Abstract
L-Asparagine is necessary and sufficient for the maximal induction of ornithine decarboxylase (ODC) (L-ornithine carboxy-lyase, EC 4.1.1.17) activity in confluent N18 mouse neuroblastoma cells in a salts/glucose medium; L-asparagine also induces maximal ODC activity when added to a tissue culture medium. L-Glutamine is about one-half as effective as asparagine. Cholera toxin and agents that are known to raise intracellular cyclic AMP concentrations have no effect on the induction of ODC activity unless suboptimal concentrations of asparagine are present in the salts/glucose medium. Whereas actinomycin D does not inhibit induction of ODC activity by asparagine, it inhibits the induction of ODC activity in association with cyclic AMP. In the salts/glucose medium, the rate of loss of ODC activity following the inhibition of protein synthesis by cycloheximide or puromycin depends upon the presence or absence of asparagine; loss is rapid only in the absence of asparagine and does not appear to be related to the inhibition of protein synthesis. These results are discussed in the context that the overlay of the growth medium tends to mask the minimal requirements for enzyme induction, because the composition of the medium defines: (a) the requirements for the induction of ODC activity; (b) the effect, or lack of effect, of cyclic AMP (and of inducers of intracellular cyclic AMP) on the induction of ODC activity; (c) the effect, or lack of effect, of actinomycin D on the induction of ODC activity; and (d) the action of puromycin and of cycloheximide on the rate of loss of ODC activity. It will be interesting to determine whether these results are uniquely applicable