Abstract
Since the discovery, over a decade and a half ago, that genetically engineered DNA can be delivered in vaccine form and elicit an immune response, there has been much progress in understanding the basic biology of this platform. A large amount of data has been generated in preclinical model systems, and more sustained cellular responses and more consistent antibody responses are being observed in the clinic. Four DNA vaccine products have recently been approved, all in the area of veterinary medicine. These results suggest a productive future for this technology as more optimized constructs, better trial designs and improved platforms are being brought into the clinic.
DNA vaccines burst into the scientific limelight in the early 1990s. Tang and Johnston described the delivery of DNA into the skin of mice using a ‘gene gun’, in an attempt to deliver human growth hormone as a gene therapy1. The authors felt that this could be a useful technique to generate antibody responses against specific transgene products. At the same time, three presentations at the annual vaccine meeting at the Cold Spring Harbor Laboratory in 1992 reported the use of DNA vectors to drive both humoral and cellular immune responses against pathogens or tumour antigens in vivo. Margaret Liu and her colleagues at the pharmaceutical company Merck reported injecting ‘naked’ plasmids intramuscularly to deliver immunogens that would generate immune responses against influenza virus antigens in mice2. Similarly, Robinson described the ability of DNA plasmids to drive immune responses against influenza virus antigens3. A presentation by Weiner described the ability of plasmids carrying HIV antigens or tumour antigens to produce immune responses and protection from tumour challenge in mice4. Although none of these studies used the same delivery method, formulation or plasmid, together they provided evidence to the scientific community that this simple technique could be developed as an immunization platform.
In the past decade and a half, the DNA vaccine concept has been tested and applied against various pathogens and tumour antigens. In theory, this conceptually safe, non-live vaccine approach is a unique and technically simple means to induce immune responses. Importantly, DNA vaccines affect not only humoral immunity but also cellular immunity — the elusive target of live infection. In particular, DNA vaccines induce killer cytotoxic T lymphocytes (CTLs), suggesting that an important shift had occurred in non-live vaccine platforms. The use of the DNA approach also promised to overcome the safety concerns associated with live vaccines — their reversion risks, as was observed in a subset of primates receiving a live but attenuated simian immunodeficiency virus (SIV) vaccine, and their potential spread to unintended individuals5. In addition, it avoids the risks linked to the manufacture of killed vaccine, as exemplified by the tainting of a polio vaccine with live polio virus owing to a production error6.
DNA vaccines have experienced a recent resurgence in interest. Several technical improvements have contributed to this achievement, including gene optimization strategies, improved RNA structural design, novel formulations and immune adjuvants, and more effective delivery approaches. For example, the use of species-specific codon optimization results in increased protein production on a per-cell basis, leading to enhanced T-cell responses7-10 and antibody induction11-14. These methods, particularly when combined, cause augmented levels of immune responses in rodents as well as large animal models. Importantly, the recent licensing of four veterinary vaccine products — for horses, dogs, pigs and fish — have served to re-energize a field that had been hampered by poor product performance in larger animal models, in non-human primate studies and in human clinical trials.
In this Review we summarize the status of the field in animals and in human clinical trials. We describe important platform optimization strategies that improve expression, potency and immunogenicity. We also highlight creative strategies and important technical achievements that continue to drive this important platform. DNA vaccines have produced enticing results in a wide array of applications, from prophylactic vaccine strategies that target viral, bacterial or parasitic infections to potential therapeutics used to treat infectious diseases, cancers, Alzheimer disease, allergy and autoimmune disorders.
Mechanism of action
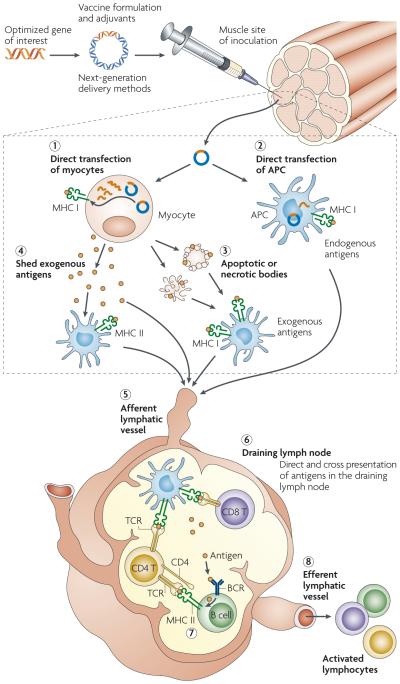
The mechanisms by which DNA vaccines produce antigen-specific immunity in vivo are under intense investigation, with an idealized model presented in BOX 1. The optimized gene sequence of interest is delivered to the skin (intradermally), subcutaneum or muscle by one of several delivery methods. using the host cellular machinery, the plasmid enters the nucleus of transfected local cells (such as myocytes or keratinocytes), including resident antigen presenting cells (APCs). Here, expression of plasmid-encoded genes is followed by generation of foreign antigens as proteins that have been converted to peptide strings. These host-synthesized antigens can become the subject of immune surveillance in the context of both major histocompatibility complex (MHC) class I and class II molecules of APCs in the vaccinated host. Antigen-loaded APCs travel to the draining lymph nodes where they ‘present’ antigenic peptide– MHC complexes in combination with signalling by co-stimulatory molecules to naive T cells. This interaction provides the necessary secondary signals to initiate an immune response and to activate and expand T cells or, alternatively, to activate b cell and antibody production cascades. Together, both humoral and cellular immune responses are engendered.
Box 1. Induction of cellular and humoral immunity by DNA vaccines.
The optimized gene sequence of interest (for example, antigenic or immune adjuvant genes) is generated synthetically or by PCR. This sequence is enzymatically inserted into the multiple cloning region of a plasmid backbone, purified, and then delivered to the inoculation site by one of several delivery methods to either the skin, subcutaneum or muscle. Using the host cellular machinery, the plasmid enters the nucleus of transfected myocytes (1) and of resident antigen presenting cells (APCs) (2); here, the plasmid components initiate gene transcription, which is followed by protein production in the cytoplasm and the consequent formation of foreign antigens as proteins or as peptide strings. The cell provides endogenous post-translational modifications to antigens that reproduce native protein conformations and the cell customizes the antigens in a similar manner to the pathways induced by live infection with recombinant vectors.
These host-synthesized antigens then can become the subject of immune surveillance in the context of both major histocompatibility complex class I (MHC I) and MHC II proteins of the vaccinated individual. APCs have a dominant role in the induction of immunity of DNA vaccines by presenting vaccine-derived endogenous peptides on MHC I molecules. This can follow either direct transfection by the plasmid vaccine (2) or cross-presentation of cell-associated exogenous antigens; for example, owing to APC engulfment of apoptotic transfected cells (3). In addition, APCs mediate the display of peptides on MHC II molecules after secreted protein antigens that have been shed from transfected cells are captured and processed within the endocytic pathway (4). Antigen-loaded APCs travel to the draining lymph node (DLN) via the afferent lymphatic vessel (5) where they present peptide antigens to naive T cells via MHC and the T cell receptor (TCR) in combination with co-stimulatory molecules, providing the necessary secondary signals to initiate an immune response and expansion of T cells (6). In response to peptide-bound MHC molecules and co-stimulatory secondary signals, activated CD4 T helper cells secrete cytokines during cell-to-cell interaction with B cells and bind to co-stimulatory molecules that are required for B cell activation (7). In addition, shed antigen can be captured by specific high affinity immunoglobulins (B cell receptors; BCLs) expressed on the surface of B cells in the DLN; these then present processed antigen to CD4 T helper cells, thereby facilitating the induction of an effective B cell response. In theory, once migrating T cells have been primed in the DLN they could be restimulated and further expanded at the site of immunization by presentation of the peptide–MHC complexes displayed by transfected muscle cells. These processes coordinately elicit specific immunity against plasmid-encoded antigen by activating both T and B cells, which, now they are ‘armed’, can travel through the efferent lymphatic system (8) and provide a surveillance system. Together, the two arms of the immune system, which are induced specifically following DNA vaccination, can create a powerful defence against most infectious diseases.
Preclinical studies
A proliferation of studies on small animals followed the initial reports on DNA vaccines. These approaches focused primarily on antibody induction in mice and included important targets, such as herpes simplex virus, hepatitis B virus, HIV, influenza virus and others, including viruses labelled as ‘bioterroristic agents’15-17. The DNA platform generated a great deal of excitement preclinically as protective immunity was induced by such vaccines against a broad range of virus families. Some examples of this application include strategies that target the Rabies virus, Filovirus, Flavivirus, Togavirus and bunyanvirus, as well as the bacterial disease agent anthrax and the malaria parasite18,19. In addition, owing to the ability of DNA vaccines to drive cellular immunity, cancer immune therapy agents were targeted, including those relevant to melanoma, lymphoma, and colon, prostate and breast cancers. For viruses and bacteria that have high genetic diversity and require CTLs for defence against infection, DNA platforms have been tested as immune therapy for different chronic viral infections, including human papilloma virus, hepatitis C virus and HIV. Furthermore, the CTL induction engendered by DNA approaches seems to be particularly relevant to such variable pathogens.
These results have led to numerous DNA vaccine clinical trials in humans for a variety of infectious agents and cancers, as well as for immune modulation strategies to treat asthma and allergy by targeting host production of immunoglobulin e (Ige). In addition, trials have tested the ability of the DNA vaccine platform to deliver gene therapy agents to treat specific chronic ailments.
Initial DNA vaccine studies in humans
The first DNA vaccine studies in humans, initiated almost 15 years ago, were limited in concept and high on optimism. The goals of the various clinical trials were to demonstrate the safety and tolerability of the candidate vaccines, and to and explore the limits of immune potency of specific DNA vaccines in humans (TABLE 1). The earliest Phase I clinical trial for a DNA vaccine was of an HIV-1 candidate tested in individuals infected by HIV-1, followed by studies in volunteers who were not infected by HIV-1 (Ref. 20). other prophylactic and therapeutic DNA vaccine trials followed, including trials that tested DNA vaccines against cancer, influenza, malaria, hepatitis b and other HIV-1 candidates21-25. These trials demonstrated that the DNA vaccine platform is well tolerated and safe, as no adverse events were reported and all studies went to completion. In this section, we focus on the results of two main applications for which prophylactic vaccines have been tested in the clinic. owing to the wealth of studies on infectious disease targets we focus on this class first, including HIV-1, and then we discuss therapeutic vaccines, including those for cancer therapy, which merit attention owing to the relevance of these studies for many diseases that involve cell proliferation.
Table 1.
Current DNA product Phase I to III open clinical trial targets
| Number of open trials | Category | Diseases and conditions treated |
|---|---|---|
| Phase I | ||
| 54 | Cancer | Melanoma, glioblastoma, lymphoma, cancer cachexia, acute lymphoblastic leukaemia, head and neck squamous cell carcinomarenal cell carcinoma, chronic lymphocytic B leukaemia, Hodgkins lymphoma, and colorectal, prostate, pancreatic, lung and breast cancer |
| 6 | Cardiovascular | Re-endothelialization, claudication, limb ischaemia, angiogenesis and Buerger disease |
| 5 | Healthy volunteers | HIV vaccine safety |
| 2 | Infectious disease | Chronic hepatitis B and HIV-1 |
| 1 | Neurological | Effect of human insulin-like growth factor for cubital tunnel syndrome |
| 1 | Ocular | Retinitis pigmentosa |
| 3 | Other | Erectile dysfunction, type I diabetes mellitus and overactive bladder syndrome |
| Phase II | ||
| 10 | Cancer | Melanoma, angioendothelioma, squamous cell carcinoma of the head and neck, lung cancer, mantle cell carcinoma and pancreatic adenocarcinoma |
| 4 | Cardiovascular | Claudication (cramp-like pains in the legs cause by poor circulation), peripheral ischaemic ulcers, limb ischaemia |
| 3 | Neurological | Relapsing remitting multiple sclerosis, diabetic neuropathy and nerve conduction velocity |
| 3 | Ocular | Atrophic macular degeneration and retinitis pigmentosa |
| Phase III | ||
| 1 | Cancer | Lung cancer |
| 1 | Cardiovascular | Limb ischaemia |
Overall, the first-generation DNA vaccines failed to demonstrate high levels of vaccine-specific immunity in humans, although we have learned a great deal about the safety and delivery of this platform. However, new modifications and improvements to the technology are encouraging.
