaments, microtubules and intermediate filaments. The leading role in this process is played by actin due to its unique dynamic properties, associated with two interchangeable protein forms, globular (G-actin) and filamentous (F-actin). There appears to be a direct association between folate metabolism and actin; the disruption of the actin cytoskeleton reversibly increases the proportion of folate receptors on the cell surface and the rate of 5-MTHF delivery (72).
Rebuilding of actin filaments is regulated by multiple actin-binding proteins including the actin-depolymerizing factor cofilin, which appears to be the major calcium-independent regulator of this process. Cofilin is a small (19 kDa) ubiquitous protein that facilitates the turnover between filamentous and globular actin (73, 74). A study of cardiac development in folate receptor knockout mice has revealed dramatic alterations in components of the actin cytoskeleton network, including down-regulation of cofilin (75). This finding is also in line with a proteomic study that showed down-regulation of cofilin in rats fed a folate-deficient diet (76). In further support of the important role of folate in metastasis, it has been recently demonstrated that the folate withdrawal inhibits migration and invasion of cultured cells by a mechanism associated with cofilin-dependent alterations of the actin cytoskeleton (77). The role of folate in the promotion of metastasis could be associated with maintaining methylation of the Rho family GTPases, a process required for their proper membrane association and activation of downstream targets controlling cytoskeleton dynamics. In support of this mechanism, treatment of cancer cells with the antifolate MTX led to a dramatic decrease of Ras methylation and its aberrant localization to the cytosol resulting in the decreased activation of MAPK/Akt (78). It has been also hypothesized that folate controls methylation of the cytoskeleton (79), which could be another mechanism to regulate migration. Curiously, the folate-metabolizing enzyme FTCD (formiminotransferase cyclodeaminase) has been shown to bind vimentin filaments perhaps controlling the assembly of intermediate filament cytoskeleton (80, 81). While such a connection may represent an important factor in epithelial-mesenchymal transition and the development of metastases, it is not clear whether the interaction of FTCD with vimentin is regulated by folate or not since the folate-dependent catalytic activity of the enzyme is not required for its function towards filaments (81).
The role of folate enzymes in tumorigenesis
An overview of the role of folate in cancer disease would be incomplete without consideration of the enzymes involved in the metabolism of the vitamin. Intracellular folate status can be defined by three major components: (i) dietary ingestion of the vitamin, (ii) its transport, and (iii) its metabolism by folate enzymes. Folate metabolism is a complex network of reactions which involves many forms of the coenzyme and numerous folate-metabolizing enzymes (simplistically depicted in Fig. 1) (82, 83). Alteration of their catalysis through activation, inhibition or expression changes could affect cellular function irrespective of folate supplementation. Folate enzymes have been implicated in tumorigenesis and cancer progression as well as in other diseases. Physiological functions of numerous folate enzymes have been evaluated by different approaches including the search for nucleotide polymorphisms as potential markers of NTDs, cardiovascular diseases and cancer (7).
Most of folate enzymes catalyze the reactions which benefit cellular proliferation. Accordingly, increased production of many folate-related proteins is often observed in tumors. The well-known examples are the folate receptor, which is highly expressed in cancer cells (84), and DHFR, which elevation in response to antifolate treatment is one of the main mechanisms of the resistance to these drugs (85). DHFR is required to convert dihydrofolate, produced in the reaction of the TMP biosynthesis, back to tetrahydrofolate, thus regenerating the active form of folate. The enzyme is elevated at the transition from G1 to S phase since TMP biosynthesis takes place during S phase while cells are preparing for division. This enzyme is also responsible for the incorporation of the dietary FA into the reduced folate pool through its conversion to dihydrofolate and then to tetrahydrofolate (Fig. 1). Of note, DHFR can be up-regulated upon increased folate consumption (86). Thus, elevated levels of FA in the cell may promote unnecessary proliferation by up-regulating the expression of DHFR thus increasing the production of tetrahydrofolate and the rate of nucleotide and methionine biosynthesis. Of note, the FA and DHFR-driven increase in dihydrofolate could inhibit MTHFR and 5-MTHF generation (31) thus diverting one-carbon groups towards nucleotide biosynthesis and enhancing proliferation. Furthermore, DHFR and another folate enzyme, thymidylate synthase (TS) have been shown to regulate translation through the direct binding of several mRNAs including their own (85, 87). In this regard, TS has been shown to repress the translation of p53 mRNA, a process which might prevent the activation of this tumor suppressor (87). Thus, through such a mechanism, TS might function in an oncoprotein-like manner. Interestingly, elevated DHFR can antagonize the proliferation control exerted by another folate enzyme, ALDH1L1 (29).
