Abstract
Diabetes is associated with several complications such as retinopathy, nephropathy, neuropathy and cardiovascular diseases. Currently, insulin is the main used medication for management of insulin-dependent diabetes mellitus (type-1 diabetes). In this metabolic syndrome, in addition to decrease of endogenous insulin, the plasma level of connecting peptide (C-peptide) is also reduced due to beta cell destruction. Studies in the past decade have shown that C-peptide is much more than a byproduct of insulin biosynthesis and possess different biological activities. Therefore, it may be possible that C-peptide deficiency be involved, at least in part, in the development of different complications of diabetes. It has been shown that a small level of remaining C-peptide is associated with significant metabolic benefit. The purpose of this review is to describe beneficial effects of C-peptide replacement on pathological features associated with insulin-dependent diabetes. Also, experimental and clinical findings on the effects of C-peptide on whole-body glucose utilization, adipose tissue metabolism and tissues blood flow are summarized and discussed. The hypoglycemic, antilipolytic and vasodilator effects of C-peptide suggest that it may contribute to fine-tuning of the tissues metabolism under different physiologic or pathologic conditions. Therefore, C-peptide replacement together with the classic insulin therapy may prevent, retard, or ameliorate diabetic complications in patients with type-1 diabetes.
Keywords: C-peptide, Diabetes, Insulin, Nephropathy, Neuropathy
Core tip: In type-1 diabetes, in addition to decrease of endogenous insulin, the plasma level of connecting peptide (C-peptide) is also reduced due to beta cell destruction. Therefore, it may be possible that C-peptide deficiency be involved in the development of diabetic complications such as retinopathy, nephropathy, neuropathy and cardiovascular diseases. In this paper, beneficial effects of C-peptide replacement on pathological features associated with type-1 diabetes are described. Also, experimental and clinical findings that support the hypoglycemic, antilipolytic and vasodilator effects of C-peptide are discussed.
INTRODUCTION
Diabetes mellitus is still an increasing health problem in both developing and developed countries. World Health Organization reported (August, 2011) that, 346 million people have diabetes worldwide and 3.4 million patients died from diabetes-related complications in the year 2004. Diabetes is generally classified into two main types: insulin-dependent diabetes mellitus [type-1 diabetes (T1D)] which is a state of insulin deficiency because of destruction of islet beta cells, and non-insulin-dependent diabetes mellitus [type-2 diabetes (T2D)] which is characterized by resistance to the action of insulin[1].
Poor control of diabetes is associated with several complications such as nephropathy, retinopathy, neur-opathy and cardiovascular diseases[1]. Currently, insulin is the main used medication for management of T1D[2]. Even though early-onset complications may be controlled by insulin therapy, it remains difficult to achieve normal glycemic control and late-onset complications occur in many of diabetic patients[3,4]. In addition to decrease of endogenous insulin, the level of connecting peptide (C-peptide) is also reduced in the plasma of patients with T1D due to autoimmune destruction of beta cell[5]. Although for many years C-peptide has been considered as a byproduct of insulin biosynthesis, data from several lines of studies reveals the beneficial actions of C-peptide replacement in prevention of metabolic changes and structural alterations in T1D[6]. Therefore, one cannot rule out the possibility that C-peptide deficiency may also be involved, at least in part, in the development of some pathological features associated with T1D. This article reviews the current understanding of biological effects of C-peptide and the beneficial actions of C-peptide replacement on preventing or ameliorating the T1D-related complications.
C-PEPTIDE SYNTHESIS AND SECRETION
In pancreatic beta cells, proinsulin is transferred in vesicles from rough endoplasmic reticulum to Golgi apparatus, where the vesicles are directed into a regulated secretion. During this transition of vesicles, three peptidases participate in proinsulin posttranslational processing to generate insulin and C-peptide[7]. First, proinsulin is cleaved by prohormone convertase type 2 at the A-chain/C-peptide junction or by prohormone convertase type 1/3 at the B-chain/C-peptide junction. Then, carboxypeptidases H removes two pairs of amino acids located at both cleaved junctions providing the des forms of proinsulin (des-31,32 and des-64, 65). Finally, endopeptidase type 1/3 and type 2 recognizes des-64,65 proinsulin and des-31, 32 proinsulin, respectively, leading to release of insulin and C-peptide from proinsulin. C-peptide facilitates the correct folding of proinsulin to allow form two disulfide bridges between A- and B-chains of insulin and therefore plays an essential role in biosynthesis of insulin[6,8]. In most species only one form of proinsulin has been described. However, in rats and mice two proinsulin isoforms I and II have been found[9].
