Abstract
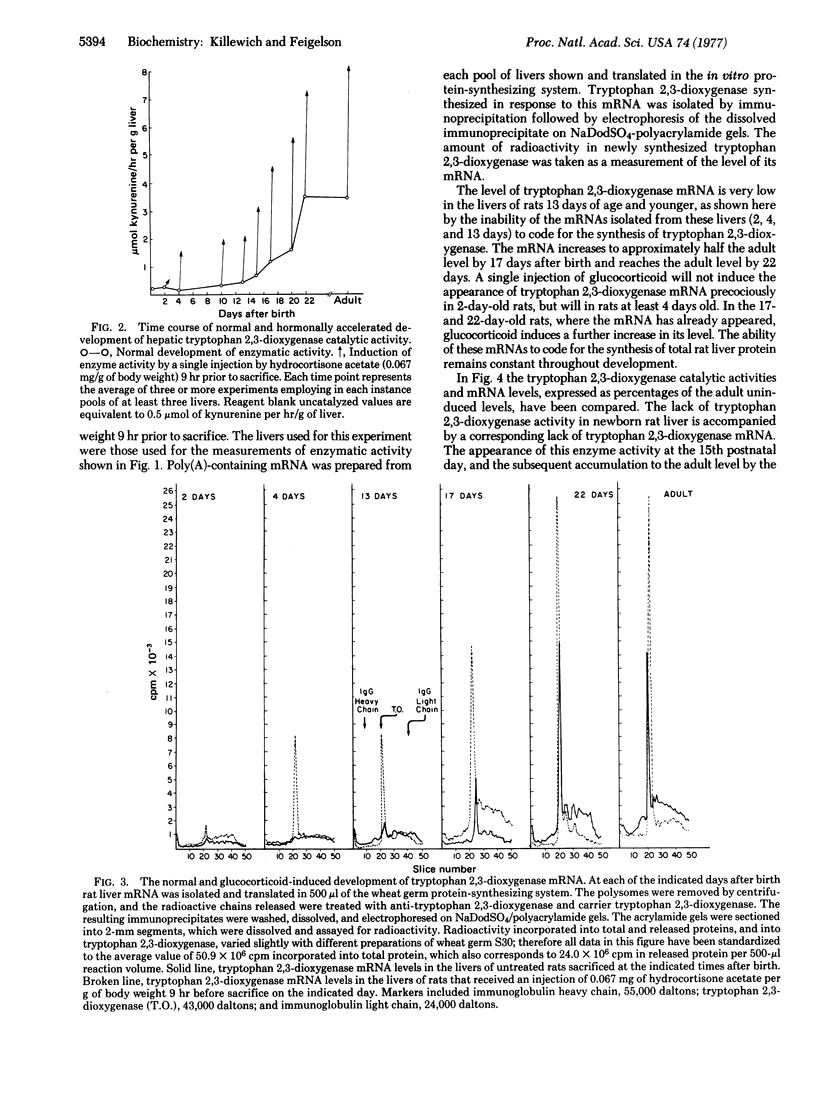
The enzyme tryptophan 2,3-dioxygenase [EC 1.13.11.11; L-tryptophan:oxygen 2,3-oxidoreductase (decyclizing)] first appears in the livers of young rats around the 15th postnatal day, and increases to the adult level by the 22nd day. Studies have shown that the appearance and subsequent development of the enzyme activity result from an increase in the rate of its synthesis and thus in the amount present in the liver. In this study, we have attempted to ascertain whether the appearance and development of tryptophan 2,3-dioxygenase mRNA coincided with, and thus led to, the development of enzyme activity, or whether the biosynthesis of this enzyme was due to a developmental event enabling translation of a preexisting, sequestered, reservoir of its mRNA. Using a cell-free protein-synthesizing system based on a wheat germ S30 supernatant, we measured the level of tryptophan 2,3-dioxygenase mRNA in the livers of rats between 0 and 22 days of age. We found that functional tryptophan 2,3-dioxygenase mRNA is not detectable in rat liver until the 15th postnatal day. It increases to the adult level by the 22nd postnatal day, in parallel with the enzyme. The appearance and development of tryptophan 2,3-dioxygenase are the direct consequence of the parallel appearance and development of its mRNA.
