Abstract
Objective
To further evaluate the sensitivity and specificity of urine aquaporin-1 (AQP1) and perilipin-2 (PLIN2) concentrations to diagnose clear cell or papillary renal cell carcinoma (RCC) we compared these unique urine biomarker concentrations in patients with RCC, non-cancer renal masses, bladder cancer and prostate cancer.
Patients and Methods
From February 1 through October 31, 2012 pre-operative urine samples were obtained from patients with a presumptive diagnosis of RCC based on an imaged renal mass, prostate cancer or transitional cell bladder cancer. Imaged renal masses were diagnosed post-nephrectomy, as cancer or benign, by histology. Urine AQP1 and PLIN2 concentrations were measured by sensitive and specific Western blot and normalized to urine creatinine concentration.
Results
Median urine AQP1 and PLIN2 in patients with clear cell and papillary RCC (n=47) were 29 and 36 relative absorbance units/mg urine creatinine. In contrast, median concentrations in bladder (n=22) and prostate cancer (n=27), patients with chromophobe tumors (n=7), and in benign renal oncocytoma (n=9) and angiomyolipomas (n=7), were all less than 10 (P <.001 vs RCC for both biomarkers, Kruskal-Wallis test) and comparable to healthy controls. The area under the receiver operating characteristic curve was 0.99 to 1.00 for both biomarkers.
Conclusions
These results further demonstrate the specificity and sensitivity of urine AQP1 and PLIN2 concentrations for RCC. These novel tumor-specific proteins have high clinical validity and substantial potential as specific screening biomarkers for clear cell and papillary RCC, and in the differential diagnosis of imaged renal masses.
INTRODUCTION
Cancers of the kidney and renal pelvis account for approximately 4% of all adult malignant tumors. The American Cancer Society anticipated 65,120 new cases and 13,680 deaths related to renal malignancies for 2013.1 There has been an increase in the diagnosis of smaller, lower stage renal cell carcinoma (RCC) that is likely due to greater use of abdominal imaging and consequently incidental detection. Thus, the fraction of incidentally detected compared with all diagnosed RCC’s increased from approximately 10% in 1970 to at least 60% by 1998.2
Pathological stage is one of the most important prognostic indicators for survival of RCC.3,4 Patients with pre-symptomatic, incidentally detected tumors have a 5-year disease-free survival of 85%, while patients with cancers detected symptomatically have a 5-year disease-free survival of only 62%.2,5 The prognosis for metastatic RCC is even worse; the 5-year RCC-specific survival ranges from about 40% with nodal metastases to about 20% with distant metastases.6,7 This clearly demonstrates that early detection is beneficial and improves outcomes. Nevertheless, there is no currently available noninvasive method to enable early diagnosis or screening for RCC.
An initial investigation in 2010 found higher urine aquaporin-1 (AQP1) and adipophilin (since renamed as perilipin-2, PLIN2) concentrations in clear cell and papillary RCC patients compared to controls.8 These biomarker elevations were normalized after tumor excision.8 To determine the specificity of AQP1 and PLIN2 for renal cancer versus common renal diseases, a second investigation compared urine AQP1 and PLIN2 concentrations in patients with RCC with those in patients with common non-cancer kidney disease (diabetic nephropathy, glomerulonephritis, urine tract infection). That investigation found significantly higher median AQP1 and PLIN2 concentrations in RCC patients compared to the patients with non-cancerous renal disease or patients without any renal disease. This second investigation also reaffirmed that AQP1 and PLIN2 concentrations were correlated with tumor size, and were decreased 83–84% following tumor excision.9 This suggests that urine concentrations of AQP1 or PLIN2 are not confounded by common non-cancer kidney diseases and do indicate tumor burden. More specifically, these urine biomarkers reflected clear cell or papillary tumor size and stage, but not grade.8–10
Our previous studies provide some degree of analytical and clinical validity to the ability of urine AQP1 and PLIN2 levels identify patients with clear cell or papillary subtypes of kidney cancer.8–10 However, the ability of AQP1 and PLIN2 to differentiate patients with clear cell or papillary RCC from patients with other urinary tract cancers is unknown. In addition, greater use of abdominal imaging has led to increased incidental detection of renal masses. Nevertheless, radiologic imaging cannot definitively differentiate all cancerous from benign renal masses.11–21 Thus the typical clinical approach is partial or radical nephrectomy of an imaged renal mass, with post-operative pathologic analysis. Unfortunately, this does result in the partial or total removal of otherwise normal kidneys in almost 20% of cases.11–21 Therefore an additional unmet clinical need is a biomarker for unambiguous differentiation of clear cell or papillary RCC from other, particularly benign, imaged renal masses. Thus, there is need for further clinical validation of AQP1 and PLIN2 as RCC biomarkers.
To address these questions, this investigation compared pre-nephrectomy (or pre-operative) urine AQP1 and PLIN2 concentrations in patients with clear cell or papillary RCC, patients with other (non-cancer) imaged renal masses, prostate or bladder cancer, to better understand the specificity and sensitivity of these two renal cancer biomarkers.