Increase of blood glucose leads to secretion of an equimolar amount of insulin and C-peptide into the portal circulation[7]. However, the liver rapidly uptakes insulin because of single pass effect and only 50% of insulin reaches to the systemic circulation with a half-life about 4 min. On the other hand, C-peptide is primarily metabolized by kidney and has a circulating half-life about 30 min which is the reason for its higher plasma concentration than insulin[5,7]. The level of C-peptide in fasting and postprandial conditions varies between 0.3-1 nM and 1.5-2.5 nmol/L, respectively[10].
Since C-peptide and insulin are secreted from beta cells in equimolar concentrations, measuring serum C-peptide is an estimate of residual beta cell function and can be used to differentiate between patients with T1D and T2D[6]. However, the mean C-peptide concentration is higher in diabetic patients with renal diseases insufficiency compared with those have normal renal function. There-fore, in sever renal failure, the serum C-peptide assay is unreliable for assess residual beta cells[6,11,12].
BIOLOGICAL EFFECTS OF C-PEPTIDE
Effects of C-peptide on glucose utilization
Experimental studies on diabetic rats showed that C-peptide prolongs the hypoglycemic effect of insulin[13] and increases whole-body glucose utilization[9,14,15]. The glucose lowering effect of C-peptide was also investigated in human. Hoogwerf et al[16] have shown no effect by C-peptide on blood glucose level in healthy subjects or patients with T1D. However, Johansson and coworkers demonstrated that infusion of physiological concentrations of C-peptide to patients with T1D augments whole body glucose utilization by approximately 25%[17]. Also, Oskarsson et al[18] showed that C-peptide hasten the insulin-induced hypoglycemia in diabetic patients. Activation of glucose metabolism by short time C-peptide infusion in healthy controls and in patients with T1D was also reported by Wilhelm et al[19].
The augmented whole body glucose utilization is most probably a result of increased muscle glucose uptake rather than inhibition of hepatic gluconeogenesis[20]. In normal rats, we observed that adipose tissue glucose consumption was not affected by C-peptide[21]. Direct examinations under in vitro condition confirmed that C-peptide stimulates the rate of glucose transport to muscle strips obtained from healthy subjects or patients with T1D[22]. Also, Zierath et al[23] showed that C-peptide dose-dependently increases glucose uptake into human skeletal muscle through a mechanism shared partly with insulin. Although the exact pathway involved in this effect of C-peptide is still unknown, incubation of isolated muscle strips with a cAMP analogue abolishes the C-peptide-stimulated glucose transport.
Regarding metabolic actions of C-peptide, it should be considered that although this peptide at low physiological concentrations mimics insulin effects, however in the presence of high level of insulin (e.g., in the postprandial condition) the concomitant elevated level of C-peptide may blunt the insulin’s peripheral effects[21,24]. It is possible that high levels of C-peptide induce a desensitization processes which may be recovered after a period of its absence.
Effect of C-peptide on adipose tissue
Soon after discovery of C-peptide, Solmon et al[25] examined the effects of pork and beef C-peptide on adrenocorticotropin-induced lipolysis in rats, but no significant effects were found. Subsequently, Yu and coworkers tested the effect of supraphysiological concentrations of porcine C-peptide on the lipolysis in isolated adipocytes from rats and found an insignificant antilipolytic effect[26]. Using an ex-vivo organ culture method, we observed a similar insignificant reduction in basal lipolysis of rat retroperitoneal adipose tissue[21]. Because it has been reported that some effects of C-peptide appear only in diabetes condition[9,27,28], we examined whether C-peptide alters lipolysis in diabetic rats. Our data showed that C-peptide like insulin significantly inhibits isoproterenol-stimulated lipolysis[29]. Therefore C-peptide may act, conditionally, as an antili-polytic hormone and may be involved in fine-tuning of lipid metabolism.
Effects of C-peptide on circulation
Patients with T1D show reduced tissues blood flow despite intensive insulin therapy and good management of glucose control[30]. C-peptide has been shown to enhance blood flow of kidney[17], nerve[31], skeletal muscle[32], myocardium[30,33] and skin[34]. The vasodilator effect of C-peptide is mediated by stimulation of nitric oxide release from endothelial cells[35-37]. Wallerath et al[35] reported that physiological postprandial concentration of C-peptide is able to activate endothelial nitric oxide synthase (eNOS) and stimulating nitric oxide production. Forst et al[38] showed that intravenous infusion of C-peptide to patients with T1D increases plasma concentration of cGMP, as an index of nitric oxide activity[38]. This finding is in agreement with earlier report that in diabetic rats the C-peptide induced glucose utilization is sensitive to eNOS inhibition[9].