Several folate enzymes involved in serine/glycine metabolism have been implicated in tumorigenesis and cancer progression as well. In a recent study, levels of SHMT2, MTHFD2 and MTHFD1L correlated with enhanced proliferation of cancer cells, and increased expression of these enzymes was associated with greater mortality in breast cancer patients (88). Another folate-related enzyme, glycine decarboxylase (GDC), was found to be elevated in tumor-initiating cells and is required for their growth and tumorigenic properties (89). Remarkably, the over-expression of GDC promotes cellular transformation, and aberrant activation of GDC correlates with poorer survival in lung cancer patients. In line with the above findings, the tumorigenic effects of GDC require catalytically active enzyme. GDC is a part of the multi-enzyme glycine degradation complex (89). The entire process utilizes glycine to donate one-carbon groups into folate pool and to produce energy, and it remains to be seen whether the above effects occur primarily through acceleration of folate metabolism or via another mechanism (such as increased energy production secondary to enhanced glycine utilization).
Two abundant folate-related enzymes with regulatory functions, ALDH1L1 and GNMT, fit the definition of type 2 tumor suppressors. ALDH1L1 (10-formyltetrahydrofolate dehydrogenase, FDH) controls the level of folate-bound one-carbon groups by removing them from the folate pool as CO2, thus restricting folate-dependent biosynthetic reactions (90). The ALDH1L1 gene is silenced in many human cancers through methylation of its promoter region (91), and its re-expression induces specific apoptotic cascades in cancer cells with JNKs and p53 as major downstream targets (92–94). GNMT (glycine-N-methyltransferase) controls the level of activated methyl groups by converting S-adenosylmethionine (SAM) and glycine to S-adenosylhomocysteine and sarcosine in a folate-dependent manner, thus limiting the methylation ability of the cell (95). GNMT is down-regulated in cancers as well and exerts an inhibitory effect on carcinogenesis (96, 97). A case-control study revealed that the enzyme has protective effects against prostate cancer (97). In agreement with these findings, GNMT knockout mice have a high tendency to develop hepatocellular carcinomas and demonstrate an increased amount of overall genomic methylation as well as promoter hypermethylation of tumor suppressors RASSF1A (inhibitor of oncoprotein Ras) and SOCS2 (inhibitors of JAK/STAT pathway) (98).
Conclusion
Folate-dependent reactions are vital for cellular division and homeostasis due to their involvement in nucleic acid biosynthesis, methylation reactions and amino acid metabolism. The widespread inception of FA supplements has been successful in the prevention of NTDs and may have additional benefits for the population as a whole. However, there is still controversy in the literature over the existence of both beneficial and harmful side effects of FA supplementation and it has become increasingly urgent to understand the long-term effects of folate on human health. The precise molecular mechanisms by which folate influences cellular functioning are just beginning to emerge. It is likely that the most immediate downstream response to folate status would be alterations in nucleotide levels/nucleic acid biosynthesis and methionine biosynthesis/SAM levels (schematically outlined in Fig. 1). Folate deficiency would lead to diminished production of nucleotides and SAM while the abundance of the vitamin might elevate their production. SAM is a universal methyl group donor in more than a hundred reactions in the cells including DNA, RNA and protein methylation, and the biosynthesis of numerous key metabolites such as polyamines, epinephrine, creatine, and phosphatidyl choline. However, the cellular response to these changes may produce versatile and not so easily predictable end-point effects. In relation to cancer, folate metabolism may play a particularly essential role in proliferation, tumorigenesis, and metastasis. Thus, it is important to discriminate between the mechanisms by which folate protects from or promotes tumorigenesis and metastasis. This knowledge will allow more specific dietary recommendations for different population groups to minimize any potential adverse effect of this vitamin.
Acknowledgments
This work was supported by the National Institutes of Heals grants DK54388 and CA095030 (to SAK). Kyle C. Strickland was supported by a Ruth L. Kirschstein National Research Service Award for Individual Predoctoral MD/PhD Fellows F30DK083215.
Abbreviations
- 5-MTHF
5-methyltetrahydrofolate
- DHFR
dihydrofolate reductase
- FA
folic acid
- NTDs
neural tube defects
Footnotes
Conflict of interests
The authors declare no conflict of interests.
