Abstract
Consistent individual differences in parenting are widespread; however, we know little about why there is variation in parenting behavior among individuals within species. One possible explanation for consistent individual differences in parenting is that individuals invest in different aspects of parental care, such as provisioning or defense. In this field study we measured consistent individual differences in parenting behavior and evaluated correlations between parenting and other behaviors in threespine stickleback (Gasterosteus aculeatus). We repeatedly measured male parenting behavior and male behavior in the presence of three different types of live intruders: a female, a conspecific male, and a predator, meant to provoke courtship, aggressive and antipredator behavior, respectively. While males plastically adjusted their reactions to different types of intruders, we found consistent individual differences in behavior (behavioral types) both within and across contexts, even after accounting for variation in body size and nest characteristics. Males that performed more parenting behavior responded faster to all types of intruders. These results suggest that in nature, individual male stickleback exhibit robust parental behavioral types, and highly parental males are more attentive to their surroundings. Future studies are needed to examine the potential causes of individual variation in parental behavior in the field.
Keywords: personality, parental behavior, territory defense, aggression, field study, behavioural syndrome
Consistent individual differences in mothering are widespread (Meaney 2001; MacColl and Hatchwell 2003; Schwagmeyer and Mock 2003; Nakagawa et al. 2007; Westneat et al. 2011). There is also evidence that individual fathers differ in how they behave as parents. For example, male sticklebacks consistently differ in rates of fanning, a direct form of parental care (Stein and Bell 2012). Individual parents also consistently differ in indirect forms of parental care, such as offspring/nest defense (Kontiainen et al. 2009; Burtka and Grindstaff 2013).
However, we know little about why there is variation in parenting behavior among individuals within species. One possibility is that parents differ because they “program” their offspring for different types of environments, and variations in parenting act as cues to offspring about current conditions (Marshall and Uller 2007; Stein and Bell 2014). Another possibility is that variation in parenting reflects an individual’s physiology – highly aggressive individuals, for example, might be less parental due to a proximate constraint such as high levels of androgens (Ketterson and Nolan 1999; Wingfield et al. 1990). There might also be tradeoffs between direct and indirect forms of care such that highly attentive parents, for example, might trade off direct care with nest defense (Rangeley and Godin 1992; Lissaker and Kvarnemo 2006; Mutzel et al. 2013). Studies in birds have also provided evidence for positive correlations between direct offspring care (e.g. provisioning) and indirect forms of care (e.g. nest defense), suggesting that the most aggressive parents are also the most attentive to their offspring (Rytkonen et al. 1995; Betini and Norris 2013; Wetzel and Westneat 2014). We might also expect courtship and parenting behaviors to be positively correlated if behavior during courtship provides an indication of future parenting behavior (Stiver and Alonzo 2009).
The majority of studies of parental care variation have been performed in the lab, where resources are abundant and predation non-existent. Fewer studies have been conducted in the field (but see Duckworth 2006; Patrick and Browning 2011; Barnett et al. 2012; Kazama et al. 2012; Mutzel et al. 2013; Cole et al. 2014; Wetzel and Westneat 2014), where there are more time and energy constraints due to few or patchy resources and predation risk. Therefore field studies might uncover constraints on the amount of care a parent can provide that are undetected in the lab. Here, we investigate male behavior on the breeding grounds in a natural population of threespine sticklebacks (Gasterosteus aculeatus).
Threespine sticklebacks are teleost fish in which the father is the sole provider of parental care, and paternal care is necessary for offspring survival (Wootton 1984). During the breeding season, male sticklebacks establish territories, build nests, and court females, while at the same time actively defending their nest from predators. Certain individual and territory qualities increase reproductive success, including large body size and nesting in deep water (Kraak et al. 1999a). After a female spawns, she leaves the territory and the male provides all of the parental care; males continue to court females to obtain more eggs for up to three days after the first spawning (Kraak et al. 1999b). During the incubation period (approximately 6 days in the population studied here), males “fan” the eggs with the pectoral fins, providing oxygen and clearing carbon dioxide (Wootton 1984) and remove rotten eggs and debris. Previous studies have shown that males that spend more time fanning their nest enjoy higher rates of hatching success (von Hippel 2000), and males are consistent in their fanning behavior both within and across clutches in the lab (Stein and Bell 2012). However, it is unknown whether males demonstrate consistent individual differences in parenting behavior in the field, where resources are limited and parenting is not immune from competing demands on males’ time and energy, such as territory defense and courtship opportunities.
Therefore, the goals of this field study were to 1) assess whether males consistently differed from one another in behavior, and 2) examine relationships between individual differences in parenting behavior and behaviors in other contexts. We observed undisturbed parenting males in the field to obtain a “baseline” measure of parenting behavior. We then recorded each male’s behavior in the courtship, aggression and antipredator contexts by presenting the male with a gravid female, rival male stickleback, and a predator, respectively. The behavior of each male was repeatedly observed in each context for three days. After testing whether males consistently differ in behavior, we used these data to examine correlations between behaviors within and across contexts to determine whether parenting behavior was part of a larger suite of correlated behaviors.
METHODS
Study area and study system
This field study was performed in the South Fork of the Navarro River (Philo, Mendocino County, CA). The Navarro River is an undammed freshwater river running northwest along the California coast. Adult sticklebacks in this population experience predation by avian and fish predators (Feliciano 2004; LRS pers. obs.). While nests and fry in this population are preyed upon by a number of predacious insects and fish, predatory fish such as coastrange sculpin (Cottus aleuticus) and prickly sculpin (C. asper) additionally pose a threat to adults (Moodie 1972; Pressley 1981). A behavioral syndrome between aggressiveness and boldness has been documented in this population (Bell 2005), opening the possibility that parental behavior may also be part of a larger suite of correlated behaviors.
