Abstract
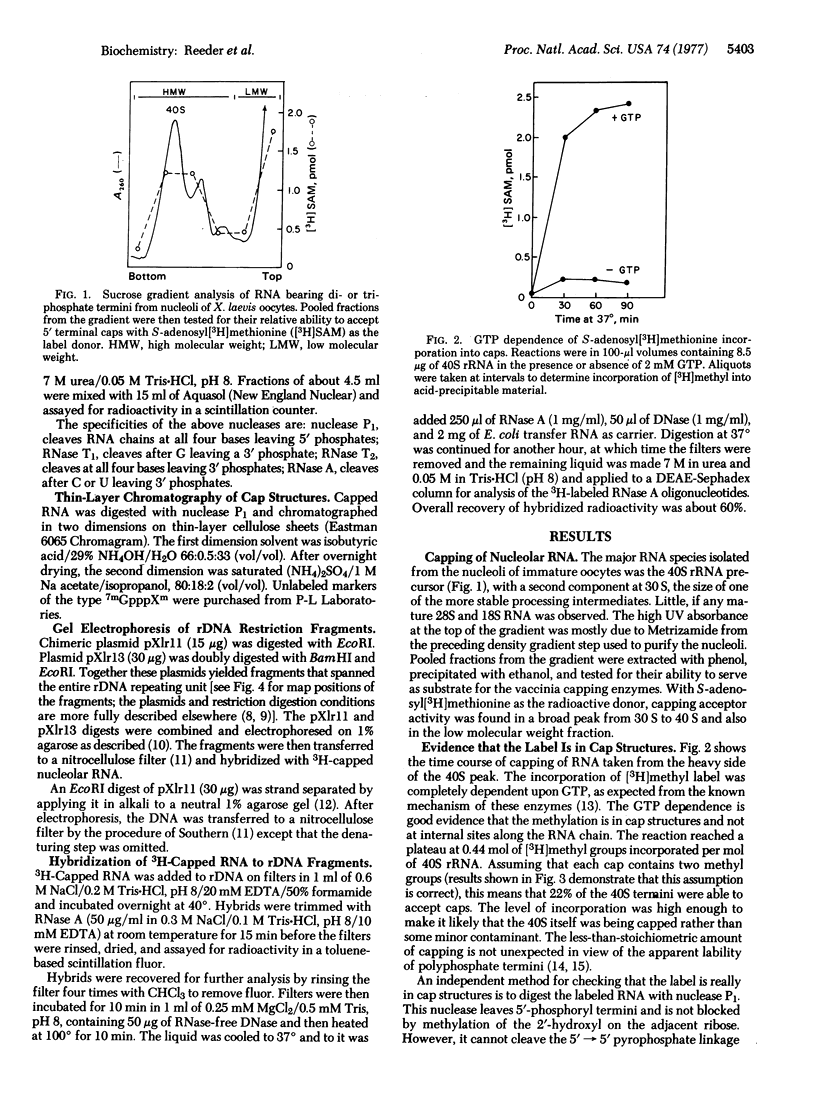
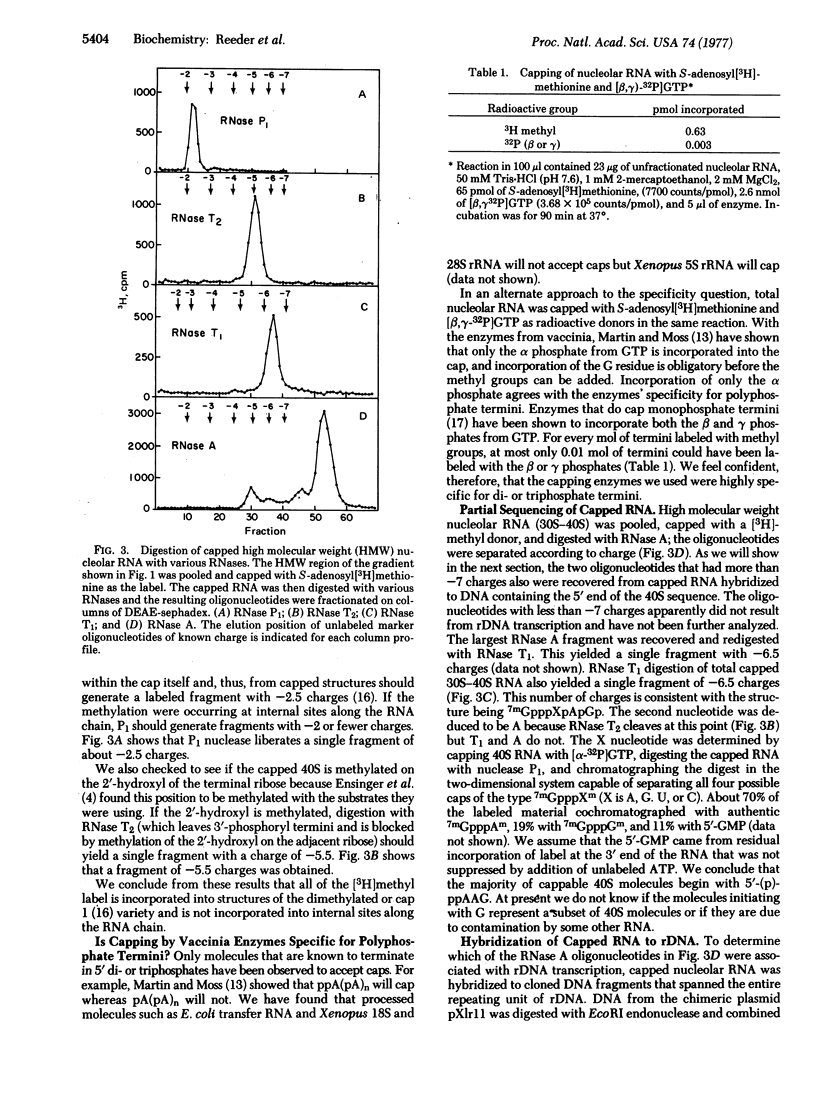
We report the results of a novel method for locating sites of transcription initiation using a complex of capping enzymes from vaccinia virions that catalyze the reaction pppG + S-adenosylmethionine + (p)ppXpYpZp..... →7mGpppXpYpZp..... [Ensinger, M. J., Martin, S. A., Paoletti, E. and Moss, B. (1975) Proc. Natl. Acad. Sci. USA 72, 2525-2529]. This enzyme complex will cap di- or triphosphate termini but will not cap monophosphate or hydroxyl termini. Xenopus laevis 40S precursor rRNA from oocytes is capped by these enzymes, and we conclude that it has 5′-polyphosphate termini. Therefore, 40S RNA must represent the primary transcript of amplified X. laevis ribosomal DNA. The majority of 40S molecules with polyphosphate termini begin with the sequence (p)ppAAG. There is evidence, however, that the 5′ terminus may be heterogeneous. The majority of all detectable initiation events were localized close to the region coding for the 5′ end of the 40S RNA. No initiation sites were detected in the nontranscribed spacer, but an apparent initiation site in the middle of the transcribed region was also observed.
Keywords: 40S rRNA precursor, primary transcript, oocyte nucleoli, RNA sequencing, capping enzymes
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham G., Rhodes D. P., Banerjee A. K. The 5' terminal structure of the methylated mRNA synthesized in vitro by vesicular stomatitis virus. Cell. 1975 May;5(1):51–58. doi: 10.1016/0092-8674(75)90091-4. [DOI] [PubMed] [Google Scholar]
- Botchan P., Reeder R. H., Dawid I. B. Restriction analysis of the nontranscribed spacers of Xenopus laevis ribosomal DNA. Cell. 1977 Jul;11(3):599–607. doi: 10.1016/0092-8674(77)90077-0. [DOI] [PubMed] [Google Scholar]
- Brownlee G. G., Cartwright E., McShane T., Williamson R. The nucleotide sequence of somatic 5 S RNA from Xenopus laevis. FEBS Lett. 1972 Sep 1;25(1):8–12. doi: 10.1016/0014-5793(72)80442-3. [DOI] [PubMed] [Google Scholar]
