Abstract
Background
Radiofrequency ablation (RFA) has become an accepted form of endoscopic treatment for Barrett’s esophagus (BE), yet reported response rates are variable. There are no accepted quality measures for performing RFA, and provider-level characteristics may influence RFA outcomes.
Objective
To determine whether endoscopist RFA volume is associated with rates of complete remission of intestinal metaplasia (CRIM) after RFA in patients with BE.
Design
Retrospective analysis of longitudinal data.
Setting
Three tertiary-care medical centers.
Patients
Patients with BE treated with RFA.
Intervention
RFA.
Main Outcome Measurements
For each endoscopist, we recorded RFA volume, defined as the number of unique patients treated as well as corresponding CRIM rates. We calculated a Spearman correlation coefficient relating these 2 measures.
Results
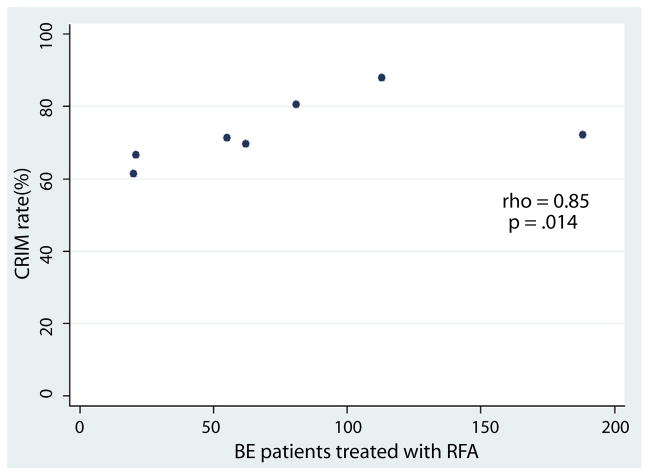
We identified 417 patients with BE treated with RFA who had at least 1 post-RFA endoscopy with biopsies. A total of 73% of the cases had pretreatment histology of high-grade dysplasia or adenocarcinoma. The procedures were performed by 7 endoscopists, who had a median RFA volume of 62 patients (range 20–188). The overall CRIM rate was 75.3% (provider range 62%–88%). The correlation between endoscopist RFA volume and CRIM rate was strong and significant (rho = 0.85; P = .014). In multivariable analysis, higher RFA volume was significantly associated with CRIM (P for trend .04).
Limitations
Referral setting may limit generalizability. Limited number of endoscopists analyzed.
Conclusion
Endoscopist RFA volume correlates with rates of successful BE eradication. Further studies are required to confirm these findings and to determine whether RFA volume is a valid predictor of treatment outcomes in BE.
Barrett’s esophagus (BE) is the premalignant lesion of esophageal adenocarcinoma (EAC), histologically characterized by the metaplastic conversion of the cells of the distal esophagus from normal squamous epithelium to intestinal-type columnar epithelium. Alarmingly, the incidence of EAC has risen dramatically over the past several decades in Western countries,1,2 and the prognosis associated with esophageal cancer continues to be poor, with 17% 5-year survival in the United States.3 As a result, endoscopic surveillance is recommended for patients with BE in an attempt to detect neoplastic changes at an early juncture, and endoscopic therapy is subsequently performed in many of those who progress to dysplasia or cancer.