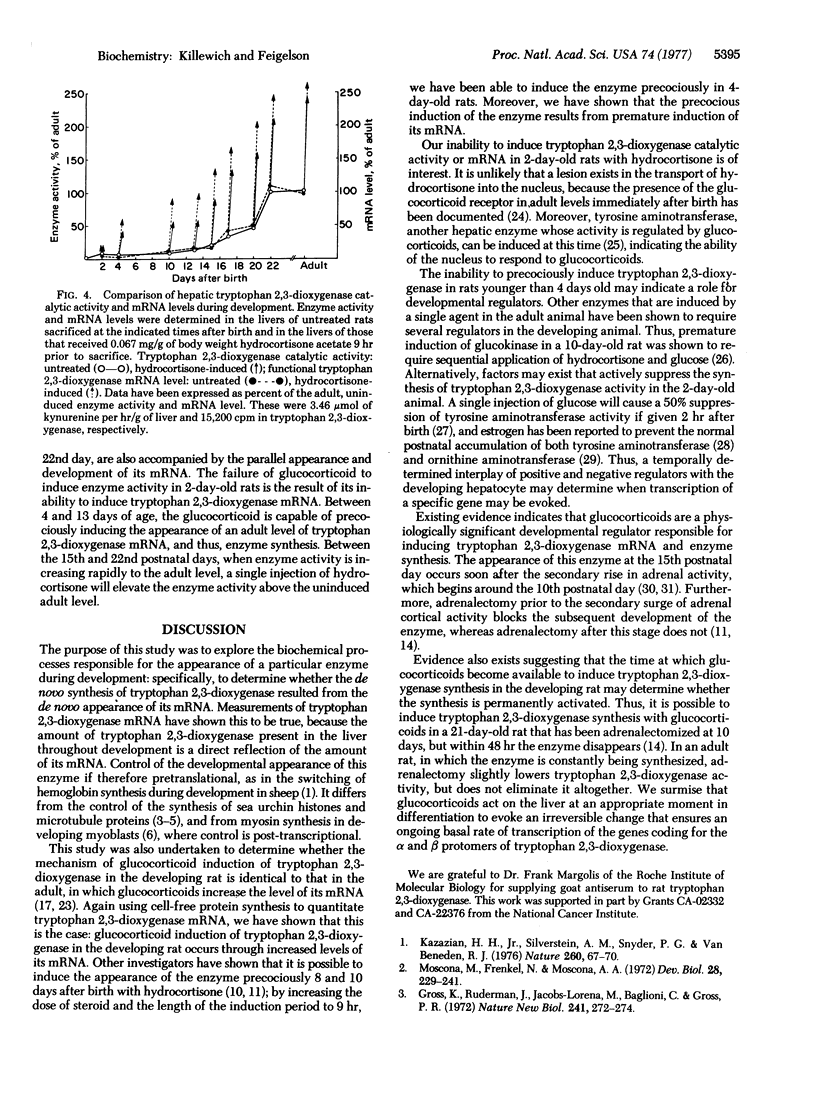
It has been shown that glucocorticoids, which induce tryptophan 2,3-dioxygenase activity in adult rats, are capable of inducing the appearance of this enzyme precociously in 8- and 10-day-old rats. We have found that it is also possible to induce tryptophan 2,3-dioxygenase catalytic activity with hydrocortisone precociously in 4-day-old rats. Moreover, precocious induction of enzyme activity and the induction that occurs during the enzyme's normal developmental rise to the adult level between 15 and 22 days, are mediated through parallel increases in the level of tryptophan 2,3-dioxygenase mRNA.
