Abstract
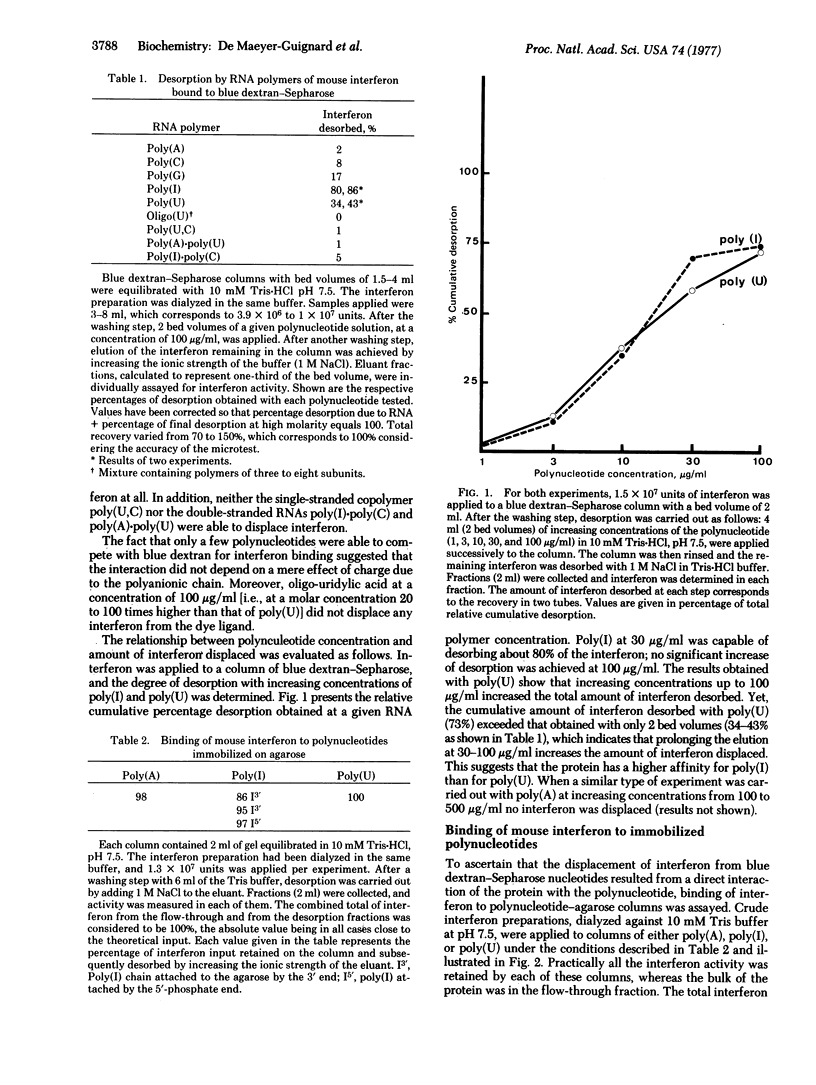
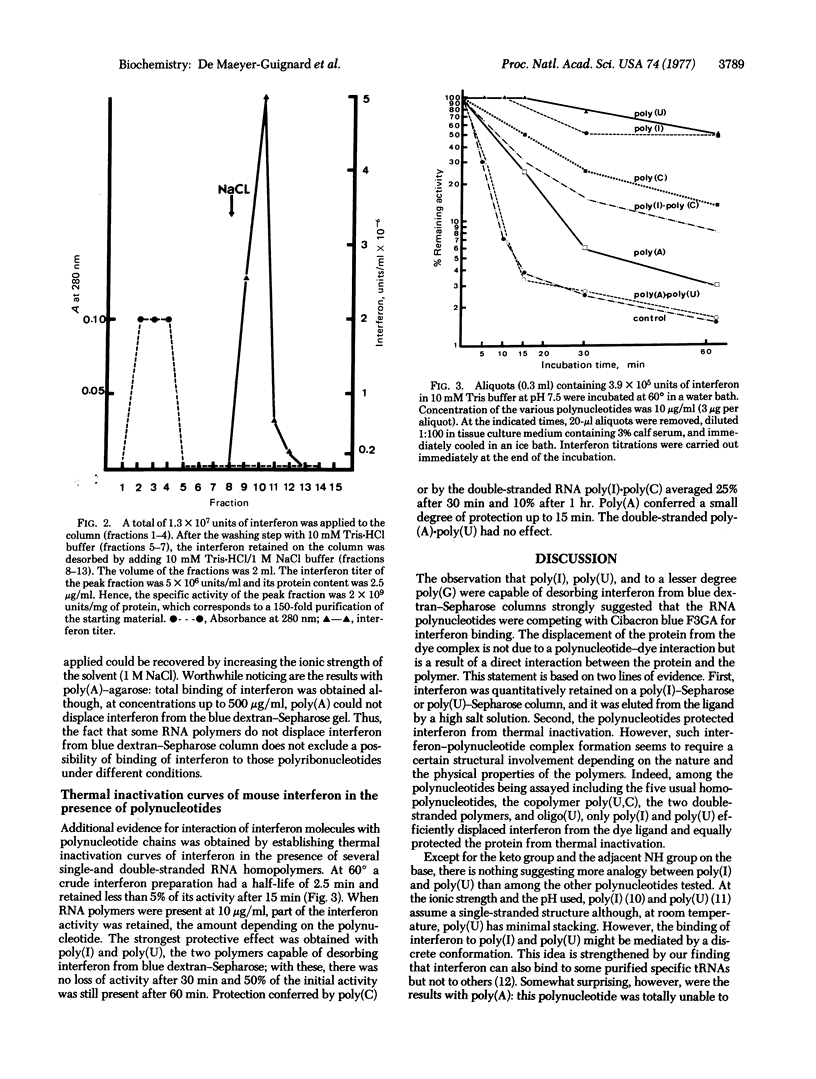
The polynucleotides poly(I) and poly(U), and to a lesser extent poly(G), are capable of desorbing mouse interferon from blue dextran-Sepharose columns, whereas poly(A) andpoly(C) are without effect. When covalently bound to agarose, poly(I), poly(U), and also poly(A) act as potent ligands for purification of interferon by affinity chromatography. Furthermore, poly(I) and poly(U) confer a significant degree of protection to interferon against thermal denaturation. Taken together, these observations point to a direct interaction of interferon with these polymers and suggest that interferon molecules have a polynucleotide attachment site. Possible implications for the concept of interferon induction are discussed.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachner L., De Clercq E., Thang M. N. Sepharose-bound poly (I)-poly (C): interaction with cells and interferon production. Biochem Biophys Res Commun. 1975 Mar 17;63(2):476–483. doi: 10.1016/0006-291x(75)90712-3. [DOI] [PubMed] [Google Scholar]
- Cesario T. C., Schryer P., Mandel A., Tilles J. G. Affinity of human fibroblast interferon for blue dextran. Proc Soc Exp Biol Med. 1976 Dec;153(3):486–489. doi: 10.3181/00379727-153-39574. [DOI] [PubMed] [Google Scholar]
- Jankowski W. J., von Muenchhausen W., Sulkowski E., Carter W. A. Binding of human interferons to immobolized Cibacron Blue F3GA: The nature of molecular interaction. Biochemistry. 1976 Nov 16;15(23):5182–5187. doi: 10.1021/bi00668a036. [DOI] [PubMed] [Google Scholar]
- Robberson D. L., Davidson N. Covalent coupling of ribonucleic acid to agarose. Biochemistry. 1972 Feb 15;11(4):533–537. doi: 10.1021/bi00754a008. [DOI] [PubMed] [Google Scholar]