OTHER BIOLOGICAL EFFECTS OF C-PEPTIDE
Interaction with insulin
In the presence of C-peptide, insulin hexamers in solution becomes undetectable. Also, subcutaneous injection of an insulin and C-peptide mixture to diabetic patients accelerates the increase of insulin levels in plasma and in comparison with injection of insulin alone utilizes more glucose. Therefore, it seems that C-peptide increases disaggregation of insulin by binding to insulin oligomers and thereby enhances the availability of monomeric (biologically active form) insulin[39].
Protection of endothelium
It has been reported that C-peptide is able to inhibit leukocyte-endothelium interaction induced by thrombin or by NG-nitro-L-arginine methyl ester. This effect of C-peptide may be important in protection of vasculature against inflammatory disorders such those observed in T1D[40].
EFFECTS OF C-PEPTIDE ON DIABETIC COMPLICATIONS
Effects on diabetic nephropathy
Different aspects of the diabetic renal pathogenic abnormalities can be improved by C-peptide in T1D (Figure 1). In diabetic rats, C-peptide decreases urinary protein excretion[41-43], reduces glomerular hyperfiltration rate and restores the renal functional reserve[42-44]. These beneficial effects have also been demonstrated in insulin-dependent diabetic patients[17,45]. In a clinical study, patients were administered insulin alone or in combination with C-peptide by subcutaneous infusion pump for 4 wk. While combination therapy led to decrease of glomerular filtration rate and protein excretion after 2 wk, the insulin alone was ineffective[45]. Johansson et al[46] extended the period of C-peptide therapy to 3 mo and reported a significant decrease in the rate of protein excretion in patients receiving combination of insulin and C-peptide. In line with these findings, Zerbini et al[47] found a decreased C-peptide/creatinine ratio in the plasma of T1D patients with nephropathy when compared with those without albuminuria[47]. Microscopic examinations have showed that in diabetic rats, C-peptide reduces the hypertrophy of mesangial matrix in glomeruli of the kidneys[48]. Several mechanisms are postulated for beneficial effects of C-peptide on renal function including inhibition of apoptosis, increase of Na+, K+-ATPase activity and interaction with the signaling pathway of growth factors[49,50]. Activation of the key signaling molecules such as phospholipase C and protein kinase C followed by phosphorylation of extracellular-signal-regulated kinase and c-Jun N-terminal kinase have been shown in human renal tubular cells treated with C-peptide[49]. Regarding beneficial effects of C-peptide, we should emphasis that some of the C-peptide beneficial effects are limited to animals or patients who show very low or missing C-peptide plasma levels[9,27,28]. Therefore, the nephroprotective effect of C-peptide may represent a therapeutic goal for patients with T1D.
Figure 1.
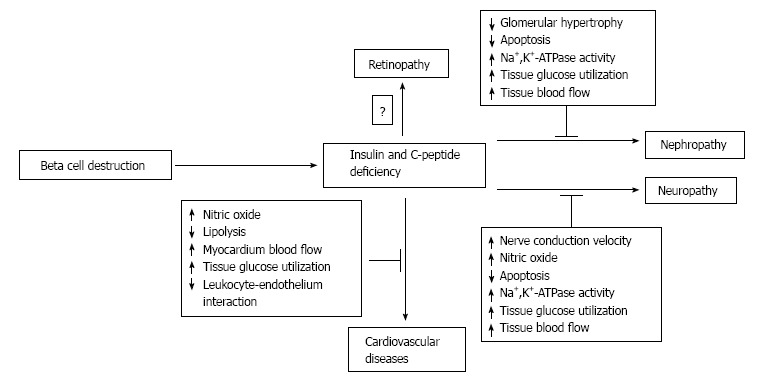
Proposed mechanisms (dashed rectangles) by which C-peptide may prevent, retard, or ameliorate diabetic complications in patient with type-1 diabetes. ↓: Decrease; ↑: Increase.