Radiofrequency ablation (RFA) is an efficacious treatment for BE with dysplasia, with excellent short-term outcomes.4 In fact, the most recent American Gastroenterological Association position statement advocates RFA as part of endoscopic therapy as the preferred management strategy for BE with high-grade dysplasia.5 The use of RFA for BE is expanding rapidly, as evidenced by data from RFA registries in the United States and the United Kingdom,6,7 and treatment with RFA has spread beyond specialized academic centers into the community setting.8
Despite this, there is a paucity of literature to date on quality and endoscopy in BE. The few studies on quality in endoscopic surveillance of BE have found wide variation in practice patterns. For example, there is poor adherence to recommended biopsy guidelines in both community and academic settings.9,10
In the quality literature examining other endoscopic procedures, especially screening colonoscopy, associations between provider-level characteristics and outcomes have been examined extensively. Most prominently, endoscopist adenoma detection rates have been recognized as an independent predictor of interval colorectal cancer after screening colonoscopy.11 Other studies have identified provider-level factors that influence adenoma detection rate, including endoscopist specialty and colonoscopy volume.12–17 Similar relationships between higher procedural volumes and improved outcomes exist for other endoscopic procedures such as ERCP18–22 as well as for surgeries such as esophagectomy.23,24 However, no studies to date have evaluated provider-level characteristics and RFA outcomes in BE.
In light of established associations between procedure volume and outcomes in various other settings, we sought to determine whether there is an association between endoscopist RFA volume and the rate of success in achieving complete remission of intestinal metaplasia in patients with BE.
METHODS
We performed a multicenter, retrospective review of patients with BE who had undergone RFA. By using a combination of electronic medical record review and a query of electronic endoscopy reporting systems (ProVation MD; ProVation Medical, Minneapolis, Minn) for Current Procedural Terminology codes 43228 and 43258, we identified 601 patients with BE who underwent RFA between January 1, 2006 and June 30, 2012 at 3 tertiary-care referral centers (Columbia University, University of Pennsylvania, and Mayo Clinic-Rochester). We collected information regarding patient characteristics including age, sex, baseline BE length, pretreatment histology, dates of first and last RFA, total number of RFAs, RFA treatment type (circumferential, focal, or both), and date of the endoscopy during which biopsy specimens demonstrated complete remission of intestinal metaplasia (CRIM), if such histology was achieved. There was no protocol for a stopping point for RFA treatment; the decision of when to perform biopsies after initiating RFA therapy was at the discretion of the individual endoscopist. We defined CRIM as the absence of intestinal metaplasia or worse pathology from all esophageal or gastroesophageal junction biopsy specimens on the first post-RFA endoscopy. The first post-RFA endoscopy represented either (1) endoscopy with no visible BE or (2) endoscopy with residual visible suspected BE. Biopsy specimens of these tissues with suspected residual BE were taken instead of retreatment with RFA (we termed these incompletely treated, because these patients were successfully retreated based on biopsy findings or were ultimately treatment failures). Because this was a retrospective analysis, there was no standardized biopsy protocol. In general, the practice of all the endoscopists included in the study was to perform random, 4-quadrant biopsies from the gastroesophageal junction and every 1 to 2 cm along the original BE length. This study was approved by the institutional review boards of Columbia University, University of Pennsylvania, and the Mayo Clinic-Rochester.
We chose to restrict analyses to providers who had performed RFA on at least 10 unique patients during the study period because we believed that CRIM rates calculated from data on fewer than 10 patients lacked sufficient precision to contribute meaningfully to the analyses. Patients were excluded from analysis if they had RFA performed by more than 1 endoscopist (n = 5) or by an endoscopist who performed RFA on fewer than 10 patients within the study period (n = 19); if their pretreatment histology was without intestinal metaplasia, dysplasia, or EAC (n = 27); if their pre-RFA biopsy results were unavailable for review (n = 10); or if their post-RFA biopsies were performed after the conclusion of the study period (n = 1). For each endoscopist, we assigned an anonymous identifier and recorded annual endoscopic volume (any procedure type) and years since completing fellowship.