to ODC, whether many of the reactions attributed to cyclic AMP in the literature may be mediated by asparagine and glutamine, and whether actinomycin D, cycloheximide, and puromycin can be relied upon to differentiate between transcriptional and post-transcriptional control.
Keywords: cyclic AMP, actinomycin D, cycloheximide, protein half-life, cholera toxin
Full text
PDF
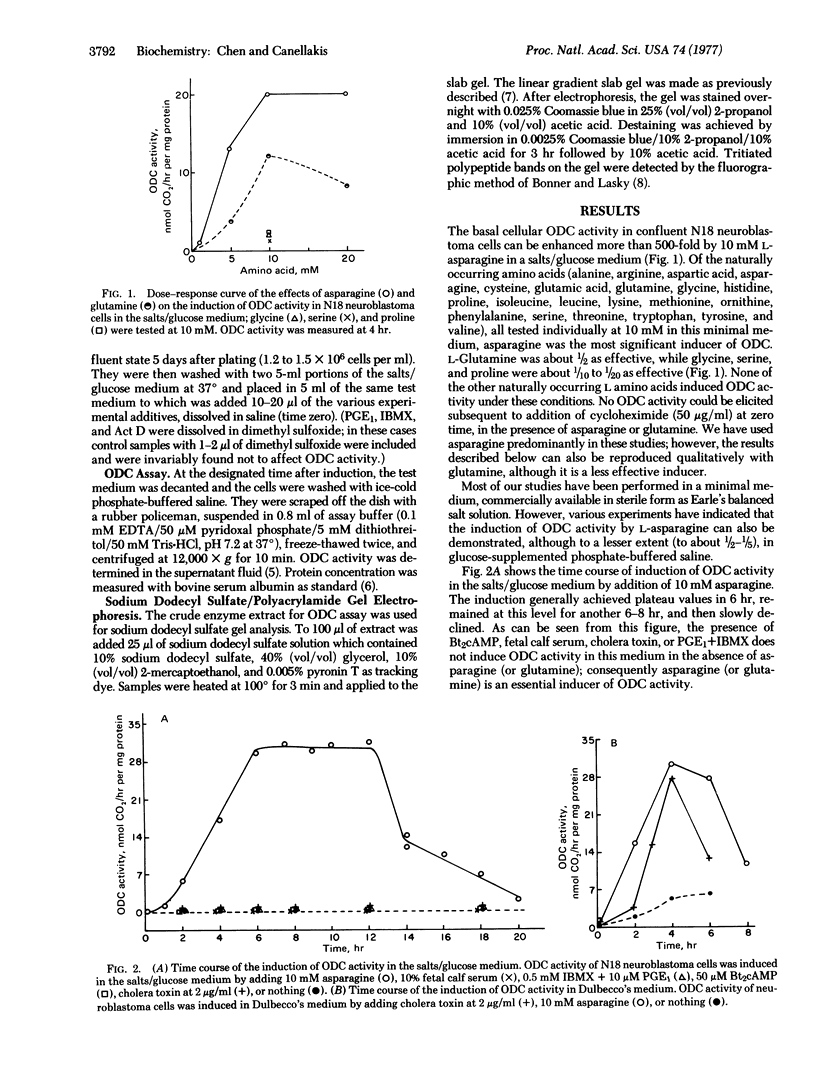
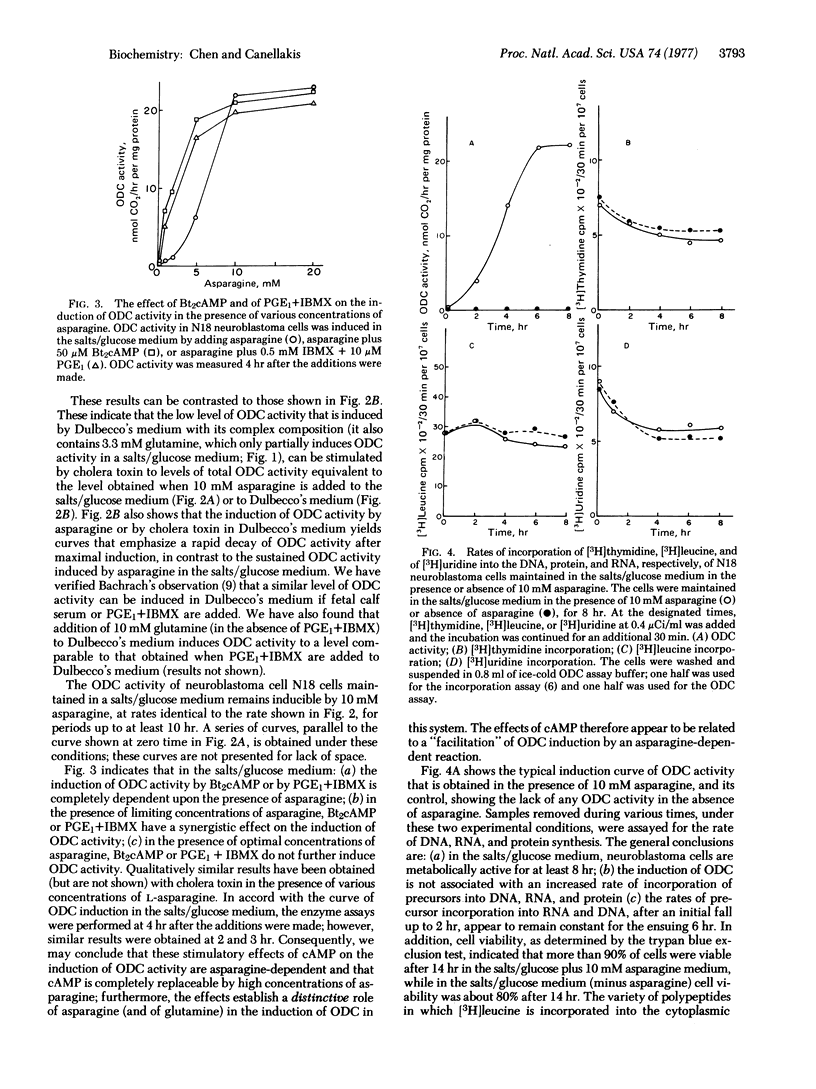
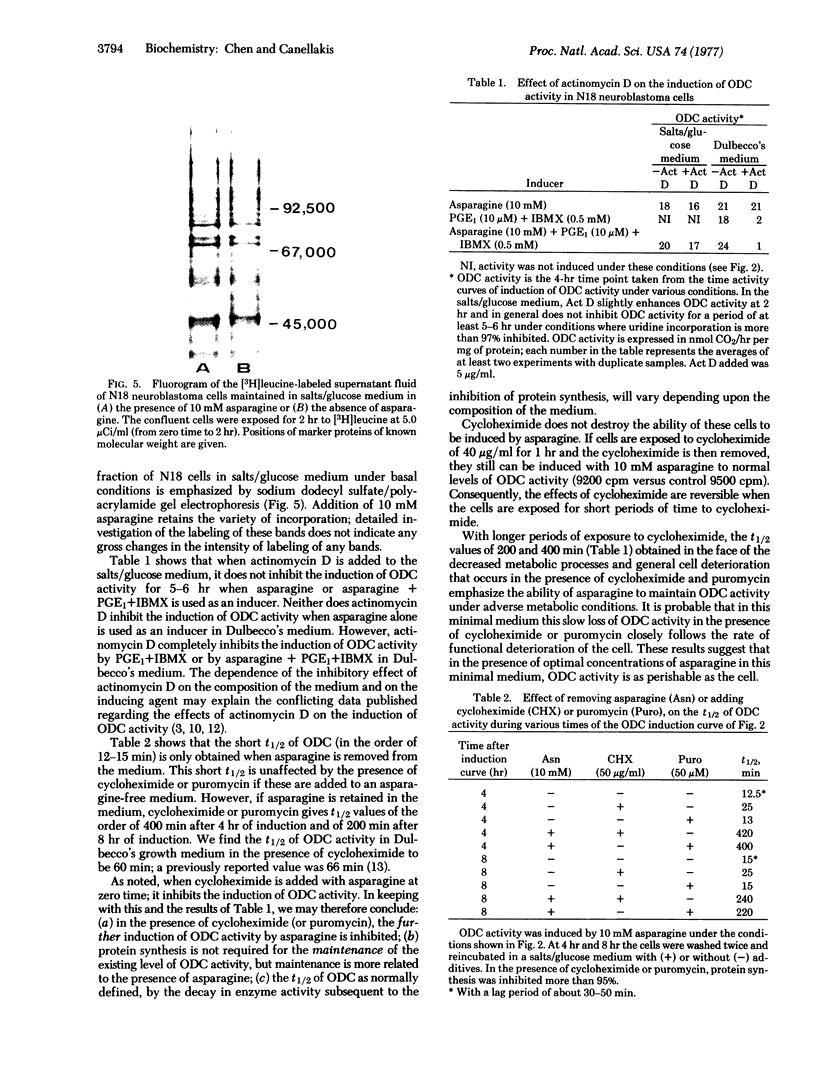

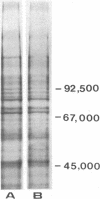
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amano T., Richelson E., Nirenberg M. Neurotransmitter synthesis by neuroblastoma clones (neuroblast differentiation-cell culture-choline acetyltransferase-acetylcholinesterase-tyrosine hydroxylase-axons-dendrites). Proc Natl Acad Sci U S A. 1972 Jan;69(1):258–263. doi: 10.1073/pnas.69.1.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachrach U. Cyclic AMP-mediated induction of ornithine decarboxylase of glioma and neuroblastoma cells. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3087–3091. doi: 10.1073/pnas.72.8.3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck W. T., Bellantone R. A., Canellakis E. S. The in vivo stimulation of rat liver ornithine decarboxylase activity by dibutyryl cyclic adenosine 3',5'-monophosphate, theophylline and dexamethasone. Biochem Biophys Res Commun. 1972 Sep 26;48(6):1649–1655. doi: 10.1016/0006-291x(72)90904-7. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Chen K., Heller J., Canellakis E. S. Studies on the regulation of ornithine decarboxylase activity by the microtubules: the effect of colchicine and vinblastine. Biochem Biophys Res Commun. 1976 Jan 26;68(2):401–408. doi: 10.1016/0006-291x(76)91159-1. [DOI] [PubMed] [Google Scholar]