Effects on diabetic neuropathy
Accumulating evidence suggests that C-peptide can prevent, retard, or ameliorate neuropathy in T1D (Figure 1)[51-57]. Decreased level of Na+, K+-ATPase activity and reduced nitric oxide formation are considered as contributor factors to pathogenesis of diabetic neuro-pathy. It has been shown that C-peptide prevents the neural Na+, K+-ATPase defect and the nerve conduction velocity reduction[52]. In study of Cotter et al[31] C-peptide at physiologic doses improved sensory and motor nerve conduction velocity in STZ-induced diabetic rats through increase of nitric oxide release. Ekberg et al[58] demonstrated that C-peptide improves vibration perce-ption in patients with T1D[58]. C-peptide also may prevent cognitive dysfunction by its antiapoptotic effect in the brain particularly in the hippocampus[51-54]. The antiapoptotic property was also confirmed by Li et al[59] who showed that C-peptide, in the presence of insulin, inhibits high glucose-induced apoptosis in neuroblastoma cells. There are also clinical evidence that autonomic dysfunction can be ameliorated by C-peptide replacement. Infusion of C-peptide to patients with T1D increases the heart rate variability during deep breathing and the heart rate brake index after tilting[55]. In contrast to insulin alone, administration of a combination of C-peptide and insulin improves heart rate during deep breathing in T1D patients[46].
CONCLUSION
According to data presented in this paper, C-peptide is much more than a byproduct of insulin synthesis and has several biological actions such as hypoglycemic, antilipolytic and vasodilator effects. These biological effects suggest that it may act as a hormone to contribute in fine-tuning of the tissues metabolism under different physiologic or pathologic conditions. In T1D diabetes, in particular, it was found that patients who conserve low but sustained secretion of endogenous C-peptide show better metabolic control and less retinopathy, nephropathy and neuropathy than patients who have become fully C-peptide and insulin deficient[56-60]. These beneficial effects are demonstrated only in T1D models. It is possible that in physiological conditions, C-peptide produces its maximum effect and induces some levels of desensitization processes in phosphorylation mediated actions, especially nitric oxide-dependent pathways. Recovering the C-peptide mechanism of action during a period of its absence is in good agreement with the experimental results in T1D models. Therefore, present data suggest the possibility of a clinically applicable role for C-peptide replacement, together with the classic insulin therapy, to prevent, retard, or ameliorate diabetic complications in patient with T1D.
Footnotes
P- Reviewer: Nakamura Y S- Editor: Qi Y L- Editor: A E- Editor: Lu YJ
Supported by Mashhad University of Medical Sciences.
Conflict-of-interest: No potential conflicts of interest relevant to this article were reported.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: September 9, 2014
First decision: October 14, 2014
Article in press: December 17, 2014
References
- 1.Deshpande AD, Harris-Hayes M, Schootman M. Epide-miology of diabetes and diabetes-related complications. Phys Ther. 2008;88:1254–1264. doi: 10.2522/ptj.20080020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aathira R, Jain V. Advances in management of type 1 diabetes mellitus. World J Diabetes. 2014;5:689–696. doi: 10.4239/wjd.v5.i5.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tzoulaki I, Molokhia M, Curcin V, Little MP, Millett CJ, Ng A, Hughes RI, Khunti K, Wilkins MR, Majeed A, et al. Risk of cardiovascular disease and all cause mortality among patients with type 2 diabetes prescribed oral antidiabetes drugs: retrospective cohort study using UK general practice research database. BMJ. 2009;339:b4731. doi: 10.1136/bmj.b4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Palmer JP, Fleming GA, Greenbaum CJ, Herold KC, Jansa LD, Kolb H, Lachin JM, Polonsky KS, Pozzilli P, Skyler JS, et al. C-peptide is the appropriate outcome measure for type 1 diabetes clinical trials to preserve beta-cell function: report of an ADA workshop, 21-22 October 2001. Diabetes. 2004;53:250–264. doi: 10.2337/diabetes.53.1.250. [DOI] [PubMed] [Google Scholar]