References
- 1.Stover PJ. Physiology of folate and vitamin B12 in health and disease. Nutr Rev. 2004;62:S3–12. doi: 10.1111/j.1753-4887.2004.tb00070.x. discussion S3. [DOI] [PubMed] [Google Scholar]
- 2.Benedek TG. Methotrexate: from its introduction to non-oncologic therapeutics to anti-TNF-alpha. Clin Exp Rheumatol. 2010;28:S3–8. [PubMed] [Google Scholar]
- 3.Herrmann W, Obeid R. The mandatory fortification of staple foods with folic acid: a current controversy in Germany. Deutsches Arzteblatt international. 2011;108:249–54. doi: 10.3238/arztebl.2011.0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Obeid R, Herrmann W. The Emerging Role of Unmetabolized Folic Acid in Human Diseases: Myth or Reality? Curr Drug Metab. 2012 doi: 10.2174/138920012802850137. [DOI] [PubMed] [Google Scholar]
- 5.CDC Grand Rounds: additional opportunities to prevent neural tube defects with folic acid fortification. MMWR Morb Mortal Wkly Rep. 2010;59:980–4. [PubMed] [Google Scholar]
- 6.Bukowski R, Malone FD, Porter FT, Nyberg DA, Comstock CH, Hankins GD, et al. Preconceptional folate supplementation and the risk of spontaneous preterm birth: a cohort study. PLoS Med. 2009;6:e1000061. doi: 10.1371/journal.pmed.1000061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carr DF, Whiteley G, Alfirevic A, Pirmohamed M. Investigation of inter-individual variability of the one-carbon folate pathway: a bioinformatic and genetic review. The pharmacogenomics journal. 2009;9:291–305. doi: 10.1038/tpj.2009.29. [DOI] [PubMed] [Google Scholar]
- 8.Bailey SW, Ayling JE. The extremely slow and variable activity of dihydrofolate reductase in human liver and its implications for high folic acid intake. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:15424–9. doi: 10.1073/pnas.0902072106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suh JR, Herbig AK, Stover PJ. New perspectives on folate catabolism. Annual review of nutrition. 2001;21:255–82. doi: 10.1146/annurev.nutr.21.1.255. [DOI] [PubMed] [Google Scholar]
- 10.Sanderson P, McNulty H, Mastroiacovo P, McDowell IF, Melse-Boonstra A, Finglas PM, et al. Folate bioavailability: UK Food Standards Agency workshop report. Br J Nutr. 2003;90:473–9. doi: 10.1079/bjn2003889. [DOI] [PubMed] [Google Scholar]
- 11.Morris MS, Jacques PF, Rosenberg IH, Selhub J. Circulating unmetabolized folic acid and 5-methyltetrahydrofolate in relation to anemia, macrocytosis, and cognitive test performance in American seniors. The American journal of clinical nutrition. 2010;91:1733–44. doi: 10.3945/ajcn.2009.28671. [DOI] [PubMed] [Google Scholar]
- 12.Bailey RL, Mills JL, Yetley EA, Gahche JJ, Pfeiffer CM, Dwyer JT, et al. Unmetabolized serum folic acid and its relation to folic acid intake from diet and supplements in a nationally representative sample of adults aged > or =60 y in the United States. The American journal of clinical nutrition. 2010;92:383–9. doi: 10.3945/ajcn.2010.29499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Obeid R, Kasoha M, Kirsch SH, Munz W, Herrmann W. Concentrations of unmetabolized folic acid and primary folate forms in pregnant women at delivery and in umbilical cord blood. The American journal of clinical nutrition. 2010;92:1416–22. doi: 10.3945/ajcn.2010.29361. [DOI] [PubMed] [Google Scholar]
- 14.Obeid R, Kirsch SH, Kasoha M, Eckert R, Herrmann W. Concentrations of unmetabolized folic acid and primary folate forms in plasma after folic acid treatment in older adults. Metabolism: clinical and experimental. 2011;60:673–80. doi: 10.1016/j.metabol.2010.06.020. [DOI] [PubMed] [Google Scholar]
- 15.Boilson A, Staines A, Kelleher CC, Daly L, Shirley I, Shrivastava A, et al. Unmetabolized folic acid prevalence is widespread in the older Irish population despite the lack of a mandatory fortification program. The American journal of clinical nutrition. 2012;96:613–21. doi: 10.3945/ajcn.111.026633. [DOI] [PubMed] [Google Scholar]
- 16.Smith AD, Kim YI, Refsum H. Is folic acid good for everyone? The American journal of clinical nutrition. 2008;87:517–33. doi: 10.1093/ajcn/87.3.517. [DOI] [PubMed] [Google Scholar]