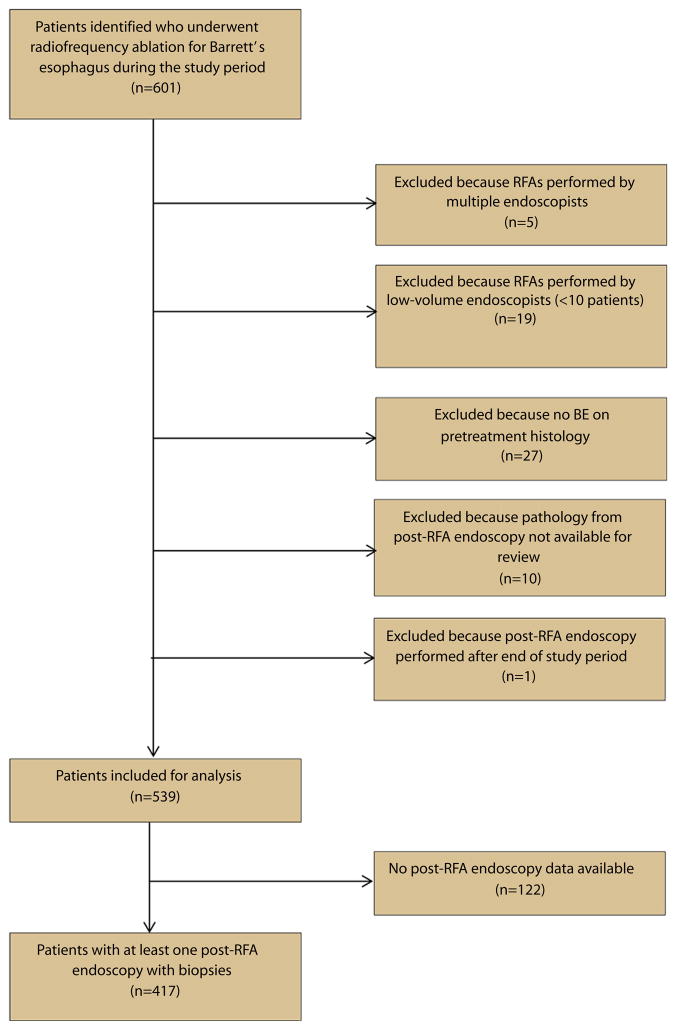
Based on the remaining 539 patients, for each endoscopist we determined RFA volume, defined as the number of unique patients on which each endoscopist performed RFA. In order to account for variability in ablation practice patterns between endoscopists, we decided to analyze the total number of RFA procedures as a secondary measure of RFA experience. We subsequently calculated each endoscopist’s CRIM rate, which equaled the proportion of unique patients treated who achieved CRIM. Patients for whom we lacked data from post-RFA endoscopies (n = 122) were not included in this calculation (Fig. 1).
Figure 1.
Flow diagram of patients reviewed for analysis of association between complete remission of intestinal metaplasia rates and endoscopist radiofrequency ablation volume. RFA, radiofrequency ablation; BE, Barrett’s esophagus.
For descriptive purposes, we calculated proportions for categorical variables. For continuous variables, we reported medians with interquartile ranges (IQRs) or, for normally distributed data, means with standard deviations. We then plotted endoscopist RFA volume by CRIM rate. We calculated a Spearman coefficient to assess the relationship between these two variables. Next, we repeated these analyses comparing CRIM rate with number of RFA procedures, our secondary measure of RFA experience. Last, we classified the endoscopists into approximate tertiles of RFA volume and performed multivariable logistic regression analyses to assess whether RFA volume was associated with CRIM after adjustment for age, sex, BE length, pre-treatment histology, RFA treatment type, and year. We defined statistical significance as P < .05.
RESULTS
We identified 12 endoscopists who had performed RFA for BE during the study period, of whom 7 had performed procedures on more than 10 patients within that period. In total, those endoscopists performed RFA on 539 patients during that period (Table 1). The patients were overwhelmingly male (83%), and the mean age was 65 years. Most had either high-grade dysplasia (60%) or adenocarcinoma (13%) as the highest histology before initiation of RFA therapy. The patients underwent a median of 2 RFA procedures (IQR 1–3).
TABLE 1.