MATERIALS AND METHODS
PATIENTS
Approval was obtained from the Washington University Institutional Review Board, (IRB ID 201202051) and written informed consent was obtained from all patients. From February through October, 2012, pre-operative urine samples were obtained on the day of surgery from consecutive patients with a) a presumptive diagnosis of kidney cancer based on an imaged renal mass, b) 27 patients with prostate cancer, or c) 22 patients with bladder cancer. Table 1 lists the demographics of the 47 patients with a preoperative imaged renal mass and a post-surgical histologically proven diagnosis of clear cell or papillary RCC, and a composite of 26 control patients undergoing surgery for non-urologic issues spanning the ages of all patient groups. Table 1 also lists the demographics of the 7 patients each with a post-surgically diagnosed chromophobe tumor or an angiomyolipomas and the 9 patients diagnosed with an oncocytoma consented between November 2009 to October 2012. The composite control cohort consists of 9 patients (mean age 61) matching the ages of the patients with RCC, chromophobe tumors, oncocytomas, angiomyolipomas and prostate cancer (One-Way ANOVA P=.65) with mean ages ranging from 56 to 64. An older control patient subgroup of 17 individuals had a mean age of 73 and closely matched the mean age of 75 years representing the patients with bladder cancer (One Way ANOVA P=.47). Table 2 summarizes the RCC tumor stage, grade, node involvement and incidence of distant metastases of the 47 patients with RCC. Supplemental eTable 1 includes the prostate specific antigen (PSA) values, pathological stage, and grade for the 27 prostate cancer patients. Supplemental eTable 2 shows pathological features of the 22 bladder cancer patients.
Table 1.
Clinical and laboratory features of control and patients with an imaged renal mass.
| Characteristics | RCC | Control | Chromophobe | Oncocytoma | AML | P-value |
|---|---|---|---|---|---|---|
| Number | 47 | 26 | 7 | 9 | 7 | |
| Age: Mean ±SD | 59±11 | 69±8 | 64±10 | 62±6 | 56±9 | .01 |
| Male, no. (%) | 13 (28) | 10 (39) | 4 (57) | 3 (33) | 2 (29) | .57 |
| Tumor Size: Mean±SD | 4.0±2.9 | N/A | 4.2±1.3 | 2.8±1.3 | 2.1±1.6 | .20 |
| Nephrometry Score: Mean±SD | 8.4±2.9 | N/A | 7.4±1.3 | 8.1±2.0 | 6.7±2.0 | .18 |
Abbreviations: RCC Renal Cell Carcinoma defined as patients with clear cell and papillary kidney cancers, AML Angiomyolipoma, SD Standard Deviation.
Statistical analysis was performed by Fisher’s Exact Test for categorical values and by One-Way ANOVA with F-Test for numerical values.
Table 2.
Pathological data for renal cell cancer patients
| Number | % of Total | |
|---|---|---|
| Tumor Histological Subtype | ||
| Clear Cell | 35 | 75 |
| Papillary | 10 | 21 |
| Mixed (clear & papillary) | 2 | 4 |
| Post-op pT Stage | ||
| pT1a | 32 | 68 |
| pT1b | 5 | 11 |
| pT2 | 2 | 4 |
| pT3 | 8 | 17 |
| Furhman Grade | ||
| 1 | 5 | 11 |
| 2 | 27 | 56 |
| 3 | 9 | 20 |
| 4 | 5 | 11 |
| Not Reported | 1 | 2 |
| Nodes | ||
| N0 | 44 | 94 |
| N1 | 2 | 4 |
| Not Reported | 1 | 2 |
| Metastasis at Nephrectomy | ||
| No | 44 | 94 |
| Yes | 3 | 4 |
Sample size calculations were based on results of the control group using the mean and standard deviation of the urine AQP1 and PLIN2 concentrations of our recent study.9 To detect a 2-fold (conservative assumption) increase in biomarker concentrations above control in the present study, based on a two-sided t-test and 80% power, would require a minimum of 7 patients for AQP1 and 5 for PLIN2 (0.05 significance). Our enrollment exceeded these minimums in all of the patient groups to bolster the statistical significance of the results.
CLINICAL AND PATHOLOGICAL DATA
Demographic data and medical history were recorded including age, sex, weight, and surgery performed. Serum creatinine concentrations were determined pre-operatively and the estimated glomerular filtration rate (eGFR) was calculated using the Modification of Diet in Renal Disease equation.22 Pre-operative computed tomography and magnetic resonance images of the renal masses were reviewed and nephrometry scores assigned.23 The most recent serum PSA concentration prior to surgery was recorded for prostate cancer patients. Post-operative pathology reports provided cell type, size, tumor stage/node metastases/distant metastases (TNM), and Fuhrman grade for RCC patients or diagnosis of other imaged renal mass types. Pathological stage and grade were recorded for patients undergoing a radical prostatectomy.
