Abstract
Despite the development of highly effective prophylactic vaccines against human papillomavirus (HPV) serotypes 16 and 18, prevention of cervical dysplasia and cancer in women infected with high-risk HPV serotypes remains an unmet medical need. We report encouraging phase 1 safety, tolerability, and immunogenicity results for a therapeutic HPV16/18 candidate vaccine, VGX-3100, delivered by in vivo electroporation (EP). Eighteen women previously treated for cervical intraepithelial neoplasia grade 2 or 3 (CIN2/3) received a three-dose (intramuscular) regimen of highly engineered plasmid DNA encoding HPV16 and HPV18 E6/E7 antigens followed by EP in a dose escalation study (0.3, 1, and 3 mg per plasmid). Immunization was well tolerated with reports of mild injection site reactions and no study-related serious or grade 3 and 4 adverse events. No dose-limiting toxicity was noted, and pain was assessed by visual analog scale, with average scores decreasing from 6.2/10 to 1.4 within 10 min. Average peak interferon-g enzyme-linked immunospot magnitudes were highest in the 3 mg cohort in comparison to the 0.3 and 1 mg cohorts, suggesting a trend toward a dose effect. Flow cytometric analysis revealed the induction of HPV-specific CD8+ T cells that efficiently loaded granzyme B and perforin and exhibited full cytolytic functionality in all cohorts. These data indicate that VGX-3100 is capable of driving robust immune responses to antigens from high-risk HPV serotypes and could contribute to elimination of HPV-infected cells and subsequent regression of the dysplastic process.
INTRODUCTION
Cervical cancer is the most common cancer among women in developing countries and ranks as the second most common cancer among women worldwide, with an estimated 493,000 new cases and about 274,000 deaths annually (1, 2). The World Health Organization recognized cervical cancer as the first cancer to be 100% attributable to infection: Human papillomavirus (HPV) DNA has been identified in more than 99.7% of tumor biopsy specimens (3). There are more than 100 genotypes of HPV, but HPV types 16 and 18 are responsible for up to 75% of all cervical cancer cases (4, 5), making these subtypes the focus of HPV vaccine development and potential treatments.
HPV preventive vaccines (Gardasil and Cervarix), which are constructed of virus-like particles, generate strong neutralizing antibodies to capsid antigen L1 in recipients; these antibodies likely mediate the observed protection against HPV infection (6–8). However, in many HPV-associated malignancies, infected cells do not express detectable levels of capsid antigen (L1 or L2). Therefore, current preventive vaccines are not likely to be effective for controlling or preventing disease progression in preexisting HPV infections or HPV-associated lesions, and there remains a pressing need to develop therapeutic HPV vaccines. CD8+ T cells play a critical role in responding to virus-infected cells; therefore, a therapeutic HPV vaccine will likely be highly dependent on generating a CD8+ T cell response against HPV-infected cells.
HPV E6 and E7 proteins are constitutively coexpressed in all HPV-infected precancerous cells and are the most abundant viral transcripts found in biopsies from HPV-related cervical carcinoma cells (9). Because of their interaction with the p53 and retinoblastoma proteins (10–12), E6 and E7 are responsible for the transformation of cells and are required for the maintenance of HPV-associated malignancies (13, 14). Notably, E6- and E7-specific cellular immune responses are associated with regression of HPV16-associated lesions (15–17). Farhat et al. reported that, compared to women with persistent cervical HPV16 infection, the percentages of positive enzyme-linked immunospot (ELISpot) responses to HPV16 E6 and E7 are significantly increased among women with recently resolved HPV infection (18). Therefore, the HPV E6 and E7 antigens are considered to be promising immunotherapeutic targets.
To date, several types of HPV therapeutic vaccines, including live vector–based vaccines (19, 20), protein/peptide-based vaccines (21–24), cell-based vaccines (25), and DNA vaccines (26, 27), have been developed with a focus on stimulating the production and activation of HPV E6 and E7–specific T cells. However, these vaccines have had modest clinical success (28), perhaps because few HPV E6 and E7–specific vaccines have demonstrated robust E6- or E7-specific cellular immune responses or effective antitumor activity.
Despite an uncertain past as a vaccine approach in antigen-specific immunotherapy, DNA vaccines afford several conceptual advantages over traditional vaccination strategies. DNA vaccines appear safe, are stable and easily produced, and can be administered repeatedly because they do not engender specific antivector serology and there are no preexisting antibodies against DNA vectors in prospective vaccinees (29, 30). Furthermore, DNA vaccines have the ability to target multiple antigens, which could optimize and amplify desirable immunologic responses. However, although DNA vaccines have been shown to induce balanced CD4+ and CD8+ T cell responses as well as humoral immune responses in small-animal models, clinical data from multiple studies show that DNA vaccines have induced poor T cell and humoral responses as a stand-alone platform in humans (31, 32). Thus, the DNA platform has been used primarily in prime-boost strategies to date, and a robust DNA vaccine platform capable of generating strong T cell immunity would be a valuable resource for the potential treatment of cervical and other cancers. Many strategies, such as codon/RNA optimization, the addition of highly efficient immunoglobulin leader sequences (33–35), and use of “centralized” immunogens (36–38), have been applied to improve the magnitude and breadth of the cellular immune responses induced by DNA vaccines. Electroporation (EP) delivery can further enhance both the cellular and humoral response induced by DNA vaccines, especially in large animals such as dogs, pigs, cattle, nonhuman primates, and humans (39–42).
Here, we use a combination of these approaches in gene optimization and gene delivery to generate immunity to E6/E7 of HPV16 and HPV18 serotypes. The DNA was delivered by EP, which can increase the immunogenicity of DNA vaccines by more than 10- to 100-fold in vivo (43). We observed that these DNA vaccines drove high levels of functional cellular immunity by both CD8+ and CD4+ T cells, including coexpression in these cells of multiple lytic markers and killing function. Furthermore, high binding titers of anti-E6/E7 antibodies were induced. These levels of E6- and E7-specific immunity induced by a DNA vaccine may be useful in treatment of cervical disease.
RESULTS
The safety and tolerability of VGX-3100 were assessed in women previously treated for high-grade cervical dysplasia
Eighteen females were enrolled in this study. The mean age was 29 years, and 16 subjects were white. No subjects discontinued early from the study, and all participants received all vaccinations and completed all study visits. Rate and severity of injection site reactions during the 7 days after each vaccination and frequency of adverse events (AEs) collected during the 28-day follow-up period are summarized in tables S1 and S2.
AEs were reported as mild to moderate, and injection site reactions and laboratory abnormalities resolved without sequelae. There were no deaths reported. One serious adverse event (SAE) (seizure) that was assessed as not likely to be related to treatment occurred in a 29-year-old subject. Three grade 3 AEs were reported in this study (tension headache, viral gastroenteritis, and wrist fracture); all were assessed to be not related to treatment. A grade 3 fever (39.0°C) was reported by one subject 5 days after her second vaccination and resolved by the following day without treatment.
Except for two subjects with hypoglycemia, all laboratory abnormalities were mild or moderate and none required treatment. Three subjects with a history of hypercholesterolemia at study entry experienced grade 3 cholesterol elevations during the study. These laboratory observations were not of clinical significance because no AEs of hypoglycemia or cholesterol elevation were reported during the study. One subject had a grade 3 creatine phosphokinase elevation that the investigator considered to be not related to treatment. No grade 3 or 4 injection site reactions were reported. The most frequent injection site reactions were grade 1 or 2 pain and tenderness and all resolved without sequelae as summarized in table S2.
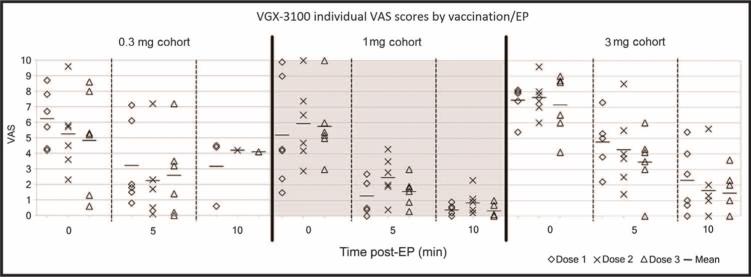
Study participants were asked to assess their pain following the vaccination/EP procedure with a visual analog scale (VAS) by making a mark on a 10-cm line, anchored with word descriptors at each end (“No Pain” and “Worst Pain”), at 0, 5, and 10 min after each vaccination. As shown in Fig. 1, most subjects reported the greatest intensity of pain immediately after the procedure, which dissipated rapidly within 10 min. No significant differences were observed between the VAS scores at the three dose levels.
Fig. 1.
Mean VAS assessment of local pain after vaccination/EP procedure. The VAS scores shown for cohort 1 at the 10 minute time point represent only those patients whose VAS scores were greater than 2 at the 5 minute time point. For cohorts 2 and 3, the protocol was amended to collect data from all patients at the 10 minute time point.
VGX-3100 induces strong, long-lived antibody responses
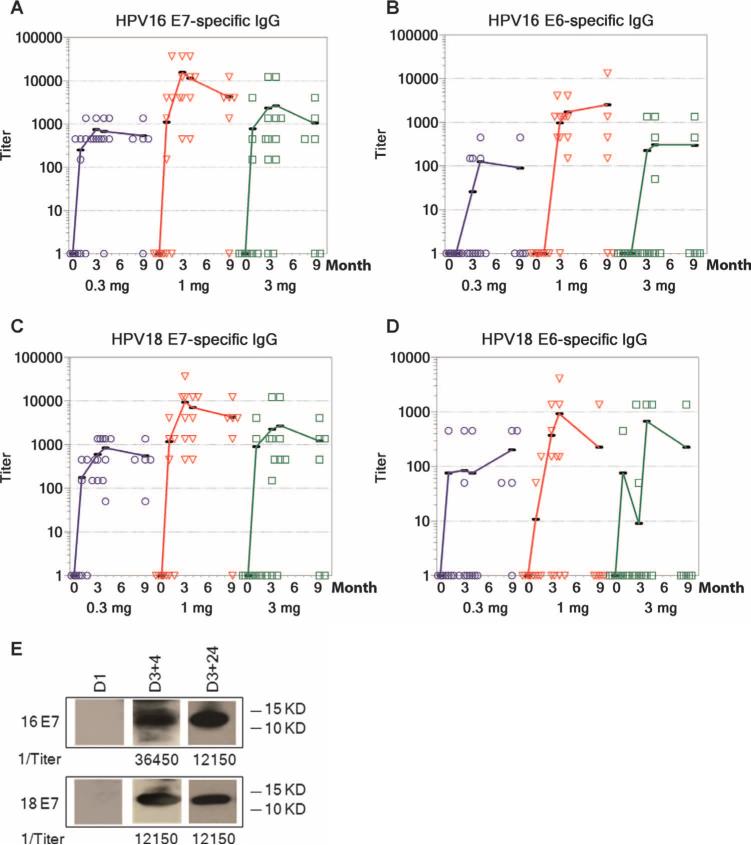
Binding antibodies were measured by enzyme-linked immunosorbent assay (ELISA) against all four vaccine antigens and appeared to peak 4 weeks after the third immunization (D3+4, refer to participants and study design section of Materials and Methods for time point nomenclature) (Fig. 2). The antibodies elicited by VGX-3100 against HPV16 or HPV18 E7 were more robust than those measured against both E6 proteins. Using an endpoint dilution ELISA, we observed an increase in HPV16 E7 titers in 17 of 18 (94%) vaccinees at D3+4 and an increase in HPV18 E7 titers in all 18 vaccinees. Titers to HPV16 and HPV18 E6 increased in 12 of 18 (67%) and 7 of 18 (39%) subjects, respectively (Table 1). As indicated in Fig. 2 (A to D), antibody titers against the four vaccine antigens remained high even 24 weeks after the last immunization, indicating that administration of VGX-3100 is capable of inducing antibodies that persist at least 6 months after the treatment regimen. Overall, 100% of the study participants (18 of 18) reported antibody positivity to at least two vaccine antigens, and 94% (17 of 18) reported positivity to three antigens; 56% (10 of 18) were positive to all four antigens.
Fig. 2.