Observations were conducted from 8 June – 1 July 2011. Individuals were observed between 1000 and 1700 PST every day. Only parenting males with eggs in the nest were used in this study. When a male stickleback was found guarding a nest, we observed the individual for up to 10 minutes for evidence of fanning, indicating the presence of eggs. We then tagged the nest using flagging tape tied on foliage or to a stick 30 cm from the nest. Flagging tape was at least 50 cm above the surface of the water to avoid attracting fish predators to the nest. There was no observed increase in avian predators to the study site after flagging tape was introduced. Parenting behavior in the absence of an intruder (“undisturbed” context) was then observed (see Behavioral Assays below). Following the observation of undisturbed parenting behavior, we gently removed the male from his nest using a dip net and placed him in a 19 liter bucket with fresh river water. We visually determined whether there were eggs in the nest and their stage of development (eyed or uneyed). In this population, fertilized eggs are uneyed for approximately three days, followed by three days in the eyed stage before hatching. Parenting behavior changes during the course of the nesting cycle (Stein and Bell 2012) and in this study we focus on the uneyed stage as males are still receptive to females during this time, allowing us to assess males’ courtship behavior. None of the clutches hatched during the three-day observation period, which suggests that nests were similar in age thereby reducing the possibility of offspring age-related parental investment. We then covered the nest with a wire cage to prevent depredation while the male was away from his nest. We quickly measured the male’s standard length and the depth of his nest in the water. Previous studies have demonstrated that larger males can fan the nest more efficiently (Kraak et al. 1999a; Künzler and Bakker 2000), and nest depth has been correlated with greater reproductive success (Kraak et al. 1999a). We did not include coloration as a measure of male parental ability here because male coloration changes over the parenting cycle and may not be a reliable indicator of male quality in every population (Candolin 2007; Boughman 2007; Sparkes et al. 2008). The male was then returned to the nest and observed until he resumed parenting. Handling time of the males took less than 10 minutes (range 4 min – 9.5 min). All males resumed parenting and there was no indication that our activities caused males to abandon their nests.
Behavioral assays
Each male was observed in four contexts (undisturbed, courtship, aggression, and antipredator) in a fixed order every day for three consecutive days (three repeats per context per male). We used a fixed order because we were primarily interested in rank order consistency between individuals (Dingemanse et al. 2007; Bell 2013). To guard against carryovers across contexts, we tested individuals with the predator last, as we expected this intruder to have the most potential for carryover due to a slower stress recovery when male stickleback encounter a predator vs a conspecific (Bell et al. 2007; Bell 2013). Every day, animals used as intruders were captured using minnow traps baited with dog biscuits approximately eight kilometers upriver from the study site and were transported in opaque buckets. Intruders were presented to the focal male inside a 10×10×10 cm wire cage with 0.64 cm openings allowing visual and olfactory cues to reach the focal male. Preliminary observations suggested that an intruder elicited the maximum behavioral response when the cage was placed on the ground 3 cm in front of the nest opening, and there was no effect of an empty cage on fanning behavior (mean empty cage ± SE: 22.09 ± 8.49 s; mean no cage ± SE: 25.73 ± 7.78 s; paired t-test: t10 = 0.39, P = 0.70). The cage was attached by string to the end of a rod and lowered remotely from the bank of the river. Individual intruders were used up to three times per day, once per male, and were returned to their point of capture at the end of the day. In total, N = 114 live gravid females (mean size ± SE: 4.5 ± 0.3 cm), N = 108 live reproductive males (mean size ± SE: 4.3 ± 0.3 cm), and N = 98 live sculpin (mean size ± SE: 8.2 ± 1.8 cm) were used as intruders throughout the study.
In total, we observed the behavior of 30 parenting males. Complete datasets (daily observations of behavior in all four contexts for three days, N = 12 observations per male) are available for 25 males. Due to disruptions at the field site, some males (N = 4) were measured on two days, and N = 1 male was measured on one day.
We first observed males for 10 minutes without an intruder to obtain an “undisturbed” measure of parenting behavior. The observer stood three feet away from the nest facing the nest opening and recorded time fanning (moving the pectoral fins over the nest). We then introduced the intruders in a fixed order with an hour in between each observation. We measured behavior (described below) for two minutes after the first orient to the intruder. Then, the cage was removed. If the focal male did not orient within ten minutes after the cage was placed at the nest, the male was recorded as “not responding” and the cage was removed (N = 6 observations in the courtship context; N = 5 observations in the aggression context; N = 6 observations in the antipredator context). Non-responding males were assigned a latency score of 301 (one second greater than the maximum latency score) and remained in the analysis of latency to orient. These individuals were not used for analysis of fanning or intruder-directed behaviors (see below).
We recorded the following behaviors that were elicited by all three types of intruders: latency to orient, the number of bites directed towards the intruder, and the time spent fanning. Males can be aggressive toward females because females often attack nests and eat fertilized eggs (Foster 1988). When the female intruder was presented, we recorded the number of zig-zags, a conspicuous courtship display (Wootton 1984). We measured two antipredator behaviors in response to the sculpin (Cottus spp): the number of times the male froze (head not moving for more than two seconds) and the number of jerky swims the male performed (quickly “darting” in one direction sensu McGhee et al. 2012).