- 17.Mason JB. Unraveling the complex relationship between folate and cancer risk. Biofactors. 2011;37:253–60. doi: 10.1002/biof.174. [DOI] [PubMed] [Google Scholar]
- 18.Ulrich CM, Potter JD. Folate and cancer--timing is everything. JAMA. 2007;297:2408–9. doi: 10.1001/jama.297.21.2408. [DOI] [PubMed] [Google Scholar]
- 19.Lucock M, Yates Z. Folic acid fortification: a double-edged sword. Curr Opin Clin Nutr Metab Care. 2009;12:555–64. doi: 10.1097/MCO.0b013e32833192bc. [DOI] [PubMed] [Google Scholar]
- 20.Elmore CL, Wu X, Leclerc D, Watson ED, Bottiglieri T, Krupenko NI, et al. Metabolic derangement of methionine and folate metabolism in mice deficient in methionine synthase reductase. Mol Genet Metab. 2007;91:85–97. doi: 10.1016/j.ymgme.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sittig LJ, Herzing LB, Xie H, Batra KK, Shukla PK, Redei EE. Excess folate during adolescence suppresses thyroid function with permanent deficits in motivation and spatial memory. Genes Brain Behav. 2012;11:193–200. doi: 10.1111/j.1601-183X.2011.00749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morris MC, Evans DA, Bienias JL, Tangney CC, Hebert LE, Scherr PA, et al. Dietary folate and vitamin B12 intake and cognitive decline among community-dwelling older persons. Arch Neurol. 2005;62:641–5. doi: 10.1001/archneur.62.4.641. [DOI] [PubMed] [Google Scholar]
- 23.Leeming RJ, Lucock M. Autism: Is there a folate connection? J Inherit Metab Dis. 2009;32:400–2. doi: 10.1007/s10545-009-1093-0. [DOI] [PubMed] [Google Scholar]
- 24.Zhou YH, Tang JY, Wu MJ, Lu J, Wei X, Qin YY, et al. Effect of folic acid supplementation on cardiovascular outcomes: a systematic review and meta-analysis. PloS one. 2011;6:e25142. doi: 10.1371/journal.pone.0025142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clarke R, Halsey J, Bennett D, Lewington S. Homocysteine and vascular disease: review of published results of the homocysteine-lowering trials. J Inherit Metab Dis. 2011;34:83–91. doi: 10.1007/s10545-010-9235-y. [DOI] [PubMed] [Google Scholar]
- 26.Heseker HB, Mason JB, Selhub J, Rosenberg IH, Jacques PF. Not all cases of neural-tube defect can be prevented by increasing the intake of folic acid. Br J Nutr. 2009;102:173–80. doi: 10.1017/S0007114508149200. [DOI] [PubMed] [Google Scholar]
- 27.Marean A, Graf A, Zhang Y, Niswander L. Folic acid supplementation can adversely affect murine neural tube closure and embryonic survival. Human molecular genetics. 2011;20:3678–83. doi: 10.1093/hmg/ddr289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Butterworth CE, Jr, Tamura T. Folic acid safety and toxicity: a brief review. The American journal of clinical nutrition. 1989;50:353–8. doi: 10.1093/ajcn/50.2.353. [DOI] [PubMed] [Google Scholar]
- 29.Oleinik NV, Krupenko NI, Reuland SN, Krupenko SA. Leucovorin-induced resistance against FDH growth suppressor effects occurs through DHFR up-regulation. Biochem Pharmacol. 2006;72:256–66. doi: 10.1016/j.bcp.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 30.Sauer J, Mason JB, Choi SW. Too much folate: a risk factor for cancer and cardiovascular disease? Curr Opin Clin Nutr Metab Care. 2009;12:30–6. doi: 10.1097/MCO.0b013e32831cec62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matthews RG, Daubner SC. Modulation of methylenetetrahydrofolate reductase activity by S-adenosylmethionine and by dihydrofolate and its polyglutamate analogues. Advances in enzyme regulation. 1982;20:123–31. doi: 10.1016/0065-2571(82)90012-7. [DOI] [PubMed] [Google Scholar]
- 32.Troen AM, Mitchell B, Sorensen B, Wener MH, Johnston A, Wood B, et al. Unmetabolized folic acid in plasma is associated with reduced natural killer cell cytotoxicity among postmenopausal women. The Journal of nutrition. 2006;136:189–94. doi: 10.1093/jn/136.1.189. [DOI] [PubMed] [Google Scholar]
- 33.Hirsch S, Miranda D, Fuentes C, Leiva L, Barrera G, Montoya M, et al. Effect of supraphysiological concentration of serum folate on natural killer cell activity in healthy subjects. e-SPAN Journal. 2012;7:e125–e8. [Google Scholar]
- 34.Farber S, Cutler EC, Hawkins JW, Harrison JH, Peirce EC, 2nd, Lenz GG. The Action of Pteroylglutamic Conjugates on Man. Science. 1947;106:619–21. doi: 10.1126/science.106.2764.619. [DOI] [PubMed] [Google Scholar]