Immunoglobulin G (IgG) responses after vaccination with VGX-3100 versus time (months). (A to D) HPV16 E7 (A), HPV16 E6 (B), HPV18 E7 (C), and HPV18 E6 (D) measured by ELISA in the 0.3, 1.0, and 3.0 mg groups at entry [dose 1 (D1)], 1 week after the second immunization (D2+1), 1 week after the third immunization (D3+1), 4 weeks after the third immunization (D3+4), and 24 weeks after the third immunization (D3+24). (E) Representative HPV16 and HPV18 E7–specific seroconversion (subject 14-4) measured by Western blot at D1, D3+4, and D3+24.
Table 1.
Response rates measured by IFN-γ ELISpot and ELISA in the 03,1.0, and 3.0 mg groups. Numbers shown represent the number of responding subjects in each cohort for each antigen out of a possible six evaluable subjects in each cohort for each antigen for both humoral (H) and cellular (C) assays.
| Dose cohort (mg) | HPV16 E6 |
HPV16 E7 |
HPV18 E6 |
HPV18 E7 |
No. of responders/total subjects |
No. of responsive antigens/total antigens |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H | C | H | C | H | C | H | C | H | C | H | C | |
| 0.3 | 3 | 4 | 5 | 3 | 1 | 3 | 6 | 2 | 6 | 4 | 15/24 | 12/24 |
| 1 | 6 | 5 | 6 | 3 | 3 | 3 | 6 | 4 | 6 | 5 | 21/24 | 15/24 |
| 3 | 3 | 5 | 5 | 5 | 3 | 5 | 6 | 4 | 6 | 5 | 17/24 | 19/24 |
| Overall | 12 | 14 | 17 | 11 | 7 | 11 | 18 | 10 | 18/18 | 14/18 | 53/72 | 46/72 |
Serum E7 antibody specificity was confirmed by Western blot (Fig. 2E and fig. S1). Bands representing both HPV E7 antigens were dem onstrated in 15 of 16 subjects (94%) at D3+4 [two subjects were excluded because of high background activity before dose 1 (D1)]. Twelve of the 14 subjects exhibiting seroconversion at D3+4 (86%) remained positive to both HPV E7 proteins by Western blot at 24 weeks after the third immunization (D3+24), recon-firming the ability of VGX-3100 to induce robust and lasting antibody responses (no samples were available at D3+24 for two subjects).
VGX-3100 induces robust HPV-specific TH 1-biased cellular immune responses
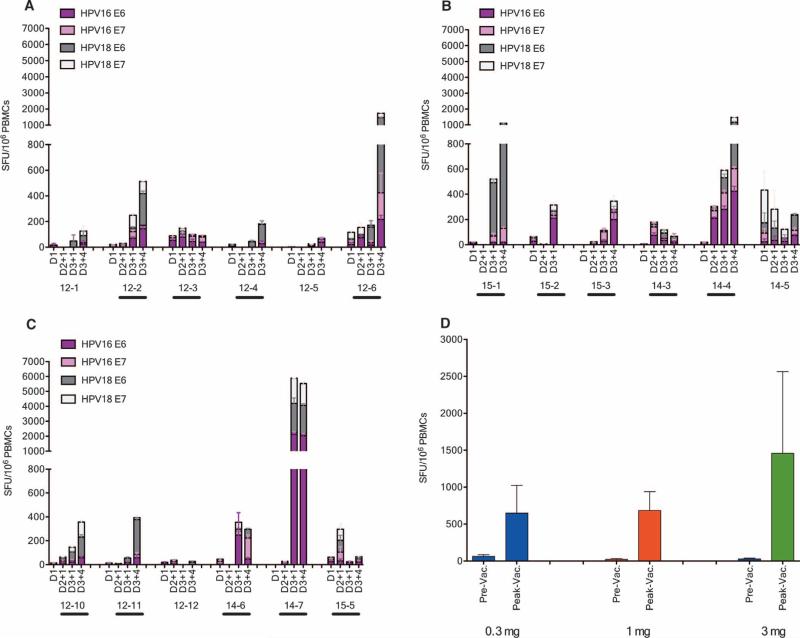
Standard vaccine interferon-γ (IFN-γ) ELISpot assays (44, 45) were performed to determine the number of antigen-specific IFN-γ-–secreting cells in response to stimulation with HPV16 or HPV18 E6 and E7 peptide pools. As shown in Fig. 3, after three vaccinations, four of six of subjects who received a dose of 0.3 mg per plasmid (Fig. 3A), five of six subjects who received a dose of 1 mg per plasmid (Fig. 3B), and five of six subjects who received a dose of 3 mg per plasmid (Fig. 3C) mounted vaccine-induced HPV16 or HPV18 E6 or E7–specific cellular immune responses. High preexisting responses [ELISpot >100 spot-forming units (SFU) per 106 peripheral blood mononuclear cells (PBMCs) at baseline] were measured in a single subject in the 1.0 mg group (14-5). Overall, 14 subjects (78%) developed positive cellular immune responses by ELISpot, with magnitudes ranging from 100 to more than 5000 SFU per 106 PBMCs. The peak average responses in low-, intermediate-, or high-dose groups were 648, 683, and 1458 SFU per 106 PBMCs, respectively, indicating a trend of vaccine dose effect (Fig. 3D).
Fig. 3.
Robust HPV16 and HPV18 E6/E7–specific TH1-biased cellular immune responses after vaccination with VGX-3100. (A to C) Antigen-specific IFN-γ ELISpot assays determine the number of antigen-specific IFN-γ–secreting cells in response to stimulation with HPV16 or HPV18 E6 and E7 peptide pools at D1, D2+1, D3+1, and D3+4 in the 0.3 mg (A), 1.0 mg (B), and 3.0 mg (C) groups. “Responders” by analytical criteria are designated with a bar below the subject ID. (D) Peak average responses in the 0.3, 1.0, and 3.0 mg groups.
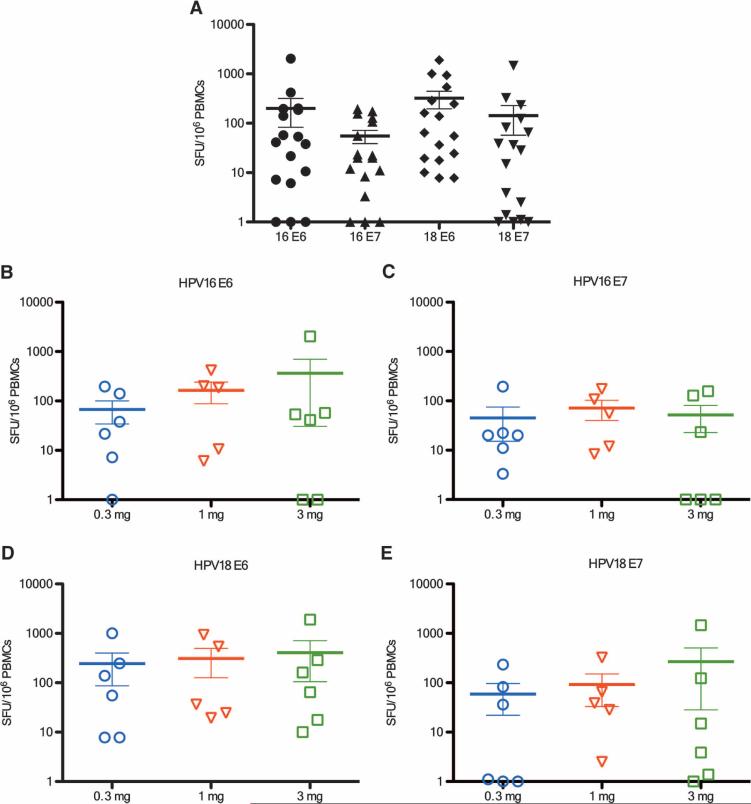
Figure 4 depicts the background-subtracted IFN-γ ELISpot magnitudes for each antigen by dose group. The highest IFN-γ ELISpot magnitudes were measured against HPV18 E6, although the differences among antigens were not statistically significant (Fig. 4A). As shown in Fig. 4 (B to E), average magnitudes trended higher in the higher-dose groups, except for HPV16 E7. Overall, 14 subjects responded to at least one antigen, 13 responded to at least two antigens, and 9 responded to all four antigens.
Fig. 4.
ELISpot responses to each vaccine antigen present at all doses. (A) Overall background-subtracted cellular immune responses against four vaccine antigens. (B to E) Background-subtracted antigen-specific cellular immune responses in the 0.3, 1.0, and 3.0 mg groups to (B) HPV16 E6, (C) HPV16 E7, (D) HPV18 E6, and (E) HPV18 E7.
Response rates ranged from 10 to 14 of 18 for the individual antigens (Table 1). Overall, vaccine-induced antigen-specific responses were measured in 46 of 72 assays. In addition, a trend was observed in dose-dependent increases in the number of antigens to which a response was detected in the three dose groups (12 of 24, 15 of 24, and 19 of 24 in the 0.3, 1.0, and 3.0 mg cohorts, respectively).
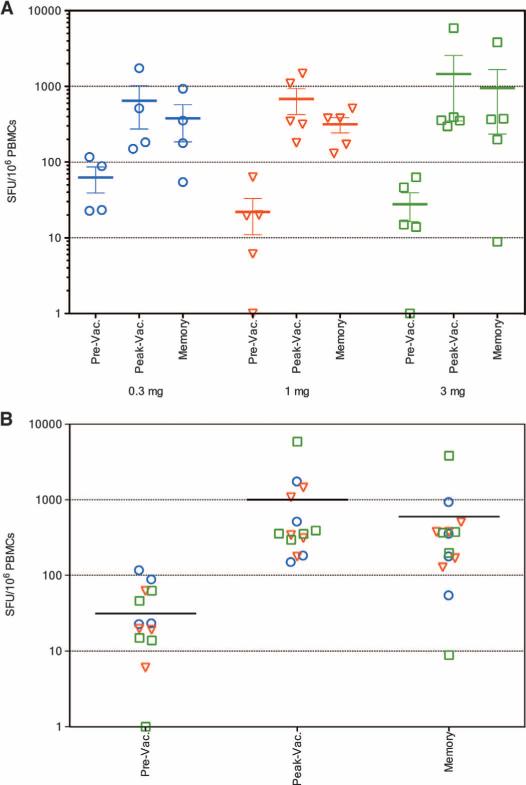
Memory T cell responses were evaluated in the IFN-γ ELISpot assay at 24 weeks after dose 3 in 11 of 14 responders with evaluable samples. As shown in Fig. 5, three of four responders in the 0.3 mg group, four of five responders in the 1 mg group, and four of five responders in the 3 mg group showed HPV16 or HPV18 E6 or E7–specific memory responses that persisted at least 6 months after final vaccination.
Fig. 5.
Strong memory T cell responses after vaccination with VGX-3100. (A) Memory T cell responses elicited in the responders in the 0.3, 1.0, and 3.0 mg groups, respectively. (B) Overall memory T cell responses elicited in all responders. IFN-γ ELISpot in response to stimulation with HPV16 or HPV18 E6 and E7 peptide pools in the 0.3 mg (circle), 1.0 mg (triangle), and 3.0 mg (square) groups. The baseline immune responses (Pre-Vac), peak responses (Peak-Vac), and memory responses (D3+24) of responders are shown for each dose group.
As noted above, 14 of 18 subjects mounted a cellular response to the vaccine, and 18 of 18 had a humoral response. On an individual an tigen basis, 100% of subjects with a vaccine-induced cellular response to the E7 antigen of HPV16 (11 of 11) and HPV18 (10 of 10) in the IFN-γ ELISpot assay also had an increase in humoral response to the same antigens after vaccination (Table 1 and Fig. 2). Similarly, 64.3% (9 of 14) and 45.5% (5 of 11) of subjects with a vaccine-induced cellularresponsetotheE6antigenofHPV16 and HPV18, respectively, had an increase in humoral response after vaccination. Overall, our data suggest that the vaccine was able to induce cellular and humoral responses in the majority of subjects, and these responses were generally concordant.
