Abstract
Pulmonary hypertension is comprised of heterogeneous diagnoses with distinct hemodynamic pathophysiology. Identifying elevated pulmonary vascular resistance (PVR) is critical for appropriate treatment. We reviewed data for patients seen at referral PH clinics who underwent echocardiography and right heart catheterization within 1 year. We derived equations to estimate PVR based on the ratio of estimated pulmonary artery (PA) systolic pressure (PASPDoppler) to RVOT VTI. We validated these equations in a separate sample and compared them to a published model based on the ratio of transtricuspid flow velocity to RVOT VTI (Model 1, Abbas et al 2003). The derived models were:
| (Model 2) |
| (Model 3) |
The cohort included 217 patients with mean PA pressure=45.3±11.9mmHg, PVR=7.3±5.0WU and PA wedge pressure=14.8±8.1mmHg; just over 1/3rd had PA wedge pressure >15mmHg (35.5%) and 82.0% had PVR>3WU. Model 1 systematically underestimated PVR, especially with high PVR. The derived models demonstrated no systematic bias. Model 3 correlated best with PVR (r=0.80 vs. 0.73 and 0.77 for Models 1 and 2 respectively). Model 3 had superior discriminatory power for PVR>3WU (AUC=0.946) and PVR>5WU (AUC=0.924), though all models discriminated well. Model 3 estimated PVR>3 was 98.3% sensitive and 61.1% specific for PVR>3WU (PPV=93%; NPV=88%). In conclusion, we present an equation to estimate PVR, using the ratio of PASPDoppler to RVOT VTI and a constant designating presence of RVOT VTI mid-systolic notching, which provides superior agreement with PVR across a wide range of values.
Keywords: pulmonary hypertension, hemodynamics, echocardiography
Introduction
Right sided heart catheterization (RHC) is the gold standard for hemodynamic evaluation of patients with pulmonary hypertension (PH) including PVR estimation. 1-3 RHC is invasive and cannot be applied repeatedly to all patients with suspected PH. Estimated pulmonary artery systolic pressure (PASPDoppler) from transtricuspid flow velocity (TTFV) is at the core of echocardiographic evaluation of suspected PH.4-6 PASP does not define PVR, however; patients with vastly different PVR may have identical PASP. Noninvasive PVR estimation would be useful for diagnosis and monitoring response to therapy. Several investigators have proposed echocardiographic PVR prediction models.7-14 Some use timing of right ventricular outflow tract (RVOT) flow to estimate PVR.12 Others use a ratio of a correlate of PA pressure to PA flow in the denominator. An early model based on the ratio of TTFV to RVOT velocity time integral (RVOT-VTI) has been validated to predict PVR in patients with cirrhosis and adverse outcomes among coronary disease patients. 8,15,16 This model, however, was derived in patients with normal or mildly elevated PVR, and has poor agreement at higher levels of PVR.17,18 Equations using transtricuspid pressure gradient (4xTTFV2) in place of TTFV have been derived for patients with markedly elevated PVR.9 Others have evaluated a multi-parameter approach involving estimation of all variables involved in calculating PVR (mean PA pressure, left atrial pressure, stroke volume and heart rate).11 This approach is time intensive and less clinically useful. Our objectives were to derive and validate a simple equation to accurately estimate PVR for widespread application in a diverse referral population of patients with PH or suspected PH.
Methods
The cohort included patients seen by the PH services at the Hospital of University of Pennsylvania, Brigham and Women's Hospital, Boston Children's Hospital, or Massachusetts General Hospital who underwent RHC and transthoracic echocardiogram (TTE) between March 2002 and January 2012. Exclusions were: >12 months between RHC and TTE, interval initiation of pulmonary vasodilator or loop diuretic or cardiovascular/abdominal surgery or change in clinical status, unrepaired congenital heart disease, or intravenous inotropes/vasopressors or positive pressure ventilation at the time of either study. We excluded 44/261(17.1%) otherwise eligible patients because of poor quality tricuspid regurgitation signals. Median time between RHC and TTE was 21.5d(IQR 5-55.5). Patients were classified into WHO PH diagnostic group.2
Patients underwent clinically indicated TTE with Philips Sonos 7500 or IE33 (Philips Medical Systems, Andover, MA) or GE Vivid 7 Ultrasound (GE, Milwaukee, WI). Measurements were made by an echocardiographer (ARO) blinded to invasive hemodynamics in accordance with American Society of Echocardiography guidelines19 using Acuson KinetDx (WS3000 Diagnostic Workstation, Siemens Medical Solutions USA Inc., Mountain View, CA) or Showcase Premier 5.3 (Trillium Technology Inc., Ann Arbor, MI). At least 3 cardiac cycles were measured (5-10 with irregular rhythms) and average values were used.