AQP1 AND PLIN2 MEASUREMENT
Urine AQP1 and PLIN2 concentrations were determined as previously described.8–10 Briefly, thawed urine was centrifuged (1800g for 10 minutes) to remove debris before processing for Western blot analysis. The urine creatinine concentration was quantified by the Jaffe reaction.24 Proteins were precipitated by 15 volumes of acetone/methanol (1:1) and then dissolved in an amount of sodium dodecyl sulfate (SDS) sample buffer such that the 5 μL of sample applied to the gel reflected the amount of urine containing 10 μg of creatinine for patients with kidney cancer or 20 μg creatinine for bladder or prostate cancer patients. The blocked membranes were incubated with 1:500 dilution of anti-AQP1 (H-55) antibody or a 1:200 dilution of anti-ADFP (PLIN2) (H-80) antibody (both from Santa Cruz Biotechnology Inc, Santa Cruz, CA) in blocking buffer that contained 0.1% Tween-20 overnight. After washing, the membranes were incubated with a 1:2000 dilution of donkey anti-rabbit IgG IRDye 680 (LICOR Biosciences, Lincoln, NE) in blocking buffer with 0.1% Tween-20 for 1 hour. Both AQP1 and PLIN2 were visualized and quantified using an infrared imager (Odyssey Infrared Imager; LI-COR) and proprietary software. The response of both biomarkers was linear over the range of concentrations found in patient urine. Both AQP1 and PLIN2 were quantified using relative absorbance units and normalized to urine creatinine excretion. The inter-assay variation from gel to gel for AQP1 was 10% while that for PLIN2 was 9%.8,9 Representative Western blots for both AQP1 and PLIN2 are included as Supplemental eFigures 1A–D showing data from patients with bladder, prostate cancer and RCC.
STATISTICAL ANALYSIS
Analysis was performed using R statistical software and Analyse-it for Excel 2010. Comparisons of age and sex were performed between RCC, prostate cancer, and bladder cancer patients. Descriptive statistics of patient demographic categorical variables were compared using Fisher’s Exact Test and their numerical values analyzed by One-Way ANOVA with F-Test. Urine AQP1 and PLIN2 concentrations were compared in patients using the Kruskal-Wallis test. Receiver operating characteristic curve analysis compared the AQP1 or PLIN2 concentrations of the 47 patients with RCC to either the 23 patients with other imaged renal masses or the 58 patients with bladder or prostate cancer. All tests were performed two-sided with statistical significance set at the 0.05 level.
RESULTS
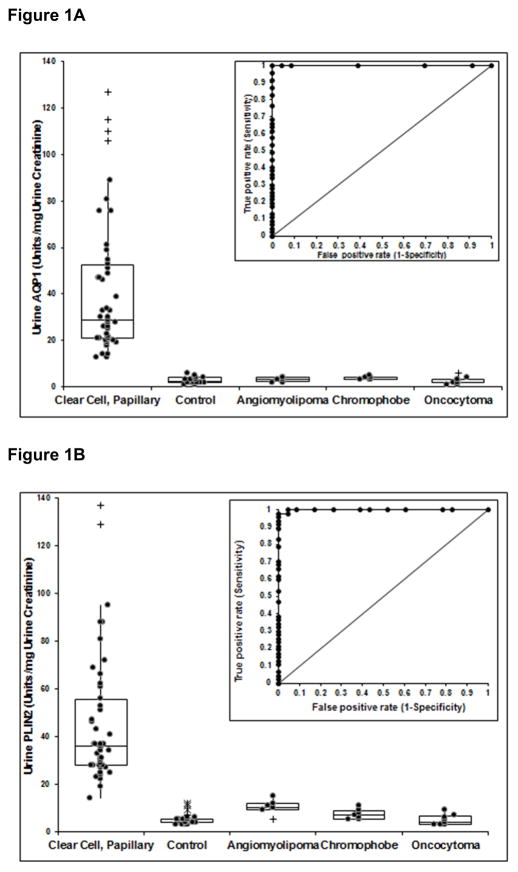
Urine AQP1 and PLIN2 concentrations for patients with clear cell or papillary RCC and all control patients are shown in Figures 1A and 1B. Both the median and 1st/3rd quartile AQP1 (29(21, 53)) and PLIN2 (36(28, 56)) concentrations were significantly greater in the urine of RCC patients (P<.001 for both), compared to all control patients AQP1 (3(2, 4)) and PLIN2 (5(4, 5)). The urine biomarker levels in patients with an angiomyolipoma AQP1 (3(2, 4) and PLIN2 (10(9, 12)), the chromophobe subtype of RCC AQP1 (4(3, 4)) and PLIN2 (7(5, 9)), and patients with an oncocytoma AQP1 (2(2, 3)) and PLIN2 (7(5, 9)) were all significantly less than those in patients with RCC (Figure 1A and 1B). The median and 1st/3rd quartile for urine PLIN2 of patients with angiomyolipomas was greater from that of the 26 composite controls (P<.001) or patients with oncocytomas (P<.001) but still significantly less than that of patients with RCC (P<.001) (Figure 1B). The nephrometry score, a comparison of anatomical characteristics of imaged renal masses,23 was not significantly different among these patient cohorts (Table 1) suggesting that the location and size of any imaged mass within the kidney has no association with urine biomarker levels. Receiver operating characteristic curve analysis (insets of Figures 1A and 1B) determined that a cut-off value of 5 absorbance units/mg urine creatinine for AQP1 and 13 absorbance units/mg urine creatinine for PLIN2 resulted in 100% sensitivity and 100% specificity for AQP1 (Figure 1A) and 100% sensitivity and 99% specificity for PLIN2 (Figure 1B).