VGX-3100 induced responses across multiple human leukocyte antigen class I and II alleles
We conducted human leukocyte antigen (HLA) typing to assess whether the immunogenicity of VGX-3100 vaccine was associated with class I HLA-A, HLA-B, and HLA-C and class II HLA-DRB and HLADQB loci. As shown in tables S3 and S4, there were no clear negative or positive associations between a particular HLA type and antigen-specific T cell responses to VGX-3100. Indeed, most alleles were represented across the 18 volunteers and most alleles engendered an immune response to at least one antigen. This analysis substantiates that the vaccines induced broad responses restricted by a diverse array of HLA class 1 and class II alleles.
VGX-3100 drives cellular responses detectable using flow cytometry
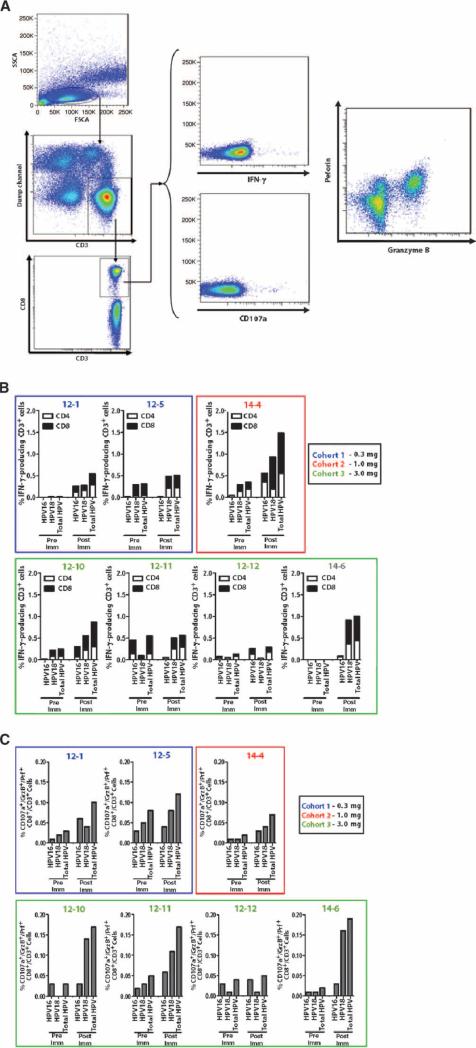
In light of the strong cellular immune response observed in the IFN-γ ELISpot assay, intracellular cytokine staining (ICS) assays were performed to analyze the cellular immune responses induced by VGX-3100 further. More specifically, as the generation of CD8+ T cells capable of killing cervical cells that are infected with HPV16 or HPV18 is considered an important immunotherapy goal, we performed ICS assays to measure markers that more clearly identify cytotoxic T lymphocytes (CTLs) as well as further evaluate IFN-γ production in T cells incubated with HPV16 and HPV18 E6/E7 (Fig. 6A). As noted in Materials and Methods, the available PBMCs were prioritized first to the ELISpot assay (18 of 18 subjects analyzed). The ICS assays described below were then performed in all subjects across the three cohorts, where sufficient PBMCs were available for further analysis.
Fig. 6.
IFN-γ and CTL responses by flow cytometry after vacci-nation with VGX-3100. (A) Multiparametic flow cytometry was used to detect HPV16- and HPV18-specific production of IFN-γ and regulation of CD107a, granzyme B, and perforin. Representative gating strategy and response to HPV peptide are shown. (B) HPV-specific IFN-γ synthesis increased after immunization and was detectable in both CD4+ and CD8+ T cell subsets in pre- and postimmunization samples. (C) Coexpression of CD107a, granzyme B, and perforin in CD8+ T cell subset in response to HPV16 and HPV18 E6/E7 was increased after immunization, suggesting the induction and expansion of CTLs.
VGX-3100 drives the induction of CD8+ T cells with CTL phenotype
As shown in Fig. 6B, HPV16 and/or HPV18 E6 and E7–specific IFN-γ production could be detected in all subjects tested (seven of seven analyzed; two from cohort 1, one from cohort 2, and four from cohort 3). Responses were detected irrespective of cohort, and synthesis of IFN-γ was seen in both CD4+ T cells (open bars) and CD8+ T cells (closed bars). Total response rates varied by subject but reached a 1.0 to 1.5% response range in postimmunization samples (Fig. 6B). To further profile IFN-γ production, we also categorized responding CD4+ and CD8+ T cells by CD27 and CD45RO cell surface expression (fig. S2A). CD4+ T cells producing IFN-γ tended to fall largely into the CD27−/CD45RO+ and CD27+/CD45RO+ subsets (putative effector memory and central memory cells, respectively), with a lower response rate seen in the CD27−/CD45RO− subset (putative effector cells) for both HPV16 and HPV18 antigens (fig. S2B). Similarly, CD8+ T cells responding to HPV16 or HPV18 antigens fell largely into the effector memory subset. Unlike CD4+ responses, however, a lower percentage of responding CD8+ T cells exhibited a central memory phenotype, whereas slightly more CD8+ T cells were found to be effector phenotype.
Although IFN-γ synthesis in response to antigen is a clear indication of a T helper 1 (TH1)–biased immune response, its production is not strictly correlated with CTL activity (46), namely, the ability of a CD8+ T cell to load granzyme B and perforin as well as release these lytic granules in response to cognate antigen in an effort to kill a target cell (47–49). Thus, additional markers were evaluated to more clearly define putative CTLs from the subset of CD8+ T cells that were able to produce IFN-γ. For the purposes of this assay, we defined a CTL as a CD8+ T cell that stained positively for granzyme B, perforin, and CD107a, a marker that is well established as a sign of cytolytic degranulation (50) (Fig. 6A). After stimulation with cognate antigen, CD8+ T cells exhibiting all three markers would represent cells capable of HPV16- and/or HPV18-specific degranulation (as determined by CD107a positivity). Such cells also exhibited the ability to load sufficient amounts of both granzyme B and perforin, which are chiefly responsible for initiating cell death in target cells with which CTLs interact (47–49). All but one subject tested in this assay (six of seven) exhibited increases in CTL frequencies after immunization (Fig. 6C), indicating that immunization with VGX-3100 consistently drove the induction of HPV-specific CD8+ T cells that express markers commonly used to identify CTLs. Subjects from the 3 mg cohort showed the most robust postvaccination CTL frequencies (0.15 to 0.20% response rate) per this analysis (Fig. 6C), although this cohort was overrepresented because of restrictions on sample availability, thus obscuring the ability to clearly attribute this to a dose effect. The CD27 and CD45RO analysis was extended to the CTL population studied in this assay, and the vast majority of responding cells fell into the CD27−/CD45RO+ effector memory subset, followed by the CD27−/CD45RO− effector subset (fig. S2C). Very few cells fell into the CD27+/CD45RO+ central memory subset.
VGX-3100 induces antigen-specific CTLs
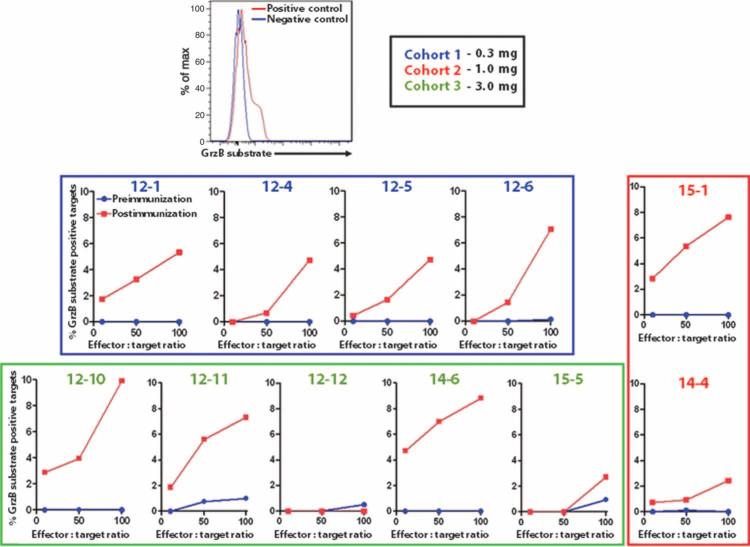
Although our phenotypic analysis had suggested the presence of CD8+ T cells resembling CTLs, a functional assay was required to confirm cytolytic activity. Using a sensitive and specific flow cytometry–based assay to measure the amount of active granzyme B delivered to target cells from antigen-specific effectors, we assessed functional effector cell killing capacity (46, 51, 52). Whole PBMCs were used as the effector number [final effector/target (E:T) ratios of 10:1, 50:1, and 100:1], that is, the assay output would be based on the quantity of antigen-specific CTLs within the PBMCs added. Postimmunization effectors exhibited CTL activity in 10 of 11 subjects tested (Fig. 7; 4 from cohort 1, 2 from cohort 2, and 5 from cohort 3) as evidenced by active granzyme B delivery to targets from HPV-specific effectors. This CTL activity was undetectable in the preimmunization samples from the same subjects. Killing activity at the 100:1 E:T ratio varied from 2.44 and 2.73% in the modest responders to 8.82 and 9.92% in the strong responders. Although immunization with VGX-3100 drove killing activity in all three cohorts, three of the four strongest responders were in the 3 mg cohort. As a possible dose effect was also observed in the IFN-γ ELISpot assay, a regression analysis was performed to determine whether the magnitude of IFN-γ ELISpot correlated with the magnitude of killing measured. As reported by other groups (46, 53), there was no significant correlation between IFN-γ and killing magnitudes (P = 0.2315; fig. S3A). Of interest, regression analysis of killing activity and CTL phenotyping showed a strong correlation between CTL frequency and killing magnitudes at the 10:1 E:T ratio (P = 0.0009; fig. S3B), supporting that CTL phenotyping by ICS may be informative with regard to killing activity in specific instances.
Fig. 7.
Detection of new HPV-specific functional CTL responses (quantitative killing assay). Delivery of active granzyme B from effectors to targets pulsed with HPV16 and HPV18 E6/E7 at varying E:T ratios: 10:1, 50:1, and 100:1. Whereas effector PBMCs isolated before immunization mediated little to no CTL activity in the form of granzyme B delivery (blue lines), postimmunization PBMCs were able to function as CTLs and deliver granzyme B to targets in an antigen-specific manner (red lines), suggesting an increased frequency of functional CTLs after immunization.
Preexisting regulatory T cells did not correlate with cellular immune responses
Previous studies have established links between the presence of regulatory T cells (Tregs; defined as CD4+/CD25hi/FoxP3+) and HPV-induced disease (54–58), as well as suggested a possible negative link between the presence of Tregs and subsequent response magnitudes to HPV vaccination in infected individuals (58). We examined whether the presence of peripheral Tregs correlated in any way with the induction of HPV16 and HPV18 E6 and E7–specific immune responses. As seen in fig. S4, preimmunization Tregs could be clearly identified with the CD25 and FoxP3 markers within the CD4+ T cell subset, with frequencies ranging from 0.21 to 5.47%. Regression analysis was performed to detect a relationship between Treg frequencies and subsequent response rates in the IFN-γ ELISpot, CTL ICS, or quantitative killing assays; no statistically significant association was observed between the presence of Tregs and response magnitude in any of the three assays used (fig. S4; P = 0.9072, 0.8343, and 0.9849, respectively), suggesting that preexisting Tregs did not influence the induction of cellular immune responses to VGX-3100.