DNA vaccines against HIV-1
Many current and recently completed trials for HIV-1 have used recombinant viral vector platforms or DNA vaccines in combination with other traditional vector-based vaccines. Immune responses that have been primed by the delivery of a DNA-encoded antigen can be boosted by the administration of recombinant protein or recombinant viruses, through so-called ‘prime-boost’ strategies. In preclinical animal models these approaches have increased the number of neutralizing antibodies and also boosted DNA-primed CTL responses.
One important viral vector platform is the modified vaccinia virus Ankara (MVA). GeoVax has used MVA in a two-pronged prime-boost approach. The DNA vaccine contains several HIV antigens as the priming immunogen, which is followed by boosting with a recombinant MVA that also contains HIV antigens; this combination approach is well tolerated and produces detectable and reproducible cellular immunity in humans26. A DNA prime followed by an MVA boost was also studied by the McMichael group27. This DNA vaccine was composed of a string of DNA epitopes for HIV and a DNA fragment encoding the subtype A HIV gag antigen as the priming immunogens; this was followed by an MVA boost that contained the same vaccine antigens. However, like other epitope vaccines delivered by different DNA approaches, only low-level T cell responses were induced by this vaccine, suggesting that the function of epitope-based vaccines in humans warrants further study.
In addition, both Novartis and the laboratory of Shan Lu at the university of Massachusetts have been exploring DNA-plasmid priming followed by homologous antigenic-protein boosting in human studies. The Novartis approach uses DNA that is formulated in polymers, for example, polylactice-coglycolides, boosted with a novel trimeric gp140 envelope protein antigen. by contrast, the university of Massachusetts group focused on a multiplasmid cocktail and a multicocktail envelopeprotein boost strategy. both studies have shown excellent safety records and strong induction of antibody responses as well as CD4+ T cell responses, but only limited neutralizing antibody responses have been induced. These studies clearly support the view that this platform combination can be useful in the clinic for vaccine targets that are CD4+ T cell dependent; however, new approaches that lead to the induction of more potent CTL responses continue to be a focus for HIV-1 studies.
The most potent recombinant vector platform in humans for generation of cellular immunity seems to be the adenovirus platform. The viral vector generated using adenovirus serotype 5 (Ad5) is the most potent, although vectors based on new and promising primate serotypes, as well as rare human serotypes, are also being developed. Much of the work on Ad5-based vaccines is being applied to the HIV-1 vaccine arena. Important studies from Merck and the Vaccine Research Center at the National Institutes of Health have directly compared CTL induction generated in humans by plasmid vectors versus the Ad5 recombinant vector. both studies showed that plasmid DNA was at least four times less potent in magnitude and response rate than Ad5 vaccines that contained similar HIV antigen cassettes. However, a recent comparison of the immune responses induced by the Ad5 viral vector approach that was stopped by Merck recently28 and that of the Vaccine Research Center, which uses a prime-Ad5 boost approach29,30, demonstrated little difference in the immune responses elicited by the two vaccination strategies, in contrast to data from preclinical animal model testing. This study by Merck again illustrates the need to improve the immune potency of the DNA approach in priming studies. The lack of translation from animal model to humans further demonstrates the need for continued improvements in delivery technology and other optimizations of the important prime-boost strategy, and underscores the limitation of naked plasmid approaches in this format.
Cancer immune therapies
Many cancer antigens are excellent immune therapy targets for DNA vaccines. As such, cancer vaccines resemble drug therapies in that they can be re-administered on a regular repetitive schedule without vector interference concerns. In mouse models, DNA vaccines have been successfully directed against a wide variety of tumours, almost exclusively by driving strong cellular immune responses in an antigen-specific fashion. Furthermore, tumour burden has been decreased or even obliterated by novel DNA vaccine strategies that deliver cytokines as plasmids directly into tumours.
For cancer immune therapies that have moved into the clinic, current candidates are well tolerated. There are also indications of immune responses in patients with melanoma and prostate cancer, and in some cases suggestive clinical benefit has been reported. on the basis of meeting clinical endpoints in a Phase II study, Vical has moved forward a Phase III study of interleukin-2 cytokine delivery as a plasmid formulation for treating melanoma. This is an important milestone for the DNA vaccine field. Furthermore, studies that include the use of electroporation to deliver antigens or cytokines directly into tumours are giving interesting results. In one important Phase I clinical trial, plasmid DNA encoding the potent cytokine interleukin-12 was delivered directly into melanoma lesions, followed by electroporation. Clinical remissions of tumours were reported, even for lesions that were distant from the injection site in some patients31. The use of helper toxoids linked to T cell epitopes delivered by electroporation to enhance immune responses against prostate cancer is also promising32,33. Some interesting newer cancer studies target viral antigens directly. These new approaches are likely to promote the growth of the DNA cancer immune therapy field.
Taken together, the selected trials for DNA vaccines described here have shown that immune responses can be generated in humans, but they also highlight the need for increased potency if this vaccine technology is to be effective. Towards this end, it will be crucial to analyse the results of ongoing research in the clinic, specifically pertaining to the success or failure of certain design and delivery methodologies for these next-generation platforms. In addition, attention to successful licensure of DNA products in the veterinary arena will provide useful information for future human clinical study designs, as described in more detail below.
Licensed veterinary DNA vaccines and therapies
In the past 3 years, four DNA products have been licensed for animal use. one against West Nile virus34 in horses, one against infectious haematopoietic necrosis virus35 in schooled salmon, one for treatment of melanoma in dogs36 and the most recent licensure, growth hormone releasing hormone (GHRH)37 product for foetal loss in swine (TABLE 2). These licensures are an important validation of the DNA vaccine platform because they illustrate its commercial potential. Moreover, the success of these products show that DNA vaccines can be manufactured to scale and at low cost, especially in the case of the fish vaccine for infectious haematopoietic necrosis virus, and that large animals under specific circumstances can be successfully protected or treated by specific DNA vaccineapproaches.
Table 2.
Current licensed DNA therapies
| Vaccine target | Product name | Company involved | Date licensed and country |
Target organisms |
Benefits |
|---|---|---|---|---|---|
| West Nile virus | West Nile Innovator |
Centers for Disease Control and Prevention and Fort Dodge Laboratories |
2005 USA | Horses | Protects against West Nile virus infection |
| Infectious haematopoietic necrosis virus |
Apex-IHN | Novartis | 2005 Canada | Salmon | Improves animal welfare, increase food quality and quantity |
| Growth hormone releasing hormone |
LifeTide-SW5 | VGX Animal Health | 2007 Australia | Swine and food animals* |
Increases the number of piglets weaned in breeding sows; significantly decreases perinatal mortality and morbidity |
| Melanoma | Canine Melanoma Vaccine |
Merial, Memorial Sloan–Kettering Cancer Center and The Animal Medical Center of New York |
2007 USA, conditional license |
Dogs | Treats aggressive forms of cancer of the mouth, nail bed, foot pad or other areas as an alternative to radiation and surgery |
Refers to agricultural animals as opposed to pet animals — a higher level of stringency is required for approval for animals that are part of the food chain.
These approvals are ground-breaking in several respects. For example, the melanoma vaccine (Canine Melanoma Vaccine) is the first licensed cellular immune therapy for cancer. GHRH (LifeTide-SW5) is the first licensed gene therapy product and the first licensed electroporation-delivered product for any application. It could be argued that the use of DNA in these animal products represents an easy target in the DNA vaccine arena, compared with potential human applications. For example, the antibody titres induced by the West Nile virus vaccine in horses are very low, suggesting a low threshold for immune protection from this specific pathogen, at least in this species. However, the canine melanoma immune therapy, a model system with clear similarity to human disease, and the electroporation delivery of GHRH in pigs, a large species, argue differently. These two clinical veterinary successes suggest that, with some adjustments, both of these products might give rise to human analogues that can be used to alleviate human disease. These product successes bode well for the sustainability and growth of the field as they are probably the tip of the iceberg regarding the practicality of DNA vaccines as formulated products for animal health.
Safety issues
Advantages of DNA vaccines
Whereas traditional vaccines rely on the production of antibodies through the injection of live attenuated virus, killed viral particles or recombinant viral proteins, DNA vaccines can be constructed to function with many encoded safety features while retaining the specificity of a subunit vaccine. As DNA vaccine plasmids are non-live, non-replicating and non-spreading, there is little risk of either reversion to a disease-causing form or secondary infection. In addition to their safety, DNA vaccines are highly flexible, encoding several types of genes including viral or bacterial antigens, and immunological and biological proteins. DNA vaccines are stable, are easily stored and can be manufactured on a large scale. They also, for example, bypass concerns that adventitial agents might be transferred from tissue-culture lines to the vaccinated individual. Many potential advantages of DNA vaccines are summarized in TABLE 3.
Table 3.
Advantages of DNA vaccination
| Commendable qualities | Attributes |
|---|---|
| Design | Synthetic and PCR methods allow simple engineering design modifications |
| Optimization of plasmids through codon and RNA structure changes | |
| Brings the power of genomics to vaccine construction | |
| Time to manufacture | Rapid production and formulation |
| Reproducibile, large-scale production and isolation | |
| Safety | Unable to revert into virulent forms, unlike live vaccines |
| In contrast to some killed vaccines, efficiency does not require use of toxic treatments | |
| No significant adverse events in any clincal trial — many thousands vaccinated so far | |
| Stability | More temperature-stable than conventional vaccines |
| Long shelf life | |
| Mobility | Ease of storage and transport |
| Likely not to require a cold chain | |
| Immunogenicity | Induction of antigen-specific T and B cell responses similar to those elicited by live attenuated platforms |
Cold chain, the requirement to keep a vaccine chilled or frozen to protect its potency from degrading at room temperature over the time frame from manufacture to use.
Potential safety concerns
Issues have been raised with regard to the safety of DNA vaccines, these include the potential to integrate into cellular DNA, the development of autoimmunity, and the possibility of antibiotic resistance. DNA vaccines that are currently being tested do not show relevant levels of integration into host cellular DNA38-43. If integration is detected at all, it typically occurs at rates that are orders of magnitude below the spontaneous mutation frequency44. However, vectors that are modified or adjuvanted with the goal of increasing immunogenicity could increase the chances of integration. A further concern is that an integrated vaccine might cause insertional mutagenesis through the activation of oncogenes or the inactivation of tumour suppressor genes. In addition, an integrated plasmid DNA vaccine could, in theory, result in chromosomal instability through the induction of chromosomal breaks or rearrangements. However, none of these concerns have been witnessed in the preclinical or clinical evaluation of DNA products.
With regards to the development of autoimmunity induced by DNA vaccination, preclinical studies in non-human primates and early studies in humans did not detect increases in anti-nuclear or anti-DNA antibodies. Participants in human trials of DNA vaccines are followed for possible signs and symptoms of autoimmunity, and laboratory markers of autoimmunity are sometimes monitored as well. To date, there has been no convincing evidence of autoimmunity developing in association with a DNA vaccine20,23,45-47.
