Abstract
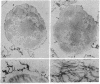
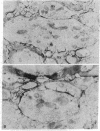
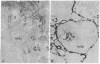
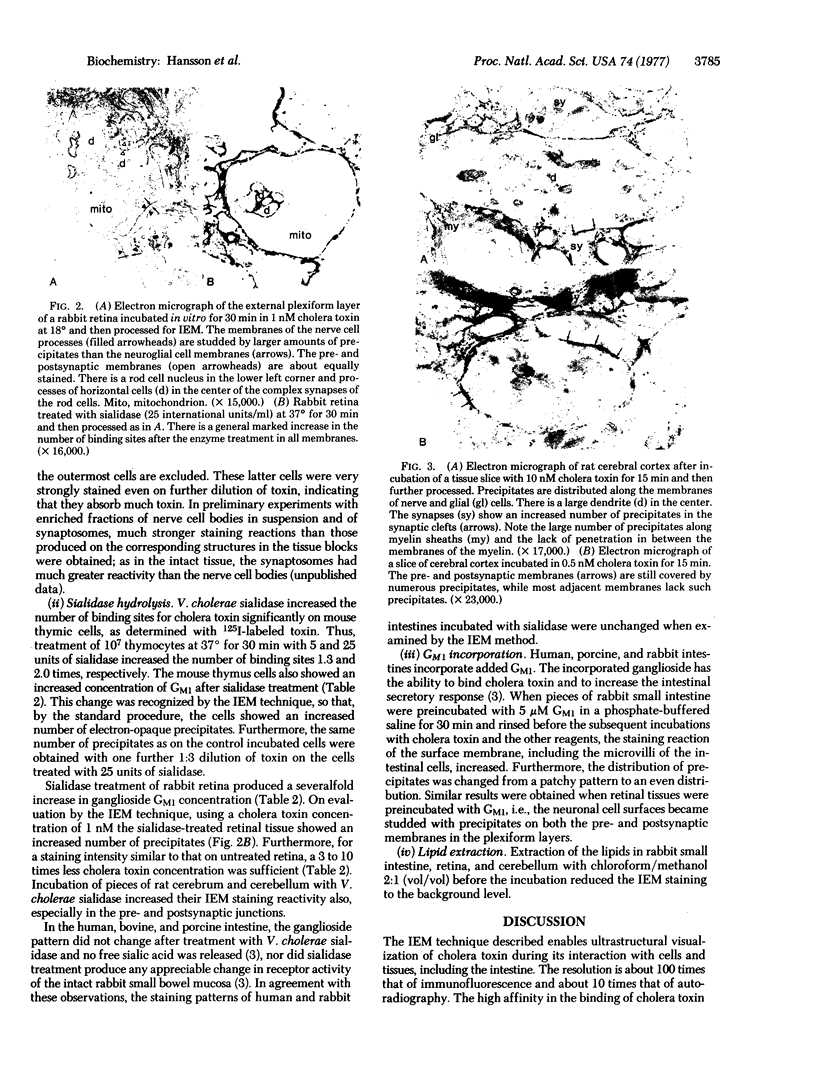
An immunoelectron microscopic method is described for sensitive high-resolution visualization of tissuebound cholera toxin. The principle is to incubate cells or tissue sections with toxin and then to localize the bound toxin with toxin-specific peroxidase (donor:hydrogen-peroxide oxidoreductase; EC 1.11.1.7)-conjugated antibody and enzyme substrate. Thin sections are examined for electron-opaque precipitates in a transmission electron microscope. Because of the specific binding of the toxin to membrane ganglioside GM1, the method can be used for ultrastructural localization of this ganglioside. Semiquantitative data are obtained by titration of the limiting concentration of cholera toxin producing specific precipitates. The specificity of the method was controlled in various ways, including analyses of the correlation between the immunoelectron microscopy results and determinations of ganglioside GM1 in tissues with different ganglioside concentrations, tissues hydrolyzed with Vibrio cholerae sialidase, tissues in which exogenous GM1 has been incorporated, and lipid-extracted tissues. The immunoelectron microscopic method demonstrates that membrane GM1 ganglioside is positioned on the external side exclusively. Cell-bound toxin remains in its original location on the plasma membrane surface of cells below 18°, but appears to be redistributed both laterally and vertically in the membrane of cells incubated at 37° for 30 min or longer. The results of this method indicate that in the central nervous system GM1 is concentrated in the pre- and postsynaptic membranes of the synaptic terminals; a further increase in reactivity of these structures after hydrolysis of the nervous tissue with V. cholerae sialidase suggests that higher gangliosides of the same series are particularly increased in the pre- and postsynaptic junctions.