VGX-3100 induces antigen-specific CD8+ T cells capable of substantial activation and lytic marker up-regulation
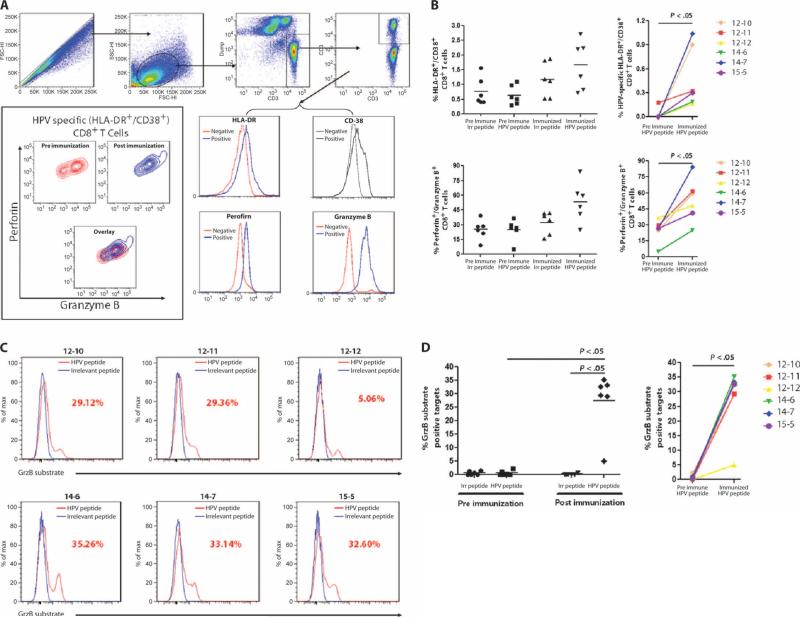
Chronic viral infections are often associated with immune incompetence and may result in an induced state of lowered reactivity to viral antigens (59–61). Moreover, in the context of HPV infection, HPV-specific Tregs may mediate suppression of virus-specific immune responses important in control or elimination of infection (57, 58). The ability of an immunotherapy for HPV infection to drive potent immune responses would therefore likely necessitate the induction of immune responses that are durable and robust even in the face of prolonged antigenic exposure, including possible suppressive activity by Tregs. Although we had interrogated immune responses in assays using short-term (24 hours or less) antigenic stimulation, we had not yet examined this response in the face of prolonged (multiple day) stimulation that could render cells more susceptible to suppressive cell effects (such as Tregs). Therefore, we exposed PBMCs to HPV16 and HPV18 peptides in vitro over the course of 5 days and measured subsequent immune activity in the form of activation markers HLA-DR and CD38 (62) and the ability of CD8+ T cells to synthesize granzyme B and perforin. These assays were performed on PBMCs from subjects in the 3 mg cohort because there appeared to be a trend toward more robust cellular immune responses in that group. As shown in Fig. 8A, we were able to detect differential regulation of HLA-DR, CD38, and both granzyme B and perforin in patient samples. Pre- and post-immunization PBMCs were stimulated with an irrelevant control peptide [ovalbumin (OVA)] or HPV16 and HPV18 peptides and assessed for cellular activation and the ability to load lytic granules. Incubation of preimmunization samples with HPV peptides did not result in a statistically significant increase in the number of activated CD8+ T cells as measured by HLA-DR and CD38 costaining when compared with stimulation by irrelevant control peptide (Fig. 8B, top left), suggesting a lack of immune reactivity before vaccination.
Fig. 8.
Lytic granule loading and CTL functional quality assessment of CTLs induced by VGX-3100 (qualitative killing assay). (A) CD8+ T cells from pre- and post-immunization PBMCs were assessed for their ability to activate and load lytic granules upon extended antigenic stimulation. Representative gating strategy is shown. For HLA-DR, CD38, granzyme B, and perforin, red lines indicate expression levels from unstimulated samples and blue lines indicate poststimulation expression. Contour plots show stimulated pre- and postimmunization samples, including an overlay. (B) Postimmunization samples show statistically significant increases in the percentage of CD8+ T cells that stained positively for the activation markers HLA-DR and CD38 upon extended stimulation with HPV peptide [raw group averages of 1.19% versus 1.66% (top left); background-corrected group averages of 0.03% versus 0.49% (top right)] and also show a statistically significant increase in the number of cells costaining for granzyme B and perforin within this activated subset [group average of 32.1% versus 53.3% (bottom)]. (C) Activated CD8+ T cells mediate robust cytotoxicity in the form of active granzyme B delivery to HPV-stimulated target cells but not to targets incubated with irrelevant peptides. Numbers in red indicate the percentage of targets receiving active granzyme B from an effector. E:T ratio was 12.5:1, where effectors were normalized on the basis of HLA-DR and CD38 costaining of CD8+ T cells. (D) Data summary for CTL activity of activated CD8+ T cells. Preimmunization CD8+ T cells mediated little to no granzyme B delivery to targets stimulated with HPV peptide, whereas postimmunization PBMCs delivered a statistically significant amount when compared to preimmunization activity as well as to activity against targets incubated with irrelevant peptide.
However, when PBMCs isolated after the third immunization were stimulated with the irrelevant control peptide or HPV peptides, a significant increase was seen in the activation status of CD8+ T cells (Fig. 8B, top). Moreover, when responses were corrected for background activation, HPV-specific CD8+ T cell activation (HLA-DR+/CD38+) was significantly increased in samples collected after immunization compared to those collected before immunization (P < 0.05; Fig. 8B, top right). These data suggest that a robust HPV-specific CD8+ T cell response was driven by VGX-3100.
We undertook additional analysis of these activated CD8+ T cells in the form of measuring HPV-specific up-regulation of granzyme B and perforin, the presence of which would further support the induction of a CTL phenotype. As shown in Fig. 8B, costaining for these lytic proteins in HLA-DR+/CD38+/CD8+ T cells was low and not significantly different in preimmunization samples, whether stimulated with irrelevant OVA peptides or HPV peptides for stimulation (Fig. 8B, top). Analysis of postimmunization samples leads to a markedly different result, whereby the percentage of activated CD8+ T cells that costained for granzyme B and perforin remained low when stimulated with irrelevant OVA peptide, but increased significantly when exposed to HPV peptides (P < 0.05) (Fig. 8B, bottom). Together, these results suggest that VGX-3100 drove the induction of CD8+ T cells capable of activation in the context of prolonged antigenic exposure and that these cells are capable of granzyme B and perforin synthesis, thus exhibiting a clear CTL phenotype.
VGX-3100 induction of CD8+ T cells with killing capacity is consistent in the 3 mg cohort (qualitative killing assay)
As differences in killing due to the frequency of CTLs within the PBMC pool had already been analyzed (Fig. 7), and because we were specifically interested in the quality of the function of HPV-specific CD8+ T cells that had been activated after prolonged antigenic exposure (Fig. 8, A and B), we again employed the killing assay but adjusted the mechanism by which we established the E:T ratio to normalize the effector number to reflect the percentage of HPV-specific CD8+ T cells present (as opposed to total PBMCs). To that end, the HLA-DR and CD38 markers were used to determine the number of total cells that needed to be added to targets to achieve a final E:T ratio of 12.5:1. This method was used to ensure that each sample would contain the same number of HPV-specific CD8+ T cells added to the same number of targets, and thus, killing activity would reflect the quality of the CTL response and not the quantity of CTLs present within the effector pool. Results from the 3 mg cohort are presented in Fig. 8C, and the analysis is presented in Fig. 8D. Although no HPV-specific CTL function was measured before immunization (Fig. 8D, left), all six subjects showed an up-regulation in activity after immunization; this increase reached statistical significance (P < 0.05, left and right). No significant killing activity was detected when preimmunization samples were used as effectors, and no killing activity was measured against targets pulsed with the irrelevant control peptide. An individual subject (12-12) previously classified as a nonresponder by ELISpot and ICS (Figs. 3, 6, and 7) showed measurable CTL activity in this qualitative killing assay (Fig. 8D). The postimmunization killing activity was both valid and specific because no activity was detectable when preimmunization samples were used (Fig. 8D), suggesting that the way this assay is performed may allow for detection of immune responses that are below the limit of detection of the ELISpot and ICS assays. Moreover, the other five subjects exhibited killing activity ranging from 29 to 36% (Fig. 8, C and D), suggesting that the quality of CTL function was consistent between these remaining subjects. The data support that the “activated CTL” phenotype exhibited by CD8+ T cells after prolonged antigenic exposure reflects the ability of these cells to kill infected targets in an antigen-specific fashion and that the quality of the CTL response driven by VGX-3100 is consistent among responders. This type of immune response is believed to be of paramount importance in the context of chronic HPV infection, in which CTLs will need to remain activated and functional in the face of prolonged antigenic exposure.
DISCUSSION
Here, we report on a dose-escalating phase 1 clinical study of a highly optimized HPV16 and HPV18 E6/E7 DNA vaccine delivered by efficient EP in women previously treated for high-grade cervical dysplasia—cervical intraepithelial neoplasia grade 2 or 3 (CIN2/3). Several features were incorporated into this vaccine design with the goal of increasing vaccine antigen immune potency, including codon/RNA optimization and the addition of a highly efficient leader and Kozak sequence. An endoproteolytic cleavage site was introduced between the E6 and the E7 sequences for proper protein folding and better CTL processing. Using these constructs, we detected increases in TH1-biased cellular immune responses in 78% of subjects by IFN-γ ELISpot. The responses were representative of a broad array of HLA class I and II alleles. The average peak T cell responses induced by VGX-3100 were 642 to 1458 SFU per 106 PBMCs in the different dose cohorts, indicating robust and broad vaccine-induced cellular immunity. Notably, no vaccine-related SAEs or grade 3 or 4 AEs or injection site reactions were observed in this first clinical trial of a therapeutic DNA vaccine expressing both E6 and E7 proteins of HPV16 and HPV18. In particular, fever, myalgia, arthralgia, or malaise—commonly associated with other vaccine modalities—were not reported. These safety findings are consistent with several previous reports from other DNA vaccine studies demonstrating a favorable safety profile, although now demonstrated with EP-mediated delivery. The immunogenicity benefits realized by EP-mediated delivery method of highly optimized DNA vaccine constructs did not adversely affect the favorable safety profile observed in previous DNA vaccine trials.
It is generally accepted that HPV-infected cells can only be eliminated by T cell–mediated immunity. Consequently, immunotherapy capable of inducing robust HPV16- or HPV18-specific cellular immune responses is highly desirable. Many efforts have been made in the development of HPV immuno-therapeutic vaccines. However, the cellular immune responses to E6 and/or E7 induced by various HPV therapeutic vaccine approaches have been modest or undetectable in clinical trials, when responses were measured by direct IFN-γ ELISpot (24, 26, 32, 63, 64). As a result, the cultured ELISpot assay has been used to amplify the HPV vaccine–induced responses and thus quantitate vaccine-induced immunity. Moreover, strong, long-lasting memory responses to HPV E6 or E7 have not been previously reported, and responses reported have been mostly limited in T cell breadth in response to other immunotherapeutic approaches. For example, the CTLs determined by 51Cr release assay after vaccination with TA-HPV (a recombinant HPV16 and HPV18 E6 and E7 vaccinia vaccine) were only transiently present in the circulation and were not detectable 4 weeks after immunization (64). The IFN-γ T cell responses induced by an HPV16 E7 protein–based vaccine appeared to be transient as well, and long-term IFN-γ responses to the vaccine antigen E7 could be detected in only 28% of the vaccinated subjects (22). In addition, E6- or E7-specific antibodies have been undetectable (HPV16 E7 DNA vaccine) (32) or transient (HPV vaccinia or protein–based vaccines) in previous clinical studies. Antibodies peaked immediately after the vaccinia boost at week 16 but declined by weeks 20 and 24 in a clinical study of HPV16 heterologous protein prime/vaccinia boost (65). Together, these findings suggest that although multiple platforms have been explored to develop effective HPV immunotherapies, the approaches studied to date do not appear to be highly potent with the currently used immunological assays.
In previous studies, vaccine-induced E6- or E7-specific CTL responses were evaluated to ascertain their contribution to any evidence of protection against the development of squamous intraepithelial lesions (66–68). It has also been reported that the occurrence of HPV-induced cancer was strongly associated with failure to mount a strong HPV-specific TH1 and CTL response (69). Induction of a persistent CTL response after vaccination was suggested to be critical. HPV-specific TH (CD4+ T cells) and cytotoxic CD8+ T cells expressing a distinct CTL phenotype (perforin, granzyme B, and CD107a) were induced in the majority of women vaccinated with VGX-3100. Moreover, the HPV-specific CD8+ T cells generated by VGX-3100 were fully capable of deploying their lytic granules to kill target cells expressing HPV16 and HPV18 E6 and E7 antigens, suggesting that they are truly functional cytotoxic lymphocytes. Additional investigationoftheseCTLs insamples fromthe3 mgcohort revealed that the quality of their activity is consistent between responders. The killing activity could be detected even 6 months after vaccination. This is the first report of an HPV vaccine demonstrating consistently robust vaccine-induced CTLs in a clinical study.