Data analysis
In order to ease interpretation of our data, we first inverted latency to orient measures. We subtracted each individual’s latency from 302, one second higher than the maximum latency score (301 s). Therefore, we interpret high latency (inverted) scores as highly attentive behavior (i.e. the male quickly oriented to the intruder). The resulting scores, along with bites at the intruder and intruder-specific behaviors, were non-Gaussian distributed and best approximated a Poisson error distribution with additive overdispersion which we used for all further analyses unless otherwise stated.
We used generalized linear mixed models (GLMM) with Markov chain Monte Carlo (MCMC) estimation for both within- and across-context analyses. MCMC is a Bayesian statistical method that is powerful for fitting non-Gaussian distributions and partitioning variance among random effects (Hadfield 2010; Dingemanse and Dochtermann 2013). We used MCMCglmm (Hadfield 2010) in R v. 3.0.1, which returns 95% credibility intervals for random effects. Throughout, we used non-informative priors (Hadfield 2010) appropriate for the relative error distributions and preliminary analyses indicated that our results were not sensitive to changes in prior settings (data not shown). We ensured convergence and adequate chain mixing by comparing the posterior distributions and auto-correlation plots of five independent chains with 500 000 iterations, a 1000 burn-in period and thinning every 100 iterations for each model.
We analyzed behaviors (time fanning, latency to orient, and bites at intruders) separately. As fanning was observed for ten minutes without an intruder and for two minutes with intruders, we examined the proportion of time spent fanning. Proportion time fanning was best approximated by a binomial distribution which was used for its models. Context (undisturbed, courtship, aggression, and antipredator) and day of observation were included as fixed effects, individual and trial number were included as random effects, and male standard length, and nest depth were included as covariates in all models.
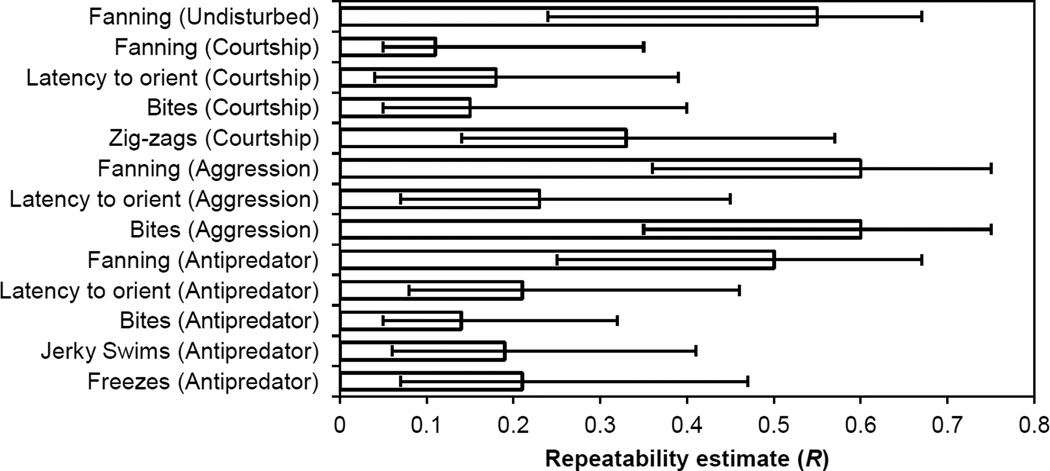
To determine whether males in the field exhibit consistent individual differences in behavior, we used variance components to estimate repeatability of each behavior within and across contexts as the proportion of total variation attributable to among-individual variation using variance components extracted from the univariate (across contexts) and multivariate (within contexts) GLMMs described below. We corrected all repeatability estimates as appropriate for the behavior’s distribution (Poisson with additive overdispersion for intruder-directed behaviors; binomial for proportion total time fanning (Nakagawa and Schielzeth 2010)). We determined whether there were consistent individual differences by visually inspecting the posterior distribution of the repeatability estimate: if the estimate (and its 95% CI) was not pressed against zero, we interpreted this as evidence of consistent individual differences.
A goal of this study was to examine whether there was evidence of correlations in behavior within or across contexts. To examine this question within each context, we first ran a multivariate model including all behaviors measured within the context (courtship: fanning, bites, zig zags; aggression: fanning, bites; antipredator: fanning, bites, jerky swims, freezes). We included day of observation, male standard length, and nest depth as fixed effects, and individual ID and trial number as random effects. We were particularly interested in correlations between fanning (our measure of direct parenting behavior) and the other variables. We partitioned the covariation into among- and within-individual variation components and converted covariance into correlations, per Dingemanse and Dochtermann (2013). Covariances are reported in Table S1. For the correlations, if the 95% CIs did not overlap zero, we interpreted this as evidence that the correlations were statistically significant.
We took a different approach to examine evidence for correlations between undisturbed parenting behavior and behavior in other contexts. Because behaviors in different contexts were measured at different times, we were only able to partition among-individual variance (Dingemanse and Dochtermann 2013). Specifically, we ran a multivariate model with proportion time fanning, latency to orient, and bites at the intruder across all contexts, with context, day of observation, male standard length, and nest depth included as fixed effects, and individual ID and trial number included as random effects. This model produced covariances, which we then converted into correlations. Covariances are reported in Table S2.
RESULTS
Consistent individual differences
Within each context, individual differences in behavior were consistent across all three days of observations, as evidenced by the statistically significant estimates of repeatability (Table 1; Fig. 1). For example, relative to other males, males that spent more time fanning when an intruding male was present on the first day continued to fan often when an intruding male was present on subsequent days. Indeed, fanning was especially repeatable compared to other behaviors, with repeatability of fanning exceeding 0.5 in all contexts except in the courtship context (Table 1; Fig. 1).
Table 1.