- 5.Eisenbarth GS, Buse JB. Type 1 diabetes mellitus. In: Melmed S, Polonsky KS, Larsen PR, editors. Williams textbook of endocrinology. Philadelphia: Elsevier; 2011. pp. 1436–1453. [Google Scholar]
- 6.Marques RG, Fontaine MJ, Rogers J. C-peptide: much more than a byproduct of insulin biosynthesis. Pancreas. 2004;29:231–238. doi: 10.1097/00006676-200410000-00009. [DOI] [PubMed] [Google Scholar]
- 7.Steiner DF, Bell GI, Rubenstein AH. Chemistry and biosynthesis of the islet hormones. In: DeGroot L, Jameson JL, editors. Endocrinology. Philadelphia: Elsevier; 2006. pp. 925–960. [Google Scholar]
- 8.Habener JF. Genetic control of peptide hormone formation. In: Melmed S, Polonsky KS, Larsen PR, editors. Williams textbook of endocrinology. Philadelphia: Elsevier; 2011. pp. 31–43. [Google Scholar]
- 9.Li L, Oshida Y, Kusunoki M, Yamanouchi K, Johansson BL, Wahren J, Sato Y. Rat C peptide I and II stimulate glucose utilization in STZ-induced diabetic rats. Diabetologia. 1999;42:958–964. doi: 10.1007/s001250051254. [DOI] [PubMed] [Google Scholar]
- 10.Kuzuya H, Blix PM, Horwitz DL, Rubenstein AH, Steiner DF, Faber OK, Binder C. Heterogeneity of circulating human C-peptide. Diabetes. 1978;27 Suppl 1:184–191. doi: 10.2337/diab.27.1.s184. [DOI] [PubMed] [Google Scholar]
- 11.Covic AM, Schelling JR, Constantiner M, Iyengar SK, Sedor JR. Serum C-peptide concentrations poorly phenotype type 2 diabetic end-stage renal disease patients. Kidney Int. 2000;58:1742–1750. doi: 10.1046/j.1523-1755.2000.00335.x. [DOI] [PubMed] [Google Scholar]
- 12.DeFronzo RA, Tobin JD, Rowe JW, Andres R. Glucose intolerance in uremia. Quantification of pancreatic beta cell sensitivity to glucose and tissue sensitivity to insulin. J Clin Invest. 1978;62:425–435. doi: 10.1172/JCI109144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wójcikowski C, Maier V, Dominiak K, Fussgänger R, Pfeiffer EF. Effects of synthetic rat C-peptide in normal and diabetic rats. Diabetologia. 1983;25:288–290. doi: 10.1007/BF00279945. [DOI] [PubMed] [Google Scholar]
- 14.Sato Y, Oshida Y, Han YQ, Morishita Y, Li L, Ekberg K, Jörnvall H, Wahren J. C-peptide fragments stimulate glucose utilization in diabetic rats. Cell Mol Life Sci. 2004;61:727–732. doi: 10.1007/s00018-003-3460-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu W, Oshida Y, Yang WP, Li L, Ohsawa I, Sato J, Iwao S, Johansson BL, Wahren J, Sato Y. Effect of C-peptide administration on whole body glucose utilization in STZ-induced diabetic rats. Acta Physiol Scand. 1996;157:253–258. doi: 10.1046/j.1365-201X.1996.489236000.x. [DOI] [PubMed] [Google Scholar]
- 16.Hoogwerf BJ, Bantle JP, Gaenslen HE, Greenberg BZ, Senske BJ, Francis R, Goetz FC. Infusion of synthetic human C-peptide does not affect plasma glucose, serum insulin, or plasma glucagon in healthy subjects. Metabolism. 1986;35:122–125. doi: 10.1016/0026-0495(86)90111-3. [DOI] [PubMed] [Google Scholar]
- 17.Johansson BL, Sjöberg S, Wahren J. The influence of human C-peptide on renal function and glucose utilization in type 1 (insulin-dependent) diabetic patients. Diabetologia. 1992;35:121–128. doi: 10.1007/BF00402543. [DOI] [PubMed] [Google Scholar]