Left atrial AP end-systolic dimension was measured from the parasternal long axis view. RVOT pulse wave (PW) Doppler interrogation was performed from the basal short axis views just proximal to the pulmonic valve. By inspecting the PW Doppler signal from the RVOT, the presence of a mid-systolic notch (MSN) was characterized as a distinct notch or nadir within the initial two-thirds of the systolic ejection period.20 TTFV was obtained from continuous wave Doppler interrogation in the view that provided the best envelope with highest estimates. LV inflow PW Doppler was used to measure peak E/A velocities. Lateral mitral annular tissue Doppler was used to measure e' velocity.21
RHC was performed using a balloon-tipped fluid filled catheter as previously described.20 No sedation was administered for most catheterizations, while minimal to moderate conscious sedation was used for a subset with spontaneous ventilation. Supplemental O2 was not administered unless the patient used chronic supplemental O2 at rest in which case the same dose was used during RHC. Cardiac output was estimated either by triplicate thermodilution (n=83) or assumed Fick (n=134). O2 consumption was assumed to be 125mL/m2 BSA. There was no difference (p=0.74) in mean cardiac output between the 2 groups, and the method used did not significantly modify the relationship between RVOT-VTI and cardiac output (i.e. no 2-way interaction).
Categorical data are expressed as proportion (%), while continuous data are presented as mean±SD or median (interquartile range) as appropriate. Unpaired T-tests and Wilcoxon rank sums were used to compare means for normally and non-normally distributed continuous variables, respectively; χ2 or Fisher's exact test were performed to compare proportions for categorical variables.
We compared derived models to a published model based on the ratio of TTFV to VTI:
| (Model 1) |
(Abbas et al)8
The overall sample was divided using random sampling into derivation and validation samples. To derive the model, we used linear regression and forward selection with p value for entry<0.10. Variables were retained if they increased r2≥0.02. Potential variables included left atrial AP dimension, lateral mitral E:e' , TTFV, 4xTTFV2, estimated PASP using fixed RA pressure=8mmHg (PASPDoppler) or variable RA pressure of 5/10/15mmHg or 3/8/15mmHg based on IVC diameter and collapse,22,23 PASPDoppler-E:e', acceleration time, RVOT mid-systolic notching (MSN), RVOT-VTI (VTI), and ratios of the following variables to VTI: (a) TTFV, (b) 4xTTFV2 and (c) PASPDoppler (=4xTTFV2+8). Given the potential for model instability in the setting of collinear parameters, we further assessed the predictive value of alternative models to confirm the derived model was associated with the highest r2.
The derived model was PVR=-0.05+1.0x(PASPDoppler/VTI)+2.8x(MSN) where MSN=1 if present and MSN=0 if absent. Partial r2=0.596 for PASPDoppler/VTI and=0.068 for the MSN term. To simplify, we rounded coefficients the nearest integer given the clinical insignificance of PVR±0.25WU. We further evaluated a model including only PASPDoppler/VTI given the potential for increased simplicity and to test the value of MSN in the validation cohort. Sensitivity analyses were performed to assess model performance in specific subsets of patients (e.g. high PAWP) and whether alternative parameters might improve the model. Bland-Altman plots were used to assess agreement between derived models and PVR. Model discrimination for clinically relevant levels of PVR (>3 or >5WU) was assessed using ROC curves and AUC, as well as sensitivity/specificity/negative/positive predictive value.
Thus, the simple PASPDoppler/VTI model was:
| (Model 2) |
The comprehensive model was:
| (Model 3) |
We also assessed agreement between PVR and a model derived by Kouzu et al in a population enriched for elevated PVR (μPVR=16.2WU): .9 For simplicity, we limit reported results on this equation to Bland-Altman plots illustrating overestimation of PVR at low values; this equation correlated less well with PVR than did Model 3 (r=0.77 vs. 0.80). Statistical analyses used SAS for Windows 9.3 (SAS, Cary, NC) and GraphPad Prism 5.02 for Windows (GraphPad Software, San Diego, CA).