Characteristics of patients with BE treated with RFA
| Total | 539 |
| Male sex, % | 83 |
| Age, mean (± SD), y | 65 ± 11 |
| Baseline BE length, cm | 4 (IQR 1–6) |
| Baseline histology, no. (%) | |
| IM, no dysplasia | 65 (12) |
| LGD (or indeterminate) | 86 (16) |
| HGD | 320 (60) |
| EAC | 68 (13) |
| Total no. RFA treatments | 2 (IQR 1–3) |
| RFA device used, no. (%) | |
| Circumferential only | 73 (14) |
| Focal only | 272 (50) |
| Both | 193 (36) |
| Year of first RFA, no. (%) | |
| 2006 | 15 (3) |
| 2007 | 73 (14) |
| 2008 | 136 (25) |
| 2009 | 119 (22) |
| 2010 | 124 (23) |
| 2011 | 72 (13) |
BE, Barrett’s esophagus; RFA, radiofrequency ablation; SD, standard deviation; IQR, interquartile range; IM, intestinal metaplasia; LGD, low-grade dysplasia; HGD, high-grade dysplasia; EAC, esophageal adenocarcinoma.
Of those 539 patients, 417 had at least 1 post-RFA EGD with biopsies. The overall rate of CRIM was 75% (provider range 62%–88%) (Table 2). There was a wide range in the number of patients on whom each endoscopist performed RFA, from 20 to 188 patients. The same was true of the number of RFA procedures per endoscopist, ranging from 54 to 470. Persistent intestinal metaplasia was found at the gastroesophageal junction only (no visible BE endoscopically) in 33 patients (34.4% of persistent intestinal metaplasia, 8.0% of all patients), and there was residual visible BE (incompletely treated) in 63 patients (65.6% of persistent intestinal metaplasia, 15.2% of all patients).
TABLE 2.
Individual endoscopist RFA volumes (no. of unique patients treated), procedure volumes (no. of RFA procedures performed), and rates of CRIM
| Endoscopist | Patient volume | Procedure volume | CRIM rate (%) |
|---|---|---|---|
| A | 20 | 54 | 61.5 |
| B | 21 | 61 | 66.7 |
| C | 55 | 151 | 71.4 |
| D | 62 | 118 | 69.6 |
| E | 81 | 196 | 80.6 |
| F | 113 | 251 | 88.0 |
| G | 188 | 470 | 72.2 |
RFA, Radiofrequency ablation; CRIM, complete remission of intestinal metaplasia.
Endoscopist RFA patient volume correlated strongly with CRIM rate (rho = 0.85; P = .014) (Fig. 2). Similarly, the total number of RFA procedures performed by each endoscopist also strongly correlated with the CRIM rate (rho = 0.89; P = .007). In multivariable logistic regression analysis, tertile of RFA volume was significantly associated with CRIM (P for trend .04) (Table 3). Older patient age (per year, odds ratio [OR] 0.95; 95% confidence interval [CI], 0.93–0.98) and longer length of BE (per cm, OR 0.80; 95% CI, 0.73–0.87) were both inversely associated with CRIM.
Figure 2.
Significant positive correlation between the total number of patients treated by radiofrequency ablation by each endoscopist and the rate of complete remission of intestinal metaplasia. CRIM, complete remission of intestinal metaplasia; BE, Barrett’s esophagus; RFA, radiofrequency ablation.
TABLE 3.
Multivariable logistic regression analysis of association between tertile of endoscopist RFA volume and CRIM rate
| Tertile | Odds ratio* | 95% CI |
|---|---|---|
| Lowest | 1.00 | Referent |
| Middle | 1.54 | 0.58–4.07 |
| Highest | 2.34 | 0.95–5.77 |
RFA, Radiofrequency ablation; CRIM, complete remission of intestinal metaplasia; CI, confidence interval.
P for trend = .04.
Adjusted for age, sex, Barrett’s esophagus length, pre-RFA histology, year, and RFA treatment type.