A third issue regarding DNA vaccines involves antibiotic resistance. Large-scale manufacture of a DNA vaccine involves enriching cultured cells for the plasmid by virtue of its antibiotic-resistant marker. Concern has been raised that resistance to the same antibiotic might be introduced in participants and transferred into carried bacteria when the plasmid is used in clinical trials. However, the antibiotic resistance genes contained by vaccine plasmids are restricted to those antibiotics — in particular the kanamycin restriction element — that are not commonly used to treat human infections. Alternative strategies that do not use antibiotic selection at all are also important48-50 and are being explored.
In response to these various safety concerns, summarized in TABLE 4, the european union51 and the US Food and Drug Association (FDA)52-56 have developed specific advice on safety and testing of DNA vaccines. efforts to examine integration, antibiotic resistance and the induction of autoimmunity are being followed even as an impressive unblemished record of clinical safety is continuing to be expanded upon. based on this outstanding safety record the focus of the field has shifted to optimizing immune induction.
Table 4.
Potential concerns regarding DNA vaccination
| Theoretical issues | Concern | Resolution |
|---|---|---|
| Integration | DNA vaccines integrate into cellular DNA owing to optimized expression plasmids, resulting in insertional mutagenesis, chromosomal instability, or activation or inactivation of tumour suppressor genes |
US Food and Drug Administration requires integration studies for new DNA products using accepted assays in animals before beginning human trials |
| Autoimmunity | Development of autoimmune disorders against patient DNA | Studies have shown this result is unlikely: no anti-nuclear or disease associated anti-DNA antibodies have been detected |
| Development of autoantibodies against immune adjuvants | Examine patients for signs of autoimmunity using laboratory markers |
|
| Antibiotic resistance | Production process involves selection of bacterial cells using antibiotic resistance, which is conferred by a plasmid gene. |
Antibiotic resistance in plasmid is driven by bacterial origin of replication (not mammalian) |
| There is a risk that antibiotic resistance is transferred to patients receiving vaccine through the unintentional transfer of bacteria |
Antibiotics used are restricted to antibiotics not commonly used to treat human infections |
|
| Low immunogenicity |
First-generation DNA plasmids elicit low levels of T cell and B cell memory |
Use novel formulations, immune plasmid adjuvants and delivery systems to enhance immunogenicity. Prime-boost approaches are common in clinical studies |
DNA vaccine platform: room for improvement
The principal issue regarding the future of DNA vaccines concerns improving their immunogenicity in larger animals and in humans. The DNA vaccine platform has driven significantly weaker immune responses in non-human primates and in humans compared with mice. It seems to be less immunogenic compared with recombinant viral vectors such as adenoviral vectors or recombinant protein for induction of antibody responses. However, DNA vaccine technology efforts are ongoing to optimize the platform to increase antigen expression and vaccine immunogenicity using several strategies that are discussed below.
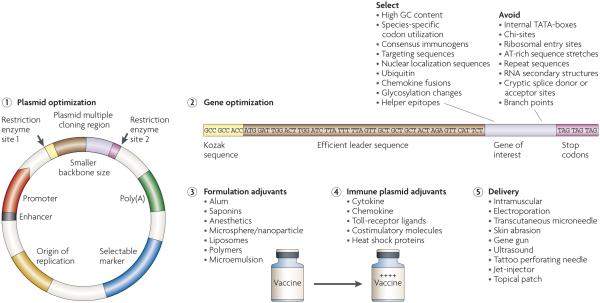
As shown in FIG. 1, there are several ways in which antigen expression and immunogenicity can be improved for the DNA vaccine platform. These include optimization of the transcriptional elements in the plasmid backbone with the aim to improve antigen expression levels, strategies to improve protein expression of the gene of interest, inclusion of adjuvants in the formulation or as immune modulators, and the use of next-generation delivery methods.
Figure 1. DNA vaccines: optimization strategies to enhance immunogenicity.
DNA vaccine technology has been the target of ongoing efforts to optimize the platform to increase antigen expression and vaccine immunogenicity (see main text for a detailed explanation of each step). There are currently several ways in which antigen expression and immunogenicity can be improved for the DNA vaccine platform. These include: optimization of the transcriptional elements on the plasmid backbone (1); strategies to improve protein expression of the gene of interest (2), including factors to avoid (for example, chi-sites, which are sequences that encourage crossing-over to occur at that site); inclusion of formulation adjuvants (3) or immune plasmid adjuvants (4); and the use of next-generation delivery methods (5). Several formulations have been developed and are being testing at all stages of preclinical and clinical development for their ability to enhance antigen expression and immunogenicity. These mechanisms include encapsulation and protection of DNA from extracellular degradation through to particle trapping and high-velocity delivery, with the ultimate goal of introducing the plasmid directly into the cytosol of target cells. In addition, immune adjuvant classes that encode immune modulatory molecules that target death receptors, growth factors, adhesion molecules, cytokines and chemokines as well as Toll receptor ligands exist. It should be noted that many of these plasmid optimization and formulation strategies, as well as the adjuvant systems, are used in combination with novel delivery mechanisms that result in an overall enhanced vaccine platform.
Optimization of transcriptional elements
Several laboratories have identified methods to optimize the transcriptional elements in the plasmid backbone so that gene transcription and expression can be improved. An important component of the plasmid is the promoter that drives expression of the gene of interest.
Microbial gene promoters are not necessarily optimized for driving optimal mammalian gene expression. early studies therefore used strong promoters from human oncogenic viruses such as Rous sarcoma virus57 or SV40 (Ref. 58). However, more recently, promoters from non-carcinogenic sources that are equally effective have been used, including one from human cytomegalovirus (CMV)59 (reviewed in Ref. 60). For most vaccine plasmids, the human CMV promoter is a common choice because it promotes high-level constitutive expression in a wide range of mammalian cells, and does not suffer from downstream read-through as might be expected from a strong promoter. Alternatively, the use of host tissue-specific promoters avoids constitutive expression of antigens in inappropriate tissues. For example, the use of the promoter of the muscle creatine kinase gene leads to the induction of antibody and T cell responses, although levels were at least tenfold lower than plasmids that contain the CMV promoter61,62. These data suggest that the use of host-cell promoters limits expression and, ultimately, immunogenicity, which might be an advantage for gene delivery. Moreover, promoters with significant homology to host-cell sequences might need to be optimized for improved human clinical outcomes.
A second important modification to the antigenic plasmids is the inclusion of a termination site, or poly(A) signal site, that is 11–30 nucleotides downstream from a conserved sequence (AAuAAA) at the 3′ end of the mRNA. This signal is required for proper termination of transcription and export of the mRNA from the nucleus. Many DNA vaccines use the bovine growth hormone terminator sequence63 or endogenous terminators that are downstream from the oRF of the gene of interest to ensure proper transcriptional termination. It remains to be seen whether modifications to the polyadenylation and termination signals influence gene expression, although early reports are promising64.
Both enhancer elements and transcriptional transactivators can enhance promoter activity when placed either upstream or downstream of the oRF. Some of the transactivator genes that have been studied are of viral origin. For example, the regulatory R region from the 5′ long terminal repeat (LTR) of human T-cell leukaemia virus type 1 acts as a transcriptional and post-transcriptional enhancer; the resulting CMV–R DNA vaccines elicit substantially higher specific cellular immune responses to HIV-1 compared with the analogous parental DNA vaccines in both mice and cynomolgus monkeys65. However, the use of regulatory enhancers of viral origin might not be well received in such platforms owing to their association with oncogenesis. Further experimentation with non-viral transactivators66 or enhancer elements17,65-68 for DNA vaccine plasmids is therefore important.
Optimization of regulatory elements would be a simple and effective strategy to augment the immunogenicity of DNA vaccines in mice and primates, and further study is needed for successful translation of this strategy in the clinic. The use of high-efficiency origins of bacterial replication relevant for the bacterial strains used for production can also markedly improve the quantity of plasmid product.
Enhancing protein production
One strategy that is used to improve the expression of the transgene product is to optimize the initiation start site for protein synthesis, because endogenous sites of viruses and bacteria might not be optimal for expression in mammalian cells. Modification of the Kozak consensus sequence69 is a focus for this purpose. ensuring correct termination is also important. Double stop-codons can be added to prevent read through, which could lead to oversized and incorrectly folded products and interfere with mRNA instability.
One of the most effective ways to increase protein production is through the use of codon optimization70. Because immunogenicity depends on the effective translation and transcription of the antigenic protein, gene expression can be greatly increased by adopting species-specific codon changes. This procedure results in increased protein production, leading to enhanced T cell7-10 and antibody11-14 responses.
RNA optimization can also lead to more efficient translation through several important modifications, including removal of instability elements that lower expression; these elements include secondary mRNA structures that can inhibit ribosomal loading and cryptic sequences that inhibit nuclear export of mRNA71-73. High antigenic expression rates and prolonged mRNA stability are not only crucial for heterologous mammalian expression, but are important for the generation of effective DNA vaccines10.
The addition of leader sequences can enhance the stability of mRNA and contribute to translational efficiency. For example, the signal sequence for the HIV-1 envelope glycoprotein (env) delays its own cleavage, therefore slowing down the rate of gp120 folding inside the endoplasmic reticulum74. A marked increase in protein production was detected when the native HIV-1 env leader75, or native leaders encoded by vaccinia-expressing Mycobacterium tuberculosis antigens76, was replaced by the human tissue plasminogen activator leader sequence. enhanced expression of plasmid antigens is also observed using the efficient leader from the IgE gene77-80.
Engineering vaccine antigens that elicit broadly cross-neutralizing antibodies has been a challenge, in particular for HIV-1. To enhance the immunogenicity of conserved neutralization epitopes on env, elimination of glycosylation sites has been investigated81. This treatment can increase antibody titre, although the effect of such structural modifications on the neutralization phenotype is not as clear. Moreover, the insertion of proteolytic cleavage sites between antigenic sequences in the gene of interest to generate fused proteins or peptides might be important in CTL processing. Similarly, recombinant proteins containing fusogenic sequences provide a promising system to induce cytotoxic T cells by live-vector vaccines by destabilizing the phagosome membrane so that epitopes can reach the cytosol82.
Recently, much attention has focused on the development of consensus immunogens as transgenes for immunization owing to their importance for targeting variable pathogens10,16,83-86. Several other strategies to determine consensus sequence include the analysis of ancestral sequences87, as well as more advanced methods for epitope scanning; for example, by using T cell assays to identify reactive epitopes10, predictive computer analysis software88,89 or phage display90. In addition, the use of DNA libraries and protein sequence data for target epitopes that have the ability to be recognized by the human immune system have been used to identify novel epitopes for potential immunotherapy targets, such as viruses, bacteria, parasites, tumour-associated antigens or other self-antigens associated with the pathogenesis of disease91-93. Such constructs can drive improved cross-reactive immune responses, particularly at the T cell level and in mouse systems. Primate and human studies of epitope strings as DNA vaccines have not indicated that these approaches are as potent as whole antigen sequences. However, it is hoped that the potency of this strategy will be improved by new approaches to link epitopes, by the inclusion of helper T cell sequences, and by new epitope selection technologies — particularly when coupled with new delivery strategies. In the cancer model, for example, tumour-specific antigen epitopes have been linked to fragments of tetanus toxoid to improve helper T cell responses94. This strategy has generated strong protective immunity in model systems and is a promising clinical approach for supplying novel T cell help.
Improving immunogenicity through formulation
One current trend in DNA vaccine formulation is the use of biodegradable polymeric microparticles (reviewed in Ref. 95) and liposomes. The prospects for the broad application of microparticle-based and liposome-based delivery systems for DNA vaccines are excellent — their utility for delivery and enhanced immunogenicity in several different host and antigenic vaccine platforms has been shown in mice, non-human primates and humans96-101.