Results
Demographic, clinical and hemodynamic data are presented in Table 1. Mean age was 60.6 years and there was a high prevalence of common chronic diseases. Mean PAP was markedly elevated (45.2±13.0mmHg), as was PVR (7.7±5.1WU). Just over 1/3rd (35.5%) had PAWP>15mmHg, and 10% had PAWP≥23mmHg. Derivation and validation cohorts were similar, though the validation cohort tended to be older with a smaller proportion of WHO group I PH.
Table 1. Descriptive statistics.
Demographics, clinical characteristics and hemodynamics for the derivation and validation cohorts. P values are for differences between derivation and validation cohorts. Categorical data presented as %; continuous data presented as mean±SD. Hypertension refers to a clinical diagnosis of systemic arterial hypertension defined clinically consistent with recommendations of the Joint National Committee 7 as either SBP≥140, DBP≥90 or treatment with an antihypertensive medication (except if prescribed for an alternative use such as calcium channel blockers for treatment of Raynaud's phenomenon); dyslipidemia is defined clinically according to ATP III guidelines and/or treatment with a lipid medication; coronary artery disease refers to atherosclerotic coronary disease, either documented significant epicardial coronary stenosis, with our without prior intervention, or history of acute coronary syndrome.
| Parameter | Overall | Derivation | Validation | P value |
|---|---|---|---|---|
|
| ||||
| n=217 | N=109 | N=108 | ||
| Age (years) | 60.6±15.2 | 58.6±15.4 | 62.6±14.8 | 0.05 |
| Male | 25.3% | 20.2% | 30.6% | 0.09 |
| White | 85.5% | 86.5% | 84.4% | 0.84 |
| Hypertension | 53.9% | 52.3% | 55.6% | 0.68 |
| Dyslipidemia | 32.3% | 29.3% | 35.2% | 0.39 |
| Coronary artery disease | 22.6% | 20.2% | 25.0% | 0.42 |
| Atrial fibrillation | 23.0% | 19.3% | 26.9% | 0.20 |
| Pacemaker or defibrillator | 7.8% | 7.3% | 8.3% | 0.81 |
| Congenital heart disease | 6.5% | 5.5% | 7.4% | 0.59 |
| Connective tissue disease | 14.7% | 13.8% | 15.7% | 0.71 |
| Sarcoidosis | 4.6% | 1.8% | 7.4% | 0.06 |
| Chronic obstructive lung disease | 21.7% | 19.3% | 24.1% | 0.41 |
| Obstructive sleep apnea | 15.2% | 16.5% | 13.9% | 0.71 |
| Interstitial lung disease | 16.2% | 16.5% | 15.9% | 1.00 |
| Asthma | 4.1% | 5.5% | 2.8% | 0.50 |
| HIV | 1.8% | 1.8% | 1.9% | 1.00 |
| Diabetes mellitus | 18.0% | 12.8% | 23.1% | 0.05 |
| Liver cirrhosis | 5.1% | 5.5% | 4.6% | 1.00 |
| Tobacco, current | 6.5% | 9.2% | 3.7% | 0.17 |
| Tobacco, former | 49.0% | 45.7% | 52.4% | 0.41 |
| WHO Group I | 30.9% | 37.6% | 24.1% | 0.04 |
| WHO Group II | 25.8% | 22.9% | 28.7% | 0.36 |
| WHO Group III | 16.1% | 12.8% | 19.4% | 0.20 |
| Heart rate (bpm) | 76.9±14.6 | 76.6±15.1 | 77.2±14.2 | 0.75 |
| Systolic blood pressure (mmHg) | 125.3±20.8 | 123.2±20.1 | 127.3±21.3 | 0.16 |
| Right atrial pressure (mmHg) | 10.7±6.1 | 10.6±6.0 | 10.8±6.2 | 0.78 |
| Systolic PA pressure (mmHg) | 72.8±21.2 | 72.8±19.7 | 72.8±22.6 | 1.00 |
| Mean PA pressure (mmHg) | 45.2±13.0 | 45.4±12.5 | 45.0±13.6 | 0.80 |
| PA wedge pressure (mmHg) | 13.8±7.9 | 13.2±7.4 | 14.4±8.2 | 0.25 |
| Pulmonary vascular resistance (WU) | 7.7±5.1 | 7.7±5.1 | 7.7±5.1 | 0.97 |
| Cardiac output (l/min) | 4.8±1.8 | 4.9±1.8 | 4.7±1.7 | 0.43 |
HIV: Human immunodeficiency virus
PA: Pulmonary artery
WHO: World Health Organization
In the derivation cohort, we found that the published model using TTFV/RVOT-VTI (Model 1) underestimated PVR, especially at higher PVR values. The best linear approximation using TTFV/ RVOT-VTI was (or PVR = 0.76 + 2.37x; x is PVR estimate per Model 1), with r2=0.531. Model 1 demonstrates significant bias (bias=-4.1) and increasingly underestimates PVR with higher PVR values (Figure 1, top). The model derived by Kouzu (Figure 1, bottom) was accurate at high PVR but overestimated normal to mildly elevated PVR (bias=+2.7, with a SD of bias of 3.4, 95% limits of agreement -3.9 to 9.3).