Repeatability estimates (R) for behaviors within each context. Male behavior was measured in every context once per day for three days. Estimates include Day, Standard Length, and Nest Depth as fixed effects. All models include Trial and Individual ID as random effects. Numbers in brackets indicate 95% credibility intervals
| Context | R [95% CI] |
|---|---|
| Undisturbed | |
| Fanning | 0.55 [0.24, 0.67] |
| Courtship | |
| Fanning | 0.11 [0.05, 0.35] |
| Orient | 0.18 [0.04, 0.39] |
| Bites | 0.15 [0.05, 0.40] |
| Zig-Zags | 0.33 [0.14, 0.57] |
| Aggression | |
| Fanning | 0.60 [0.36, 0.75] |
| Orient | 0.23 [0.07, 0.45] |
| Bites | 0.60 [0.35, 0.75] |
| Antipredator | |
| Fanning | 0.50 [0.25, 0.67] |
| Orient | 0.21 [0.08, 0.46] |
| Bites | 0.14 [0.05, 0.32] |
| Jerky swims | 0.19 [0.06, 0.41] |
| Freezes | 0.21 [0.07, 0.47] |
Fig. 1.
All behaviors measured were repeatable, indicating consistent individual differences in behavior the field. If the estimate (and its 95% credibility interval) is not pressed against zero, we interpreted this as evidence of consistent individual differences. Error bars are 95% credibility intervals
Males also exhibited consistent individual differences in behavior across contexts, i.e. even in the presence of different ecologically relevant stimuli. The repeatability (R [95% CI]) of proportion of time fanning across all four contexts was R = 0.18 [0.10, 0.34]. There were also consistent individual differences in latency to orient (R = 0.24 [0.11, 0.41]) and number of bites at the intruder (R = 0.16 [0.08, 0.34]) across contexts.
Average differences in behavior across contexts
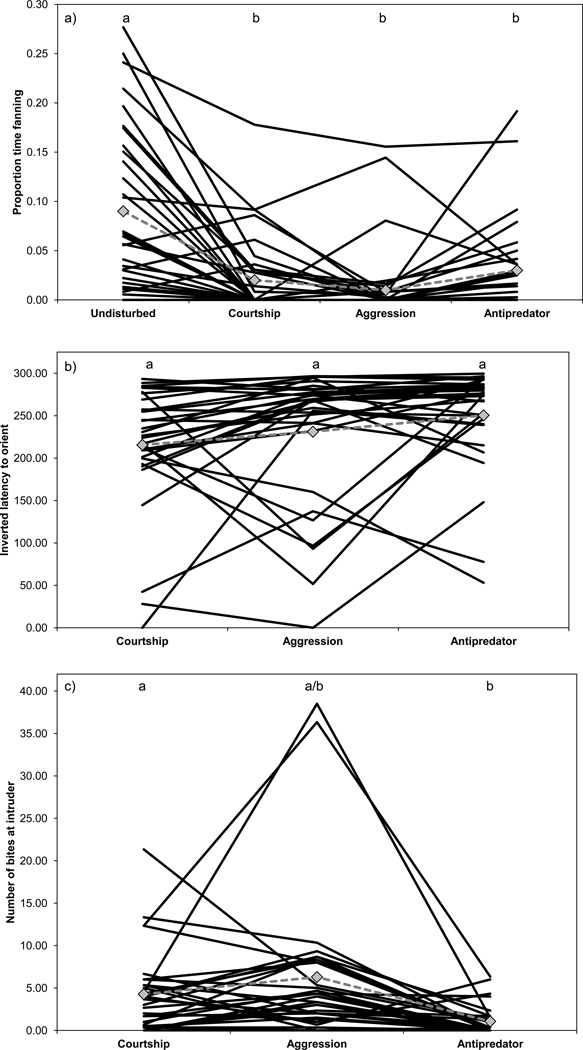
As expected, different types of intruders provoked different behavioral reactions (Table 2; Fig. 2). Males fanned less when an intruder was present (proportion time fanning, mean ± SE: undisturbed: 0.09 ± 0.01; courtship: 0.02 ± 0.005; aggression: 0.01 ± 0.004; antipredator: 0.02 ± 0.006), and at comparable levels in the presence of different types of intruders (Fig. 2a). Males oriented faster to some intruders compared to others (Fig. 2b); for example, males oriented especially quickly to the predator (inverted latency to orient, mean ± SE: courtship: 218.98 ± 11.30 s; aggression: 229.27 ± 12.67 s; antipredator: 249.02 ± 9.69 s). Different types of intruders elicited variable levels of aggression, with the predator provoking the fewest bites (mean ± SE: courtship: 4.46 ± 0.86 bites; aggression: 6.01 ± 1.10 bites; antipredator: 1.05 ± 0.32 bites). We also found that males with nests in deeper water spent a greater proportion of time fanning and were quicker to orient to intruders (Table 2).
Table 2.
GLMM table for fixed effects.
| Factor | Proportion time fanning | Latency to orient | Number of bites |
|---|---|---|---|
| Context | 3.41 [2.38, 4.67] | 0.12 [0.02, 0.20] | 1.76 [−2.54, −1.02] |
| Day | − 0.23 [−1.21, 0.84] | −0.31 [−0.55, −0.04] | −0.22 [−0.82, 0.46] |
| Length | 0.36 [−0.10, 0.83] | 0.07 [−0.12, 0.26] | 0.05 [−0.24, 0.32] |
| Depth | 0.18 [0.07, 0.30] | 0.07 [0.22, 1.22] | 0.03 [−0.76, 1.80] |
Numbers in brackets indicate 95% credibility intervals.