- 18.Oskarsson P, Johansson BL, Adamson U, Lins PE. Effects of C-peptide on insulin-induced hypoglycaemia and its counterregulatory responses in IDDM patients. Diabet Med. 1997;14:655–659. doi: 10.1002/(SICI)1096-9136(199708)14:8<655::AID-DIA435>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 19.Wilhelm B, Weber MM, Ekberg K, Ries C, Kugler M, Pfuetzner A, Kann PH, Wahren J, Forst T. Activation of glucose metabolism by C-peptide in patients with type 1 diabetes mellitus and in healthy control subjects. Diabetes. 2007;56:A337. [Google Scholar]
- 20.Johansson BL, Linde B, Wahren J. Effects of C-peptide on blood flow, capillary diffusion capacity and glucose utilization in the exercising forearm of type 1 (insulin-dependent) diabetic patients. Diabetologia. 1992;35:1151–1158. doi: 10.1007/BF00401369. [DOI] [PubMed] [Google Scholar]
- 21.Ghorbani A, Omrani GR, Hadjzadeh MA, Varedi M. Effects of rat C-peptide-II on lipolysis and glucose consumption in cultured rat adipose tissue. Exp Clin Endocrinol Diabetes. 2011;119:343–347. doi: 10.1055/s-0031-1275662. [DOI] [PubMed] [Google Scholar]
- 22.Zierath JR, Galuska D, Johansson BL, Wallberg-Henriksson H. Effect of human C-peptide on glucose transport in in vitro incubated human skeletal muscle. Diabetologia. 1991;34:899–901. doi: 10.1007/BF00400197. [DOI] [PubMed] [Google Scholar]
- 23.Zierath JR, Handberg A, Tally M, Wallberg-Henriksson H. C-peptide stimulates glucose transport in isolated human skeletal muscle independent of insulin receptor and tyrosine kinase activation. Diabetologia. 1996;39:306–313. doi: 10.1007/BF00418346. [DOI] [PubMed] [Google Scholar]
- 24.Grunberger G, Qiang X, Li Z, Mathews ST, Sbrissa D, Shisheva A, Sima AA. Molecular basis for the insulinomimetic effects of C-peptide. Diabetologia. 2001;44:1247–1257. doi: 10.1007/s001250100632. [DOI] [PubMed] [Google Scholar]
- 25.Solomon SS, Brush JS, Kitabchi AE. Antilipolytic activity of insulin and proinsulin on ACTH and cyclic nucleotide-induced lipolysis in the isolated adipose cell of rat. Biochim Biophys Acta. 1970;218:167–169. doi: 10.1016/0005-2760(70)90104-9. [DOI] [PubMed] [Google Scholar]
- 26.Yu SS, Kitbachi AE. Biological activity of proinsulin and related polypeptides in the fat tissue. J Biol Chem. 1973;248:3753–3761. [PubMed] [Google Scholar]
- 27.Kunt T, Schneider S, Pfützner A, Goitum K, Engelbach M, Schauf B, Beyer J, Forst T. The effect of human proinsulin C-peptide on erythrocyte deformability in patients with Type I diabetes mellitus. Diabetologia. 1999;42:465–471. doi: 10.1007/s001250051180. [DOI] [PubMed] [Google Scholar]
- 28.Nordquist L, Moe E, Sjöquist M. The C-peptide fragment EVARQ reduces glomerular hyperfiltration in streptozotocin-induced diabetic rats. Diabetes Metab Res Rev. 2007;23:400–405. doi: 10.1002/dmrr.704. [DOI] [PubMed] [Google Scholar]
- 29.Ghorbani A, Omrani GR, Hadjzadeh MA, Varedi M. Proinsulin C-peptide inhibits lipolysis in diabetic rat adipose tissue through phosphodiestrase-3B enzyme. Horm Metab Res. 2013;45:221–225. doi: 10.1055/s-0032-1323764. [DOI] [PubMed] [Google Scholar]
- 30.Johansson BL, Sundell J, Ekberg K, Jonsson C, Seppänen M, Raitakari O, Luotolahti M, Nuutila P, Wahren J, Knuuti J. C-peptide improves adenosine-induced myocardial vasodilation in type 1 diabetes patients. Am J Physiol Endocrinol Metab. 2004;286:E14–E19. doi: 10.1152/ajpendo.00236.2003. [DOI] [PubMed] [Google Scholar]
- 31.Cotter MA, Ekberg K, Wahren J, Cameron NE. Effects of proinsulin C-peptide in experimental diabetic neuropathy: vascular actions and modulation by nitric oxide synthase inhibition. Diabetes. 2003;52:1812–1817. doi: 10.2337/diabetes.52.7.1812. [DOI] [PubMed] [Google Scholar]
- 32.Jensen ME, Messina EJ. C-peptide induces a concentration-dependent dilation of skeletal muscle arterioles only in presence of insulin. Am J Physiol. 1999;276:H1223–H1228. doi: 10.1152/ajpheart.1999.276.4.H1223. [DOI] [PubMed] [Google Scholar]