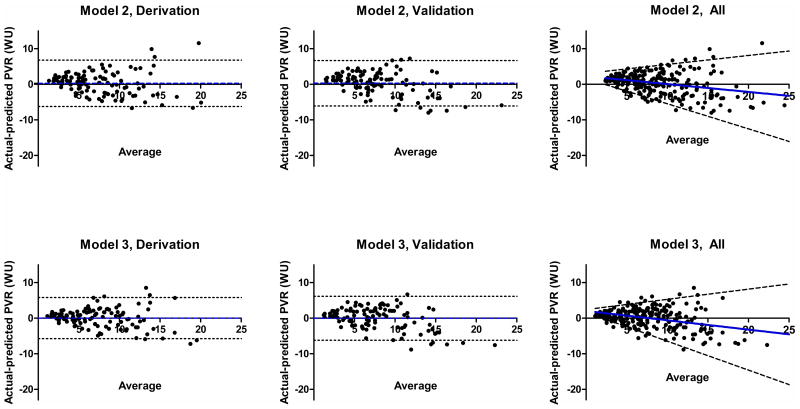
Figure 1.
Bland-Altman plots for the derivation (left) and validation (middle) cohorts for Model Model 1 (top) and Kouzu's model (bottom). The average of the PVR value from catheterization and the respective PVR estimation model is plotted on the x axis, while the difference between catheterization PVR and the PVR estimated by the model is plotted on the y axis. The blue dotted line specifies mean bias while black dotted lines represent the 95% limits of agreement. At right, data for all subjects are plotted for Model 1 and Kouzou's model with 95% LOA using regression of the absolute value of the residuals to estimate the standard deviation at varying levels of average PVR. These plots demonstrate that Model 1 has greater negative bias at higher values of PVR, while Kouzou's model overestimates PVR at low PVR values.
Model 2 correlated more closely with PVR(r2=0.593). Model 3 correlated better still (r2=0.669). The left-hand column of Figure 2 shows Bland-Altman plots for Model 2 and Model 3 in the derivation cohort. As shown in Figure 3a (data for the entire cohort; equivalent results for derivation and validation cohorts), lines approximating PVR with and without MSN are parallel (p=0.07 for difference in slope) but y intercepts at x=0 differed by ∼3WU (p<0.0001). Including the additional term for MSN resulted in equivalent intercepts (p=0.34)(Figure 3b).
Figure 2.
Bland-Altman plots for the derivation (left) and validation (middle) cohorts for Model Model 2 (top) and Model 3 (bottom). The average of the PVR value from catheterization and the respective PVR estimation models is plotted on the x axis, while the difference between catheterization PVR and the PVR estimated by the model is plotted on the y axis. The blue dotted line specifies mean bias while black dotted lines represent 95% limits of agreement. The right-most panels plot data for all subjects and 95% LOA using regression of the absolute value of the residuals to estimate the standard deviation at varying levels of average PVR for Model 2 and 3, top and bottom respectively. While both models have minimal bias at all levels of PVR, because of its small positive bias and narrow 95% LOA at low PVR values, Model 3 is unlikely to estimate PVR<3WU for a patient with elevated PVR.
Figure 3.