Keywords: immunoelectron microscopy, membrane receptor, Vibrio cholerae sialidase, ganglioside titration
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMINOFF D. Methods for the quantitative estimation of N-acetylneuraminic acid and their application to hydrolysates of sialomucoids. Biochem J. 1961 Nov;81:384–392. doi: 10.1042/bj0810384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig S. W., Cuatrecasas P. Mobility of cholera toxin receptors on rat lymphocyte membranes. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3844–3848. doi: 10.1073/pnas.72.10.3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuatrecasas P. Gangliosides and membrane receptors for cholera toxin. Biochemistry. 1973 Aug 28;12(18):3558–3566. doi: 10.1021/bi00742a032. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. Interaction of Vibrio cholerae enterotoxin with cell membranes. Biochemistry. 1973 Aug 28;12(18):3547–3558. doi: 10.1021/bi00742a031. [DOI] [PubMed] [Google Scholar]
- Field M: Intestinal secretion: effect of cyclic AMP and its role in cholera. N Engl J Med. 1971 May 20;284(20):1137–1144. doi: 10.1056/NEJM197105202842008. [DOI] [PubMed] [Google Scholar]
- Holm M., Månsson J. E., Vanier M. T., Svennerholm L. Gangliosides of human, bovine and rabbit retina. Biochim Biophys Acta. 1972 Oct 5;280(2):356–364. doi: 10.1016/0005-2760(72)90104-x. [DOI] [PubMed] [Google Scholar]
- Holmgren J., Lindholm L., Lönnroth I. Interaction of cholera toxin and toxin derivatives with lymphocytes. I. Binding properties and interference with lectin-induced cellular stimulation. J Exp Med. 1974 Apr 1;139(4):801–819. doi: 10.1084/jem.139.4.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren J., Lonnroth I. Oligomeric structure of cholera toxin: characteristics of the H and L subunits. J Gen Microbiol. 1975 Jan;86(1):49–65. doi: 10.1099/00221287-86-1-49. [DOI] [PubMed] [Google Scholar]
- Holmgren J., Lönnroth I. Cholera toxin and the adenylate cyclase-activating signal. J Infect Dis. 1976 Mar;133 (Suppl):64–74. doi: 10.1093/infdis/133.supplement_1.s64. [DOI] [PubMed] [Google Scholar]
- Holmgren J., Lönnroth I., Månsson J., Svennerholm L. Interaction of cholera toxin and membrane GM1 ganglioside of small intestine. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2520–2524. doi: 10.1073/pnas.72.7.2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren J., Lönnroth I., Svennerholm L. Tissue receptor for cholera exotoxin: postulated structure from studies with GM1 ganglioside and related glycolipids. Infect Immun. 1973 Aug;8(2):208–214. doi: 10.1128/iai.8.2.208-214.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren J., Svennerholm A. M. Enzyme-linked immunosorbent assays for cholera serology. Infect Immun. 1973 May;7(5):759–763. doi: 10.1128/iai.7.5.759-763.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y. T., Månsson J. E., Vanier M. T., Svennerholm L. Structure of the major glucosamine-containing ganglioside of human tissues. J Biol Chem. 1973 Apr 10;248(7):2634–2636. [PubMed] [Google Scholar]
- Mullin B. R., Aloj S. M., Fishman P. H., Lee G., Kohn L. D., Brady R. O. Cholera toxin interactions with thyrotropin receptors on thyroid plasma membranes. Proc Natl Acad Sci U S A. 1976 May;73(5):1679–1683. doi: 10.1073/pnas.73.5.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Révész T., Greaves M. Ligand-induced redistribution of lymphocyte membrane ganglioside GM1. Nature. 1975 Sep 11;257(5522):103–106. doi: 10.1038/257103a0. [DOI] [PubMed] [Google Scholar]
- SVENNERHOLM L. CHROMATOGRAPHIC SEPARATION OF HUMAN BRAIN GANGLIOSIDES. J Neurochem. 1963 Sep;10:613–623. doi: 10.1111/j.1471-4159.1963.tb08933.x. [DOI] [PubMed] [Google Scholar]
- Vanier M. T., Holm M., Ohman R., Svennerholm L. Developmental profiles of gangliosides in human and rat brain. J Neurochem. 1971 Apr;18(4):581–592. doi: 10.1111/j.1471-4159.1971.tb11988.x. [DOI] [PubMed] [Google Scholar]