- Ensinger M. J., Martin S. A., Paoletti E., Moss B. Modification of the 5'-terminus of mRNA by soluble guanylyl and methyl transferases from vaccinia virus. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2525–2529. doi: 10.1073/pnas.72.7.2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatlen L. E., Amaldi F., Attardi G. Oligonucleotide pattern after pancreatic ribonuclease digestion and the 3' and 5' termini of 5S ribonucleic acid from HeLa cells. Biochemistry. 1969 Dec;8(12):4989–5005. doi: 10.1021/bi00840a048. [DOI] [PubMed] [Google Scholar]
- Hayward G. S. Gel electrophoretic separation of the complementary strands of bacteriophage DNA. Virology. 1972 Jul;49(1):342–344. doi: 10.1016/s0042-6822(72)80042-4. [DOI] [PubMed] [Google Scholar]
- Higashinakagawa T., Wahn H., Reeder R. H. Isolation of ribosomal gene chromatin. Dev Biol. 1977 Feb;55(2):375–386. doi: 10.1016/0012-1606(77)90180-4. [DOI] [PubMed] [Google Scholar]
- Kominami R., Muramatsu M. Heterogeneity of 5' -termini of nucleolar 45S, 32S and 28S RNA in mouse hepatoma. Nucleic Acids Res. 1977 Jan;4(1):229–240. doi: 10.1093/nar/4.1.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landesman R., Gross P. R. Patterns of macromolecule synthesis during development of Xenopus laevis. I. Incorporation of radioactive precursors into dissociated embryos. Dev Biol. 1968 Dec;18(6):571–589. doi: 10.1016/0012-1606(68)90027-4. [DOI] [PubMed] [Google Scholar]
- Martin S. A., Moss B. Modification of RNA by mRNA guanylyltransferase and mRNA (guanine-7-)methyltransferase from vaccinia virions. J Biol Chem. 1975 Dec 25;250(24):9330–9335. [PubMed] [Google Scholar]
- Miller O. L., Jr, Beatty B. R. Visualization of nucleolar genes. Science. 1969 May 23;164(3882):955–957. doi: 10.1126/science.164.3882.955. [DOI] [PubMed] [Google Scholar]
- Moss B. Utilization of the guanylyltransferase and methyltransferases of vaccinia virus to modify and identify the 5'-terminals of heterologous RNA species. Biochem Biophys Res Commun. 1977 Jan 24;74(2):374–383. doi: 10.1016/0006-291x(77)90314-x. [DOI] [PubMed] [Google Scholar]