- 35.Choi SW, Mason JB. Folate and carcinogenesis: an integrated scheme. The Journal of nutrition. 2000;130:129–32. doi: 10.1093/jn/130.2.129. [DOI] [PubMed] [Google Scholar]
- 36.Rock CL, Lampe JW, Patterson RE. Nutrition, genetics, and risks of cancer. Annu Rev Public Health. 2000;21:47–64. doi: 10.1146/annurev.publhealth.21.1.47. [DOI] [PubMed] [Google Scholar]
- 37.Mason JB. Folate status: effects on carcinogenesis. In: Bailey LB, editor. Folate in Health and Disease. New York: Marcel Dekker, Inc; 1995. pp. 361–78. [Google Scholar]
- 38.Goldman ID, Chattopadhyay S, Zhao R, Moran R. The antifolates: evolution, new agents in the clinic, and how targeting delivery via specific membrane transporters is driving the development of a next generation of folate analogs. Curr Opin Investig Drugs. 2010;11:1409–23. [PubMed] [Google Scholar]
- 39.Ly A, Lee H, Chen J, Sie KK, Renlund R, Medline A, et al. Effect of maternal and postweaning folic acid supplementation on mammary tumor risk in the offspring. Cancer research. 2011;71:988–97. doi: 10.1158/0008-5472.CAN-10-2379. [DOI] [PubMed] [Google Scholar]
- 40.McKay JA, Williams EA, Mathers JC. Gender-specific modulation of tumorigenesis by folic acid supply in the Apc mouse during early neonatal life. Br J Nutr. 2008;99:550–8. doi: 10.1017/S0007114507819131. [DOI] [PubMed] [Google Scholar]
- 41.Ebbing M, Bonaa KH, Nygard O, Arnesen E, Ueland PM, Nordrehaug JE, et al. Cancer incidence and mortality after treatment with folic acid and vitamin B12. JAMA. 2009;302:2119–26. doi: 10.1001/jama.2009.1622. [DOI] [PubMed] [Google Scholar]
- 42.Figueiredo JC, Grau MV, Haile RW, Sandler RS, Summers RW, Bresalier RS, et al. Folic acid and risk of prostate cancer: results from a randomized clinical trial. Journal of the National Cancer Institute. 2009;101:432–5. doi: 10.1093/jnci/djp019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Larsson SC, Giovannucci E, Wolk A. Folate intake, MTHFR polymorphisms, and risk of esophageal, gastric, and pancreatic cancer: a meta-analysis. Gastroenterology. 2006;131:1271–83. doi: 10.1053/j.gastro.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 44.Cole BF, Baron JA, Sandler RS, Haile RW, Ahnen DJ, Bresalier RS, et al. Folic acid for the prevention of colorectal adenomas: a randomized clinical trial. JAMA. 2007;297:2351–9. doi: 10.1001/jama.297.21.2351. [DOI] [PubMed] [Google Scholar]
- 45.Kim YI. Folic acid supplementation and cancer risk: point. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2008;17:2220–5. doi: 10.1158/1055-9965.EPI-07-2557. [DOI] [PubMed] [Google Scholar]
- 46.Kawakita D, Matsuo K, Sato F, Oze I, Hosono S, Ito H, et al. Association between dietary folate intake and clinical outcome in head and neck squamous cell carcinoma. Annals of oncology : official journal of the European Society for Medical Oncology/ESMO. 2012;23:186–92. doi: 10.1093/annonc/mdr057. [DOI] [PubMed] [Google Scholar]
- 47.Ibiebele TI, Hughes MC, Pandeya N, Zhao Z, Montgomery G, Hayward N, et al. High intake of folate from food sources is associated with reduced risk of esophageal cancer in an Australian population. The Journal of nutrition. 2011;141:274–83. doi: 10.3945/jn.110.131235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Welzel TM, Katki HA, Sakoda LC, Evans AA, London WT, Chen G, et al. Blood folate levels and risk of liver damage and hepatocellular carcinoma in a prospective high-risk cohort. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2007;16:1279–82. doi: 10.1158/1055-9965.EPI-06-0853. [DOI] [PubMed] [Google Scholar]
- 49.French AE, Grant R, Weitzman S, Ray JG, Vermeulen MJ, Sung L, et al. Folic acid food fortification is associated with a decline in neuroblastoma. Clinical pharmacology and therapeutics. 2003;74:288–94. doi: 10.1016/S0009-9236(03)00200-5. [DOI] [PubMed] [Google Scholar]
- 50.Vargas AJ, Thompson PA. Diet and nutrient factors in colorectal cancer risk. Nutr Clin Pract. 2012;27:613–23. doi: 10.1177/0884533612454885. [DOI] [PubMed] [Google Scholar]