Bolded estimates are significant;
indicates marginal significance
Fig. 2.
Males adjusted their behavior across contexts. Each line represents an individual male; gray dotted line and diamonds indicate average levels of behavior. N = 30. Letters indicate significant differences between contexts
Correlations between parenting behavior and reactions to intruders
Although parenting behavior decreased in the presence of intruders, indicating a time or energy constraint (Fig. 2a), we did not find evidence for within-context correlations at either the among- or within-individual level between parenting behavior and reaction to intruders (Table 3). For example, if a male started to perform more fanning behavior when a female was present, it didn’t come at the expense of courtship behavior. Similarly, males that fanned less in the presence of a female didn’t necessarily perform more zig-zags.
Table 3.
Correlations (among- and within-individual) between fanning within the context and other behaviors measured within the same context. Estimates include Day, Standard Length, and Nest Depth as fixed effects. All models include Trial and Individual ID as random effects. “Among” indicates among-individual correlations; “Within” indicates within-individual correlations.
| Context | Correlations [95% CI] | |
|---|---|---|
| Among | Within | |
| Courtship | ||
| Fanning*Orient | 0.09 [−0.16, 0.30] | 0.11 [−0.12, 0.33] |
| Fanning*Bites | 0.006 [−0.25, 0.25] | −0.13 [−0.35, 0.10] |
| Fanning*Zig zags | 0.11 [−0.11, 0.44] | 0.11 [−0.08, 0.34]* |
| Aggression | ||
| Fanning*Orient | 0.08 [−0.18, 0.45] | 0.03 [−0.12, 0.19] |
| Fanning*Bites | −0.07 [−0.40, 0.29] | −0.06 [−0.12, 0.17] |
| Antipredator | ||
| Fanning * Orient | 0.06 [−0.27, 0.35] | 0.05 [−0.12, 0.23] |
| Fanning * Bites | 0.07 [−0.18, 0.40] | −0.09 [−0.31, 0.07]* |
| Fanning * Jerky swims | −0.01 [−0.32, 0.26] | −0.15 [−0.35, 0.03]* |
| Fanning * Freezes | 0.01 [−0.36, 0.28] | −0.10 [−0.32, 0.06]* |
Numbers in brackets indicate 95% credibility intervals.
Bolded correlations are significant;
indicates marginal significance
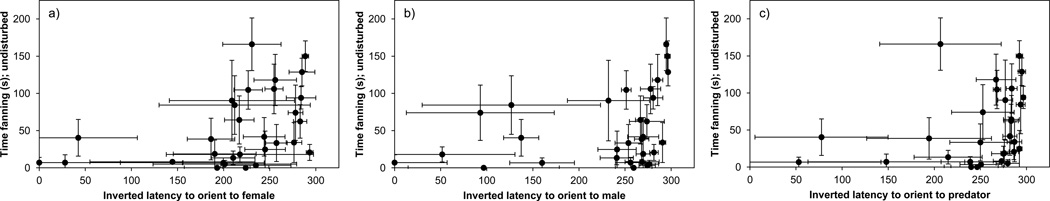
We detected some evidence for behavioral correlations across contexts: males that provided more care (undisturbed fanning) oriented faster to all intruder types (Table 4, Fig. 3). Surprisingly, fanning was not related to other behaviors, and direct (fanning) and indirect (biting at the intruder) forms of parental care were unrelated both within and across contexts (Table 3; Table 4).
Table 4.
Correlations (among-individual) between undisturbed parenting behavior (fanning) and behavior in different contexts. Estimates include Day, Standard Length, and Nest Depth as fixed effects. All models include Trial and Individual ID as random effects.
| Context | Correlations [95% CI] |
|---|---|
| Courtship | |
| Fanning*Orient | 0.10 [0.01, 0.52]* |
| Fanning*Bites | 0.07 [−0.60, 0.81] |
| Fanning*Zig zags | 0.18 [−0.68, 0.73] |
| Aggression | |
| Fanning*Orient | 0.69 [0.07, 0.91] |
| Fanning*Bites | 0.52 [−0.32, 0.86] |
| Antipredator | |
| Fanning * Orient | 0.23 [0.10, 0.45] |
| Fanning * Bites | 0.26 [−0.75, 0.85] |
| Fanning * Jerky swims | −0.30 [−0.75, 0.68] |
| Fanning * Freezes | −0.36 [−0.72, 0.64] |
Numbers in brackets indicate 95% credibility intervals.
Statistically significant correlations are indicated in bold,
indicates marginal significance
Fig. 3.
Correlations between undisturbed parenting behavior (fanning) and inverted latency to orient to an intruder. Males that spent more time fanning without an intruder were quicker to orient to all intruders. N = 30 for (a) courtship and (b) aggression contexts; N = 28 for the (c) antipredator context. Error bars are ± 1 SE
DISCUSSION
Male sticklebacks showed consistent individual differences in parenting, courtship, aggressive, and antipredator behaviors in the field, as they do in the lab (Stein and Bell 2012), suggesting wild stickleback exhibit robust behavioral types. We found little evidence that males exhibited correlations between parental and other behaviors within contexts, suggesting that over short timescales, males are capable of managing competing demands without sacrificing parental care. Between contexts, we found evidence that parental care was positively correlated with attentiveness toward intruders, suggesting a behavioral syndrome encompassing attention to offspring and attention to the surrounding environment.
Males showed consistent individual differences in behavior within and across contexts
There were consistent individual differences in parenting behavior. In general, males performing high levels of fanning on the first day of behavioral observations also showed high levels of fanning relative to other males on the second and third days. Although all males decreased fanning in the presence of an intruder, individuals that fanned the most when an intruder was absent also fanned the most when an intruder was present. Altogether, these results suggest a strong parental behavioral type in wild threespine sticklebacks that is robust to different ecological challenges.