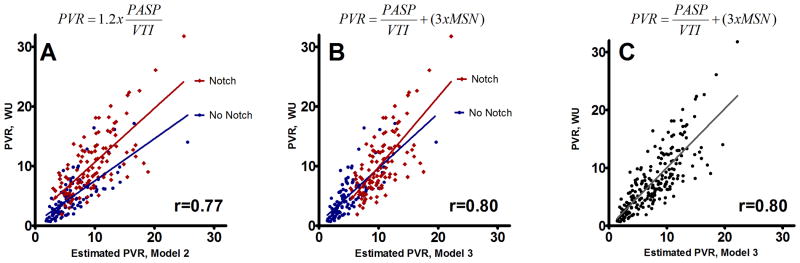
Figure 3A: PVR versus estimated PVR by Model 2, with red/blue designating the presence of mid-systolic notching (MSN). The best-fit line for patients with MSN is significantly higher for a given value of estimated PVR (p=<0.0001 for intercept=0). Figures 3B/C show PVR versus Model 3 estimated PVR (red/blue indicated presence MSN in panel B). Adding a constant of 3 for patients with notching results in overlapping best-fit lines with equal intercepts (p=0.34). Figure 3C shows the best fit line for Model 3.
We evaluated whether including estimates of PAWP improved the predictive models. Neither LA dimension nor lateral mitral annular E:e' met criteria to be included in the model. Given the theoretical benefit of including a PAWP estimate, however, we explored various ways to estimate TPG (difference between PASPDoppler and correlates of PAWP). No resulting equation provided statistically significant improvement in estimates. For example, using a previously proposed method (numerator=PASPDoppler-E/e'),13 we found the correlation for n=87 with E/e' data(r2=0.595) was the same as for Model 2, with a numerator of PASPDoppler alone(r2=0.596) In order to understand whether this was due to variability in estimating PAWP or to limited intrinsic value to including PAWP, we estimated TPG as PASPDoppler -actual (catheterization) PAWP. Using this as the numerator in Model 2, the correlation improved modestly (r2=0.593→r2=0.622), but there was no improvement in Model 3(r2=0.669→r2=0.662).
We examined how well any 2 of 3 component variables (mPAP, PAWP, CO) of catheter PVR could explain PVR by substituting the mean value for each parameter as a constant in the equation. CO and mPAP provided much more information on PVR than PAWP did. Setting PAWP=15, the equation (PVR=(mPAP-15)/CO) had r2=0.876. Equations substituting a constant for mPAP and CO, respectively, produced r2=0.653 and r2=0.687. Likewise, mPAP and CO correlated with PVR much better than PAWP did (r2=0.575, 0.342 and 0.129 respectively). No single catheter-derived variable predicted PVR as well as the derived echocardiographic models; even perfect echocardiographic estimation of one parameter would not predict PVR as well as a combination.
In the validation cohort, Model 3 correlated better with PVR (r2=0.622) than did Model 2 (r2=0.597) or Model 1 (r2=0.551). Intercept and coefficients of the best-fit line did not differ significantly from the derivation cohort for either Model 2 or 3. No additional predictors of PVR achieved statistical significance (all p>0.15) or provided additional explanatory power (Δr2<0.02).
The middle column of Figure 1 shows Bland-Altman plots in the validation cohort for Model 1 and Kouzu's model. Model 1 again displays bias (bias=-4.1, SD of bias=4.1, 95% limits of agreement=-12.2 to 4.0) and progressively underestimates PVR with higher PVR values. Kouzu's model tended to overestimate lower PVR (bias=+2.7, SD=3.2, 95% limits=-3.7 to 9.0). Figure 2 shows the equivalent data for Model 2 and Model 3, neither of which demonstrated systematic bias (Model 2: bias=-0.29, SD=3.3, 95% limits=-6.7 to 6.1; Model 3: bias=+0.03, SD=3.2, 95% limits=-6.2 to 6.2). The right-hand columns of Figures 1 and 2 show data for all subjects, but the 95% LOA are estimated using regression of the absolute value of the residuals to estimate the standard deviation at varying levels of average PVR as opposed to assuming a model will have the same bias and LOA across the spectrum of PVR values. It can be seen (Figure 1) progressively underestimates PVR at higher values, while Kouzu's model overestimates PVR at low levels. On the other hand, Models 2 and 3 demonstrate narrow LOA at low PVR values with a slight positive bias (Figure 2), which is consistent with a high sensitivity for PVR>3WU.