Because the threshold for deciding whether RFA is or is not working for a particular patient may differ markedly between endoscopists, we repeated the analyses excluding all of the incompletely treated patients. There remained a positive but nonsignificant correlation between endoscopist RFA volume and CRIM rate (rho = .52; P = .23). We subsequently analyzed incomplete RFA rates by endoscopist and found a nonsignificant inverse association between RFA volume and incomplete RFA rates (rho = −0.57; P = .18).
We also assessed whether overall endoscopist experience could be associated with RFA treatment outcomes. We found no correlation between CRIM rate and yearly endoscopic volume (P = .76) or with years since completing gastroenterology fellowship (P = .59).
DISCUSSION
In this study of patients with BE treated with RFA at 3 tertiary-care referral centers, we found that greater endoscopist RFA volume was associated with increased rates of achieving complete remission of intestinal metaplasia. Both the number of patients treated with RFA, our primary measure of endoscopist experience, and the total number of RFA procedures performed by each endoscopist correlated strongly and significantly with CRIM rate. Tertile of endoscopist volume also was associated with CRIM after adjusting for age, sex, BE length, pre-RFA histology, and year. We found a relatively broad range of CRIM rates by provider, indicative of significant provider-level variability with regard to outcomes of RFA. Older age and greater BE length were inversely associated with CRIM, consistent with results of prior studies.6,25,26 Additionally, the overall CRIM rate of 75% among the patients we studied is similar to previously reported rates.4,6,25,26
To our knowledge, this is the first report of a correlation between endoscopist experience with RFA and outcomes in the ablation of BE. Prior studies have reported correlations between provider volume and outcomes for endoscopic procedures spanning the range of technical difficulty from colonoscopy12–17 to ERCP.18,19 Although findings in colonoscopy studies are heterogeneous, there is a common trend of an association of experience with markers of colonoscopy quality, such as adenoma detection rate or procedure completion. In studies of ERCP, data on volume and outcomes are more homogenous, showing correlations between ERCP volume and higher procedural success rates and fewer adverse events.18,21,22 These studies linking volume and outcomes have led to changes in standards for endoscopy practice, such as minimum acceptable adenoma detection rates27 and defining the difficulty of a planned ERCP to identify procedures that should be referred to more experienced endoscopists.28 The literature in surgery also supports a volume-outcome relationship, leading to similar standards. For esophagectomy, centers performing at least 20 resections per year had markedly reduced operative mortality rates, leading to a recommendation that only higher-volume centers perform esophagectomy.24
It is unclear whether the endoscopist CRIM rate is an appropriate metric of RFA quality in BE, because the CRIM rate for the provider with the highest volume was modestly lower than that for other high-volume endoscopists. Patients treated by endoscopist G were not older and did not have longer BE segments compared with patients treated by other endoscopists. There may have been patient-level differences, such as greater number of comorbidities, resulting in a more conservative treatment strategy in which dysplasia eradication might have been the goal of care. However, this and other patient-related factors that may have affected endoscopic management decisions were not accounted for in this retrospective analysis. It is also plausible that there is a CRIM plateau around 80% (a figure consistent with other published data4,6,8) and that the 88% data point for endoscopist F is itself an outlier. Future studies with data from larger numbers of endoscopists can help shed light on these questions.
There may be other factors to explain variable CRIM rates. If one endoscopist systematically performed biopsies less in the treated esophagus, then the CRIM rates could be higher simply because of less sampling. In this case, we might expect to see variable rates of detection of subsquamous intestinal metaplasia after RFA. However, only 2 patients had subsquamous intestinal metaplasia on post-RFA esophageal biopsies. Therefore, we do not believe that between-endoscopist differences in sampling intensity of the neosquamous esophagus explain the observed differences in CRIM rates. Fellow involvement in cases also may have impacted CRIM rates, but this was not captured in our analyses.