Male size and nest depth have previously been shown to affect reproductive success (Kraak 1999a) and fanning behavior (Künzler and Bakker 2000) in sticklebacks. Our statistical results suggest that males with nests in deeper water fanned more often (Table 2). As oxygen concentrations decrease with water depths, males in deeper water might compensate for low oxygen availability by fanning. Males in deeper water also were quicker to orient to all types of intruders. The causal relationship between territory attributes and behavioral type is not straightforward. Having a nest in deeper water, for example, allow an individual to have a highly parental behavioral type because they are safer from bird predators. Alternatively, other aspects of the male’s behavioral type (e.g. being more aggressive) may allow an individual to secure a more resource-rich territory. These relationships should be examined in future studies.
Different intruders provoked different behavioral reactions
Each intruder represented a different ecological challenge, and males adjusted their behavior accordingly. Given that intruders were presented in a fixed order, average differences in behavior across contexts might reflect a carryover effect such as habituation (Bell 2013). However, there was not a systematic change in average behavior over time, as one would predict for a carryover effect, and the patterns were biologically reasonable. For example, males showed high levels of aggression toward intruding males, consistent with other studies (Huntingford 1976; Wootton 1984), and with the hypothesis that males are a serious threat because they might steal eggs or a territory. In addition, males oriented especially quickly to the predator compared to the other intruders, which is consistent with the observation that parenting males are particularly vulnerable to predators (Candolin 1998). Interestingly, males did not increase fanning during the courtship context, even though fanning can be a courtship display (Tinbergen and van Iersel 1947). Males in this study performed zig-zags, a behavior only observed during courtship, indicating the males in this study were receptive to females. Overall rates of fanning were lower in the presence of an intruder. It is possible that males might compensate for a reduction in fanning during periods when they were not observed; however, males do not compensate for a reduction in fanning in the lab (Stein and Bell 2012).
Little evidence for behavioral syndromes within contexts
We predicted that individual differences in parenting behavior reflect part of a behavioral syndrome, and that trade-offs between behaviors within contexts might explain variation in parenting behavior. For example, males that fan the nest the most might be relatively unaggressive (Lissaker and Kvarnemo 2006), or relatively timid around predators (Budaev et al. 1999). Alternatively, nest defense and direct parental care might be positively correlated (Betini and Norris 2013; Wetzel and Westneat 2014). This may be especially evident within contexts, where time budget tradeoffs are more pronounced.
We found little evidence in support for these ideas within contexts, although it is possible that high-parenting behavioral types might experience trade-offs with behavioral traits that were not measured in this study, such as those having to do with immunity (Sabat 1994). A lack of evidence for correlations within contexts suggests that males are able to juggle multiple competing demands over very short timescales (here, a two minute intrusion occurring three times a day) without sacrificing direct or indirect care. Over longer timescales, or over multiple intrusions, time budget tradeoffs may become more evident. Additionally, with a small sample size in this study, the possibility of type II errors are inflated (Bell 2005), and it is possible that with a larger dataset correlations between these behaviors may be revealed.
It is interesting to note that within-individual correlations within the antipredator context were marginally significant (i.e. half of the model runs produced 95% CIs that did not overlap zero) (Table 3). Within-individual correlations can reveal tradeoffs that may not be detectable at the population (among-individual) level (van Noordwijk and de Jong 1986; Reznick et al. 2000). As a male increased fanning, he decreased nest defense (biting at the predator) and jerky swimming and freezing (antipredator behavior). This suggests that parents in this population may face a tradeoff between survival and reproduction (Cole et al. 2014).
Parenting-attentiveness behavioral syndrome across contexts
Males performing high levels of direct care appear to be more attentive to intruders into their territory. For example, males that fanned the nest more often oriented more quickly to a rival male stickleback, a female, and a predator. These across context correlations may reflect a larger “attentive” behavioral type: individuals that are overall more sensitive to cues from their environment may also be more sensitive to cues from their eggs or offspring.
Within-context correlations are likely strongly influenced by time budgets: if a male is currently fanning, he cannot attack an intruder. Across-context correlations, on the other hand, can reveal whether behaviors in one context are correlated to behaviors in an unrelated context, without the constraint of time. Such across-context correlations may be important in uncovering ecological or evolutionary constraints (Bell 2005; Dochtermann and Dingemanse 2013). A boldness-aggressive syndrome has been previously established in this population (Bell 2005). The discovery of a parenting-attentiveness syndrome suggests a potential new personality axis in this population, and future studies should examine whether these behaviors are correlated in other populations and systems.
ETHICAL NOTE
All focal males resumed parenting behavior following measurements and observations. Intruders were placed in a wire cage with openings too small for either the focal male or the intruder to injure each other. All intruder individuals were held for less than five hours and released at their point of capture following observations; all intruders resumed normal behavior upon release. This study was conducted under California Fish and Game Permit SC-11131 and approved by the Institutional Animal Care and Use Committee at the University of Illinois at Urbana-Champaign (protocol #09204).
Supplementary Material
ACKNOWLEDGEMENTS
We are grateful to A. Fritzsche, L. Hostert, and M. Grobis for their help in the field. We also thank K. Laskowski, G. Kozak, K. McGhee, members of the Bell lab, and three reviewers for valuable comments on the manuscript. This project was supported by the University of Illinois, National Science Foundation Doctoral Dissertation Improvement Grant (IOS 1210696) and an NSF Graduate Research Fellowship to LRS, and NSF IOS 1121980 and National Institutes of Health R01 GM082937 to AMB.