Plasmid DNA is trapped on the surface of the polymers, for example, polylactice-coglycolides or chitosan, and is delivered systemically or directly to mucosal surfaces (orally or via the respiratory tract), where the complex is taken up by denritic cells (DCs). This results in upregulation of DC activation markers and further augments systemic and mucosal immune responses102,103. examples of important molecules moving forward as formulation adjuvant or delivery methods for DNA vaccines include polyethyleneimine, amine-functionalized polymethacrylates, cationic poly(β-amino esters), poloxamers and polyvinylpyrrolidone polymers. These have proven to be successful in disease models — for example, in the delivery of gene therapeutic agents for cystic fibrosis104-108. The poloaxmer CRL1005 has been successful in preclinical models for simian HIV vaccine strategies109. Importantly, Vaxfectin, a related molecule, has enhanced antibody responses to a DNA vaccine targeting influenza in human clinical studies110. Most of these types of formulation molecules have not shown significant clinical benefit, although recent data from the Vaxfectin trial (performed by Vi cal) indicate a clear improvement in immune responses in humans. other results from trials are eagerly awaited.
In addition to polymers, ongoing studies have shown that liposome vehicles can protect DNA from degradation by serum proteins during transfer of DNA across membranes and after the release of genetic material following fusion with endosomes111,112. Because liposomes can be prepared with significant structural versatility, including vesicle surface charge (both cationic and anionic liposomes can be made), size, lipid content and co-delivery with other adjuvants, they offer considerable flexibility113-115 towards vaccine optimization and have been shown to induce cellular and humoral immunity110,116-119.
Improving immunogenicity by including immune modulatory adjuvants
In addition to optimizing the DNA antigenic plasmid construct, a separate approach is to include immune modulatory genes as cassettes as part of the plasmid vaccines cocktail. Multiple laboratories have reported that co-injection of plasmids encoding cytokines, chemokines or co-stimulatory molecules can have a substantial effect on the immune response to plasmid-encoded antigen; these include studies in multiple viral and cancer antigen systems. For example, in non-human primates interleukin-12 is a potent DNA vaccine adjuvant for cellular immunity. In addition, many other classes of immune modulatory molecule exist that target death receptors, growth factors, adhesion molecules, other cytokines120 and chemokines as well as Toll-receptor ligands. The growing number of immune adjuvant formulations described above can be used individually or in combination to maximize effects. In addition, synthetic oligodeoxynucleotides containing unmethylated CpG motifs act as immune adjuvants in mice, as they boost the humoral and cellular response to co-administered antigens121. Moreover, the large number of ongoing preclinical studies underscores the importance and usefulness of these adjuvants owing to their pleiotropic effects on the growth, differentiation, maturation, survival and proliferation of many different cell types. DNA vaccination strategies that incorporate immune modulatory formulation adjuvants should improve responses in the clinic, in both prophylactic and therapeutic studies.
Improving immunogenicity by using next-generation delivery strategies
The most common route of immunization used in DNA vaccine studies is the intramuscular route. However, several studies have demonstrated the importance of direct transfection of APCs122,123; by contrast, following intramuscular immunizations, the pre-dominant cell type transfected with the DNA vaccine is myocytes124. Therefore, increasing the transfection efficiency of target cells through various physical delivery methods is an area that is being heavily investigated.
Some of the more recent delivery methods, including the transcutaneous microneedle, have the ability to bypass the stratum corneum layer of the skin, thus reaching Langerhans cells — the APCs of the skin (reviewed in Ref. 125). Further progress in microneedle array design, microneedle application apparatus and formulation will probably confirm that this methodology is a realistic clinical strategy for delivering DNA to and through skin. In addition, the use of low-frequency ultrasound as a potent physical adjuvant for successful transcutaneous immunization has been developed126. Another topical application method includes ‘painting’ a DNA vaccine with cytokine-expression plasmids onto the skin of mice after elimination of the keratinocyte layers; this method induced marked immune responses, both cellular and humoral, against the HIV-1 env protein127. A second group is examining a novel vaccine for HIV called Dermavir, which is topically administered under a patch containing plasmid DNA that is chemically formulated into a nanoparticle and delivered into epidermal Langerhans cells128,129.
New improvements in particle-mediated epidermal delivery (PMeD) technology and vector design, including co-formulation of PMeD DNA vaccines with adjuvants, are in progress to further enhance the potency of particle-mediated DNA vaccines (further reviewed in Ref. 130). These efforts are expected to accelerate the commercialization of PMeD as an effective approach for vaccination against infectious diseases. Jet-injection mechanical devices aim to deliver protein and DNA vaccines into the viable epidermis, thus providing potentially safer alternatives to needle injection, and this method promises increased efficacy in the prevention and/or therapy of infectious diseases, allergic disorders and cancer131-136. The tattoo perforating needle device has a bundle of fine metal needles that oscillate at a constant high frequency and puncture the skin, leading to DNA transfer to skin-associated cells and the expression of reporter genes in mice137, which results in the induction of T cell responses. A recent novel approach that was reported in small animals is to target epitope-based DNA vaccines to DCs using DC-targeting ligands138. It will be crucial to determine how these technologies will ultimately translate to the clinic.
Among the most impressive preclinical delivery strategies is electroporation, a technique that has been studied for two decades as a method to improve delivery of chemotherapy drugs to kill specific tumour cells139-141. electroporation has been extensively studied in large animal species such as dogs, pigs, cattle and non-human primates to deliver therapeutic genes that encode a variety of hormones, cytokines, enzymes or antigens142-146. In vivo expression levels improved markedly using this approach — levels increased by several fold over plasmid injection alone. This method might allow for less frequent immunizations with the DNA platform, and can improve both cellular and humoral responses. Again, this has been shown mostly in smaller animal models and, in particular, tumour systems32,141,144,147-159. However, recent studies have moved these delivery strategies to the non-human primate model142,149,153,160-162. Several different strategies of this technology are being pursued, including: those that deliver an electric current at the same time as the DNA injection; devices that use constant voltage or monitored constant current; devices fitted with penetrating probes that target the muscle or the skin; and strategies that use callipers to deliver an electric pulse through the skin. However, too little is currently known about several of these devices and much additional research in this area is warranted.
Recently, several devices have been moved into clinical evaluation, and the results are eagerly awaited. Important pressing issues include a comparison of the immune responses induced by these strategies with the best recombinant viral platforms and their tolerability in humans. As DNA vaccines delivered by electrostimulation deliver a higher bolus of antigen produced by a lower dose of plasmid, there are conceptual positive safety aspects of this technology, as well as additional concerns that arise from our unfamiliarity with this technology in humans. The strong results produced by this approach in non-human primates and large animals including pigs, horses and cattle are encouraging.
Conclusions
It has been more than 16 years since DNA vaccines stepped into the scientific limelight. During this time DNA vaccine technologies have generated great deal of excitement as well as disappointment. This situation, however, is similar to the development of a different breakthrough technology platform, that of monoclonal antibodies. More than 20 years elapsed between Kohler and Millstein”s pioneering report of hybridoma technology163 to the commercialization of the first human therapeutic antibody product. New technologies seem to be simple and straightforward but, as illustrated in this Review, they are deceptively so. An initial idea is transformed into a workable platform only through subtle and gradual improvements. In this regard it is clear that, after several years of frustration, the DNA platform is back on a productive path. In fact, as shown in BOX 2, the DNA platform represents almost one quarter of all gene therapy vector systems under clinical evaluation. This opinion is strengthened by recent licenses in the area of animal health and by the improvements in immune potency reported in the non-human primate model systems.
Box 2. Growing interest in the DNA platform.
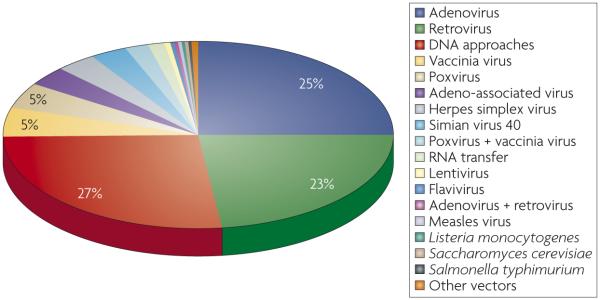
Interestingly, plasmid DNA vectors make up approximately 27% (354 out of 1,311 trials) of all gene-therapy vector platforms studied in Phase I to Phase III trials in 2007 (see figure; data modified from Gene Therapy Trials Worldwide provided by the Journal of Gene Medicine). These data support the overall preclinical success and Phase I safety of the platform. This platform has been growing steadily: before 1998, DNA plasmid vectors constituted an average of only 4% of all gene-therapy platform trials.
As shown in TABLE 1, when all open enrolling trials that make up the 154 Phase I to Phase III clinical trials that use the DNA platform are examined, 68% are trials testing DNA therapies for cancer, whereas 11% are for treatment of cardiovascular diseases. Infectious disease trials make up approximately 5% of the current open human clinical Phase I to Phase III trials testing the DNA vaccine platform and include vaccines against HIV-1, cytomegalovirus, and influenza virus. Most open Phase I to Phase III clinical trials for the DNA platform are being tested in the United States (68%), followed by the United Kingdom (15%) and Germany (8%).
However, the next 2 years of clinical testing of new and more complex DNA vaccines will be pivotal for either generating a true clinical success based on immune potency, or for telling us that we still have much further to go. Previous clinical disappointments highlight the likelihood that complexity in DNA vaccine design — including better and improved formulations, better delivery technologies, enhanced plasmid and delivery approaches, and more judicious clinical implementation — will be a staple for the continued enhancement of this platform. The further advancement of the DNA platform will continue to be an exciting and highly productive adventure that illustrates the best in academic creativity and translational science. Its success will be built on a high level of cooperation between industry, the regulatory authorities, funding by non-governmental organizations, the public and academicians.
Acknowledgements
We would like to thank members of the Weiner laboratory for helpful discussions, including M.P. Morrow, D. Hokey, D. Laddy and K. Schoenly. We apologize for any important work that was not cited in this article owing to space limitations. The work in D.B.W.”s laboratory is supported in part by funding from the National Institutes of Health.
Glossary
- Formulation
A mixture of one or more active ingredients is made safe and easy to store, transport, dilute or apply through the presence of other materials (for example, vehicles or solvents).
- Cytotoxic T lymphocyte
(CTL. Also known as Tc, T-killer cell or killer T cell). Belongs to a sub-group of T lymphocytes that are capable of inducing the death of infected somatic or tumour cells. They target and kill cells that are infected with other pathogens or that are otherwise damaged or dysfunctional.
- Adjuvant
An agent that can stimulate the immune system and increase the response to a vaccine, without having any specific antigenic immune response.
- Codon optimization
The preference that different organisms show for one of the several codons that encode a particular amino acid. Translationally optimal codons are those that are recognized by abundant tRNAs. Within a phylogenetic group, the frequency of particular codons in a gene is highly correlated with higher translation rates and accuracy.
- Subcutaneum
The layer of tissue that lies just under the surface of the skin.
- Antigen presenting cell
(APC). Specialized cell that can prime naive T cells through the expression of MHC class I molecules (which are expressed by most cells and can prime CD8+ cytotoxic T cells) as well as MHC class II molecules (which prime CD4+ T helper cells).
- Vector interference
The observation that re-administration of the same bacterial or viral vector leads to a reduction in its potency. This occurs because the host”s immune response develops neutralizing antibody responses against the vector when it is first administered. DNA does not contain protein targets so there is no vector interference or loss of potency following DNA re-administration.
- Electroporation
A physical process that exposes the target tissue to a brief electric-field pulse in order to induce temporary and reversible pores in the cell membrane. During the period of membrane destabilization, molecules such as plasmids can gain intracellular access.
- Adventitial agents
Unknown pathogens that are present in the cell lines that are used to produce vaccines and that can become part of the final vaccine preparation. SV40 was discovered this way, as a contaminant of the polioviral vaccine. As DNA vaccines are not produced in mammalian cell culture they can not contain these agents.