The current study has several strengths. We analyzed data from a large number of BE patients who underwent treatment with RFA at 1 of 3 major referral centers. The large majority of patients included in the analyses had high-grade dysplasia or intramucosal adenocarcinoma, reflecting the current recommended practice for this patient population. We examined data for specific endoscopists, which provided a much more informative picture of outcomes related to procedure volume than would analyses of hospital or center volume.
Our study also has limitations to be considered. Despite examining all RFAs performed at 3 major referral centers, we identified only 7 endoscopists who performed RFA for BE on more than 10 patients during the nearly 6-year study period, potentially limiting generalizability of the findings. This study was a retrospective review rather than a prospective trial with a uniform treatment and follow-up protocol. Therefore, although similar standards were practiced by the endoscopists at all 3 centers, slight variations in management style may have influenced CRIM rates as measured in this study. Last, in our study, the number of patients treated by each endoscopist varied greatly (20–188), limiting our ability to identify a learning curve for RFA and a threshold above which CRIM rates may plateau.
Additional research is warranted to evaluate associations between provider-level characteristics and outcomes of RFA. Analysis of larger numbers of endoscopists is needed to validate RFA volume as a predictor of CRIM. Whether CRIM rate is an appropriate metric for quality in RFA is open for debate, because durable remission of intestinal metaplasia and ultimately the prevention of EAC are the most meaningful outcomes. Recent data examining the durability of CRIM after RFA have found differing rates of CRIM maintenance,26,29,30 suggesting that provider-level differences may influence not only response but recurrence as well.
In this study of patients treated with RFA for BE, we found that both endoscopist patient volume and endoscopist case volume were associated with the rate of CRIM. Nonetheless, further research involving larger numbers of endoscopists is required to confirm our findings, perhaps ultimately leading to the creation of quality metrics for RFA.
Take-home Message.
Increased endoscopist radiofrequency ablation volume correlates with response rates for the eradication of Barrett’s esophagus.
Provider-level factors likely play an important role in outcomes of ablation for Barrett’s esophagus.
Abbreviations
- BE
Barrett’s esophagus
- CRIM
complete remission of intestinal metaplasia
- EAC
esophageal adenocarcinoma
- RFA
radiofrequency ablation
Footnotes
DISCLOSURE: This study was supported in part by the National Institutes of Health U54 award (C.J.L., J.M.P., G.G.G., G.W.F., M.D., P.G.I., L.L., K.K.W., J.A.A.; U54 CA163004) and a Career Development Award (J.A.A.; K07 CA132892) C. Lightdale is on the advisory board of CDx Diagnostics, received product royalties from Cook Medical, and is a consultant for Covidien. J. Poneros is a consultant for Boston Scientific. G. Ginsberg is a consultant for Olympus and CDx. P. Iyer received research funding from Takeda and is a consultant for Olympus. K. Wang received research support from Covidien, Pinnacle Pharmaceuticals, and CSA Medical. J. Abrams Received research support from Covidien, C2 Therapeutics, and Trio Medicines. No other financial relationships relevant to this publication were disclosed.