T cell memory has been suggested to be critical in the clearance of HPV because the presence of memory responses is associated with the rapid clearance of infected cells (70). In this trial, IFN-γ T cell responses were measured 24 weeks after the last immunization to gauge memory responses. Eleven of 14 responders exhibited persistent memory responses. Moreover, this response was not limited to secretion of IFN-γ: Fully functional HPV-specific CTLs also persisted long-term (up to 24 weeks after the final vaccination) in several subjects as measured in the killing assays. These responses—CTL induction and long-lasting cellular immune response—induced by VGX-3100 are in accordance with the types of responses believed to be necessary to eliminate persistent infection and are thus indicative of the positive potential of this vaccine for the immunotherapy of HPV16- and HPV18-induced persistent infection, dysplasia, and malignancy.
CD4+ Tregs have been shown to play a critical role in curtailing effective antitumor immunity (71). The presence of HPV-specific CD4+ Tregs at the interface of tumor and the immune system indicates that anti-tumor immunity in cervical cancer subjects can be suppressed at both the induction and effector levels. Preexisting E6- and E7-specific CD4+ Tregs suggest the possibility that vaccination might result in activation and expansion of this Treg subset and therefore affect host tolerance to tumorantigens(72, 73).Weobserved that womenwithCD4+CD25hi/FoxP3+ T cells before immunization did not exhibit a negative influence on the induction of cellular immune responses induced by VGX-3100. Further study is needed to investigate this issue more thoroughly and to examine HPV E6 or E7–specific tumor-induced Tregs (73).
Further, although the humoral responses induced against the E6 or E7 oncoproteins may not play a protective role against HPV-associated cervical neoplasms, E6- or E7-specific antibodies may serve as a surrogate marker for vaccine potency and the induction of antigen-specific cellular immune responses. The strong and persistent antibodies induced by VGX-3100 in this study are encouraging for the further development of antibody-dependent DNA vaccine strategies. In this regard, we note that all 18 (100%) of the vaccinated subjects across the three cohorts exhibited enhanced antibody responses to at least two of the antigens. On an individual antigen basis, we noted that the majority of subjects mounting a cellular response as measured by IFN-γ ELISpot also produced a humoral response to the same antigen (ranging from 45.5 to 100% across the different antigens). The differences in immune reactivity to these proteins are currently unclear but may be due to a number of factors, including innate immunogenic potential of these antigens and their relative ordering on the plasmid constructs, which may affect the expression, processing, or presentation of the antigens.
Although it has been possible to screen for and treat early-stage disease for more than 5 decades and highly effective preventative HPV prophylactic vaccines exist, cervical cancer remains the second most common cause of worldwide cancer death in women. Cervical cancer incidence remains high, and compared to other cancers, there is a relatively early peak risk of invasive cervical cancer (74). Methods to achieve broad coverage, especially in developing countries where more than 80% of all cases of cervical cancers occur (1), and expanding uptake of preventative vaccines remains a significant challenge. It is estimatedthat itwilltakedecadesforpreventive HPV vaccines to affect cervical cancer rates because of the prevalence of significant population with existing HPV infections and the slow process of carcinogenesis (75). An estimated 5 million cervical cancer deaths will occur in the next 20 years because of existing HPV infection (76). Therefore, HPV immunotherapeutic development remains of great importance. The data generated from this phase 1 trial of VGX-3100 are important in terms of the safety and tolerability of the DNA-plus-EP platform as well as in terms of the magnitude and types of immune responses generated. The results presented here indi- cate not only that DNA delivered by EP is safe and tolerable but also that it can induce potent humoral and cellular responses in the absence of normally required prime-boost regimens in humans. In particular, robust cellular responses noted in this study display functional cell-killing ability, which is believed to be key in the control and elimination of chronic HPV infection. It will thus be of considerable interest to determine whether this immunotherapeutic approach is capable of reducing lesion size, slowing progression to carcinoma, and, ultimately, driving disease clearance. A placebo-controlled, randomized phase 2 clinical trial of VGX-3100 is under way to determine whether this approach has an effect on clearance of HPV and high-grade dysplasia inwomenwith HPV16- or HPV18-related CIN2 and CIN3. The clear demonstration of antigen-specific humoral and functional cellular immune responses in this study opens up the likely use of this combined technology to treat or prevent other human infectious diseases and cancers.
MATERIALS AND METHODS
Ethics statement
The phase 1 clinical study was conducted in accordance with Good Clinical Practice Guidelines and approved by the institutional re- view board of each of the participating sites: Lyndhurst Clinical Research (Winston-Salem, NC); Laurel Highlands, OB/GYN, P.C. (Hopwood, PA); and Clinical Research Puerto Rico (San Juan, Puerto Rico). All study participants provided written informed consent before participation. This trial is registered at ClinicalTrials.gov (NCT00685412).
Participants and study design
Eighteen postresection CIN2/3 subjects were enrolled in this phase 1, open-label clinical trial designed to assess the safety, tolerability, and immunogenicity of escalating doses of VGX-3100 SynCon DNA vaccine delivered intramuscularly followed by EP with the CELLECTRA constant current device. Eligible subjects who consented to participate were assigned to a treatment cohort and received a three-dose series of VGX-3100 at one of three dosage levels (0.6, 2, or 6 mg of DNA per dose) administered as a 1.0-ml intramuscular injection in the deltoid on day 0, month 1, and month 3.
Participants were evaluated by study staff for a minimum of 30 min after each vaccination, and they were instructed to record oral temperatures and any local or systemic AEs on diary cards for 7 days after each dose. All safety data, including injection site reactions (such as pain, tenderness, erythema, and induration) and AEs from the 0.6 mg cohort, were submitted to the U.S. Food and Drug Administration for review before escalation to the 2 mg cohort. Additional safety reviews were performed by medical monitor in conjunction with the investigators before the second dose escalation. All subjects remained in the study for safety monitoring until the month 9 discharge visit.
Immunogenicity was assessed by HPV16 and HPV18 E6/E7–specific IFN-γ ELISpot assay and flow cytometric assays for IFN-γ, granzyme B, and perforin and killing functions with cryopreserved PBMCs obtained at prescreening, entry [dose 1 (D1)], 1 week after the second immunization (D2+1), 1 week after the third immunization (D3+1), and 4 weeks after the third immunization (D3+4). IFN-γ ELISpot responses were also assayed at 24 weeks after the third immunization (D3+24). Binding antibodies to HPV16 and HPV18 E6/E7 were measured by ELISA (all time points) and Western blot at D1, D3+4, and D3+24.
VGX-3100 SynCon DNA vaccine
VGX-3100 SynCon DNA vaccine is a mixture of two plasmids that encode the optimized consensus E6 and E7 genes of HPV serotypes 16 and 18, respectively. These two plasmids were developed as described previously (77, 78), manufactured under current Good Manufacturing Practices at VGXI Inc., and metal acceptance criteria for release. The bulkplasmidswereproducedathighconcentrationandblended to a final dose formulation of 6 mg/ml DNA (3 mg of each plasmid).
Immunological analyses and prioritization of PBMC samples
Sufficient quantities of sera and plasma were available to conduct ELISA and Western blot analyses from all 18 enrolled subjects at pre- and post-vaccination time points. The ELISA assays were conducted in triplicate measurements, and all time points from each patient were analyzed at the same time as a batch.
For the cellular assays, the available PBMCs were prioritized first to the IFN-γ ELISpot assay. Thus, we analyzed all 18 of 18 subjects as such using peptides spanning the four antigens separately to allow us to estimate individual responses against each antigen. The remaining samples, where available, were used for ICS assays. All subjects were included in the ICS analyses where sample was available. No subject was excluded for reasons other than sample availability. Thus, on the basis of the amount of viable PBMCs available, the ICS assays were prioritized as follows: quantitative killing assay—samples from 11 of 18 subjects available for analysis; lytic granule loading assay—6 of 6 high-dose cohort samples; qualitative killing assay—6 of 6 high-dose cohort samples; IFN-γ ICS assay—7 of 18 subjects (this assay yielded complementary data to the ELISpot assay and the functional assays above and hence was performed on the remaining samples for completeness).
IFN-γ ELISpot assay
The ELISpot assay was performed by the University of Pennsylvania Human Immunology Core Facility using a qualified protocol as previously described (79). The standard ELISpot protocol with 24-hour peptide stimulation was previously cross-validated across different laboratories (80) and was adopted here for use with HPV-specific peptide pools. Differences between the above-referenced protocol and the protocol used in the current study relate only to the use of HPV peptides as the stimulating antigen. Specifically, the current protocol used two sets of peptides, each containing 15–amino acid residues overlapping by 8 amino acids representing the entire consensus E6/E7 fusion protein sequence of HPV16 or HPV18, and were pooled at a concentration of 2 mg/ml per peptide into two pools, spanning the length of the E6 and E7 antigens, respectively (77, 78). The average number of SFU counted in R10 wells was subtracted from the average in individual HPV peptide wells and then adjusted to 1 × 106 PBMCs for each HPV peptide pool.
Intracellular cytokine staining
ICS was performed as previously described (52) using the following markers: CD107a-PECy7, CD14–Pacific Blue, CD16–Pacific Blue, CD8-APC (allophycocyanin), CD4-PerCPCy5.5, IFN-γ–FITC (fluorescein isothiocyanate), and CD45RO-AF700 (BD Biosciences); CD19– Pacific Blue and granzyme B–PE (phycoerythrin) Texas Red (Invitrogen); CD27-PECy5 (eBioscience); and perforin-PE (Abcam). Prepared cells were acquired with an LSR II flow cytometer equipped with BD FACSDiva software (BD Biosciences). Acquired data were analyzed with FlowJo software version 7.6.3 (Tree Star). All samples used for this assay were from a preimmunization time point (all subjects tested) and from the D3+1 time point, except for subject 14-6, for whom a D3+24 sample was used in place of D3+4. Staining was performed once per time point per sample listed.
Quantitative killing assay
The quantitative killing assay used the GranToxiLux PLUS kit (OncoImmunin) to measure antigen-specific delivery of active granzyme B from effector cells to target cells per manufacturer's instructions. Whole PBMCs obtained before or after immunization were stimulated 5 days in vitro with HPV16 and HPV18 E6/E7 peptides and used as effectors, whereas target cells were PBMCs collected from subjects before immunization that had been incubated with HPV16 and HPV18 E6 and E7 peptides for 1 hour at 37°C before their co-incubation with effectors. To ensure that killing was truly antigen-specific, we also incubated effectors with targets that had been incubated with an irrelevant peptide (OVA; GenScript). Effectors collected before immunization were also used as a control to detect any possible killing responses not attributable to VGX-3100 (for example, preexisting responses) to compare with responses obtained from effectors taken after immunization. Effectors were combined with autologous targets at three different E:T ratios: 10:1, 50:1, and 100:1. Nonspecific killing with the OVA peptide was measured and subtracted from the final reported killing activity. Overall, 11 of 18 subjects had sufficient sample available at either D3+4 or D3+24 (subjects 12-4, 14-4, 12-12, and 15-5). Staining was performed once per time point per sample listed.
Lytic granule loading assay
The lytic granule loading assay was performed by plating 1 × 106 PBMCs into a 96-well plate. For antigen-specific responses, cells were stimulated 5 days with a combination of HPV16 and HPV18 E6 and E7 peptides; an irrelevant peptide (OVA) was used as a negative control and concanavalin A was used as a positive control (Sigma-Aldrich). At the end of the 5-day incubation period, all samples were washed with phosphate-buffered saline (PBS) and subjected to staining for CD3-APCCy7, CD4-PerCPCy5.5, CD8-APC, CD38-PECy5, HLA-DR–AF700, granzyme B–PETR, and perforin-PE following the same protocol as described above. Staining was performed once per time point per sample listed.
Qualitative killing assay
The qualitative killing assay used the GranToxiLux PLUS kit as above before adjusting the E:T ratio. Unlike the quantitative killing assay, E:T ratios for the qualitative killing assay were determined with the HLADR and CD38 markers as a measure of HPV-specific effectors. Stimulated PBMCs were normalized for the presence of CD8+/CD38+/HLA-DR+ effector cells and added to targets to achieve a final E:T ratio of 12.5:1. Effectors collected before immunization were used as a control to detect preexisting killing responses attributable to previous HPV infection and not immunization with VGX-3100 for comparison with killing activity measured from effectors taken after immunization. We performed this assay on the six subjects from the 3 mg cohort using the D3+4 samples, except on subjects 14-7 and 15-5 (D3+24). Staining was performed once per time point per sample listed.