- 33.Hansen A, Johansson BL, Wahren J, von Bibra H. C-peptide exerts beneficial effects on myocardial blood flow and function in patients with type 1 diabetes. Diabetes. 2002;51:3077–3082. doi: 10.2337/diabetes.51.10.3077. [DOI] [PubMed] [Google Scholar]
- 34.Forst T, Kunt T, Pohlmann T, Goitom K, Engelbach M, Beyer J, Pfützner A. Biological activity of C-peptide on the skin microcirculation in patients with insulin-dependent diabetes mellitus. J Clin Invest. 1998;101:2036–2041. doi: 10.1172/JCI2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wallerath T, Kunt T, Forst T, Closs EI, Lehmann R, Flohr T, Gabriel M, Schäfer D, Göpfert A, Pfützner A, et al. Stimulation of endothelial nitric oxide synthase by proinsulin C-peptide. Nitric Oxide. 2003;9:95–102. doi: 10.1016/j.niox.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 36.Kitamura T, Kimura K, Makondo K, Furuya DT, Suzuki M, Yoshida T, Saito M. Proinsulin C-peptide increases nitric oxide production by enhancing mitogen-activated protein-kinase-dependent transcription of endothelial nitric oxide synthase in aortic endothelial cells of Wistar rats. Diabetologia. 2003;46:1698–1705. doi: 10.1007/s00125-003-1232-3. [DOI] [PubMed] [Google Scholar]
- 37.Joshua IG, Zhang Q, Falcone JC, Bratcher AP, Rodriguez WE, Tyagi SC. Mechanisms of endothelial dysfunction with development of type 1 diabetes mellitus: role of insulin and C-peptide. J Cell Biochem. 2005;96:1149–1156. doi: 10.1002/jcb.20620. [DOI] [PubMed] [Google Scholar]
- 38.Forst T, De La Tour DD, Kunt T, Pfützner A, Goitom K, Pohlmann T, Schneider S, Johansson BL, Wahren J, Löbig M, et al. Effects of proinsulin C-peptide on nitric oxide, microvascular blood flow and erythrocyte Na+,K+-ATPase activity in diabetes mellitus type I. Clin Sci (Lond) 2000;98:283–290. [PubMed] [Google Scholar]
- 39.Shafqat J, Melles E, Sigmundsson K, Johansson BL, Ekberg K, Alvelius G, Henriksson M, Johansson J, Wahren J, Jörnvall H. Proinsulin C-peptide elicits disaggregation of insulin resulting in enhanced physiological insulin effects. Cell Mol Life Sci. 2006;63:1805–1811. doi: 10.1007/s00018-006-6204-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scalia R, Coyle KM, Levine BJ, Booth G, Lefer AM. C-peptide inhibits leukocyte-endothelium interaction in the microcirculation during acute endothelial dysfunction. FASEB J. 2000;14:2357–2364. doi: 10.1096/fj.00-0183com. [DOI] [PubMed] [Google Scholar]
- 41.Rebsomen L, Pitel S, Boubred F, Buffat C, Feuerstein JM, Raccah D, Vague P, Tsimaratos M. C-peptide replacement improves weight gain and renal function in diabetic rats. Diabetes Metab. 2006;32:223–228. doi: 10.1016/s1262-3636(07)70272-0. [DOI] [PubMed] [Google Scholar]
- 42.Sjöquist M, Huang W, Johansson BL. Effects of C-peptide on renal function at the early stage of experimental diabetes. Kidney Int. 1998;54:758–764. doi: 10.1046/j.1523-1755.1998.00074.x. [DOI] [PubMed] [Google Scholar]
- 43.Huang DY, Richter K, Breidenbach A, Vallon V. Human C-peptide acutely lowers glomerular hyperfiltration and proteinuria in diabetic rats a dose-response study. Naunyn-Schmiedebergs Arch Pharmacol. 2002;365:67–73. doi: 10.1007/s00210-001-0502-1. [DOI] [PubMed] [Google Scholar]
- 44.Samnegård B, Jacobson SH, Jaremko G, Johansson BL, Sjöquist M. Effects of C-peptide on glomerular and renal size and renal function in diabetic rats. Kidney Int. 2001;60:1258–1265. doi: 10.1046/j.1523-1755.2001.00964.x. [DOI] [PubMed] [Google Scholar]
- 45.Johansson BL, Kernell A, Sjöberg S, Wahren J. Influence of combined C-peptide and insulin administration on renal function and metabolic control in diabetes type 1. J Clin Endocrinol Metab. 1993;77:976–981. doi: 10.1210/jcem.77.4.8408474. [DOI] [PubMed] [Google Scholar]