- Chen K., Tsai C., Canellakis E. S. Comparison of physical and immunological properties of plasma membranes of two mouse leukemia cell lines, P388 and L1210. Cancer Res. 1975 Sep;35(9):2403–2412. [PubMed] [Google Scholar]
- Clark J. L. Specific induction of ornithine decarboxylase in 3T3 mouse fibroblasts by pituitary growth factors: cell density-dependent biphasic response and alteration of half-life. Biochemistry. 1974 Oct 22;13(22):4668–4674. doi: 10.1021/bi00719a031. [DOI] [PubMed] [Google Scholar]
- Cooney D. A., Handschumacher R. E. L-asparaginase and L-asparagine metabolism. Annu Rev Pharmacol. 1970;10:421–440. doi: 10.1146/annurev.pa.10.040170.002225. [DOI] [PubMed] [Google Scholar]
- Fausto N. The control of ornithine decarboxylase activity during liver regeneration. Biochim Biophys Acta. 1971 Apr 29;238(1):116–128. doi: 10.1016/0005-2787(71)90015-3. [DOI] [PubMed] [Google Scholar]
- Fidler I. J., Montgomery P. C., Cesarini J. P. Modification of surface topography of lymphocytes by L-asparaginase. Cancer Res. 1973 Dec;33(12):3176–3180. [PubMed] [Google Scholar]
- Heller J. S., Fong W. F., Canellakis E. S. Induction of a protein inhibitor to ornithine decarboxylase by the end products of its reaction. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1858–1862. doi: 10.1073/pnas.73.6.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershko A., Tomkins G. M. Studies on the degradation of tyrosine aminotransferase in hepatoma cells in culture. Influence of the composition of the medium and adenosine triphosphate dependence. J Biol Chem. 1971 Feb 10;246(3):710–714. [PubMed] [Google Scholar]