Binding antibody assay
A standardized binding ELISA was performed to measure the anti–HPV16/18 E6 or E7 antibody response induced by VGX-3100 SynCon DNA vaccine. Endpoint titers of antibodies were determined by coating 96-well enzyme immunoassay plates with HPV16 or HPV18 E6 or E7 proteins (1 μg/ml) (recombinant HPV16/18 E7 and HPV16 E6 were procured from ProteinX Lab; recombinant HPV18 E6 was cloned and purified from Escherichia coli expression vector by our group). Sera were diluted with 1% bovine serum albumin in PBS and tested in trip-licate with an automated and calibrated plate washer and read on a kinetic microplate reader (Molecular Devices). Positivity was considered if the average optical density (OD) of a sample was greater than 0.15 absorbance units and greater than the average OD before D1 (preimmunization) plus 2.5 times SD of OD before D1 at the same dilution. Number “1” instead of “0” was used for negative results to be able to display the data on a log scale.
Western blot assay
Five micrograms of HPV16 E7 or 18 E7 was loaded on an SDS–polyacrylamide electrophoresis gradient gel (4 to 12%), and Western blot analyses were performed with the plasma samples collected before D1, D3+4, and D3+24 from each subject at 1:200 dilution and visualized with horseradish peroxidase–conjugated goat anti-human IgG (GE Healthcare) with an ECL Western blot analysis system (GE Healthcare).
Statistical analyses
Standard and paired Student's t tests were performed to analyze statistical significance of all quantitative data produced in this study. Un-less otherwise indicated, P values were calculated to determine statistical significance at various confidence levels.
To summarize the T cell ELISpot data, immune responses to each individual antigen were reported. For each time point, the mean numbers of SFU from triplicate wells with PBMCs incubated with medium alone (background) were subtracted from the means of PBMCs stimulated with HPV16 or HPV18 E6 or E7 peptides. After subtracting medium control, the mean difference in the wells with the PBMCs collected after vaccination and at D1 had to exceed 20 SFU per 106 PBMCs and be greater than two times SD of D1 antigen-specific response to be characterized as a positive response.
Supplementary Material
Acknowledgments
We dedicate this manuscript in memory of D. Sewell. Sewell's work with J.Y. and D.B.W. was instrumental to the development of VGX-3100, and the clinical promise of this approach is a tribute to Sewell's early contributions. We thank E. Thompson for assistance with the ICS assay, J. Morales and J. Sunyecz and their staff for their clinical efforts, VGXI Inc. for manufacturing VGX-3100 clinical material, and the HPV-001 clinical team and the study volunteers for their participation.
Funding: Supported by NIH, Flu (R01 AI092843), and HIV Vaccine Design and Development Teams (U19 AI078675) awarded to D.B.W. and Sponsored Research Agreement funding to D.B.W. for Consensus Immunogen Design.
Footnotes
Author contributions: R.D.-A., C.J.W., J.J.K., and N.Y.S. contributed to the design of the clinical study, whereas D.B.W. provided invaluable input for the overall program. M.L.B., R.L.P., J.C.L., M.G., A.S.K., K.E.B., C.J.W., J.J.K., and N.Y.S. contributed to the implementation of the clinical protocol and analysis of the safety data. M.L.B., J.Y., M.P.M., X.S., A.S.K., K.E.B., C.K., F.L., J.D.B., D.B.W., and N.Y.S. contributed to the immune analysis and interpretation of the immune data. M.L.B., J.Y., M.P.M., J.C.L., J.J.K., D.B.W., and N.Y.S. contributed to the manuscript writing. All authors approved the final manuscript.
Competing interests: M.L.B., J.Y., M.P.M., X.S., J.C.L., M.G., A.S.K., K.E.B., C.K., F.L., R.D.-A., C.J.W., J.J.K., and N.Y.S. are current or former employees of Inovio Pharmaceuticals Inc., the sponsor of the clinical study. D.B.W. notes the following relationships are associated with this work and should be acknowledged. D.B.W. has received consulting fees, has stock ownership, or has served on Advisory Boards or Review Board Service or has received speaking support from the following companies: Pfizer, Inovio, BMS, VGXI, Virxsys, Ichor, Merck, Althea, Aldevron, Novartis, and possibly others. The VGX-3100 vaccine is covered under U.S. patent 8,168,769 and published application no. 2010-0189730 and their related multiple foreign counterparts.
Citation: M. L. Bagarazzi, J. Yan, M. P. Morrow, X. Shen, R. L. Parker, J. C. Lee, M. Giffear, P. Pankhong, A. S. Khan, K. E. Broderick, C. Knott, F. Lin, J. D. Boyer, R. Draghia-Akli, C. J. White, J. J. Kim, D. B. Weiner, N. Y. Sardesai, Immunotherapy against HPV16/18 generates potent TH1 and cytotoxic cellular immune responses. Sci. Transl. Med. 4, 155ra138 (2012).
REFERENCES AND NOTES
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J. Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Franceschi S, Rajkumar T, Vaccarella S, Gajalakshmi V, Sharmila A, Snijders PJ, Muñoz N, Meijer CJ, Herrero R. Human papillomavirus and risk factors for cervical cancer in Chennai, India: A case-control study. Int. J. Cancer. 2003;107:127–133. doi: 10.1002/ijc.11350. [DOI] [PubMed] [Google Scholar]
- 3.Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, Snijders PJ, Peto J, Meijer CJ, Muñoz N. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J. Pathol. 1999;189:12–19. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 4.Muñoz N, Bosch FX, Castellsagué X, Díaz M, de Sanjose S, Hammouda D, Shah KV, Meijer CJ. Against which human papillomavirus types shall we vaccinate and screen? The international perspective. Int. J. Cancer. 2004;111:278–285. doi: 10.1002/ijc.20244. [DOI] [PubMed] [Google Scholar]
- 5.Bosch FX, de Sanjosé S. Human papillomavirus in cervical cancer. Curr. Oncol. Rep. 2002;4:175–183. doi: 10.1007/s11912-002-0079-y. [DOI] [PubMed] [Google Scholar]
- 6.Villa LL, Costa RL, Petta CA, Andrade RP, Ault KA, Giuliano AR, Wheeler CM, Koutsky LA, Malm C, Lehtinen M, Skjeldestad FE, Olsson SE, Steinwall M, Brown DR, Kurman RJ, Ronnett BM, Stoler MH, Ferenczy A, Harper DM, Tamms GM, Yu J, Lupinacci L, Railkar R, Taddeo FJ, Jansen KU, Esser MT, Sings HL, Saah AJ, Barr E. Prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in young women: A randomised double-blind placebo-controlled multicentre phase II efficacy trial. Lancet Oncol. 2005;6:271–278. doi: 10.1016/S1470-2045(05)70101-7. [DOI] [PubMed] [Google Scholar]
- 7.Harper DM. Prevention of human papillomavirus infections and associated diseases by vaccination: A new hope for global public health. Public Health Genomics. 2009;12:319–330. doi: 10.1159/000214922. [DOI] [PubMed] [Google Scholar]
- 8.Paavonen J, Jenkins D, Bosch FX, Naud P, Salmerón J, Wheeler CM, Chow SN, Apter DL, Kitchener HC, Castellsague X, de Carvalho NS, Skinner SR, Harper DM, Hedrick JA, Jaisamrarn U, Limson GA, Dionne M, Quint W, Spiessens B, Peeters P, Struyf F, Wieting SL, Lehtinen MO, Dubin G, HPV PATRICIA study group Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: An interim analysis of a phase III double-blind, randomised controlled trial. Lancet. 2007;369:2161–2170. doi: 10.1016/S0140-6736(07)60946-5. [DOI] [PubMed] [Google Scholar]
- 9.Seedorf K, Oltersdorf T, Krämmer G, Röwekamp W. Identification of early proteins of the human papilloma viruses type 16 (HPV 16) and type 18 (HPV 18) in cervical carcinoma cells. EMBO J. 1987;6:139–144. doi: 10.1002/j.1460-2075.1987.tb04731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pim D, Storey A, Thomas M, Massimi P, Banks L. Mutational analysis of HPV-18 E6 identifies domains required for p53 degradation in vitro, abolition of p53 transactivation in vivo and immortalisation of primary BMK cells. Oncogene. 1994;9:1869–1876. [PubMed] [Google Scholar]
- 11.Crook T, Tidy JA, Vousden KH. Degradation of p53 can be targeted by HPV E6 sequences distinct from those required for p53 binding and trans-activation. Cell. 1991;67:547–556. doi: 10.1016/0092-8674(91)90529-8. [DOI] [PubMed] [Google Scholar]
- 12.Boursnell ME, Rutherford E, Hickling JK, Rollinson EA, Munro AJ, Rolley N, McLean CS, Borysiewicz LK, Vousden K, Inglis SC. Construction and characterisation of a recombinant vaccinia virus expressing human papillomavirus proteins for immunotherapy of cervical cancer. Vaccine. 1996;14:1485–1494. doi: 10.1016/S0264-410X(96)00117-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Münger K, Howley PM. Human papillomavirus immortalization and transformation functions. Virus Res. 2002;89:213–228. doi: 10.1016/s0168-1702(02)00190-9. [DOI] [PubMed] [Google Scholar]
- 14.Eiben GL, Velders MP, Kast WM. The cell-mediated immune response to human papillomavirus-induced cervical cancer: Implications for immunotherapy. Adv. Cancer Res. 2002;86:113–148. doi: 10.1016/s0065-230x(02)86004-3. [DOI] [PubMed] [Google Scholar]
- 15.Sarkar AK, Tortolero-Luna G, Follen M, Sastry KJ. Inverse correlation of cellular immune responses specific to synthetic peptides from the E6 and E7 oncoproteins of HPV-16 with recurrence of cervical intraepithelial neoplasia in a cross-sectional study. Gynecol. Oncol. 2005;99:S251–S261. doi: 10.1016/j.ygyno.2005.07.099. [DOI] [PubMed] [Google Scholar]
- 16.Kadish AS, Timmins P, Wang Y, Ho GY, Burk RD, Ketz J, He W, Romney SL, Johnson A, Angeletti R, Abadi M. Albert Einstein Cervix Dysplasia Clinical Consortium, Regression of cervical intraepithelial neoplasia and loss of human papillomavirus (HPV) infection is associated with cell-mediated immune responses to an HPV type 16 E7 peptide. Cancer Epidemiol. Bio-markers Prev. 2002;11:483–488. [PubMed] [Google Scholar]
- 17.Peng S, Trimble C, Wu L, Pardoll D, Roden R, Hung CF, Wu TC. HLA-DQB1*02–restricted HPV-16 E7 peptide–specific CD4+ T-cell immune responses correlate with regression of HPV-16–associated high-grade squamous intraepithelial lesions. Clin. Cancer Res. 2007;13:2479–2487. doi: 10.1158/1078-0432.CCR-06-2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Farhat S, Nakagawa M, Moscicki AB. Cell-mediated immune responses to human papillomavirus 16 E6 and E7 antigens as measured by interferon gamma enzyme-linked immunospot in women with cleared or persistent human papillomavirus infection. Int. J. Gynecol. Cancer. 2009;19:508–512. doi: 10.1111/IGC.0b013e3181a388c4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maciag PC, Radulovic S, Rothman J. The first clinical use of a live-attenuated Listeria monocytogenes vaccine: A phase I safety study of Lm-LLO-E7 in patients with advanced carcinoma of the cervix. Vaccine. 2009;27:3975–3983. doi: 10.1016/j.vaccine.2009.04.041. [DOI] [PubMed] [Google Scholar]