Figure 1.
Relative concentrations (normalized to urine creatinine concentration) of AQP1 (A) and PLIN2 (B) in the urine of patients with clear cell and papillary kidney cancers, all 26 control patients, and patients with an angiomyolipomas, chromophobe kidney cancer or an oncocytoma. Box plots show the median along with the 1st and 3rd quartile. (+) outlier > 1.5 and < 3 of the interquartile range. (*) outlier > 3 of the interquartile range. The median urine AQP1 and PLIN2 concentrations are significantly greater in RCC patients compared to the controls or other patient groups (P<0.001 for both, Kruskal-Wallis test with Bonferroni correction). The median and 1st/3rd quartile for urine PLIN2 of patients with an angiomyolipoma were significantly greater than that of controls (P<.001) or patients with oncocytoma (P<.001) (Kruskal-Wallis test). The insert shows ROC plots comparing the AQP1 or PLIN2 concentrations of the patients with RCC to that of all patients with AMLs, chromophobes and oncocytomas. The area under the ROC curves are 1.00 for Figures 1A and 0.99 for Figure 1B.
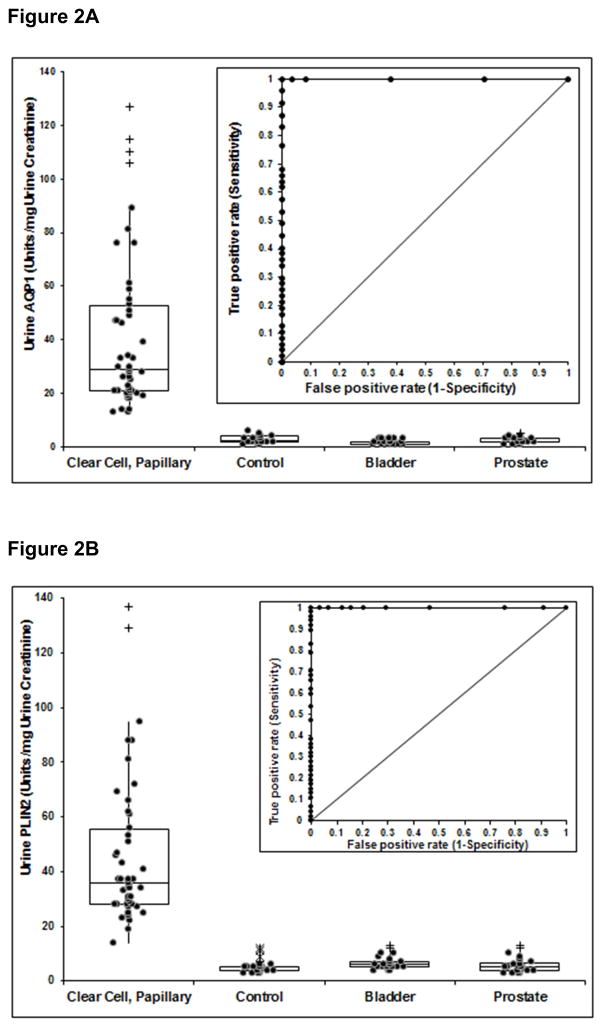
Urine AQP1 and PLIN2 concentrations for patients with clear cell or papillary RCC, all 26 control patients, and patients with bladder or prostate cancers are shown in Figures 2A and 2B. Patients with clear cell or papillary RCC or the controls were the same as in Figures 1A and 1B. Both the median and 1st/3rd quartile AQP1 concentrations were significantly greater in the RCC patients compared to patients with prostate cancer AQP1 (3(2, 3)) and PLIN2 5(4, 6), or bladder cancer AQP1 (2(1, 2) and PLIN2 (6(5, 7). Similarly, urine PLIN2 concentrations were significantly greater in RCC patients compared and to those with bladder or prostate cancer (P<.001 for both,). There is no overlap of either AQP1 or PLIN2 concentrations in the RCC group compared with controls or patients with prostate cancer or bladder cancer (Figure 2). Independent receiver operating characteristic curve analysis (insets of Figures 2A and 2B) from those determined for Figures 1A and 1B determined that a cut-off value of 5 absorbance units/mg creatinine for AQP1 and 13 absorbance units/mg creatinine for PLIN2 resulted in 100% sensitivity and specificity for both biomarkers.