- Hogan B. L., Murden S. Effect of growth conditions on the activity of ornithine decarboxylase in cultured hepatoma cells. I. Effect of amino acid supply. J Cell Physiol. 1974 Jun;83(3):345–351. doi: 10.1002/jcp.1040830304. [DOI] [PubMed] [Google Scholar]
- KNOX W. E. Two mechanisms which increase in vivo the liver tryptophan peroxidase activity: specific enzyme adaptation and stimulation of the pituitary adrenal system. Br J Exp Pathol. 1951 Oct;32(5):462–469. [PMC free article] [PubMed] [Google Scholar]
- Lee K. L., Kenney F. T. Regulation of tyrosine- -ketoglutarate transaminase in rat liver. J Biol Chem. 1971 Dec 25;246(24):7595–7601. [PubMed] [Google Scholar]
- Morris D. R., Fillingame R. H. Regulation of amino acid decarboxylation. Annu Rev Biochem. 1974;43(0):303–325. doi: 10.1146/annurev.bi.43.070174.001511. [DOI] [PubMed] [Google Scholar]
- Prouty W. F. Ornithine decarboxylase inactivation in HeLa cells. J Cell Physiol. 1976 Sep;89(1):65–76. doi: 10.1002/jcp.1040890107. [DOI] [PubMed] [Google Scholar]
- Robinson A. B., Rudd C. J. Deamidation of glutaminyl and asparaginyl residues in peptides and proteins. Curr Top Cell Regul. 1974;8(0):247–295. doi: 10.1016/b978-0-12-152808-9.50013-4. [DOI] [PubMed] [Google Scholar]
- Ross E., Schatz G. Assay of protein in the presence of high concentrations of sulfhydryl compounds. Anal Biochem. 1973 Jul;54(1):304–306. doi: 10.1016/0003-2697(73)90280-7. [DOI] [PubMed] [Google Scholar]
- Russell D. H., Snyder S. H. Amine synthesis in regenerating rat liver: extremely rapid turnover of ornithine decarboxylase. Mol Pharmacol. 1969 May;5(3):253–262. [PubMed] [Google Scholar]