- Cynomolgus monkey
(Macaca fascicularis). A primarily arboreal macaque that is native to Southeast Asia. It is also called the long-tailed macaque.
- Kozak consensus sequence
A specific sequence that occurs on eukaryotic mRNA. This sequence is recognized and required by the ribosome as the translational start site.
- Cross-neutralizing antibody
An antibody that recognizes a wide range of antigenic epitopes.
- Consensus immunogens
Immunogens that are designed using computer analysis and then coded in DNA vaccines. They are synthesized so that the genes represent the most common amino acid at any position in a sequence, based on a population of viral isolate sequences.
- Phage display
A method that uses bacteriophage for high-throughput screening of protein interactions with other proteins, DNA or peptides.
- Liposome
Made up of bilayered membranes consisting of polar and non-polar portions of phospholipids that form multilayered shells containing a charged DNA-binding region.
Footnotes
Competing interests statement
The authors declare competing financial interests: see web version for details
References
- 1.Tang DC, DeVit M, Johnston SA. Genetic immunization is a simple method for eliciting an immune response. Nature. 1992;356:152–154. doi: 10.1038/356152a0. This is the first paper to report that introducing a protein-coding gene directly into the skin of mice (by propelling DNA-coated gold microprojectiles into cells of a living animal) could elicit antibody responses against the delivered antigen. [DOI] [PubMed] [Google Scholar]
- 2.Ulmer JB, et al. Heterologous protection against influenza by injection of DNA encoding a viral protein. Science. 1993;259:1745–1749. doi: 10.1126/science.8456302. This report, and references 3 and 4, were the original articles that described the ability to deliver by plasmid a viral antigen for presentation to the immune system without the limitations of direct peptide delivery or viral vectors. [DOI] [PubMed] [Google Scholar]
- 3.Fynan EF, et al. DNA vaccines: protective immunizations by parenteral, mucosal, and gene-gun inoculations. Proc. Natl Acad. Sci. USA. 1993;90:11478–11482. doi: 10.1073/pnas.90.24.11478. The authors report that different sites of plasmid delivery supported plasmid-driven immune responses to DNA-encoding influenza virus antigen; a gene gun was used to deliver low levels of plasmid immunogenes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang B, et al. Gene inoculation generates immune responses against human immunodeficiency virus type 1. Proc. Natl Acad. Sci. USA. 1993;90:4156–4160. doi: 10.1073/pnas.90.9.4156. This is the first report of a DNA vaccine approach for HIV-1. It shows that the gene inoculation technique mimics features of vaccination with live attenuated viruses, including induction of CTL and T helper cell responses, and type-specific antibody responses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ruprecht RM. Live attenuated AIDS viruses as vaccines: promise or peril? Immunol. Rev. 1999;170:135–149. doi: 10.1111/j.1600-065x.1999.tb01335.x. [DOI] [PubMed] [Google Scholar]
- 6.Offit PA. The Cutter incident, 50 years later. N. Engl. J. Med. 2005;352:1411–1412. doi: 10.1056/NEJMp048180. [DOI] [PubMed] [Google Scholar]
- 7.Ramakrishna L, Anand KK, Mohankumar KM, Ranga U. Codon optimization of the tat antigen of human immunodeficiency virus type 1 generates strong immune responses in mice following genetic immunization. J. Virol. 2004;78:9174–9189. doi: 10.1128/JVI.78.17.9174-9189.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frelin L, et al. Codon optimization and mRNA amplification effectively enhances the immunogenicity of the hepatitis C virus nonstructural 3/4A gene. Gene Ther. 2004;11:522–533. doi: 10.1038/sj.gt.3302184. [DOI] [PubMed] [Google Scholar]
- 9.Gao F, et al. Codon usage optimization of HIV type 1 subtype C gag, pol, env, and nef genes: in vitro expression and immune responses in DNA-vaccinated mice. AIDS Res. Hum. Retroviruses. 2003;19:817–823. doi: 10.1089/088922203769232610. [DOI] [PubMed] [Google Scholar]
- 10.Yan J, et al. Enhanced cellular immune responses elicited by an engineered HIV-1 subtype B consensus-based envelope DNA vaccine. Mol. Ther. 2007;15:411–421. doi: 10.1038/sj.mt.6300036. [DOI] [PubMed] [Google Scholar]
- 11.Cheung YK, Cheng SC, Sin FW, Xie Y. Plasmid encoding papillomavirus Type 16 (HPV16) DNA constructed with codon optimization improved the immunogenicity against HPV infection. Vaccine. 2004;23:629–638. doi: 10.1016/j.vaccine.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 12.Narum DL, et al. Codon optimization of gene fragments encoding Plasmodium falciparum merzoite proteins enhances DNA vaccine protein expression and immunogenicity in mice. Infect. Immun. 2001;69:7250–7253. doi: 10.1128/IAI.69.12.7250-7253.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yadava A, Ockenhouse CF. Effect of codon optimization on expression levels of a functionally folded malaria vaccine candidate in prokaryotic and eukaryotic expression systems. Infect. Immun. 2003;71:4961–4969. doi: 10.1128/IAI.71.9.4961-4969.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith JM, et al. DNA/MVA vaccine for HIV type 1: effects of codon-optimization and the expression of aggregates or virus-like particles on the immunogenicity of the DNA prime. AIDS Res. Hum. Retroviruses. 2004;20:1335–1347. doi: 10.1089/aid.2004.20.1335. [DOI] [PubMed] [Google Scholar]
- 15.Laddy DJ, et al. Heterosubtypic protection against pathogenic human and avian influenza viruses via in vivo electroporation of synthetic consensus DNA antigens. PLoS ONE. 2008;3:e2517. doi: 10.1371/journal.pone.0002517. The authors report that by combining RNA and codon optimization, novel leader sequence design, formulation, and synthetic consensus influenza antigens, with electroporation delivery, both protective cellular and humoral immune responses can be achieved in mice, ferrets and non-human primates. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laddy DJ, et al. Immunogenicity of novel consensus-based DNA vaccines against avian influenza. Vaccine. 2007;25:2984–2989. doi: 10.1016/j.vaccine.2007.01.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kwissa M, et al. Efficient vaccination by intradermal or intramuscular inoculation of plasmid DNA expressing hepatitis B surface antigen under desmin promoter/ enhancer control. Vaccine. 2000;18:2337–2344. doi: 10.1016/s0264-410x(00)00030-x. [DOI] [PubMed] [Google Scholar]
- 18.Wang R, et al. Induction of antigen-specific cytotoxic T lymphocytes in humans by a malaria DNA vaccine. Science. 1998;282:476–480. doi: 10.1126/science.282.5388.476. The authors report that healthy, malaria-naive volunteers vaccinated with plasmid DNA encoding a malaria protein develop antigen-specific, genetically restricted, CD8+ T cell-dependent CTLs. However, the high (almost 50%) response rates reported here have been difficult to replicate. [DOI] [PubMed] [Google Scholar]
- 19.McConkey SJ, et al. Enhanced T-cell immunogenicity of plasmid DNA vaccines boosted by recombinant modified vaccinia virus Ankara in humans. Nature Med. 2003;9:729–735. doi: 10.1038/nm881. This article reports on a heterologous prime-boost regime involving the vaccination of malaria antigenic DNA followed by intradermal delivery of recombinant modified vaccinia virus Ankara. Such heterologous prime-boost immunization approaches have since come into favour. [DOI] [PubMed] [Google Scholar]
- 20.MacGregor RR, et al. First human trial of a DNA-based vaccine for treatment of human immunodeficiency virus type 1 infection: safety and host response. J. Infect. Dis. 1998;178:92–100. doi: 10.1086/515613. This paper describes the first human trial of a DNA-based vaccine for HIV-1 infection; immune responses were weaker than expected based on preclinical data. Similar findings for other DNA vaccine clinical studies would be reported, stimulating concerns about the technology’s immune potency. [DOI] [PubMed] [Google Scholar]
- 21.Mincheff M, et al. Naked DNA and adenoviral immunizations for immunotherapy of prostate cancer: a Phase I/II clinical trial. Eur. Urol. 2000;38:208–217. doi: 10.1159/000020281. [DOI] [PubMed] [Google Scholar]
- 22.Tacket CO, et al. Phase 1 safety and immune response studies of a DNA vaccine encoding hepatitis B surface antigen delivered by a gene delivery device. Vaccine. 1999;17:2826–2829. doi: 10.1016/s0264-410x(99)00094-8. [DOI] [PubMed] [Google Scholar]
- 23.Le TP, et al. Safety, tolerability and humoral immune responses after intramuscular administration of a malaria DNA vaccine to healthy adult volunteers. Vaccine. 2000;18:1893–1901. doi: 10.1016/s0264-410x(99)00407-7. [DOI] [PubMed] [Google Scholar]
- 24.Liu MA, Ulmer JB. Human clinical trials of plasmid DNA vaccines. Adv. Genet. 2005;55:25–40. doi: 10.1016/S0065-2660(05)55002-8. [DOI] [PubMed] [Google Scholar]
- 25.Ulmer JB, Wahren B, Liu MA. Gene-based vaccines: recent technical and clinical advances. Trends Mol. Med. 2006;12:216–222. doi: 10.1016/j.molmed.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 26.Peters BS, et al. Studies of a prophylactic HIV-1 vaccine candidate based on modified vaccinia virus Ankara (MVA) with and without DNA priming: effects of dosage and route on safety and immunogenicity. Vaccine. 2007;25:2120–2127. doi: 10.1016/j.vaccine.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 27.Dorrell L, et al. Safety and tolerability of recombinant modified vaccinia virus Ankara expressing an HIV-1 gag/multiepitope immunogen (MVA.HIVA) in HIV-1-infected persons receiving combination antiretroviral therapy. Vaccine. 2007;25:3277–3283. doi: 10.1016/j.vaccine.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 28.Kresge KJ. A STEP back? Additional data released from the STEP trial raises questions about whether the vaccine may have increased the risk of HIV infection. IAVI Report. [online], < http://www.iavireport. org/Issues/Issue11-5/Step.asp >.