- 20.Davidson EJ, Boswell CM, Sehr P, Pawlita M, Tomlinson AE, McVey RJ, Dobson J, Roberts JS, Hickling J, Kitchener HC, Stern PL. Immunological and clinical responses in women with vulval intraepithelial neoplasia vaccinated with a vaccinia virus encoding human papillomavirus 16/18 oncoproteins. Cancer Res. 2003;63:6032–6041. [PubMed] [Google Scholar]
- 21.Muderspach L, Wilczynski S, Roman L, Bade L, Felix J, Small LA, Kast WM, Fascio G, Marty V, Weber J. A phase I trial of a human papillomavirus (HPV) peptide vaccine for women with high-grade cervical and vulvar intraepithelial neoplasia who are HPV 16 positive. Clin. Cancer Res. 2000;6:3406–3416. [PubMed] [Google Scholar]
- 22.Hallez S, Simon P, Maudoux F, Doyen J, Noël JC, Beliard A, Capelle X, Buxant F, Fayt I, Lagrost AC, Hubert P, Gerday C, Burny A, Boniver J, Foidart JM, Delvenne P, Jacobs N. Phase I/II trial of immunogenicity of a human papillomavirus (HPV) type 16 E7 protein-based vaccine in women with oncogenic HPV-positive cervical intraepithelial neoplasia. Cancer Immunol. Immunother. 2004;53:642–650. doi: 10.1007/s00262-004-0501-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roman LD, Wilczynski S, Muderspach LI, Burnett AF, O'Meara A, Brinkman JA, Kast WM, Facio G, Felix JC, Aldana M, Weber JS. A phase II study of Hsp-7 (SGN-00101) in women with high-grade cervical intraepithelial neoplasia. Gynecol. Oncol. 2007;106:558–566. doi: 10.1016/j.ygyno.2007.05.038. [DOI] [PubMed] [Google Scholar]
- 24.Kenter GG, Welters MJ, Valentijn AR, Lowik MJ, Berends-van der Meer DM, Vloon AP, Drijfhout JW, Wafelman AR, Oostendorp J, Fleuren GJ, Offringa R, van der Burg SH, Melief CJ. Phase I immunotherapeutic trial with long peptides spanning the E6 and E7 sequences of high-risk human papillomavirus 16 in end-stage cervical cancer patients shows low toxicity and robust immunogenicity. Clin. Cancer Res. 2008;14:169–177. doi: 10.1158/1078-0432.CCR-07-1881. [DOI] [PubMed] [Google Scholar]
- 25.Santin AD, Bellone S, Palmieri M, Zanolini A, Ravaggi A, Siegel ER, Roman JJ, Pecorelli S, Cannon MJ. Human papillomavirus type 16 and 18 E7-pulsed dendritic cell vaccination of stage IB or IIA cervical cancer patients: A phase I escalating-dose trial. J. Virol. 2008;82:1968–1979. doi: 10.1128/JVI.02343-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garcia F, Petry KU, Muderspach L, Gold MA, Braly P, Crum CP, Magill M, Silverman M, Urban RG, Hedley ML, Beach KJ. ZYC101a for treatment of high-grade cervical intraepithelial neoplasia: A randomized controlled trial. Obstet. Gynecol. 2004;103:317–326. doi: 10.1097/01.AOG.0000110246.93627.17. [DOI] [PubMed] [Google Scholar]
- 27.Kim D, Gambhira R, Karanam B, Monie A, Hung CF, Roden R, Wu TC. Generation and characterization of a preventive and therapeutic HPV DNA vaccine. Vaccine. 2008;26:351–360. doi: 10.1016/j.vaccine.2007.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kenter GG, Welters MJ, Valentijn AR, Lowik MJ, Berends-van der Meer DM, Vloon AP, Essahsah F, Fathers LM, Offringa R, Drijfhout JW, Wafelman AR, Oostendorp J, Fleuren GJ, van der Burg SH, Melief CJ. Vaccination against HPV-16 oncoproteins for vulvar intraepithelial neoplasia. N. Engl. J. Med. 2009;361:1838–1847. doi: 10.1056/NEJMoa0810097. [DOI] [PubMed] [Google Scholar]
- 29.Martin T, Parker SE, Hedstrom R, Le T, Hoffman SL, Norman J, Hobart P, Lew D. Plasmid DNA malaria vaccine: The potential for genomic integration after intramuscular injection. Hum. Gene Ther. 1999;10:759–768. doi: 10.1089/10430349950018517. [DOI] [PubMed] [Google Scholar]
- 30.Chattergoon M, Boyer J, Weiner DB. Genetic immunization: A new era in vaccines and immune therapeutics. FASEB J. 1997;11:753–763. doi: 10.1096/fasebj.11.10.9271360. [DOI] [PubMed] [Google Scholar]
- 31.Kennedy JS, Co M, Green S, Longtine K, Longtine J, O'Neill MA, Adams JP, Rothman AL, Yu Q, Johnson-Leva R, Pal R, Wang S, Lu S, Markham P. The safety and tolerability of an HIV-1 DNA prime-protein boost vaccine (DP6-001) in healthy adult volunteers. Vaccine. 2008;26:4420–4424. doi: 10.1016/j.vaccine.2008.05.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trimble CL, Peng S, Kos F, Gravitt P, Viscidi R, Sugar E, Pardoll D, Wu TC. A phase I trial of a human papillomavirus DNA vaccine for HPV16+ cervical intraepithelial neoplasia 2/3. Clin. Cancer Res. 2009;15:361–367. doi: 10.1158/1078-0432.CCR-08-1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Deml L, Bojak A, Steck S, Graf M, Wild J, Schirmbeck R, Wolf H, Wagner R. Multiple effects of codon usage optimization on expression and immunogenicity of DNA candidate vaccines encoding the human immunodeficiency virus type 1 Gag protein. J. Virol. 2001;75:10991–11001. doi: 10.1128/JVI.75.22.10991-11001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muthumani K, Zhang D, Dayes NS, Hwang DS, Calarota SA, Choo AY, Boyer JD, Weiner DB. Novel engineered HIV-1 East African Clade-A gp160 plasmid construct induces strong humoral and cell-mediated immune responses in vivo. Virology. 2003;314:134–146. doi: 10.1016/s0042-6822(03)00459-8. [DOI] [PubMed] [Google Scholar]
- 35.Schneider R, Campbell M, Nasioulas G, Felber BK, Pavlakis GN. Inactivation of the human immunodeficiency virus type 1 inhibitory elements allows Rev-independent expression of Gag and Gag/protease and particle formation. J. Virol. 1997;71:4892–4903. doi: 10.1128/jvi.71.7.4892-4903.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gaschen B, Taylor J, Yusim K, Foley B, Gao F, Lang D, Novitsky V, Haynes B, Hahn BH, Bhattacharya T, Korber B. Diversity considerations in HIV-1 vaccine selection. Science. 2002;296:2354–2360. doi: 10.1126/science.1070441. [DOI] [PubMed] [Google Scholar]
- 37.Yan J, Yoon H, Kumar S, Ramanathan MP, Corbitt N, Kutzler M, Dai A, Boyer JD, Weiner DB. Enhanced cellular immune responses elicited by an engineered HIV-1 subtype B consensus-based envelope DNA vaccine. Mol. Ther. 2007;15:411–421. doi: 10.1038/sj.mt.6300036. [DOI] [PubMed] [Google Scholar]
- 38.Laddy DJ, Yan J, Corbitt N, Kobasa D, Kobinger GP, Weiner DB. Immunogenicity of novel consensus-based DNA vaccines against avian influenza. Vaccine. 2007;25:2984–2989. doi: 10.1016/j.vaccine.2007.01.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hirao LA, Wu L, Khan AS, Satishchandran A, Draghia-Akli R, Weiner DB. Intradermal/subcutaneous immunization by electroporation improves plasmid vaccine delivery and potency in pigs and rhesus macaques. Vaccine. 2008;26:440–448. doi: 10.1016/j.vaccine.2007.10.041. [DOI] [PubMed] [Google Scholar]
- 40.Luckay A, Sidhu MK, Kjeken R, Megati S, Chong SY, Roopchand V, Garcia-Hand D, Abdullah R, Braun R, Montefiori DC, Rosati M, Felber BK, Pavlakis GN, Mathiesen I, Israel ZR, Eldridge JH, Egan MA. Effect of plasmid DNA vaccine design and in vivo electroporation on the resulting vaccine-specific immune responses in Rhesus macaques. J. Virol. 2007;81:5257–5269. doi: 10.1128/JVI.00055-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vasan S, Hurley A, Schlesinger SJ, Hannaman D, Gardiner DF, Dugin DP, Boente-Carrera M, Vittorino R, Caskey M, Andersen J, Huang Y, Cox JH, Tarragona-Fiol T, Gill DK, Cheeseman H, Clark L, Dally L, Smith C, Schmidt C, Park HH, Kopycinski JT, Gilmour J, Fast P, Bernard R, Ho DD. In vivo electroporation enhances the immunogenicity of an HIV-1 DNA vaccine candidate in healthy volunteers. PLoS One. 2011;6:e19252. doi: 10.1371/journal.pone.0019252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Low L, Mander A, McCann K, Dearnaley D, Tjelle T, Mathiesen I, Stevenson F, Ottensmeier CH. DNA vaccination with electroporation induces increased antibody responses in patients with prostate cancer. Hum. Gene Ther. 2009;20:1269–1278. doi: 10.1089/hum.2009.067. [DOI] [PubMed] [Google Scholar]
- 43.Sardesai NY, Weiner DB. Electroporation delivery of DNA vaccines: Prospects for success. Curr. Opin. Immunol. 2011;23:421–429. doi: 10.1016/j.coi.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dubey S, Clair J, Fu TM, Guan L, Long R, Mogg R, Anderson K, Collins KB, Gaunt C, Fernandez VR, Zhu L, Kierstead L, Thaler S, Gupta SB, Straus W, Mehrotra D, Tobery TW, Casimiro DR, Shiver JW. Detection of HIV vaccine-induced cell-mediated immunity in HIV-seronegative clinical trial participants using an optimized and validated enzyme-linked immunospot assay. J. Acquir. Immune Defic. Syndr. 2007;45:20–27. doi: 10.1097/QAI.0b013e3180377b5b. [DOI] [PubMed] [Google Scholar]
- 45.Buchbinder SP, Mehrotra DV, Duerr A, Fitzgerald DW, Mogg R, Li D, Gilbert PB, Lama JR, Marmor M, Del Rio C, McElrath MJ, Casimiro DR, Gottesdiener KM, Chodakewitz JA, Corey L, Robertson MN, Step Study Protocol Team Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): A double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet. 2008;372:1881–1893. doi: 10.1016/S0140-6736(08)61591-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Migueles SA, Rood JE, Berkley AM, Guo T, Mendoza D, Patamawenu A, Hallahan CW, Cogliano NA, Frahm N, Duerr A, McElrath MJ, Connors M. Trivalent adenovirus type 5 HIV recombinant vaccine primes for modest cytotoxic capacity that is greatest in humans with protective HLA class I alleles. PLoS Pathog. 2011;7:e1002002. doi: 10.1371/journal.ppat.1002002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cullen SP, Brunet M, Martin SJ. Granzymes in cancer and immunity. Cell Death Differ. 2010;17:616–623. doi: 10.1038/cdd.2009.206. [DOI] [PubMed] [Google Scholar]
- 48.Berke G. The CTL's kiss of death. Cell. 1995;81:9–12. doi: 10.1016/0092-8674(95)90365-8. [DOI] [PubMed] [Google Scholar]
- 49.Malyguine A, Strobl S, Zaritskaya L, Baseler M, Shafer-Weaver K. New approaches for monitoring CTL activity in clinical trials. Adv. Exp. Med. Biol. 2007;601:273–284. doi: 10.1007/978-0-387-72005-0_29. [DOI] [PubMed] [Google Scholar]