REFERENCES
- Barnett CA, Thompson CF, Sakaluk SK. Aggressiveness, boldness and parental food provisioning in male house wrens (Troglodytes aedon) Ethology. 2012;118:984–993. [Google Scholar]
- Bell AM. Behavioural differences between individuals and two populations of stickleback (Gasterosteus aculeatus) J Evol Biol. 2005;18:464–473. doi: 10.1111/j.1420-9101.2004.00817.x. [DOI] [PubMed] [Google Scholar]
- Bell AM. Randomized or fixed order for studies of behavioral syndromes? Behav Ecol. 2013;24:16–20. doi: 10.1093/beheco/ars148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell AM, Backstrom T, Huntingford FA, Pottinger TG, Winberg S. Variable neuroendocrine responses to ecologically-relevant challenges in sticklebacks. Physiol Behav. 2007;91:15–25. doi: 10.1016/j.physbeh.2007.01.012. [DOI] [PubMed] [Google Scholar]
- Betini GS, Norris DR. The relationship between personality and plasticity in tree swallow aggression and the consequences for reproductive success. Anim Behav. 2012;83:137–143. [Google Scholar]
- Boughman JW. Condition-dependent expression of red colour differs between stickleback species. J Evol Biol. 2007;20:1577–1590. doi: 10.1111/j.1420-9101.2007.01324.x. [DOI] [PubMed] [Google Scholar]
- Budaev SV, Zworykin DD, Mochek AD. Individual differences in parental care and behavior profile in the convict cichlid: A correlation study. Anim Behav. 1999;58:195–202. doi: 10.1006/anbe.1999.1124. [DOI] [PubMed] [Google Scholar]
- Burtka JL, Grindstaff JL. Repeatable nest defense behavior in a wild population of Eastern bluebirds (Sialia sialis) as evidence of personality. Acta Ethol. 2013;16:135–146. [Google Scholar]
- Candolin U. Reproduction under predation risk and the trade-off between current and future reproduction in the threespine stickleback. Proc R Soc Lond B. 1998;265:1171–1175. [Google Scholar]
- Candolin U. Changes in expression and honesty of sexual signalling over the reproductive lifetime of sticklebacks. Proc R Soc Lond B. 2007;267:2425–2430. doi: 10.1098/rspb.2000.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole EF, Quinn JL. Shy birds play it safe: personality in captivity predicts risk responsiveness during reproduction in the wild. Biol Lett. 2014;10:20140178. doi: 10.1098/rsbl.2014.0178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingemanse NJ, Dochtermann N. Quantifying individual variation in behavior: mixed-effect modelling approaches. J Anim Ecol. 2013;82:39–54. doi: 10.1111/1365-2656.12013. [DOI] [PubMed] [Google Scholar]
- Dingemanse NJ, Wright J, Kazem AJN, Thomas DK, Hickling R, Dawnay N. Behavioral syndromes differ predictably between 12 populations of three-spined stickleback. J Anim Ecol. 2007;76:1128–1138. doi: 10.1111/j.1365-2656.2007.01284.x. [DOI] [PubMed] [Google Scholar]
- Dochtermann NA, Dingemanse NJ. Behavioral syndromes as evolutionary constraints. Behav Ecol. 2013;24:806–811. [Google Scholar]
- Duckworth RA. Behavioral correlations across breeding contexts provide a mechanism for a cost of aggression. Behav Ecol. 2006;17:1011–1019. [Google Scholar]
- Feliciano JB. Dissertation. University of California Davis; 2004. A study of the salmonid populations in the Navarro River Watershed: Patterns of decline and tools for monitoring. [Google Scholar]
- Foster SA. Diversionary displays of paternal stickleback – defenses against cannibalistic groups. Behav Ecol Sociobiol. 1988;22:335–340. [Google Scholar]
- Hadfield JD. MCMC methods for Multi-response Generalised Linear Mixed Models: The MCMCglmm R Package. J Stat Softw. 2010;33:1–22. [Google Scholar]
- Huntingford FA. The relationship between anti-predator behavior and aggression among conspecifics in the three-spined stickleback, Gasterosteus aculeatus. Anim Behav. 1976;24:245–260. [Google Scholar]
- Kazama K, Niizuma Y, Watanuki Y. Consistent individual variations in aggressiveness and a behavioral syndrome across breeding contexts in different environments in the Black-tailed Gull. J Ethol. 2012;30:279–288. [Google Scholar]
- Ketterson ED, Nolan V. Adaptation, exaptation, and constraint: A hormonal perspective. Am Nat. 1999;154:S4–S25. doi: 10.1086/303280. [DOI] [PubMed] [Google Scholar]
- Kontiainen P, Pietiainen H, Huttunen K, Karell P, Kolunen H, Brommer JE. Aggressive Ural owl mothers recruit more offspring. Behav Ecol. 2009;20:789–796. [Google Scholar]
- Kraak SBM, Bakker TCM, Mundwiler B. Sexual selection in sticklebacks in the field: Correlates of reproductive, mating, and paternal success. Behav Ecol. 1999a;10:696–706. [Google Scholar]
- Kraak SBM, Bakker TCM, Mundwiler B. Correlates of the duration of the egg collecting phase in the three-spined stickleback. J Fish Biol. 1999b;54:1038–1049. [Google Scholar]
- Künzler R, Bakker TCM. Pectoral fins and paternal quality in sticklebacks. Proc R Soc Lond B. 2000;267:999–1004. doi: 10.1098/rspb.2000.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissaker M, Kvarnemo C. Ventilation or nest defense – parental care trade-offs in a fish with male care. Behav Ecol Sociobiol. 2006;60:864–873. [Google Scholar]