- 29.Catanzaro AT, et al. Phase 1 safety and immunogenicity evaluation of a multiclade HIV-1 candidate vaccine delivered by a replication-defective recombinant adenovirus vector. J. Infect. Dis. 2006;194:1638–1649. doi: 10.1086/509258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Graham BS, et al. Phase 1 safety and immunogenicity evaluation of a multiclade HIV-1 DNA candidate vaccine. J. Infect. Dis. 2006;194:1650–1660. doi: 10.1086/509259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Daud Adil I, et al. First-in-man trial of in vivo electroporation mediated gene transfer: safety and efficacy of IL-12 plasmid dose escalation in metastatic melanoma. J. Clin. Oncol. in the press. [Google Scholar]
- 32.Buchan S, et al. Electroporation as a ‘prime/boost’ strategy for naked DNA vaccination against a tumor antigen. J. Immunol. 2005;174:6292–6298. doi: 10.4049/jimmunol.174.10.6292. Elegantly designed vectors are used in a novel homologous prime-boost approach that combines DNA fusion vaccines with electroporation. Superior anti-tumour immune responses are generated, supporting the idea that boosting might not require viral vectors, but simply changing method of delivery. [DOI] [PubMed] [Google Scholar]
- 33.Rice J, Ottensmeier CH, Stevenson FK. DNA vaccines: precision tools for activating effective immunity against cancer. Nature Rev. Cancer. 2008;8:108–120. doi: 10.1038/nrc2326. [DOI] [PubMed] [Google Scholar]
- 34.Davidson AH, et al. Immunologic responses to West Nile virus in vaccinated and clinically affected horses. J. Am. Vet. Med. Assoc. 2005;226:240–245. doi: 10.2460/javma.2005.226.240. [DOI] [PubMed] [Google Scholar]
- 35.Garver KA, LaPatra SE, Kurath G. Efficacy of an infectious hematopoietic necrosis (IHN) virus DNA vaccine in Chinook Oncorhynchus tshawytscha and sockeye O. nerka salmon. Dis. Aquat. Organ. 2005;64:13–22. doi: 10.3354/dao064013. [DOI] [PubMed] [Google Scholar]
- 36.Bergman PJ, et al. Development of a xenogeneic DNA vaccine program for canine malignant melanoma at the Animal Medical Center. Vaccine. 2006;24:4582–4585. doi: 10.1016/j.vaccine.2005.08.027. This clinical study shows that xenogeneic DNA vaccination with tyrosinase family members can produce immune responses that result in tumour rejection or protection and prolongation of survival. These findings have led to a licensed veterinary product. [DOI] [PubMed] [Google Scholar]
- 37.Thacker EL, et al. Plasmid-mediated growth hormone-releasing hormone efficacy in reducing disease associated with Mycoplasma hyopneumoniae and porcine reproductive and respiratory syndrome virus infection. J. Anim. Sci. 2006;84:733–742. doi: 10.2527/2006.843733x. [DOI] [PubMed] [Google Scholar]
- 38.Kurth R. Risk potential of the chromosomal insertion of foreign DNA. Ann. N. Y Acad. Sci. 1995;772:140–151. doi: 10.1111/j.1749-6632.1995.tb44739.x. [DOI] [PubMed] [Google Scholar]
- 39.Manam S, et al. Plasmid DNA vaccines: tissue distribution and effects of DNA sequence, adjuvants and delivery method on integration into host DNA. Intervirology. 2000;43:273–281. doi: 10.1159/000053994. [DOI] [PubMed] [Google Scholar]
- 40.Ledwith BJ, et al. Plasmid DNA vaccines: investigation of integration into host cellular DNA following intramuscular injection in mice. Intervirology. 2000;43:258–272. doi: 10.1159/000053993. [DOI] [PubMed] [Google Scholar]
- 41.Temin HM. Overview of biological effects of addition of DNA molecules to cells. J. Med. Virol. 1990;31:13–17. doi: 10.1002/jmv.1890310105. [DOI] [PubMed] [Google Scholar]
- 42.Pal R, et al. Definitive toxicology and biodistribution study of a polyvalent DNA prime/protein boost human immunodeficiency virus type 1 (HIV-1) vaccine in rabbits. Vaccine. 2006;24:1225–1234. doi: 10.1016/j.vaccine.2005.07.112. [DOI] [PubMed] [Google Scholar]
- 43.Sheets RL, et al. Biodistribution of DNA plasmid vaccines against HIV-1, Ebola, Severe Acute Respiratory Syndrome, or West Nile virus is similar, without integration, despite differing plasmid backbones or gene inserts. Toxicol. Sci. 2006;91:610–619. doi: 10.1093/toxsci/kfj169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ledwith BJ, et al. Plasmid DNA vaccines: assay for integration into host genomic DNA. Dev. Biol. (Basel) 2000;104:33–43. [PubMed] [Google Scholar]
- 45.Klinman DM, et al. DNA vaccines: capacity to induce auto-immunity and tolerance. Dev. Biol. (Basel) 2000;104:45–51. [PubMed] [Google Scholar]
- 46.MacGregor RR, et al. Safety and immune responses to a DNA-based human immunodeficiency virus (HIV) type I env/rev vaccine in HIV-infected recipients: follow-up data. J. Infect. Dis. 2000;181:406. doi: 10.1086/315199. [DOI] [PubMed] [Google Scholar]
- 47.Bagarazzi ML, et al. Safety and immunogenicity of intramuscular and intravaginal delivery of HIV-1 DNA constructs to infant chimpanzees. J. Med. Primatol. 1997;26:27–33. doi: 10.1111/j.1600-0684.1997.tb00316.x. [DOI] [PubMed] [Google Scholar]
- 48.Mairhofer J, Pfaffenzeller I, Merz D, Grabherr R. A novel antibiotic free plasmid selection system: advances in safe and efficient DNA therapy. Biotechnol. J. 2007;3:83–89. doi: 10.1002/biot.200700141. [DOI] [PubMed] [Google Scholar]
- 49.Cranenburgh RM, Hanak JA, Williams SG, Sherratt DJ. Escherichia coli strains that allow antibiotic-free plasmid selection and maintenance by repressor titration. Nucleic Acids Res. 2001;29:E26. doi: 10.1093/nar/29.5.e26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Garmory HS, et al. Antibiotic-free plasmid stabilization by operator-repressor titration for vaccine delivery by using live Salmonella enterica Serovar typhimurium. Infect. Immun. 2005;73:2005–2011. doi: 10.1128/IAI.73.4.2005-2011.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Robertson JS, Cichutek K. European Union guidance on the quality, safety and efficacy of DNA vaccines and regulatory requirements. Dev. Biol. (Basel) 2000;104:53–56. [PubMed] [Google Scholar]
- 52.Nichols WW, Ledwith BJ, Manam SV, Troilo PJ. Potential DNA vaccine integration into host cell genome. Ann. N. Y Acad. Sci. 1995;772:30–39. doi: 10.1111/j.1749-6632.1995.tb44729.x. [DOI] [PubMed] [Google Scholar]
- 53.Robertson JS, Griffiths E. Assuring the quality, safety, and efficacy of DNA vaccines. Methods Mol. Med. 2006;127:363–374. doi: 10.1385/1-59745-168-1:363. [DOI] [PubMed] [Google Scholar]
- 54.Medjitna TD, et al. DNA vaccines: safety aspect assessment and regulation. Dev. Biol. (Basel) 126:261–270. discussion 126, 327 (2006) [PubMed] [Google Scholar]
- 55.Minor PD. Regulatory issues in the use of DNA vaccines. Ann. N. Y Acad. Sci. 1995;772:170–177. doi: 10.1111/j.1749-6632.1995.tb44742.x. [DOI] [PubMed] [Google Scholar]
- 56.US Department of Health and Human Services Guidance for Industry: Considerations for Plasmid DNA Vaccines for Infectious Disease Indications. Food and Drug Administration. 2007 [online], < http://www.fda. gov/CbER/gdlns/plasdnavac.pdf >.
- 57.Gorman CM, et al. The Rous sarcoma virus long terminal repeat is a strong promoter when introduced into a variety of eukaryotic cells by DNA-mediated transfection. Proc. Natl Acad. Sci. USA. 1982;79:6777–6781. doi: 10.1073/pnas.79.22.6777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Moreau P, et al. The SV40 72 base repair repeat has a striking effect on gene expression both in SV40 and other chimeric recombinants. Nucleic Acids Res. 1981;9:6047–6068. doi: 10.1093/nar/9.22.6047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Boshart M, et al. A very strong enhancer is located upstream of an immediate early gene of human cytomegalovirus. Cell. 1985;41:521–530. doi: 10.1016/s0092-8674(85)80025-8. [DOI] [PubMed] [Google Scholar]
- 60.Donnelly JJ, Ulmer JB, Shiver JW, Liu MA. DNA vaccines. Annu. Rev. Immunol. 1997;15:617–648. doi: 10.1146/annurev.immunol.15.1.617. [DOI] [PubMed] [Google Scholar]
- 61.Bojak A, Hammer D, Wolf H, Wagner R. Muscle specific versus ubiquitous expression of Gag based HIV-1 DNA vaccines: a comparative analysis. Vaccine. 2002;20:1975–1979. doi: 10.1016/s0264-410x(02)00081-6. [DOI] [PubMed] [Google Scholar]
- 62.Cazeaux N, et al. Comparative study of immune responses induced after immunization with plasmids encoding the HIV-1 Nef protein under the control of the CMV-IE or the muscle-specific desmin promoter. Vaccine. 2002;20:3322–3331. doi: 10.1016/s0264-410x(02)00310-9. [DOI] [PubMed] [Google Scholar]
- 63.Montgomery DL, et al. Heterologous and homologous protection against influenza A by DNA vaccination: optimization of DNA vectors. DNA Cell Biol. 1993;12:777–783. doi: 10.1089/dna.1993.12.777. [DOI] [PubMed] [Google Scholar]
- 64.Hartikka J, et al. An improved plasmid DNA expression vector for direct injection into skeletal muscle. Hum. Gene Ther. 1996;7:1205–1217. doi: 10.1089/hum.1996.7.10-1205. [DOI] [PubMed] [Google Scholar]
- 65.Barouch DH, et al. A human T-cell leukemia virus type 1 regulatory element enhances the immunogenicity of human immunodeficiency virus type 1 DNA vaccines in mice and nonhuman primates. J. Virol. 2005;79:8828–8834. doi: 10.1128/JVI.79.14.8828-8834.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ito H, et al. Promoter sequences of varicella-zoster virus glycoprotein I targeted by cellular transactivating factors Sp1 and USF determine virulence in skin and T cells in SCIDhu mice in vivo. J. Virol. 2003;77:489–498. doi: 10.1128/JVI.77.1.489-498.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Garg S, Oran AE, Hon H, Jacob J. The hybrid cytomegalovirus enhancer/chicken beta-actin promoter along with woodchuck hepatitis virus posttranscriptional regulatory element enhances the protective efficacy of DNA vaccines. J. Immunol. 2004;173:550–558. doi: 10.4049/jimmunol.173.1.550. [DOI] [PubMed] [Google Scholar]
- 68.Li HS, et al. Enhancement of DNA vaccine-induced immune responses by a 72-bp element from SV40 enhancer. Chin. Med. J. (Engl.) 2007;120:496–502. [PubMed] [Google Scholar]
- 69.Kozak M. Recognition of AUG and alternative initiator codons is augmented by G. in position +4 but is not generally affected by the nucleotides in positions +5 and +6. EMBO J. 1997;16:2482–2492. doi: 10.1093/emboj/16.9.2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gustafsson C, Govindarajan S, Minshull J. Codon bias and heterologous protein expression. Trends Biotechnol. 2004;22:346–353. doi: 10.1016/j.tibtech.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 71.Zhou W, et al. Multiple RNA splicing and the presence of cryptic RNA splice donor and acceptor sites may contribute to low expression levels and poor immunogenicity of potential DNA vaccines containing the env gene of equine infectious anemia virus (EIAV) Vet. Microbiol. 2002;88:127–151. doi: 10.1016/s0378-1135(02)00099-8. [DOI] [PubMed] [Google Scholar]