The present findings indicate that glucocorticoids are developmental hormones that act upon the postnatal hepatocyte to evoke elevated levels of the mRNA species coding for tryptophan 2,3-dioxygenase; the findings are compatible with the hypothesis that such hormones act by initiating and accelerating transcription of the structural genes coding for the α and β protomers of this enzyme.
Keywords: hormonal control of development, hydrocortisone, enzyme epigenesis
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buckingham M. E., Caput D., Cohen A., Whalen R. G., Gros F. The synthesis and stability of cytoplasmic messenger RNA during myoblast differentiation in culture. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1466–1470. doi: 10.1073/pnas.71.4.1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan S. J., Keim P., Steiner D. F. Cell-free synthesis of rat preproinsulins: characterization and partial amino acid sequence determination. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1964–1968. doi: 10.1073/pnas.73.6.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz J. M., Knox W. E. The effect of development and hydrocortisone on tryptophan oxygenase, formamidase, and tyrosine aminotransferase in the livers of young rats. Biochemistry. 1967 Nov;6(11):3464–3471. doi: 10.1021/bi00863a017. [DOI] [PubMed] [Google Scholar]
- GOLDSTEIN L., KNOX W. E. THE RESPONSE OF TRYPTOPHAN PYRROLASE TO ADMINISTRATION OF CORTISOL IN DEVELOPING AND ADULT RATS. Ann N Y Acad Sci. 1963 Dec 30;111:233–242. doi: 10.1111/j.1749-6632.1963.tb36964.x. [DOI] [PubMed] [Google Scholar]
- GREENGARD O., FEIGELSON P. RELATIONSHIPS OF THE APO-ENZYME AND COENZYME OF TRYPTOPHAN PYRROLASE IN DEVELOPING AND REGENERATING RAT LIVER. Ann N Y Acad Sci. 1963 Dec 30;111:227–232. doi: 10.1111/j.1749-6632.1963.tb36963.x. [DOI] [PubMed] [Google Scholar]
- Giannopoulos G. Ontogeny of glucocorticoid receptors in rat liver. J Biol Chem. 1975 Aug 10;250(15):5847–5851. [PubMed] [Google Scholar]
- Greengard O., Dewey H. K. Initiation by glucagon of the premature development of tyrosine aminotransferase, serine dehydratase, and glucose-6-phosphatase in fetal rat liver. J Biol Chem. 1967 Jun 25;242(12):2986–2991. [PubMed] [Google Scholar]
- Greengard O., Dewey H. K. The prematurely evoked synthesis of liver tryptophan oxygenase. Proc Natl Acad Sci U S A. 1971 Aug;68(8):1698–1701. doi: 10.1073/pnas.68.8.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greengard O. Enzymic differentiation in mammalian liver injection of fetal rats with hormones causes the premature formation of liver enzymes. Science. 1969 Feb 28;163(3870):891–895. doi: 10.1126/science.163.3870.891. [DOI] [PubMed] [Google Scholar]
- Gross K., Ruderman J., Jacobs-Lorena M., Baglioni C., Gross P. R. Cell-free synthesis of histones directed by messenger RNA from sea urchin embryos. Nat New Biol. 1973 Feb 28;241(113):272–274. doi: 10.1038/newbio241272a0. [DOI] [PubMed] [Google Scholar]
- Herzfeld A., Greengard O. Aspartate aminotransferase in fat tissues: changes with growth and hormones. Biochim Biophys Acta. 1971 Apr 20;237(1):88–98. doi: 10.1016/0304-4165(71)90033-x. [DOI] [PubMed] [Google Scholar]
- Herzfeld A., Greengard O. Endocrine modification of the developmental formation of ornithine aminotransferase in rat tissues. J Biol Chem. 1969 Sep 25;244(18):4894–4898. [PubMed] [Google Scholar]
- Jamdar S. C., Greengard O. Premature formation of glucokinase in developing rat liver. J Biol Chem. 1970 Jun 10;245(11):2779–2783. [PubMed] [Google Scholar]