- 51.Kotsopoulos J, Kim YI, Narod SA. Folate and breast cancer: what about high-risk women? Cancer causes & control : CCC. 2012;23:1405–20. doi: 10.1007/s10552-012-0022-y. [DOI] [PubMed] [Google Scholar]
- 52.Donkena KV, Karnes RJ, Young CY. Vitamins and prostate cancer risk. Molecules. 2010;15:1762–83. doi: 10.3390/molecules15031762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Roswall N. Folate and lung cancer risk. Lung Cancer. 2010;67:380–1. doi: 10.1016/j.lungcan.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 54.Ulrich CM. Folate and cancer prevention: a closer look at a complex picture. The American journal of clinical nutrition. 2007;86:271–3. doi: 10.1093/ajcn/86.2.271. [DOI] [PubMed] [Google Scholar]
- 55.Ulrich CM, Neuhouser M, Liu AY, Boynton A, Gregory JF, 3rd, Shane B, et al. Mathematical modeling of folate metabolism: predicted effects of genetic polymorphisms on mechanisms and biomarkers relevant to carcinogenesis. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2008;17:1822–31. doi: 10.1158/1055-9965.EPI-07-2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Han SS, Sue LY, Berndt SI, Selhub J, Burdette LA, Rosenberg PS, et al. Associations between genes in the one-carbon metabolism pathway and advanced colorectal adenoma risk in individuals with low folate intake. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2012;21:417–27. doi: 10.1158/1055-9965.EPI-11-0782. [DOI] [PubMed] [Google Scholar]
- 57.Liu AY, Scherer D, Poole E, Potter JD, Curtin K, Makar K, et al. Gene-diet-interactions in folate-mediated one-carbon metabolism modify colon cancer risk. Molecular nutrition & food research. 2012 doi: 10.1002/mnfr.201200180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jhaveri MS, Wagner C, Trepel JB. Impact of extracellular folate levels on global gene expression. Mol Pharmacol. 2001;60:1288–95. doi: 10.1124/mol.60.6.1288. [DOI] [PubMed] [Google Scholar]
- 59.Blount BC, Mack MM, Wehr CM, MacGregor JT, Hiatt RA, Wang G, et al. Folate deficiency causes uracil misincorporation into human DNA and chromosome breakage: implications for cancer and neuronal damage. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:3290–5. doi: 10.1073/pnas.94.7.3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kruman II, Kumaravel TS, Lohani A, Pedersen WA, Cutler RG, Kruman Y, et al. Folic acid deficiency and homocysteine impair DNA repair in hippocampal neurons and sensitize them to amyloid toxicity in experimental models of Alzheimer’s disease. J Neurosci. 2002;22:1752–62. doi: 10.1523/JNEUROSCI.22-05-01752.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Duthie SJ, Hawdon A. DNA instability (strand breakage, uracil misincorporation, and defective repair) is increased by folic acid depletion in human lymphocytes in vitro. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 1998;12:1491–7. [PubMed] [Google Scholar]
- 62.Duthie SJ. Folic acid deficiency and cancer: mechanisms of DNA instability. Br Med Bull. 1999;55:578–92. doi: 10.1258/0007142991902646. [DOI] [PubMed] [Google Scholar]
- 63.Bistulfi G, Foster BA, Karasik E, Gillard B, Miecznikowski J, Dhiman VK, et al. Dietary folate deficiency blocks prostate cancer progression in the TRAMP model. Cancer Prev Res (Phila) 2011;4:1825–34. doi: 10.1158/1940-6207.CAPR-11-0140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Khanna D, Park GS, Paulus HE, Simpson KM, Elashoff D, Cohen SB, et al. Reduction of the efficacy of methotrexate by the use of folic acid: post hoc analysis from two randomized controlled studies. Arthritis Rheum. 2005;52:3030–8. doi: 10.1002/art.21295. [DOI] [PubMed] [Google Scholar]
- 65.Carter JY, Loolpapit MP, Lema OE, Tome JL, Nagelkerke NJ, Watkins WM. Reduction of the efficacy of antifolate antimalarial therapy by folic acid supplementation. Am J Trop Med Hyg. 2005;73:166–70. [PubMed] [Google Scholar]
- 66.van Eijk AM, Ouma PO, Williamson J, Ter Kuile FO, Parise M, Otieno K, et al. Plasma folate level and high-dose folate supplementation predict sulfadoxine-pyrimethamine treatment failure in pregnant women in Western kenya who have uncomplicated malaria. J Infect Dis. 2008;198:1550–3. doi: 10.1086/592715. [DOI] [PubMed] [Google Scholar]