- 72.Wu X, Jornvall H, Berndt KD, Oppermann U. Codon optimization reveals critical factors for high level expression of two rare codon genes in Escherichia coli: RNA stability and secondary structure but not tRNA abundance. Biochem. Biophys. Res. Commun. 2004;313:89–96. doi: 10.1016/j.bbrc.2003.11.091. [DOI] [PubMed] [Google Scholar]
- 73.Zhang W, et al. mRNA secondary structure at start AUG codon is a key limiting factor for human protein expression in Escherichia coli. Biochem. Biophys. Res. Commun. 2006;349:69–78. doi: 10.1016/j.bbrc.2006.07.209. [DOI] [PubMed] [Google Scholar]
- 74.Li Y, Luo L, Thomas DY, Kang CY. The HIV-1 Env protein signal sequence retards its cleavage and down-regulates the glycoprotein folding. Virology. 2000;272:417–428. doi: 10.1006/viro.2000.0357. [DOI] [PubMed] [Google Scholar]
- 75.Xu ZL, et al. Optimization of transcriptional regulatory elements for constructing plasmid vectors. Gene. 2001;272:149–156. doi: 10.1016/s0378-1119(01)00550-9. [DOI] [PubMed] [Google Scholar]
- 76.Malin AS, et al. Vaccinia expression of Mycobacterium tuberculosis-secreted proteins: tissue plasminogen activator signal sequence enhances expression and immunogenicity of M. tuberculosis Ag85. Microbes Infect. 2000;2:1677–1685. doi: 10.1016/s1286-4579(00)01323-x. [DOI] [PubMed] [Google Scholar]
- 77.Kutzler MA, et al. Coimmunization with an optimized IL-15 plasmid results in enhanced function and longevity of CD8 T cells that are partially independent of CD4 T cell help. J. Immunol. 2005;175:112–123. doi: 10.4049/jimmunol.175.1.112. [DOI] [PubMed] [Google Scholar]
- 78.Yang JS, et al. Induction of inflammation by West Nile virus capsid through the caspase-9 apoptotic pathway. Emerg. Infect. Dis. 2002;8:1379–1384. doi: 10.3201/eid0812.020224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kumar S, et al. Immunogenicity testing of a novel engineered HIV-1 envelope gp140 DNA vaccine construct. DNA Cell Biol. 2006;25:383–392. doi: 10.1089/dna.2006.25.383. [DOI] [PubMed] [Google Scholar]
- 80.Wang S, et al. Relative contributions of codon usage, promoter efficiency and leader sequence to the antigen expression and immunogenicity of HIV-1 Env DNA vaccine. Vaccine. 2006;24:4531–4540. doi: 10.1016/j.vaccine.2005.08.023. [DOI] [PubMed] [Google Scholar]
- 81.Hu SL, Stamatatos L. Prospects of HIV Env modification as an approach to HIV vaccine design. Curr. HIV Res. 2007;5:507–513. doi: 10.2174/157016207782418542. [DOI] [PubMed] [Google Scholar]
- 82.Luria-Perez R, et al. A fusogenic peptide expressed on the surface of Salmonella enterica elicits CTL responses to a dengue virus epitope. Vaccine. 2007;25:5071–5085. doi: 10.1016/j.vaccine.2007.03.047. [DOI] [PubMed] [Google Scholar]
- 83.Hanke T, et al. Development of a DNA-MVA/HIVA vaccine for Kenya. Vaccine. 2002;20:1995–1998. doi: 10.1016/s0264-410x(02)00085-3. [DOI] [PubMed] [Google Scholar]
- 84.Ellenberger DL, et al. Generation of a consensus sequence from prevalent and incident HIV-1 infections in West Africa to guide AIDS vaccine development. Virology. 2002;302:155–163. doi: 10.1006/viro.2002.1577. [DOI] [PubMed] [Google Scholar]
- 85.Malm M, et al. Cross-clade protection induced by human immunodeficiency virus-1 DNA immunogens expressing consensus sequences of multiple genes and epitopes from subtypes A, B, C, and FGH. Viral Immunol. 2005;18:678–688. doi: 10.1089/vim.2005.18.678. [DOI] [PubMed] [Google Scholar]
- 86.Kothe DL, et al. Antigenicity and immunogenicity of HIV-1 consensus subtype B envelope glycoproteins. Virology. 2007;360:218–234. doi: 10.1016/j.virol.2006.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kothe DL, et al. Ancestral and consensus envelope immunogens for HIV-1 subtype C. Virology. 2006;352:438–449. doi: 10.1016/j.virol.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 88.De Groot AS, et al. HIV vaccine development by computer assisted design: the GAIA vaccine. Vaccine. 2005;23:2136–2148. doi: 10.1016/j.vaccine.2005.01.097. [DOI] [PubMed] [Google Scholar]
- 89.Bublil EM, Yeger-Azuz S, Gershoni JM. Computational prediction of the cross-reactive neutralizing epitope corresponding to the [corrected] monclonal [corrected] antibody b12 specific for HIV-1 gp120. FASEB J. 2006;20:1762–1774. doi: 10.1096/fj.05-5509rev. [DOI] [PubMed] [Google Scholar]
- 90.Gnanasekar M, et al. Novel phage display-based subtractive screening to identify vaccine candidates of Brugia malayi. Infect. Immun. 2004;72:4707–4715. doi: 10.1128/IAI.72.8.4707-4715.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fikes JD, Sette A. Design of multi-epitope, analogue-based cancer vaccines. Expert Opin. Biol. Ther. 2003;3:985–993. doi: 10.1517/14712598.3.6.985. [DOI] [PubMed] [Google Scholar]
- 92.McKinney DM, et al. Recognition of variant HIV-1 epitopes from diverse viral subtypes by vaccine-induced CTL. J. Immunol. 2004;173:1941–1950. doi: 10.4049/jimmunol.173.3.1941. [DOI] [PubMed] [Google Scholar]
- 93.Livingston B, et al. A rational strategy to design multiepitope immunogens based on multiple Th lymphocyte epitopes. J. Immunol. 2002;168:5499–5506. doi: 10.4049/jimmunol.168.11.5499. [DOI] [PubMed] [Google Scholar]
- 94.Stevenson FK, et al. DNA fusion gene vaccines against cancer: from the laboratory to the clinic. Immunol. Rev. 2004;199:156–180. doi: 10.1111/j.0105-2896.2004.00145.x. [DOI] [PubMed] [Google Scholar]
- 95.O’Hagan DT, Singh M, Ulmer JB. Microparticle-based technologies for vaccines. Methods. 2006;40:10–19. doi: 10.1016/j.ymeth.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 96.Herrmann JE, et al. Immune responses and protection obtained by oral immunization with rotavirus VP4 and VP7 DNA vaccines encapsulated in microparticles. Virology. 1999;259:148–153. doi: 10.1006/viro.1999.9751. [DOI] [PubMed] [Google Scholar]
- 97.Kaur R, Rauthan M, Vrati S. Immunogenicity in mice of a cationic microparticle-adsorbed plasmid DNA encoding Japanese encephalitis virus envelope protein. Vaccine. 2004;22:2776–2782. doi: 10.1016/j.vaccine.2004.01.040. [DOI] [PubMed] [Google Scholar]
- 98.Otten GR, et al. Enhanced potency of plasmid DNA microparticle human immunodeficiency virus vaccines in rhesus macaques by using a priming-boosting regimen with recombinant proteins. J. Virol. 2005;79:8189–8200. doi: 10.1128/JVI.79.13.8189-8200.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wang F, et al. Enhanced immunogenicity of microencapsulated multiepitope DNA vaccine encoding T and B cell epitopes of foot-and-mouth disease virus in mice. Vaccine. 2006;24:2017–2026. doi: 10.1016/j.vaccine.2005.11.042. [DOI] [PubMed] [Google Scholar]
- 100.Pai Kasturi S, et al. Prophylactic anti-tumor effects in a B cell lymphoma model with DNA vaccines delivered on polyethylenimine (PEI) functionalized PLGA microparticles. J. Control. Release. 2006;113:261–270. doi: 10.1016/j.jconrel.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 101.Minigo G, et al. Poly-L-lysine-coated nanoparticles: a potent delivery system to enhance DNA vaccine efficacy. Vaccine. 2007;25:1316–1327. doi: 10.1016/j.vaccine.2006.09.086. [DOI] [PubMed] [Google Scholar]
- 102.Jilek S, Merkle HP, Walter E. DNA-loaded biodegradable microparticles as vaccine delivery systems and their interaction with dendritic cells. Adv. Drug Deliv. Rev. 2005;57:377–390. doi: 10.1016/j.addr.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 103.Bhavsar MD, Amiji MM. Polymeric nano-and microparticle technologies for oral gene delivery. Expert Opin. Drug Deliv. 2007;4:197–213. doi: 10.1517/17425247.4.3.197. [DOI] [PubMed] [Google Scholar]
- 104.Densmore CL. Polyethyleneimine-based gene therapy by inhalation. Expert Opin. Biol. Ther. 2003;3:1083–1092. doi: 10.1517/14712598.3.7.1083. [DOI] [PubMed] [Google Scholar]
- 105.Garzon MR, et al. Induction of gp120-specific protective immune responses by genetic vaccination with linear polyethylenimine-plasmid complex. Vaccine. 2005;23:1384–1392. doi: 10.1016/j.vaccine.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 106.Bos GW, et al. Cationic polymers that enhance the performance of HbsAg DNA in vivo. Vaccine. 2004;23:460–469. doi: 10.1016/j.vaccine.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 107.Anderson DG, et al. A polymer library approach to suicide gene therapy for cancer. Proc. Natl Acad. Sci. USA. 2004;101:16028–16033. doi: 10.1073/pnas.0407218101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Anwer K, et al. Synergistic effect of formulated plasmid and needle-free injection for genetic vaccines. Pharm. Res. 1999;16:889–895. doi: 10.1023/a:1018834305079. [DOI] [PubMed] [Google Scholar]
- 109.Shiver JW, et al. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature. 2002;415:331–335. doi: 10.1038/415331a. [DOI] [PubMed] [Google Scholar]
- 110.Jimenez GS, et al. Vaxfectin-formulated influenza DNA vaccines encoding NP and M2 viral proteins protect mice against lethal viral challenge. Hum. Vaccin. 2007;3:157–164. doi: 10.4161/hv.3.5.4175. Many reported DNA formulations have had limited success in improving immune responses of DNA vaccines in humans. The recent data generated with the Vaxfectin adjuvant in the influenza system show increased response rates and are therefore encouraging. [DOI] [PubMed] [Google Scholar]
- 111.Nakanishi M, Noguchi A. Confocal and probe microscopy to study gene transfection mediated by cationic liposomes with a cationic cholesterol derivative. Adv. Drug Deliv. Rev. 2001;52:197–207. doi: 10.1016/s0169-409x(01)00207-1. [DOI] [PubMed] [Google Scholar]
- 112.Gao X, Huang L. Cationic liposome-mediated gene transfer. Gene Ther. 1995;2:710–722. [PubMed] [Google Scholar]
- 113.Mastrobattista E, et al. Lipid-coated polyplexes for targeted gene delivery to ovarian carcinoma cells. Cancer Gene Ther. 2001;8:405–413. doi: 10.1038/sj.cgt.7700311. [DOI] [PubMed] [Google Scholar]
- 114.Perrie Y, Gregoriadis G. Liposome-entrapped plasmid DNA: characterisation studies. Biochim. Biophys. Acta. 2000;1475:125–132. doi: 10.1016/s0304-4165(00)00055-6. [DOI] [PubMed] [Google Scholar]
- 115.Yamano T, et al. Enhancement of immunity by a DNA melanoma vaccine against TRP2 with CCL21 as an adjuvant. Mol. Ther. 2006;13:194–202. doi: 10.1016/j.ymthe.2005.05.018. [DOI] [PubMed] [Google Scholar]
- 116.Zhou WZ, et al. Protective immunization against melanoma by gp100 DNA-HVJ-liposome vaccine. Gene Ther. 1999;6:1768–1773. doi: 10.1038/sj.gt.3300998. [DOI] [PubMed] [Google Scholar]
- 117.Nabel GJ, et al. Direct gene transfer with DNA-liposome complexes in melanoma: expression, biologic activity, and lack of toxicity in humans. Proc. Natl Acad. Sci. USA. 1993;90:11307–11311. doi: 10.1073/pnas.90.23.11307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wang D, et al. Intranasal immunization with liposome-encapsulated plasmid DNA encoding influenza virus hemagglutinin elicits mucosal, cellular and humoral immune responses. J. Clin. Virol. 2004;31(Suppl 1):S99–S106. doi: 10.1016/j.jcv.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 119.Wang J, et al. Strong cellular and humoral immune responses induced by transcutaneous immunization with HBsAg DNA-cationic deformable liposome complex. Exp. Dermatol. 2007;16:724–729. doi: 10.1111/j.1600-0625.2007.00584.x. [DOI] [PubMed] [Google Scholar]