- Jones R. E., Pulkrabek P., Grunberger D. Mouse pituitary tumor mRNA directed cell-free synthesis of polypeptides that are cross-reactive with adrenocorticotropic hormone antiserum. Biochem Biophys Res Commun. 1977 Feb 21;74(4):1490–1495. doi: 10.1016/0006-291x(77)90610-6. [DOI] [PubMed] [Google Scholar]
- Kazazian H. H., Jr, Silverstein A. M., Snyder P. G., VanBeneden R. J. Increasing haemoglobin beta-chain syntheses in foetal development is associated with a declining gamma-to alpha-mRNA ratio. Nature. 1976 Mar 4;260(5546):67–70. doi: 10.1038/260067a0. [DOI] [PubMed] [Google Scholar]
- Killewich L., Schutz G., Feigelson P. Functional level of rat liver tryptophan 2,3-dixoygenase messenger RNA during superinduction of enzyme with actinomycin D. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4285–4287. doi: 10.1073/pnas.72.11.4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine S., Glick D., Nakane P. K. Adrenal and plasma corticosterone and vitamin A in rat adrenal glands during postnatal development. Endocrinology. 1967 May;80(5):910–914. doi: 10.1210/endo-80-5-910. [DOI] [PubMed] [Google Scholar]
- Moscona M., Frenkel N., Moscona A. A. Regulatory mechanisms in the induction of glutamine synthetase in the embryonic retina: immunochemical studies. Dev Biol. 1972 May;28(1):229–241. doi: 10.1016/0012-1606(72)90140-6. [DOI] [PubMed] [Google Scholar]
- NEMETH A. M. Mechanisms controlling changes in tryptophan peroxidase activity in developing mammalian liver. J Biol Chem. 1959 Nov;234:2921–2924. [PubMed] [Google Scholar]
- Palmiter R. D., Oka T., Schimke R. T. Modulation of ovalbumin synthesis by estradiol-17 beta and actinomycin D as studied in explants of chick oviduct in culture. J Biol Chem. 1971 Feb 10;246(3):724–737. [PubMed] [Google Scholar]
- Pulkrabek P., Klier K., Grundberger D. Isolation of poly(A)-containing RNA on synthetic fluorophlogopite (Mica). Anal Biochem. 1975 Sep;68(1):25–35. doi: 10.1016/0003-2697(75)90675-2. [DOI] [PubMed] [Google Scholar]
- Raff R. A., Colot H. V., Selvig S. E., Gross P. R. Oogenetic origin of messenger RNA for embryonic synthesis of microtubule proteins. Nature. 1972 Jan 28;235(5335):211–214. doi: 10.1038/235211a0. [DOI] [PubMed] [Google Scholar]
- Roper M. D., Franz J. M. Glucocorticoid control of the development of tryptophan oxygenase in the young rat. J Biol Chem. 1977 Jun 25;252(12):4354–4360. [PubMed] [Google Scholar]
- Schutz G., Beato M., Feigelson P. Messenger RNA for hepatic tryptophan oxygenase: its partial purification, its translation in a heterologous cell-free system, and its control by glucocorticoid hormones. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1218–1221. doi: 10.1073/pnas.70.4.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutz G., Feigelson P. Purification and properties of rat liver tryptophan oxygenase. J Biol Chem. 1972 Sep 10;247(17):5327–5332. [PubMed] [Google Scholar]
- Schutz G., Killewich L., Chen G., Feigelson P. Control of the mRNA for hepatic tryptophan oxygenase during hormonal and substrate induction. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1017–1020. doi: 10.1073/pnas.72.3.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoultchi A., Gross P. R. Maternal histone messenger RNA: detection by molecular hybridization. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2840–2844. doi: 10.1073/pnas.70.10.2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuwiler A., Geller E. Rat liver tryptophan oxygenase induced by neonatal corticoid administration and its effect on brain serotonin. Enzyme. 1973;15(1):161–168. [PubMed] [Google Scholar]