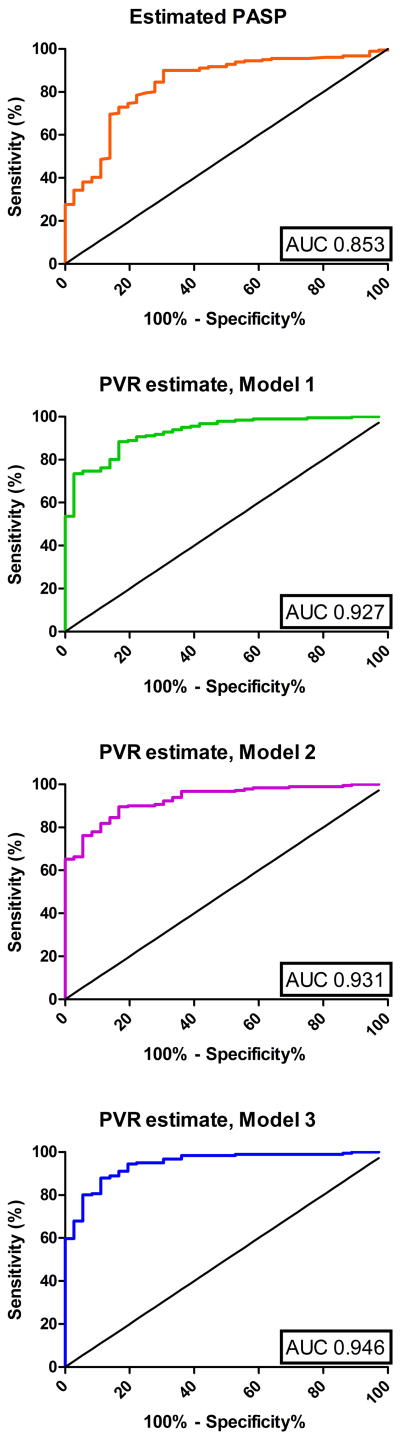
Figure 4 shows ROC curves for PASPDoppler and Models 1-3 as predictors of PVR>3. Results were similar in derivation and validation cohorts. Model 3 estimated PVR>3 had 98.3% sensitivity and 61.1% specificity for PVR>3WU (PPV=93%; NPV=88%), while estimated PVR>7 was 63.0% sensitive and 97.2% specific for PVR>3 (PPV=99%; NPV=34%). All subjects with estimated PVR>8(n=95) had PVR>3WU, as did 94.3%(50/53) with estimated PVR between 5 and 8.
Figure 4.
ROC curves for discrimination of PVR>3WU.
Figure 5 gives examples of Doppler tracings, along with PVR estimates for each model and catheterization PVR.
Figure 5.
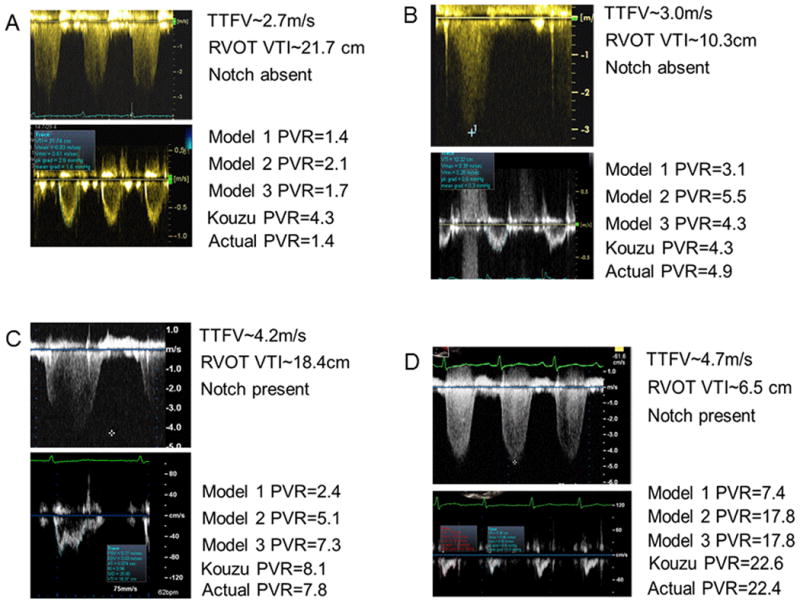
Tracings from 4 subjects, along with PVR estimates by each model and catheter PVR. Panel (A): normal TTFV and RVOT-VTI without MSN. Models 1 and 3 accurately estimate PVR, while Kouzu's model overestimates PVR. Panel (B): TTFV is likely underestimated because of signal quality. All models predict PVR>3WU, although Model 1 underestimates PVR most. Panel (C): high TTFV with high RVOT-VTI. Panel (D): Model 1 markedly underestimates PVR with high PVR.