Figure 2.
Relative concentrations (normalized to urine creatinine concentration) of AQP1 (A) and PLIN2 (B) in the urine of patients with clear cell and papillary kidney cancers, all 26 control patients, and patients with bladder or prostate cancer. Box plots show the median along with the 1st and 3rd quartile. (+) outlier > 1.5 and < 3 of the interquartile range. (*) outlier > 3 of the interquartile range. The median urine AQP1 and PLIN2 concentrations are significantly greater in RCC patients compared to the controls, and the patients with either bladder or prostate cancer (P<.01 for both, Kruskal-Wallis test). The insert shows ROC plots comparing the AQP1 or PLIN2 concentrations of the patients with RCC to that of all patients with bladder or prostate cancers. The area under the ROC curves is 1.00 each for Figures 2A and 2B.
DISCUSSION
The goal of this investigation was to investigate further the sensitivity and specificity of urine AQP1 and PLIN2 concentrations in clear cell and papillary subtypes of RCC, and to determine the overlap, or lack thereof, for biomarker concentrations between patients with RCC, and those with other imaged cancerous and noncancerous renal masses, and bladder or prostate cancer. The major finding was that median urine AQP1 and PLIN2 concentrations in patients with RCC were significantly greater than in patients with other (non-cancer) imaged renal masses (7 to 14- and nearly 4 to 9-fold, respectively), bladder cancer (14- and 9-fold, respectively) or prostate cancer (nearly 10- and 7-fold, respectively). AQP1 and PLIN2 urine concentrations for patients with other imaged renal masses, bladder or prostate cancer were not significantly different from those of the composite controls or age-related subgroups.
Both AQP1 and PLIN2 were sensitive and specific for RCC. There was no overlap in biomarker concentrations between patients with RCC and bladder or prostate cancer. Only one patient with an angiomyolipomas had a PLIN2 concentration that barely exceeded that of one patient with RCC (Figure 1B). Since there was no overlap of urine AQP1 or PLIN2 concentrations in RCC patients and those with other imaged renal masses, these biomarkers are 99–100% sensitive and specific for clear cell and papillary RCC compared to other imaged renal masses. Due to the lack of overlap in urine AQP1 or PLIN2 concentrations between patients with RCC and patients with bladder or prostate cancer, both biomarkers were 100% sensitive and specific for RCC. Clearly, these biomarkers of clear cell and papillary RCC are not confounded by bladder or prostate cancers.
The specificity of urine AQP1 or PLIN2, particularly compared with bladder and prostate cancer, adds to previous data on biomarker specificity.9 AQP1 and PLIN2 were not increased by common non-cancer kidney disease (diabetic nephropathy, glomerulonephritis, urine tract infection).9 The sensitivity and specificity of these biomarkers supports their analytical and clinical validity, and their potential application in screening and early diagnosis RCC. They may potentially also be used in the evaluation of hematuria and differential diagnosis of RCC versus bladder or prostate cancer. Eight of the 47 patients with RCC described in this study had metastases at the time of nephrectomy/partial nephrectomy. Of these 8 patients, 2 had primary tumors under 7 cm (3.2 and 4.2 cm) and 6 had primary tumors over 7cm. The concentrations of urine AQP1 and PLIN2 were reflective of tumor size, consistent with our previous study.10 Presently, of the 39 patients without noted metastases at the time of “curative” nephrectomy, 27 are in surveillance, 11 have been lost to follow-up, and one has died. Long-term follow up will be required to determine if either marker has the potential to predict local recurrences, distant metastasis, or survival differences.
Additionally, AQP1 or PLIN2 can be used to differentiate clear cell or papillary RCC from other imaged renal masses. Although the clear cell subtype of RCC represents about 68% of imaged renal masses, approximately 15–20% are benign.25,26 Imaging alone cannot reliably differentiate potentially malignant from benign renal masses. Since the common clinical approach to such masses is partial or total nephrectomy, this results in a 15–20% chance of partially or fully excising an entirely normal kidney, owing to the inability to identify a benign lesion based on imaging alone. Indeed, in one study, about 1/3 of patients with a benign tumor had underwent a radical nephrectomy.7 There debate on the value of renal mass biopsy ranges having a high diagnostic yield to the optimal use is yet to be determined.27,28 It should be noted that 2 separate studies of renal mass biopsy results found that about 80% were of diagnostic value while the remaining 20% were non-diagnostic.18,29 Nevertheless, the sensitivity and specificity of AQP1 and PLIN2 for RCC versus other renal masses supports their analytical and clinical validity, and their potential application in the differential diagnosis and further evaluation of incidentally identified renal masses.