- 120.Barouch DH, et al. Control of viremia and prevention of clinical AIDS in rhesus monkeys by cytokine-augmented DNA vaccination. Science. 2000;290:486–492. doi: 10.1126/science.290.5491.486. This report is an important example of inclusion of a plasmid encoding a molecular adjuvant in a vaccine against the simian human immunodeficiency virus in rhesus monkeys. Its administration resulted in the augmentation of antigen-specific immune responses. [DOI] [PubMed] [Google Scholar]
- 121.Higgins D, et al. Immunostimulatory DNA as a vaccine adjuvant. Expert Rev. Vaccines. 2007;6:747–759. doi: 10.1586/14760584.6.5.747. [DOI] [PubMed] [Google Scholar]
- 122.Porgador A, et al. Predominant role for directly transfected dendritic cells in antigen presentation to CD8+ T cells after gene gun immunization. J. Exp. Med. 1998;188:1075–1082. doi: 10.1084/jem.188.6.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Condon C, et al. DNA-based immunization by in vivo transfection of dendritic cells. Nature Med. 1996;2:1122–1128. doi: 10.1038/nm1096-1122. [DOI] [PubMed] [Google Scholar]
- 124.Danko I, et al. High expression of naked plasmid DNA in muscles of young rodents. Hum. Mol. Genet. 1997;6:1435–1443. doi: 10.1093/hmg/6.9.1435. [DOI] [PubMed] [Google Scholar]
- 125.Vandervoort J, Ludwig A. Microneedles for transdermal drug delivery: a minireview. Front. Biosci. 2008;13:1711–1715. doi: 10.2741/2794. [DOI] [PubMed] [Google Scholar]
- 126.Tezel A, Paliwal S, Shen Z, Mitragotri S. Low-frequency ultrasound as a transcutaneous immunization adjuvant. Vaccine. 2005;23:3800–3807. doi: 10.1016/j.vaccine.2005.02.027. [DOI] [PubMed] [Google Scholar]
- 127.Liu LJ, et al. Topical application of HIV DNA vaccine with cytokine-expression plasmids induces strong antigen-specific immune responses. Vaccine. 2001;20:42–48. doi: 10.1016/s0264-410x(01)00324-3. [DOI] [PubMed] [Google Scholar]
- 128.Calarota SA, Weiner DB, Lori F, Lisziewicz J. Induction of HIV-specific memory T-cell responses by topical DermaVir vaccine. Vaccine. 2007;25:3070–3074. doi: 10.1016/j.vaccine.2007.01.024. [DOI] [PubMed] [Google Scholar]
- 129.Cristillo AD, et al. HIV-1 prophylactic vaccine comprised of topical DermaVir prime and protein boost elicits cellular immune responses and controls pathogenic R5 SHIV162P3. Virology. 2007;366:197–211. doi: 10.1016/j.virol.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 130.Fuller DH, Loudon P, Schmaljohn C. Preclinical and clinical progress of particle-mediated DNA vaccines for infectious diseases. Methods. 2006;40:86–97. doi: 10.1016/j.ymeth.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 131.Chen D, Maa YF, Haynes JR. Needle-free epidermal powder immunization. Expert Rev. Vaccines. 2002;1:265–276. doi: 10.1586/14760584.1.3.265. [DOI] [PubMed] [Google Scholar]
- 132.Lundholm P, et al. Induction of mucosal IgA by a novel jet delivery technique for HIV-1 DNA. Vaccine. 1999;17:2036–2042. doi: 10.1016/s0264-410x(98)00404-6. [DOI] [PubMed] [Google Scholar]
- 133.Epstein JE, et al. Safety, tolerability, and lack of antibody responses after administration of a PfCSP DNA malaria vaccine via needle or needle-free jet injection, and comparison of intramuscular and combination intramuscular/intradermal routes. Hum. Gene Ther. 2002;13:1551–1560. doi: 10.1089/10430340260201644. [DOI] [PubMed] [Google Scholar]
- 134.Cui Z, Baizer L, Mumper RJ. Intradermal immunization with novel plasmid DNA-coated nanoparticles via a needle-free injection device. J. Biotechnol. 2003;102:105–115. doi: 10.1016/s0168-1656(03)00029-4. [DOI] [PubMed] [Google Scholar]
- 135.Imoto J, Konishi E. Needle-free jet injection of a mixture of Japanese encephalitis DNA and protein vaccines: a strategy to effectively enhance immunogenicity of the DNA vaccine in a murine model. Viral Immunol. 2005;18:205–212. doi: 10.1089/vim.2005.18.205. [DOI] [PubMed] [Google Scholar]
- 136.Roberts LK, et al. Clinical safety and efficacy of a powdered hepatitis B nucleic acid vaccine delivered to the epidermis by a commercial prototype device. Vaccine. 2005;23:4867–4878. doi: 10.1016/j.vaccine.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 137.Ciernik IF, Krayenbuhl BH, Carbone DP. Puncture-mediated gene transfer to the skin. Hum. Gene Ther. 1996;7:893–899. doi: 10.1089/hum.1996.7.8-893. [DOI] [PubMed] [Google Scholar]
- 138.Nchinda G, et al. The efficacy of DNA vaccination is enhanced in mice by targeting the encoded protein to dendritic cells. J. Clin. Invest. 2008;118:1427–1436. doi: 10.1172/JCI34224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gothelf A, Mir LM, Gehl J. Electrochemotherapy: results of cancer treatment using enhanced delivery of bleomycin by electroporation. Cancer Treat Rev. 2003;29:371–387. doi: 10.1016/s0305-7372(03)00073-2. [DOI] [PubMed] [Google Scholar]
- 140.Giardino R, et al. Electrochemotherapy a novel approach to the treatment of metastatic nodules on the skin and subcutaneous tissues. Biomed. Pharmacother. 2006;60:458–462. doi: 10.1016/j.biopha.2006.07.016. [DOI] [PubMed] [Google Scholar]
- 141.Heller L, et al. In vivo electroporation of plasmids encoding GM-CSF or interleukin-2 into existing B16 melanomas combined with electrochemotherapy induces long-term antitumour immunity. Melanoma Res. 2000;10:577–583. doi: 10.1097/00008390-200012000-00010. [DOI] [PubMed] [Google Scholar]
- 142.Hirao LA, et al. Intradermal/subcutaneous immunization by electroporation improves plasmid vaccine delivery and potency in pigs and rhesus macaques. Vaccine. 2008;26:440–448. doi: 10.1016/j.vaccine.2007.10.041. [DOI] [PubMed] [Google Scholar]
- 143.van Drunen Littel-van den Hurk S, Babiuk SL, Babiuk LA. Strategies for improved formulation and delivery of DNA vaccines to veterinary target species. Immunol. Rev. 2004;199:113–125. doi: 10.1111/j.0105-2896.2004.00140.x. [DOI] [PubMed] [Google Scholar]
- 144.Roos AK, et al. Enhancement of cellular immune response to a prostate cancer DNA vaccine by intradermal electroporation. Mol. Ther. 2006;13:320–327. doi: 10.1016/j.ymthe.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 145.Medi BM, Singh J. Skin targeted DNA vaccine delivery using electroporation in rabbits II. Safety. Int. J. Pharm. 2006;308:61–68. doi: 10.1016/j.ijpharm.2005.10.035. [DOI] [PubMed] [Google Scholar]
- 146.Babiuk S, et al. A single HBsAg DNA vaccination in combination with electroporation elicits long-term antibody responses in sheep. Bioelectrochemistry. 2007;70:269–274. doi: 10.1016/j.bioelechem.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 147.Kadowaki S, et al. Protection against influenza virus infection in mice immunized by administration of hemagglutinin-expressing DNAs with electroporation. Vaccine. 2000;18:2779–2788. doi: 10.1016/s0264-410x(00)00087-6. [DOI] [PubMed] [Google Scholar]
- 148.Babiuk S, van Drunen Littel-van den Hurk S, Babiuk LA. Delivery of DNA vaccines using electroporation. Methods Mol. Med. 2006;127:73–82. doi: 10.1385/1-59745-168-1:73. [DOI] [PubMed] [Google Scholar]
- 149.Capone S, et al. Modulation of the immune response induced by gene electrotransfer of a hepatitis C virus DNA vaccine in nonhuman primates. J. Immunol. 2006;177:7462–7471. doi: 10.4049/jimmunol.177.10.7462. [DOI] [PubMed] [Google Scholar]
- 150.Chen Y, et al. Preparation and characterization of a monoclonal antibody against CKLF1 using DNA immunization with in vivo electroporation. Hybridoma (Larchmt) 2005;24:305–308. doi: 10.1089/hyb.2005.24.305. [DOI] [PubMed] [Google Scholar]
- 151.Dobano C, Widera G, Rabussay D, Doolan DL. Enhancement of antibody and cellular immune responses to malaria DNA vaccines by in vivo electroporation. Vaccine. 2007;25:6635–6645. doi: 10.1016/j.vaccine.2007.06.036. [DOI] [PubMed] [Google Scholar]
- 152.Hooper JW, Golden JW, Ferro AM, King AD. Smallpox DNA vaccine delivered by novel skin electroporation device protects mice against intranasal poxvirus challenge. Vaccine. 2007;25:1814–1823. doi: 10.1016/j.vaccine.2006.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Li Z, et al. DNA electroporation prime and protein boost strategy enhances humoral immunity of tuberculosis DNA vaccines in mice and non-human primates. Vaccine. 2006;24:4565–4568. doi: 10.1016/j.vaccine.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 154.Miller M, et al. The efficacy of electroporated plasmid vaccines correlates with long-term antigen production in vivo. Vaccine. 2004;22:2517–2523. doi: 10.1016/j.vaccine.2004.01.019. [DOI] [PubMed] [Google Scholar]
- 155.Peng B, Zhao Y, Xu L, Xu Y. Electric pulses applied prior to intramuscular DNA vaccination greatly improve the vaccine immunogenicity. Vaccine. 2007;25:2064–2073. doi: 10.1016/j.vaccine.2006.11.042. [DOI] [PubMed] [Google Scholar]
- 156.Quaglino E, et al. Electroporated DNA vaccine clears away multifocal mammary carcinomas in her-2/neu transgenic mice. Cancer Res. 2004;64:2858–2864. doi: 10.1158/0008-5472.can-03-2962. [DOI] [PubMed] [Google Scholar]
- 157.Shiau YT, et al. Analysis of humoral immunity of hepatitis D virus DNA vaccine generated in mice by using different dosage, gene gun immunization, and in vivo electroporation. J. Chin. Med. Assoc. 2006;69:7–13. doi: 10.1016/s1726-4901(09)70104-2. [DOI] [PubMed] [Google Scholar]
- 158.Spadaro M, et al. Cure of mammary carcinomas in Her-2 transgenic mice through sequential stimulation of innate (neoadjuvant interleukin-12) and adaptive (DNA vaccine electroporation) immunity. Clin. Cancer Res. 2005;11:1941–1952. doi: 10.1158/1078-0432.CCR-04-1873. [DOI] [PubMed] [Google Scholar]
- 159.Wu CJ, Lee SC, Huang HW, Tao MH. In vivo electroporation of skeletal muscles increases the efficacy of Japanese encephalitis virus DNA vaccine. Vaccine. 2004;22:1457–1464. doi: 10.1016/j.vaccine.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 160.Luckay A, et al. Effect of plasmid DNA vaccine design and in vivo electroporation on the resulting vaccine-specific immune responses in rhesus macaques. J. Virol. 2007;81:5257–5269. doi: 10.1128/JVI.00055-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Otten GR, et al. Potent immunogenicity of an HIV-1 gag-pol fusion DNA vaccine delivered by in vivo electroporation. Vaccine. 2006;24:4503–4509. doi: 10.1016/j.vaccine.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 162.Hirao LA, et al. Combined effects of IL-12 and electroporation enhances the potency of DNA vaccination in macaques. Vaccine. 2008;26:3112–3120. doi: 10.1016/j.vaccine.2008.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kohler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256:495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]