- 67.Salim A, Tan E, Ilchyshyn A, Berth-Jones J. Folic acid supplementation during treatment of psoriasis with methotrexate: a randomized, double-blind, placebo-controlled trial. Br J Dermatol. 2006;154:1169–74. doi: 10.1111/j.1365-2133.2006.07289.x. [DOI] [PubMed] [Google Scholar]
- 68.Greenhalgh J, McLeod C, Bagust A, Boland A, Fleeman N, Dundar Y, et al. Pemetrexed for the maintenance treatment of locally advanced or metastatic non-small cell lung cancer. Health Technol Assess. 2010;14:33–9. doi: 10.3310/hta14suppl2/05. [DOI] [PubMed] [Google Scholar]
- 69.Tisman G, Garcia A. Control of prostate cancer associated with withdrawal of a supplement containing folic acid, L-methyltetrahydrofolate and vitamin B12: a case report. Journal of medical case reports. 2011;5:413. doi: 10.1186/1752-1947-5-413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Crott JW, Choi SW, Ordovas JM, Ditelberg JS, Mason JB. Effects of dietary folate and aging on gene expression in the colonic mucosa of rats: implications for carcinogenesis. Carcinogenesis. 2004;25:69–76. doi: 10.1093/carcin/bgg150. [DOI] [PubMed] [Google Scholar]
- 71.Crott JW, Liu Z, Keyes MK, Choi SW, Jang H, Moyer MP, et al. Moderate folate depletion modulates the expression of selected genes involved in cell cycle, intracellular signaling and folate uptake in human colonic epithelial cell lines. The Journal of nutritional biochemistry. 2008;19:328–35. doi: 10.1016/j.jnutbio.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lewis CM, Smith AK, Kamen BA. Receptor-mediated folate uptake is positively regulated by disruption of the actin cytoskeleton. Cancer research. 1998;58:2952–6. [PubMed] [Google Scholar]
- 73.Oser M, Condeelis J. The cofilin activity cycle in lamellipodia and invadopodia. Journal of cellular biochemistry. 2009;108:1252–62. doi: 10.1002/jcb.22372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bernstein BW, Bamburg JR. ADF/cofilin: a functional node in cell biology. Trends in cell biology. 2010;20:187–95. doi: 10.1016/j.tcb.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhu H, Cabrera RM, Wlodarczyk BJ, Bozinov D, Wang D, Schwartz RJ, et al. Differentially expressed genes in embryonic cardiac tissues of mice lacking Folr1 gene activity. BMC Dev Biol. 2007;7:128. doi: 10.1186/1471-213X-7-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chanson A, Sayd T, Rock E, Chambon C, Sante-Lhoutellier V, Potier de Courcy G, et al. Proteomic analysis reveals changes in the liver protein pattern of rats exposed to dietary folate deficiency. The Journal of nutrition. 2005;135:2524–9. doi: 10.1093/jn/135.11.2524. [DOI] [PubMed] [Google Scholar]
- 77.Oleinik NV, Krupenko NI, Krupenko SA. ALDH1L1 inhibits cell motility via dephosphorylation of cofilin by PP1 and PP2A. Oncogene. 2010;29:6233–44. doi: 10.1038/onc.2010.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Winter-Vann AM, Kamen BA, Bergo MO, Young SG, Melnyk S, James SJ, et al. Targeting Ras signaling through inhibition of carboxyl methylation: an unexpected property of methotrexate. Proc Natl Acad Sci U S A. 2003;100:6529–34. doi: 10.1073/pnas.1135239100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bjorklund NK, Gordon R. A hypothesis linking low folate intake to neural tube defects due to failure of post-translation methylations of the cytoskeleton. Int J Dev Biol. 2006;50:135–41. doi: 10.1387/ijdb.052102nb. [DOI] [PubMed] [Google Scholar]
- 80.Gao Y, Sztul E. A novel interaction of the Golgi complex with the vimentin intermediate filament cytoskeleton. The Journal of cell biology. 2001;152:877–94. doi: 10.1083/jcb.152.5.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gao YS, Vrielink A, MacKenzie R, Sztul E. A novel type of regulation of the vimentin intermediate filament cytoskeleton by a Golgi protein. Eur J Cell Biol. 2002;81:391–401. doi: 10.1078/0171-9335-00260. [DOI] [PubMed] [Google Scholar]
- 82.Fox JT, Stover PJ. Folate-mediated one-carbon metabolism. Vitam Horm. 2008;79:1–44. doi: 10.1016/S0083-6729(08)00401-9. [DOI] [PubMed] [Google Scholar]
- 83.Tibbetts AS, Appling DR. Compartmentalization of Mammalian folate-mediated one-carbon metabolism. Annual review of nutrition. 2010;30:57–81. doi: 10.1146/annurev.nutr.012809.104810. [DOI] [PubMed] [Google Scholar]