Our two previous studies found that urine concentrations of AQP1 and PLIN2 are progressively greater with larger renal cell tumor size, and were decreased 83–97% after tumor removal.8,9 The ability of these biomarkers to identify patients with clear cell or papillary subtypes of RCC was not confounded by common non-cancerous kidney diseases, such as diabetic nephropathy and glomerulonephritis.9 These findings, together with the present results, therefore support the conclusion that AQP1 and PLIN2 are sensitive and specific biomarkers for clear cell and papillary RCC, and are not confounded by either common non-renal urinary tract malignancies, other imaged renal masses, or common non-cancer renal disease providing broad clinical validity to their use. This further supports the concept that AQP1 and PLIN2 are biomarkers with potential applicability for early and non-invasive detection and potential screening for RCC. In addition, based on these results, paradigms for differentially diagnosing patients with imaged renal masses may be developed for clinical use.
Expression and excretion of AQP1 or PLIN2 relates to the nephron segment origin of the various tumor types. Since the clear cell and papillary subtypes of renal cell carcinomas are of proximal tubular origin they express markers consistent with this nephron segment, specifically, AQP1 and PLIN2.8 The chromophobe and oncocytoma subtypes of RCC arises from the distal nephron segment and would not be expected to express proximal tubule markers, particularly AQP1.8 AQP1 and PLIN2 were not increased by chromophobes in this cohort, consistent with our previous observation.8 Similarly, urine biomarker concentrations in patients with an oncocytoma were not different from controls, consistent with our previous study.8 These results reinforce the concept of urine biomarkers reflecting the nephron segment of tumor origin.
Abnormal increases in any one of various serum laboratory values such as erythrocyte sedimentation rate, C-reactive protein, LDH, alkaline phosphatase, anemia, thrombocytosis, elevated neutrophils, and hypercalcemia usually carries a negative prognosis for RCC, however, these markers lack specificity for RCC in that they are elevated in a wide variety of other diseases.30,31 In contrast, the sensitivity and specificities of urine AQP1 and PLIN2 are excellent. Thus, increased urine concentrations of AQP1 and PLIN2 have the sensitivity and specificity to diagnose RCC, which is lacking with other biomarkers.
One limitation to implementing large scale investigations of AQP1 and PLIN2 for renal cancer screening is the cumbersome nature of the Western blot procedure. The arrival of a sensitive and specific ELISA for each protein will increase efficiency. Also, ELISAs, particularly for PLIN2 will enable monitoring the biomarkers in the plasma to detect metastases and possibly monitor treatment of metastatic disease as well as differentiating benign from malignant tumors. Another significant limitation is the inability of these biomarkers to identify patients with chromophobe tumors.
CONCLUSION
Importantly, this study found 99–100% sensitivity and specificity of urine AQP1 and PLIN2 concentrations to distinguish between clear cell and papillary subtypes of RCC versus other imaged renal masses, and prostate cancer or bladder cancer indicating clinical validity.32,33 These results support the conclusion that urine AQP1 and PLIN2 have high potential to be used as screening biomarkers for clear cell and papillary RCC and differentiating these patients from those with other urologic abnormalities. Additionally, AQP1 and PLIN2 may have clinical utility34 in differentiating potentially malignant from benign imaged renal masses.
Supplementary Material
Supplemental eFigure 1. Representative Western blots of urine AQP1 (1A and 1C) and PLIN2 (1B and 1D) of patients with bladder cancer (1A and 1B) or prostate cancer (1C and 1D). Each lane represents one patient with bladder (B) or prostate (P) cancer or RCC (R). RCC patients 61 and 63 were common to each gel.
Supplemental eTable 1. Laboratory and pathological data for prostate cancer patients.
Supplemental eTable 2. Pathological data for bladder cancer patients
Acknowledgments
Support for this study was provided by the Departments of Anesthesiology, Washington University in St. Louis School of Medicine, the Bear Cub Fund of Washington University, a grant from the Barnes-Jewish Hospital Foundation as a sub award of Washington University Institute of Clinical and Translational Science UL1 TR000448, and a grant from the National Cancer, Institute R01CA141521 each to JJM and the Department of Urology, Washington University in St. Louis School of Medicine.
The authors would like to thank Anesthesiology Clinical Coordinators Megan Kalin, B.A. and Karen Frey, C.C.P.R. and Urology Clinical Coordinators Alethia Paradis, M.S., Janise Webb, C.T.R., along with Goutham Vemana M.D., and Joseph Song B.S. for their help in the study.
ABREVIATIONS
- AQP1
aquaporin 1
- PLIN2
perilipin 2
- RCC
clear cell and papillary renal cell carcinoma
- ROC
receiver operating characteristic
Footnotes
Trial Registration: clinical trials.gov Identifier: NCT00851994 to collect biomaterials and archive samples for biomarker analysis.