- MacColl ADC, Hatchwell BJ. Heritability of parental effort in a passerine bird. Evolution. 2003;57:2191–2195. doi: 10.1111/j.0014-3820.2003.tb00398.x. [DOI] [PubMed] [Google Scholar]
- Marshall DJ, Uller T. When is a maternal effect adaptive? Oikos. 2007;116:1957–1963. [Google Scholar]
- McGhee KE, Pintor L, Suhr E, Bell AM. A nonadaptive maternal effect in sticklebacks. Funct Ecol. 2012;26:932–940. doi: 10.1111/j.1365-2435.2012.02008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney MJ. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annu Rev Neurosci. 2001;24:1161–1192. doi: 10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- Moodie GEE. Predation, natural selection and adaptation in an unusual 3 spine stickleback. Heredity. 1972;28:155–167. [Google Scholar]
- Mutzel A, Blom MPK, Spagopoulou F, Wright J, Dingemanse NJ, Kempenaers B. Temporal trade-offs between nestling provisioning and defense against nest predators in blue tits. Anim Behav. 2013;85:1459–1469. [Google Scholar]
- Nakagawa S, Schielzeth H. Repeatability for Gaussian and non-Gaussian data: A practical guide for biologists. Biol Rev. 2010;85:935–956. doi: 10.1111/j.1469-185X.2010.00141.x. [DOI] [PubMed] [Google Scholar]
- Nakagawa S, Gillespie DOS, Hatchwell BJ, Burke T. Predictable males and unpredictable females: Sex difference in repeatability of parental care in a wild bird population. J Evolution Biol. 2007;20:1674–1681. doi: 10.1111/j.1420-9101.2007.01403.x. [DOI] [PubMed] [Google Scholar]
- Pressley PH. Parental effort and the evolution of nest-guarding tactics in the threespine stickleback, Gasterosteus aculeatus L. Evolution. 1981;35:282–295. doi: 10.1111/j.1558-5646.1981.tb04887.x. [DOI] [PubMed] [Google Scholar]
- Patrick SC, Browning LE. Exploration behaviour is not associated with chick provisioning in great tits. PLoS ONE. 2011;6:e26383. doi: 10.1371/journal.pone.0026383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangeley RW, Godin J-GJ. The effects of a trade-off between foraging and brood defense on parental behavior in the convict cichlid fish, Cichlasoma nigrofasciatum. Behaviour. 1992;120:123–138. [Google Scholar]
- Reznick D, Nunney L, Tessier A. Big houses, big cars, superfleas and the costs of reproduction. Trend Ecol Evol. 2000;15:421–425. doi: 10.1016/s0169-5347(00)01941-8. [DOI] [PubMed] [Google Scholar]
- Rytkonen S, Orell M, Koivula K, Soppela M. Correlation between 2 components of parental investment – nest defense intensity and nestling provisioning effort of willow tits. Oecologia. 1995;104:386–393. doi: 10.1007/BF00328375. [DOI] [PubMed] [Google Scholar]
- Sabat AM. Costs and benefits of parental effort in a brood-guarding fish (Ambloplites rupestris, Centrarchidae) Behav Ecol. 1994;5:195–201. [Google Scholar]
- Schwagmeyer PL, Mock DW. How consistently are good parents good parents? Repeatability of parental care in the house sparrow, Passer domesticus. Ethology. 2003;109:303–313. [Google Scholar]
- Sparkes TC, Rush V, Foster SA. Reproductive costs, condition and carotenoid-based colour in natural populations of threespine stickleback (Gasterosteus aculeatus) Ecol Freshw Fish. 2008;17:292–302. [Google Scholar]
- Stein LR, Bell AM. Consistent individual differences in fathering in threespine stickleback Gasterosteus aculeatus. Curr Zool. 2012;58:45–52. doi: 10.1093/czoolo/58.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein LR, Bell AM. Paternal programming in sticklebacks. Anim Behav. 2014;95:161–175. doi: 10.1016/j.anbehav.2014.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiver KA, Alonzo SH. Parental and mating effort: Is there necessarily a trade-off? Ethology. 2009;115:1101–1126. [Google Scholar]
- Tinbergen N, van Iersel JJA. “Displacement reactions” in the three-spined stickleback. Behaviour. 1947;1:56–63. [Google Scholar]
- van Noordwijk AJ, de Jong G. Acquisition and allocation of resources - Their influence on variation in life-history tactics. Am Nat. 1986;128:137–142. [Google Scholar]
- von Hippel FA. Vigorously courting male sticklebacks are poor fathers. Acta Ethol. 2000;2:83–89. [Google Scholar]
- Westneat DF, Hatch MI, Wetzel DP, Ensminger AL. Individual variation in parental care reaction norms: Integration of personality and plasticity. Am Nat. 2011;178:652–667. doi: 10.1086/662173. [DOI] [PubMed] [Google Scholar]
- Wetzel DP, Westneat DF. Parental care syndromes in house sparrows: positive covariance between provisioning and defense linked to parent identity. Ethology. 2014;10:249–257. [Google Scholar]
- Wingfield JC, Hegner RE, Dufty AM, Ball GF. The challenge hypothesis – theoretical implications for patterns of testosterone secretion, mating systems, and breeding strategies. Am Nat. 1990;136:829–846. [Google Scholar]
- Wootton RJ. A Functional Biology of Sticklebacks. Berkeley: University of California Press; 1984. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.