- Pace N. R. Structure and synthesis of the ribosomal ribonucleic acid of prokaryotes. Bacteriol Rev. 1973 Dec;37(4):562–603. doi: 10.1128/br.37.4.562-603.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penman S., Smith I., Holtzman E. Ribosomal RNA synthesis and processing in a particulate site in the HeLa cell nucleus. Science. 1966 Nov 11;154(3750):786–789. doi: 10.1126/science.154.3750.786. [DOI] [PubMed] [Google Scholar]
- Rungger D., Crippa M. The primary ribosomal DNA transcript in eukaryotes. Prog Biophys Mol Biol. 1977;31(3):247–269. doi: 10.1016/0079-6107(78)90010-x. [DOI] [PubMed] [Google Scholar]
- Scheer U., Trendelenburg M. F., Krohne G., Franke W. W. Lengths and patterns of transcriptional units in the amplified nucleoli of oocytes of Xenopus laevis. Chromosoma. 1977 Mar 16;60(2):147–167. doi: 10.1007/BF00288462. [DOI] [PubMed] [Google Scholar]
- Shatkin A. J. Capping of eucaryotic mRNAs. Cell. 1976 Dec;9(4 Pt 2):645–653. doi: 10.1016/0092-8674(76)90128-8. [DOI] [PubMed] [Google Scholar]
- Slack J. M., Loening U. E. 5'-Ends of ribosomal and ribosomal precursor RNAs form Xenopus laevis. Eur J Biochem. 1974 Mar 15;43(1):59–67. doi: 10.1111/j.1432-1033.1974.tb03384.x. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Wellauer P. K., Dawid I. B., Brown D. D., Reeder R. H. The molecular basis for length heterogeneity in ribosomal DNA from Xenopus laevis. J Mol Biol. 1976 Aug 25;105(4):461–486. doi: 10.1016/0022-2836(76)90229-1. [DOI] [PubMed] [Google Scholar]
- Wellauer P. K., Dawid I. B. Secondary structure maps of ribosomal RNA and DNA. I. Processing of Xenopus laevis ribosomal RNA and structure of single-stranded ribosomal DNA. J Mol Biol. 1974 Oct 25;89(2):379–395. doi: 10.1016/0022-2836(74)90526-9. [DOI] [PubMed] [Google Scholar]
- Wellauer P. K., Reeder R. H. A comparison of the structural organization of amplified ribosomal DNA from Xenopus mulleri and Xenopus laevis. J Mol Biol. 1975 May 15;94(2):151–161. doi: 10.1016/0022-2836(75)90074-1. [DOI] [PubMed] [Google Scholar]