Washington University has a patent application for the use of AQP1 and PLIN2 to diagnose kidney cancer.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pantuck AJ, Zisman A, Belldegrun AS. The changing natural history of renal cell carcinoma. J Urol. 2001 Nov;166(5):1611–1623. [PubMed] [Google Scholar]
- 2.Frank I, Blute ML, Cheville JC, Lohse CM, Weaver AL, Zincke H. Solid renal tumors: an analysis of pathological features related to tumor size. J Urol. 2003 Dec;170(6 Pt 1):2217–2220. doi: 10.1097/01.ju.0000095475.12515.5e. [DOI] [PubMed] [Google Scholar]
- 3.Hartman DS, Choyke PL, Hartman MS. From the RSNA refresher courses: a practical approach to the cystic renal mass. Radiographics: a review publication of the Radiological Society of North America, Inc. 2004 Oct;24(Suppl 1):S101–115. doi: 10.1148/rg.24si045515. [DOI] [PubMed] [Google Scholar]
- 4.Schachter LR, Cookson MS, Chang SS, et al. Second prize: frequency of benign renal cortical tumors and histologic subtypes based on size in a contemporary series: what to tell our patients. J Endourology. 2007 Aug;21(8):819–823. doi: 10.1089/end.2006.9937. [DOI] [PubMed] [Google Scholar]
- 5.Duchene DA, Lotan Y, Cadeddu JA, Sagalowsky AI, Koeneman KS. Histopathology of surgically managed renal tumors: analysis of a contemporary series. Urology. 2003 Nov;62(5):827–830. doi: 10.1016/s0090-4295(03)00658-7. [DOI] [PubMed] [Google Scholar]
- 6.Karakiewicz PI, Trinh QD, Bhojani N, et al. Renal cell carcinoma with nodal metastases in the absence of distant metastatic disease: prognostic indicators of disease-specific survival. European Urol. 2007 Jun;51(6):1616–1624. doi: 10.1016/j.eururo.2006.12.015. [DOI] [PubMed] [Google Scholar]
- 7.Steffens S, Junker K, Roos FC, et al. Small renal cell carcinomas--how dangerous are they really? Results of a large multicenter study. European journal of cancer. 2014 Mar;50(4):739–745. doi: 10.1016/j.ejca.2013.11.020. [DOI] [PubMed] [Google Scholar]
- 8.Morrissey JJ, London AN, Luo J, Kharasch ED. Urinary biomarkers for the early diagnosis of kidney cancer. Mayo Clin Proc. 2010 May;85(5):413–421. doi: 10.4065/mcp.2009.0709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morrissey JJ, Kharasch ED. The specificity of urinary aquaporin 1 and perilipin 2 to screen for renal cell carcinoma. J Urol. 2013 May;189(5):1913–1920. doi: 10.1016/j.juro.2012.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morrissey JJ, Mobley J, Song J, et al. Urinary concentrations of aquaporin-1 and perilipin-2 in patients with renal cell carcinoma correlate with tumor size and stage but not grade. Urology. 2014 Jan;83(1):256 e259–214. doi: 10.1016/j.urology.2013.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akdogan B, Gudeloglu A, Inci K, Gunay LM, Koni A, Ozen H. Prevalence and predictors of benign lesions in renal masses smaller than 7 cm presumed to be renal cell carcinoma. Clinical genitourinary cancer. 2012 Jun;10(2):121–125. doi: 10.1016/j.clgc.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 12.Halverson SJ, Kunju LP, Bhalla R, et al. Accuracy of determining small renal mass management with risk stratified biopsies: confirmation by final pathology. J Urol. 2013 Feb;189(2):441–446. doi: 10.1016/j.juro.2012.09.032. [DOI] [PubMed] [Google Scholar]
- 13.Jeon HG, Lee SR, Kim KH, et al. Benign lesions after partial nephrectomy for presumed renal cell carcinoma in masses 4 cm or less: prevalence and predictors in Korean patients. Urology. 2010 Sep;76(3):574–579. doi: 10.1016/j.urology.2009.11.082. [DOI] [PubMed] [Google Scholar]
- 14.Khan AA, Shergill IS, Quereshi S, Arya M, Vandal MT, Gujral SS. Percutaneous needle biopsy for indeterminate renal masses: a national survey of UK consultant urologists. BMC Urol. 2007;7:10. doi: 10.1186/1471-2490-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kutikov A, Fossett LK, Ramchandani P, et al. Incidence of benign pathologic findings at partial nephrectomy for solitary renal mass presumed to be renal cell carcinoma on preoperative imaging. Urology. 2006 Oct;68(4):737–740. doi: 10.1016/j.urology.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 16.Lane BR, Samplaski MK, Herts BR, Zhou M, Novick AC, Campbell SC. Renal mass biopsy--a renaissance? J Urol. 2008 Jan;179(1):20–27. doi: 10.1016/j.juro.2007.08.124. [DOI] [PubMed] [Google Scholar]