- Thang M. N., De Maeyer-Guignard J., De Maeyer E. Interaction of interferon with tRNA. FEBS Lett. 1977 Aug 15;80(2):365–370. doi: 10.1016/0014-5793(77)80477-8. [DOI] [PubMed] [Google Scholar]
- Thang M. N., Graffe M., Grunberg-Manago M. Synthesis of polyguanylic acid by polynucleotide phosphorylase of Escherichia coli. Biochim Biophys Acta. 1965 Sep 6;108(1):125–131. doi: 10.1016/0005-2787(65)90114-0. [DOI] [PubMed] [Google Scholar]
- Thiele D., Guschlbauer W. The structures of polyinosinic acid. Biophysik. 1973 May 30;9(3):261–277. doi: 10.1007/BF01184691. [DOI] [PubMed] [Google Scholar]
- Thompson S. T., Cass K. H., Stellwagen E. Blue dextran-sepharose: an affinity column for the dinucleotide fold in proteins. Proc Natl Acad Sci U S A. 1975 Feb;72(2):669–672. doi: 10.1073/pnas.72.2.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thrierr J. C., Dourlent M., Leng M. A study of polyuridylic acid. J Mol Biol. 1971 Jun 28;58(3):815–830. doi: 10.1016/0022-2836(71)90042-8. [DOI] [PubMed] [Google Scholar]
- Tovey M. G., Begon-Lours J., Gresser I. A method for the large scale production of potent interferon preparations. Proc Soc Exp Biol Med. 1974 Jul;146(3):809–815. doi: 10.3181/00379727-146-38196. [DOI] [PubMed] [Google Scholar]
- Wagner A. F., Bugianesi R. L., Shen T. Y. Preparation of sepharose-bound poly (rI:rC). Biochem Biophys Res Commun. 1971 Oct 1;45(1):184–189. doi: 10.1016/0006-291x(71)90067-2. [DOI] [PubMed] [Google Scholar]
- de Maeyer-Guignard J., de Maeyer E. Le chromophore du bleu dextran: un ligand puissant pour la purification de l'interféron par chromatographie d'affinité. C R Acad Sci Hebd Seances Acad Sci D. 1976 Sep 27;283(6):709–711. [PubMed] [Google Scholar]