- 84.Kelemen LE. The role of folate receptor alpha in cancer development, progression and treatment: cause, consequence or innocent bystander? International journal of cancer Journal international du cancer. 2006;119:243–50. doi: 10.1002/ijc.21712. [DOI] [PubMed] [Google Scholar]
- 85.Banerjee D, Mayer-Kuckuk P, Capiaux G, Budak-Alpdogan T, Gorlick R, Bertino JR. Novel aspects of resistance to drugs targeted to dihydrofolate reductase and thymidylate synthase. Biochimica et biophysica acta. 2002;1587:164–73. doi: 10.1016/s0925-4439(02)00079-0. [DOI] [PubMed] [Google Scholar]
- 86.Gao L, Chalupsky K, Stefani E, Cai H. Mechanistic insights into folic acid-dependent vascular protection: dihydrofolate reductase (DHFR)-mediated reduction in oxidant stress in endothelial cells and angiotensin II-infused mice: a novel HPLC-based fluorescent assay for DHFR activity. Journal of molecular and cellular cardiology. 2009;47:752–60. doi: 10.1016/j.yjmcc.2009.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Liu J, Schmitz JC, Lin X, Tai N, Yan W, Farrell M, et al. Thymidylate synthase as a translational regulator of cellular gene expression. Biochimica et biophysica acta. 2002;1587:174–82. doi: 10.1016/s0925-4439(02)00080-7. [DOI] [PubMed] [Google Scholar]
- 88.Jain M, Nilsson R, Sharma S, Madhusudhan N, Kitami T, Souza AL, et al. Metabolite profiling identifies a key role for glycine in rapid cancer cell proliferation. Science. 2012;336:1040–4. doi: 10.1126/science.1218595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhang WC, Shyh-Chang N, Yang H, Rai A, Umashankar S, Ma S, et al. Glycine decarboxylase activity drives non-small cell lung cancer tumor-initiating cells and tumorigenesis. Cell. 2012;148:259–72. doi: 10.1016/j.cell.2011.11.050. [DOI] [PubMed] [Google Scholar]
- 90.Krupenko SA. FDH: an aldehyde dehydrogenase fusion enzyme in folate metabolism. Chemico-biological interactions. 2009;178:84–93. doi: 10.1016/j.cbi.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Oleinik NV, Krupenko NI, Krupenko SA. Epigenetic silencing of ALDH1L1, a metabolic regulator of cellular proliferation, in cancers. Genes and Cancer. 2011;2:130–9. doi: 10.1177/1947601911405841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Oleinik NV, Krupenko NI, Priest DG, Krupenko SA. Cancer cells activate p53 in response to 10-formyltetrahydrofolate dehydrogenase expression. The Biochemical journal. 2005;391:503–11. doi: 10.1042/BJ20050533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Oleinik NV, Krupenko NI, Krupenko SA. Cooperation between JNK1 and JNK2 in activation of p53 apoptotic pathway. Oncogene. 2007;26:7222–30. doi: 10.1038/sj.onc.1210526. [DOI] [PubMed] [Google Scholar]
- 94.Ghose S, Oleinik NV, Krupenko NI, Krupenko SA. 10-formyltetrahydrofolate dehydrogenase-induced c-Jun-NH2-kinase pathways diverge at the c-Jun-NH2-kinase substrate level in cells with different p53 status. Mol Cancer Res. 2009;7:99–107. doi: 10.1158/1541-7786.MCR-08-0309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Luka Z, Mudd SH, Wagner C. Glycine N-methyltransferase and regulation of S-adenosylmethionine levels. The Journal of biological chemistry. 2009;284:22507–11. doi: 10.1074/jbc.R109.019273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tseng TL, Shih YP, Huang YC, Wang CK, Chen PH, Chang JG, et al. Genotypic and phenotypic characterization of a putative tumor susceptibility gene, GNMT, in liver cancer. Cancer research. 2003;63:647–54. [PubMed] [Google Scholar]
- 97.Huang YC, Lee CM, Chen M, Chung MY, Chang YH, Huang WJ, et al. Haplotypes, loss of heterozygosity, and expression levels of glycine N-methyltransferase in prostate cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2007;13:1412–20. doi: 10.1158/1078-0432.CCR-06-1551. [DOI] [PubMed] [Google Scholar]
- 98.Martinez-Chantar ML, Vazquez-Chantada M, Ariz U, Martinez N, Varela M, Luka Z, et al. Loss of the glycine N-methyltransferase gene leads to steatosis and hepatocellular carcinoma in mice. Hepatology. 2008;47:1191–9. doi: 10.1002/hep.22159. [DOI] [PMC free article] [PubMed] [Google Scholar]