- 17.Lim A, O’Neil B, Heilbrun ME, Dechet C, Lowrance WT. The contemporary role of renal mass biopsy in the management of small renal tumors. Frontiers in Oncology. 2012;2:106. doi: 10.3389/fonc.2012.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Menogue SR, O’Brien BA, Brown AL, Cohen RJ. Percutaneous core biopsy of small renal mass lesions: a diagnostic tool to better stratify patients for surgical intervention. BJU Inter. 2013 Apr;111(4 Pt B):E146–151. doi: 10.1111/j.1464-410X.2012.11384.x. [DOI] [PubMed] [Google Scholar]
- 19.Schlomer B, Figenshau RS, Yan Y, Venkatesh R, Bhayani SB. Pathological features of renal neoplasms classified by size and symptomatology. J Urol. 2006 Oct;176(4 Pt 1):1317–1320. doi: 10.1016/j.juro.2006.06.005. discussion 1320. [DOI] [PubMed] [Google Scholar]
- 20.Siemer S, Hack M, Lehmann J, Becker F, Stockle M. Outcome of renal tumors in young adults. J Urol. 2006 Apr;175(4):1240–1243. doi: 10.1016/S0022-5347(05)00696-8. discussion 1243–1244. [DOI] [PubMed] [Google Scholar]
- 21.Wang R, Wolf JS, Jr, Wood DP, Jr, Higgins EJ, Hafez KS. Accuracy of percutaneous core biopsy in management of small renal masses. Urology. 2009 Mar;73(3):586–590. doi: 10.1016/j.urology.2008.08.519. discussion 590–581. [DOI] [PubMed] [Google Scholar]
- 22.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999 Mar 16;130(6):461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 23.Kutikov A, Uzzo RG. The R.E.N.A.L. nephrometry score: a comprehensive standardized system for quantitating renal tumor size, location and depth. J Urol. 2009 Sep;182(3):844–853. doi: 10.1016/j.juro.2009.05.035. [DOI] [PubMed] [Google Scholar]
- 24.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 25.Corcoran AT, Russo P, Lowrance WT, et al. A review of contemporary data on surgically resected renal masses--benign or malignant? Urology. 2013 Apr;81(4):707–713. doi: 10.1016/j.urology.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 26.Umbreit EC, Shimko MS, Childs MA, et al. Metastatic potential of a renal mass according to original tumour size at presentation. BJU Intern. 2012 Jan;109(2):190–194. doi: 10.1111/j.1464-410X.2011.10184.x. discussion 194. [DOI] [PubMed] [Google Scholar]
- 27.Caoili EM, Davenport MS. Role of Percutaneous Needle Biopsy for Renal Masses. Seminars in interventional radiology. 2014 Mar;31(1):20–26. doi: 10.1055/s-0033-1363839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leppert JT, Hanley J, Wagner TH, et al. Utilization of renal mass biopsy in patients with renal cell carcinoma. Urology. 2014 Apr;83(4):774–780. doi: 10.1016/j.urology.2013.10.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leveridge MJ, Finelli A, Kachura JR, et al. Outcomes of small renal mass needle core biopsy, nondiagnostic percutaneous biopsy, and the role of repeat biopsy. European Urol. 2011 Sep;60(3):578–584. doi: 10.1016/j.eururo.2011.06.021. [DOI] [PubMed] [Google Scholar]
- 30.Lane BR, Kattan MW. Prognostic models and algorithms in renal cell carcinoma. The Urologic clinics of North America. 2008 Nov;35(4):613–625. vii. doi: 10.1016/j.ucl.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 31.Motzer RJ, Mazumdar M, Bacik J, Berg W, Amsterdam A, Ferrara J. Survival and prognostic stratification of 670 patients with advanced renal cell carcinoma. Journal Clinical Oncology. 1999 Aug;17(8):2530–2540. doi: 10.1200/JCO.1999.17.8.2530. [DOI] [PubMed] [Google Scholar]
- 32.Kerr KF, Wang Z, Janes H, McClelland RL, Psaty BM, Pepe MS. Net reclassification indices for evaluating risk prediction instruments: a critical review. Epidemiology. 2014 Jan;25(1):114–121. doi: 10.1097/EDE.0000000000000018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pepe MS, Feng Z, Janes H, Bossuyt PM, Potter JD. Pivotal evaluation of the accuracy of a biomarker used for classification or prediction: standards for study design. J Natl Cancer Inst. 2008 Oct 15;100(20):1432–1438. doi: 10.1093/jnci/djn326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parkinson DR, McCormack RT, Keating SM, et al. Evidence of clinical utility: an unmet need in molecular diagnostics for patients with cancer. Clinical Cancer Res. 2014 Mar 15;20(6):1428–1444. doi: 10.1158/1078-0432.CCR-13-2961. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental eFigure 1. Representative Western blots of urine AQP1 (1A and 1C) and PLIN2 (1B and 1D) of patients with bladder cancer (1A and 1B) or prostate cancer (1C and 1D). Each lane represents one patient with bladder (B) or prostate (P) cancer or RCC (R). RCC patients 61 and 63 were common to each gel.
Supplemental eTable 1. Laboratory and pathological data for prostate cancer patients.
Supplemental eTable 2. Pathological data for bladder cancer patients