References
- 1.Abrams JA, Sharaiha RZ, Gonsalves L, et al. Dating the rise of esophageal adenocarcinoma: analysis of Connecticut Tumor Registry data, 1940–2007. Cancer Epidemiol Biomarkers Prev. 2011;20:183–6. doi: 10.1158/1055-9965.EPI-10-0802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown LM, Devesa SS, Chow WH. Incidence of adenocarcinoma of the esophagus among white Americans by sex, stage, and age. J Natl Cancer Inst. 2008;100:1184–7. doi: 10.1093/jnci/djn211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 4.Shaheen NJ, Sharma P, Overholt BF, et al. Radiofrequency ablation in Barrett’s esophagus with dysplasia. N Engl J Med. 2009;360:2277–88. doi: 10.1056/NEJMoa0808145. [DOI] [PubMed] [Google Scholar]
- 5.Spechler SJ, Sharma P, Souza RF, et al. American Gastroenterological Association medical position statement on the management of Barrett’s esophagus. Gastroenterology. 2011;140:1084–91. doi: 10.1053/j.gastro.2011.01.030. [DOI] [PubMed] [Google Scholar]
- 6.Haidry RJ, Dunn JM, Butt MA, et al. Radiofrequency ablation (RFA) and endoscopic mucosal resection for dysplastic Barrett’s esophagus and early esophageal adenocarcinoma: outcomes of UK National Halo RFA Registry. Gastroenterology. 2013;145:87–95. doi: 10.1053/j.gastro.2013.03.045. [DOI] [PubMed] [Google Scholar]
- 7.Ganz RA, Overholt BF, Sharma VK, et al. Circumferential ablation of Barrett’s esophagus that contains high-grade dysplasia: a U.S. Multicenter Registry. Gastrointest Endosc. 2008;68:35–40. doi: 10.1016/j.gie.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 8.Lyday WD, Corbett FS, Kuperman DA, et al. Radiofrequency ablation of Barrett’s esophagus: outcomes of 429 patients from a multicenter community practice registry. Endoscopy. 2010;42:272–8. doi: 10.1055/s-0029-1243883. [DOI] [PubMed] [Google Scholar]
- 9.Abrams JA, Kapel RC, Lindberg GM, et al. Adherence to biopsy guidelines for Barrett’s esophagus surveillance in the community setting in the United States. Clin Gastroenterol Hepatol. 2009;7:736–42. doi: 10.1016/j.cgh.2008.12.027. quiz 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ofman JJ, Shaheen NJ, Desai AA, et al. The quality of care in Barrett’s esophagus: endoscopist and pathologist practices. Am J Gastroenterol. 2001;96:876–81. doi: 10.1111/j.1572-0241.2001.03637.x. [DOI] [PubMed] [Google Scholar]
- 11.Kaminski MF, Regula J, Kraszewska E, et al. Quality indicators for colonoscopy and the risk of interval cancer. N Engl J Med. 2010;362:1795–803. doi: 10.1056/NEJMoa0907667. [DOI] [PubMed] [Google Scholar]
- 12.Adler A, Wegscheider K, Lieberman D, et al. Factors determining the quality of screening colonoscopy: a prospective study on adenoma detection rates, from 12,134 examinations (Berlin colonoscopy project 3, BECOP-3) Gut. 2013;62:236–41. doi: 10.1136/gutjnl-2011-300167. [DOI] [PubMed] [Google Scholar]
- 13.Bretagne JF, Hamonic S, Piette C, et al. Variations between endoscopists in rates of detection of colorectal neoplasia and their impact on a regional screening program based on colonoscopy after fecal occult blood testing. Gastrointest Endosc. 2010;71:335–41. doi: 10.1016/j.gie.2009.08.032. [DOI] [PubMed] [Google Scholar]
- 14.Ko CW, Dominitz JA, Green P, et al. Specialty differences in polyp detection, removal, and biopsy during colonoscopy. Am J Med. 2010;123:528–35. doi: 10.1016/j.amjmed.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 15.Rabeneck L, Paszat LF, Saskin R. Endoscopist specialty is associated with incident colorectal cancer after a negative colonoscopy. Clin Gastroenterol Hepatol. 2010;8:275–9. doi: 10.1016/j.cgh.2009.10.022. [DOI] [PubMed] [Google Scholar]