- 46.Johansson BL, Pernow J. C-peptide potentiates the vasoconstrictor effect of neuropeptide Y in insulin-dependent diabetic patients. Acta Physiol Scand. 1999;165:39–44. doi: 10.1046/j.1365-201x.1999.00475.x. [DOI] [PubMed] [Google Scholar]
- 47.Zerbini G, Mangili R, Luzi L. Higher post-absorptive C-peptide levels in Type 1 diabetic patients without renal complications. Diabet Med. 1999;16:1048. doi: 10.1046/j.1464-5491.1999.00181.x. [DOI] [PubMed] [Google Scholar]
- 48.Samnegård B, Jacobson SH, Jaremko G, Johansson BL, Ekberg K, Isaksson B, Eriksson L, Wahren J, Sjöquist M. C-peptide prevents glomerular hypertrophy and mesangial matrix expansion in diabetic rats. Nephrol Dial Transplant. 2005;20:532–538. doi: 10.1093/ndt/gfh683. [DOI] [PubMed] [Google Scholar]
- 49.Maezawa Y, Yokote K, Sonezaki K, Fujimoto M, Kobayashi K, Kawamura H, Tokuyama T, Takemoto M, Ueda S, Kuwaki T, et al. Influence of C-peptide on early glomerular changes in diabetic mice. Diabetes Metab Res Rev. 2006;22:313–322. doi: 10.1002/dmrr.612. [DOI] [PubMed] [Google Scholar]
- 50.Al-Rasheed NM, Willars GB, Brunskill NJ. C-peptide signals via Galpha i to protect against TNF-alpha-mediated apoptosis of opossum kidney proximal tubular cells. J Am Soc Nephrol. 2006;17:986–995. doi: 10.1681/ASN.2005080797. [DOI] [PubMed] [Google Scholar]
- 51.Li ZG, Zhang W, Sima AA. C-peptide prevents hippocampal apoptosis in type 1 diabetes. Int J Exp Diabetes Res. 2002;3:241–245. doi: 10.1080/15604280214936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sima AA, Zhang W, Sugimoto K, Henry D, Li Z, Wahren J, Grunberger G. C-peptide prevents and improves chronic Type I diabetic polyneuropathy in the BB/Wor rat. Diabetologia. 2001;44:889–897. doi: 10.1007/s001250100570. [DOI] [PubMed] [Google Scholar]
- 53.Kamiya H, Zhang W, Ekberg K, Wahren J, Sima AA. C-Peptide reverses nociceptive neuropathy in type 1 diabetes. Diabetes. 2006;55:3581–3587. doi: 10.2337/db06-0396. [DOI] [PubMed] [Google Scholar]
- 54.Sima AA, Li ZG. The effect of C-peptide on cognitive dysfunction and hippocampal apoptosis in type 1 diabetic rats. Diabetes. 2005;54:1497–1505. doi: 10.2337/diabetes.54.5.1497. [DOI] [PubMed] [Google Scholar]
- 55.Johansson BL, Borg K, Fernqvist-Forbes E, Odergren T, Remahl S, Wahren J. C-peptide improves autonomic nerve function in IDDM patients. Diabetologia. 1996;39:687–695. doi: 10.1007/BF00418540. [DOI] [PubMed] [Google Scholar]
- 56.Sjöberg S, Gunnarsson R, Gjötterberg M, Lefvert AK, Persson A, Ostman J. Residual insulin production, glycaemic control and prevalence of microvascular lesions and polyneuropathy in long-term type 1 (insulin-dependent) diabetes mellitus. Diabetologia. 1987;30:208–213. doi: 10.1007/BF00270417. [DOI] [PubMed] [Google Scholar]
- 57.Sjöberg S, Gjötterberg M, Berglund L, Möller E, Ostman J. Residual C-peptide excretion is associated with a better long-term glycemic control and slower progress of retinopathy in type I (insulin-dependent) diabetes mellitus. J Diabet Complications. 1991;5:18–22. doi: 10.1016/0891-6632(91)90005-a. [DOI] [PubMed] [Google Scholar]
- 58.Ekberg K, Brismar T, Johansson BL, Jonsson B, Lindström P, Wahren J. Amelioration of sensory nerve dysfunction by C-Peptide in patients with type 1 diabetes. Diabetes. 2003;52:536–541. doi: 10.2337/diabetes.52.2.536. [DOI] [PubMed] [Google Scholar]
- 59.Li ZG, Zhang W, Sima AA. C-peptide enhances insulin-mediated cell growth and protection against high glucose-induced apoptosis in SH-SY5Y cells. Diabetes Metab Res Rev. 2003;19:375–385. doi: 10.1002/dmrr.389. [DOI] [PubMed] [Google Scholar]
- 60.Epidemiology of severe hypoglycemia in the diabetes control and complications trial. The DCCT Research Group. Am J Med. 1991;90:450–459. [PubMed] [Google Scholar]