- 50.Betts MR, Nason MC, West SM, De Rosa SC, Migueles SA, Abraham J, Lederman MM, Benito JM, Goepfert PA, Connors M, Roederer M, Koup RA. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood. 2006;107:4781–4789. doi: 10.1182/blood-2005-12-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Migueles SA, Osborne CM, Royce C, Compton AA, Joshi RP, Weeks KA, Rood JE, Berkley AM, Sacha JB, Cogliano-Shutta NA, Lloyd M, Roby G, Kwan R, McLaughlin M, Stallings S, Rehm C, O'Shea MA, Mican J, Packard BZ, Komoriya A, Palmer S, Wiegand AP, Maldarelli F, Coffin JM, Mellors JW, Hallahan CW, Follman DA, Connors M. Lytic granule loading of CD8+ T cells is required for HIV-infected cell elimination associated with immune control. Immunity. 2008;29:1009–1021. doi: 10.1016/j.immuni.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Morrow MP, Yan J, Pankhong P, Shedlock DJ, Lewis MG, Talbott K, Toporovski R, Khan AS, Sardesai NY, Weiner DB. IL-28B/IFN-λ3 drives granzyme B loading and significantly increases CTL killing activity in macaques. Mol. Ther. 2010;18:1714–1723. doi: 10.1038/mt.2010.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Varadarajan N, Julg B, Yamanaka YJ, Chen H, Ogunniyi AO, McAndrew E, Porter LC, Piechocka-Trocha A, Hill BJ, Douek DC, Pereyra F, Walker BD, Love JC. A high-throughput single-cell analysis of human CD8+ T cell functions reveals discordance for cytokine secretion and cytolysis. J. Clin. Invest. 2011;121:4322–4331. doi: 10.1172/JCI58653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Molling JW, de Gruijl TD, Glim J, Moreno M, Rozendaal L, Meijer CJ, van den Eertwegh AJ, Scheper RJ, von Blomberg ME, Bontkes HJ. CD4+CD25hi regulatory T-cell frequency correlates with persistence of human papillomavirus type 16 and T helper cell responses in patients with cervical intraepithelial neoplasia. Int. J. Cancer. 2007;121:1749–1755. doi: 10.1002/ijc.22894. [DOI] [PubMed] [Google Scholar]
- 55.Cao Y, Zhao J, Yang Z, Cai Z, Zhang B, Zhou Y, Shen GX, Chen X, Li S, Huang B. CD4+FOXP3+ regulatory T cell depletion by low-dose cyclophosphamide prevents recurrence in patients with large condylomata acuminata after laser therapy. Clin. Immunol. 2010;136:21–29. doi: 10.1016/j.clim.2010.02.020. [DOI] [PubMed] [Google Scholar]
- 56.Scott ME, Ma Y, Kuzmich L, Moscicki AB. Diminished IFN-γ and IL-10 and elevated Foxp3 mRNA expression in the cervix are associated with CIN 2 or 3. Int. J. Cancer. 2009;124:1379–1383. doi: 10.1002/ijc.24117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.van der Burg SH, Piersma SJ, de Jong A, van der Hulst JM, Kwappenberg KM, van den Hende M, Welters MJ, Van Rood JJ, Fleuren GJ, Melief CJ, Kenter GG, Offringa R. Association of cervical cancer with the presence of CD4+ regulatory T cells specific for human papillomavirus antigens. Proc. Natl. Acad. Sci. U.S.A. 2007;104:12087–12092. doi: 10.1073/pnas.0704672104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Welters MJ, Kenter GG, de Vos van Steenwijk PJ, Löwik MJ, Berends-van der Meer DM, Essahsah F, Stynenbosch LF, Vloon AP, Ramwadhdoebe TH, Piersma SJ, van der Hulst JM, Valentijn AR, Fathers LM, Drijfhout JW, Franken KL, Oostendorp J, Fleuren GJ, Melief CJ, van der Burg SH. Success or failure of vaccination for HPV16-positive vulvar lesions correlates with kinetics and phenotype of induced T-cell responses. Proc. Natl. Acad. Sci. U.S.A. 2010;107:11895–11899. doi: 10.1073/pnas.1006500107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hofmeyer KA, Jeon H, Zang X. The PD-1/PD-L1 (B7-H1) pathway in chronic infection-induced cytotoxic T lymphocyte exhaustion. J. Biomed. Biotechnol. 2011;2011:451694. doi: 10.1155/2011/451694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sakthivel P, Gereke M, Bruder D. Therapeutic intervention in cancer and chronic viral infections: Antibody mediated manipulation of PD-1/PD-L1 interaction. Rev. Recent Clin. Trials. 2012;7:10–23. doi: 10.2174/157488712799363262. [DOI] [PubMed] [Google Scholar]
- 61.Ingram JT, Yi JS, Zajac AJ. Exhausted CD8 T cells downregulate the IL-18 receptor and become unresponsive to inflammatory cytokines and bacterial co-infections. PLoS Pathog. 2011;7:e1002273. doi: 10.1371/journal.ppat.1002273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Querec TD, Akondy RS, Lee EK, Cao W, Nakaya HI, Teuwen D, Pirani A, Gernert K, Deng J, Marzolf B, Kennedy K, Wu H, Bennouna S, Oluoch H, Miller J, Vencio RZ, Mulligan M, Aderem A, Ahmed R, Pulendran B. Systems biology approach predicts immunogenicity of the yellow fever vaccine in humans. Nat. Immunol. 2009;10:116–125. doi: 10.1038/ni.1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.de Jong A, O'Neill T, Khan AY, Kwappenberg KM, Chisholm SE, Whittle NR, Dobson JA, Jack LC, St Clair Roberts JA, Offringa R, van der Burg SH, Hickling JK. Enhancement of human papillomavirus (HPV) type 16 E6 and E7-specific T-cell immunity in healthy volunteers through vaccination with TA-CIN, an HPV16 L2E7E6 fusion protein vaccine. Vaccine. 2002;20:3456–3464. doi: 10.1016/s0264-410x(02)00350-x. [DOI] [PubMed] [Google Scholar]
- 64.Kaufmann AM, Stern PL, Rankin EM, Sommer H, Nuessler V, Schneider A, Adams M, Onon TS, Bauknecht T, Wagner U, Kroon K, Hickling J, Boswell CM, Stacey SN, Kitchener HC, Gillard J, Wanders J, Roberts JS, Zwierzina H. Safety and immunogenicity of TA-HPV, a recombinant vaccinia virus expressing modified human papillomavirus (HPV)-16 and HPV-18 E6 and E7 genes, in women with progressive cervical cancer. Clin. Cancer Res. 2002;8:3676–3685. [PubMed] [Google Scholar]
- 65.Smyth LJ, Van Poelgeest MI, Davidson EJ, Kwappenberg KM, Burt D, Sehr P, Pawlita M, Man S, Hickling JK, Fiander AN, Tristram A, Kitchener HC, Offringa R, Stern PL, Van Der Burg SH. Immunological responses in women with human papillomavirus type 16 (HPV-16)-associated anogenital intraepithelial neoplasia induced by heterologous prime-boost HPV-16 oncogene vaccination. Clin. Cancer Res. 2004;10:2954–2961. doi: 10.1158/1078-0432.ccr-03-0703. [DOI] [PubMed] [Google Scholar]
- 66.Nakagawa M, Stites DP, Palefsky JM, Kneass Z, Moscicki AB. CD4-positive and CD8-positive cytotoxic T lymphocytes contribute to human papillomavirus type 16 E6 and E7 responses. Clin. Diagn. Lab. Immunol. 1999;6:494–498. doi: 10.1128/cdli.6.4.494-498.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nakagawa M, Stites DP, Patel S, Farhat S, Scott M, Hills NK, Palefsky JM, Moscicki AB. Persistence of human papillomavirus type 16 infection is associated with lack of cytotoxic T lymphocyte response to the E6 antigens. J. Infect. Dis. 2000;182:595–598. doi: 10.1086/315706. [DOI] [PubMed] [Google Scholar]
- 68.Nakagawa M, Stites DP, Farhat S, Sisler JR, Moss B, Kong F, Moscicki AB, Palefsky JM. Cytotoxic T lymphocyte responses to E6 and E7 proteins of human papillomavirus type 16: Relationship to cervical intraepithelial neoplasia. J. Infect. Dis. 1997;175:927–931. doi: 10.1086/513992. [DOI] [PubMed] [Google Scholar]
- 69.Welters MJ, Kenter GG, Piersma SJ, Vloon AP, Löwik MJ, Berends-van der Meer DM, Drijfhout JW, Valentijn AR, Wafelman AR, Oostendorp J, Fleuren GJ, Offringa R, Melief CJ, van der Burg SH. Induction of tumor-specific CD4+ and CD8+ T-cell immunity in cervical cancer patients by a human papillomavirus type 16 E6 and E7 long peptides vaccine. Clin. Cancer Res. 2008;14:178–187. doi: 10.1158/1078-0432.CCR-07-1880. [DOI] [PubMed] [Google Scholar]
- 70.Nakagawa M, Viscidi R, Deshmukh I, Costa MD, Palefsky JM, Farhat S, Moscicki AB. Time course of humoral and cell-mediated immune responses to human papillomavirus type 16 in infected women. Clin. Diagn. Lab. Immunol. 2002;9:877–882. doi: 10.1128/CDLI.9.4.877-882.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tanaka H, Tanaka J, Kjaergaard J, Shu S. Depletion of CD4+ CD25+ regulatory cells augments the generation of specific immune T cells in tumor-draining lymph nodes. J. Immunother. 2002;25:207–217. doi: 10.1097/00002371-200205000-00003. [DOI] [PubMed] [Google Scholar]
- 72.van der Burg SH, Piersma SJ, de Jong A, van der Hulst JM, Kwappenberg KM, van den Hende M, Welters MJ, Van Rood JJ, Fleuren GJ, Melief CJ, Kenter GG, Offringa R. Association of cervical cancer with the presence of CD4+ regulatory T cells specific for human papillomavirus antigens. Proc. Natl. Acad. Sci. U.S.A. 2007;104:12087–12092. doi: 10.1073/pnas.0704672104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhou G, Drake CG, Levitsky HI. Amplification of tumor-specific regulatory T cells following therapeutic cancer vaccines. Blood. 2006;107:628–636. doi: 10.1182/blood-2005-07-2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schiffman M, Castle PE, Jeronimo J, Rodriguez AC, Wacholder S. Human papillomavirus and cervical cancer. Lancet. 2007;370:890–907. doi: 10.1016/S0140-6736(07)61416-0. [DOI] [PubMed] [Google Scholar]
- 75.Ma B, Xu Y, Hung CF, Wu TC. HPV and therapeutic vaccines: Where are we in 2010? Curr. Cancer Ther. Rev. 2010;6:81–103. [Google Scholar]
- 76.Frazer IH, Quinn M, Nicklin JL, Tan J, Perrin LC, Ng P, O'Connor VM, White O, Wendt N, Martin J, Crowley JM, Edwards SJ, McKenzie AW, Mitchell SV, Maher DW, Pearse MJ, Basser RL. Phase 1 study of HPV16-specific immunotherapy with E6E7 fusion protein and ISCOMATRIX adjuvant in women with cervical intraepithelial neoplasia. Vaccine. 2004;23:172–181. doi: 10.1016/j.vaccine.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 77.Yan J, Harris K, Khan AS, Draghia-Akli R, Sewell D, Weiner DB. Cellular immunity induced by a novel HPV18 DNA vaccine encoding an E6/E7 fusion consensus protein in mice and rhesus macaques. Vaccine. 2008;26:5210–5215. doi: 10.1016/j.vaccine.2008.03.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yan J, Reichenbach DK, Corbitt N, Hokey DA, Ramanathan MP, McKinney KA, Weiner DB, Sewell D. Induction of antitumor immunity in vivo following delivery of a novel HPV-16 DNA vaccine encoding an E6/E7 fusion antigen. Vaccine. 2009;27:431–440. doi: 10.1016/j.vaccine.2008.10.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Boyer JD, Robinson TM, Kutzler MA, Parkinson R, Calarota SA, Sidhu MK, Muthumani K, Lewis M, Pavlakis G, Felber B, Weiner D. SIV DNA vaccine co-administered with IL-12 expression plasmid enhances CD8 SIV cellular immune responses in cynomolgus macaques. J. Med. Primatol. 2005;34:262–270. doi: 10.1111/j.1600-0684.2005.00124.x. [DOI] [PubMed] [Google Scholar]
- 80.Janetzki S, Panageas KS, Ben-Porat L, Boyer J, Britten CM, Clay TM, Kalos M, Maecker HT, Romero P, Yuan J, Kast WM, Hoos A, Elispot Proficiency Panel of the CVC Immune Assay Working Group Results and harmonization guidelines from two large-scale international Elispot proficiency panels conducted by the Cancer Vaccine Consortium (CVC/SVI). Cancer Immunol. Immunother. 2008;57:303–315. doi: 10.1007/s00262-007-0380-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.