- 16.Singh A, Kuo YF, Riall TS, et al. Predictors of colorectal cancer following a negative colonoscopy in the Medicare population. Dig Dis Sci. 2011;56:3122–8. doi: 10.1007/s10620-011-1788-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wexner SD, Garbus JE, Singh JJ. A prospective analysis of 13,580 colonoscopies. Reevaluation of credentialing guidelines. Surg Endosc. 2001;15:251–61. doi: 10.1007/s004640080147. [DOI] [PubMed] [Google Scholar]
- 18.Jowell PS, Baillie J, Branch MS, et al. Quantitative assessment of procedural competence: a prospective study of training in endoscopic retrograde cholangiopancreatography. Ann Intern Med. 1996;125:983–9. doi: 10.7326/0003-4819-125-12-199612150-00009. [DOI] [PubMed] [Google Scholar]
- 19.Testoni PA, Mariani A, Giussani A, et al. Risk factors for post-ERCP pancreatitis in high- and low-volume centers and among expert and non-expert operators: a prospective multicenter study. Am J Gastroenterol. 2010;105:1753–61. doi: 10.1038/ajg.2010.136. [DOI] [PubMed] [Google Scholar]
- 20.Rabenstein T, Schneider HT, Nicklas M, et al. Impact of skill and experience of the endoscopist on the outcome of endoscopic sphincterotomy techniques. Gastrointest Endosc. 1999;50:628–36. doi: 10.1016/s0016-5107(99)80010-8. [DOI] [PubMed] [Google Scholar]
- 21.Kapral C, Duller C, Wewalka F, et al. Case volume and outcome of endoscopic retrograde cholangiopancreatography: results of a nationwide Austrian benchmarking project. Endoscopy. 2008;40:625–30. doi: 10.1055/s-2008-1077461. [DOI] [PubMed] [Google Scholar]
- 22.Freeman ML, Nelson DB, Sherman S, et al. Complications of endoscopic biliary sphincterotomy. N Engl J Med. 1996;335:909–19. doi: 10.1056/NEJM199609263351301. [DOI] [PubMed] [Google Scholar]
- 23.Birkmeyer JD, Stukel TA, Siewers AE, et al. Surgeon volume and operative mortality in the United States. N Engl J Med. 2003;349:2117–27. doi: 10.1056/NEJMsa035205. [DOI] [PubMed] [Google Scholar]
- 24.Metzger R, Bollschweiler E, Vallbohmer D, et al. High volume centers for esophagectomy: What is the number needed to achieve low postoperative mortality? Dis Esophagus. 2004;17:310–4. doi: 10.1111/j.1442-2050.2004.00431.x. [DOI] [PubMed] [Google Scholar]
- 25.Korst RJ, Santana-Joseph S, Rutledge JR, et al. Effect of hiatal hernia size and columnar segment length on the success of radiofrequency ablation for Barrett’s esophagus: a single-center, phase II clinical trial. J Thorac Cardiovasc Surg. 2011;142:1168–73. doi: 10.1016/j.jtcvs.2011.07.037. [DOI] [PubMed] [Google Scholar]
- 26.Gupta M, Iyer PG, Lutzke L, et al. Recurrence of esophageal intestinal metaplasia after endoscopic mucosal resection and radiofrequency ablation of Barrett’s esophagus: results from a US multicenter consortium. Gastroenterology. 2013;145:79–86. doi: 10.1053/j.gastro.2013.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cohen J, Safdi MA, Deal SE, et al. Quality indicators for esophagogas-troduodenoscopy. Gastrointest Endosc. 2006;63:S10–5. doi: 10.1016/j.gie.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 28.Baron TH, Petersen BT, Mergener K, et al. Quality indicators for endoscopic retrograde cholangiopancreatography. Gastrointest Endosc. 2006;63:S29–34. doi: 10.1016/j.gie.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 29.Orman ES, Kim HP, Bulsiewicz WJ, et al. Intestinal metaplasia recurs infrequently in patients successfully treated for Barrett’s esophagus with radiofrequency ablation. Am J Gastroenterol. 2013;108:187–95. doi: 10.1038/ajg.2012.413. quiz 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Phoa KN, Pouw RE, van Vilsteren FGI, et al. Remission of Barrett’s esophagus with early neoplasia 5 years after radiofrequency ablation with endoscopic resection: a Netherlands cohort study. Gastroenterology. 2013;145:96–104. doi: 10.1053/j.gastro.2013.03.046. [DOI] [PubMed] [Google Scholar]