We performed sensitivity analyses to assess model performance in specific subsets of patients and to determine whether specific physiologic findings were associated with model performance. A) Among patients with PASPDoppler<60mmHg (TTFV≤3.6m/s), in whom this equation is most likely to be used clinically, PASPDoppler had lower discriminatory ability for PVR>3 (AUC=0.712), while Model 1 and 2 had intermediate discriminatory power (AUC=0.849 and 0.846) and Model 3 had AUC=0.873. B) Among patients with PVR<8WU, Model 3 again demonstrated the highest correlation with PVR>3 (r=0.70 vs. 0.58 and 0.62 for Models 1 and 2 respectively), (Table 2, bottom). C) Excluding PVR outliers, patients with PVR in the top or bottom 5%, (n=195, PVR 1.5-17.4WU) produced similar results, albeit with slightly lower correlation coefficients (r=0.55, 0.64, 0.67 and 0.73 for PASPDoppler and Models 1-3 respectively). D) To assess the effect of the time delay between echocardiography and catheterization we limited the analysis to studies performed <22d apart (median); this had little effect on the results (n=109, for Models 1-3 respectively, r=0.67, 0.70 and 0.76). E) Among patients with PAWP>15mmHg (n=77 correlation with PVR was slightly lower (r=0.63, 0.70, 0.74 and 0.75 for PASPDoppler and models 1-3 respectively). This was also true for the subset with a diagnosis of WHO Group II PH (n=56, r=0.62, 0.74, 0.79, 0.81 for PASPDoppler and models 1-3 respectively). Bland-Altman analysis for Model 3 for patients with PAWP>15mmHg suggested minimal systematic bias (bias+0.46, SD= 2.62). F) To determine whether heart rate affected the model, we repeated assessment of Model 2, using the product of VTI and heart rate as the denominator. This product and VTI alone correlated similarly with cardiac output among the 209 subjects with heart rate data (r = 0.49→0.50); resulting PVR estimates performed less well (r2=0.530 vs. 0.590).
Table 2.
Pearson correlation coefficients between PVR and various predictors in the derivation (top) and validation (middle) cohorts. The bottom row includes the subset of the combined cohort with PVR<8WU (n=135).
| Derivation | Acceleration time | RVOT-VTI | TTFV | Estimated PASP | Model 1 | Model 2 | Model 3 |
|---|---|---|---|---|---|---|---|
| PVR | −0.514 | −0.549 | 0.624 | 0.633 | 0.733 | 0.768 | 0.803 |
| Acceleration time | 0.512 | -0.457 | −0.444 | −0.546 | −0.538 | −0.604 | |
| RVOT-VTI | −0.264 | −0.258 | −0.827 | −0.736 | −0.722 | ||
| TTFV | 0.993 | 0.607 | 0.733 | 0.710 | |||
| Estimated PASP | 0.602 | 0.738 | 0.712 | ||||
| Model 1 | 0.978 | 0.922 | |||||
| Model 2 | 0.939 |
| Validation | Acceleration time | RVOT-VTI | TTFV | Estimated PASP | Model 1 | Model 2 | Model 3 |
|---|---|---|---|---|---|---|---|
| PVR | −0.576 | −0.545 | 0.603 | 0.601 | 0.743 | 0.772 | 0.789 |
| Acceleration time | 0.546 | −0.528 | −0.510 | −0.618 | −0.622 | −0.666 | |
| RVOT-VTI | −0.263 | −0.253 | −0.854 | −0.765 | −0.727 | ||
| TTFV | 0.994 | 0.586 | 0.731 | 0.707 | |||
| Estimated PASP | 0.577 | 0.732 | 0.706 | ||||
| Model 1 | 0.974 | 0.909 | |||||
| Model 2 | 0.934 |
| PVR<8WU | Acceleration time | RVOT-VTI | TTFV | Estimated PASP | Model 1 | Model 2 | Model 3 |
|---|---|---|---|---|---|---|---|
| PVR | -0.578 | -0.397 | 0.564 | 0.564 | 0.579 | 0.622 | 0.697 |
| Acceleration time | 0.407 | -0.368 | -0.362 | -0.485 | -0.483 | -0.583 | |
| RVOT-VTI | -0.054 | -0.047 | -0.796 | -0.656 | -0.603 | ||
| TTFV | 0.995 | 0.552 | 0.726 | 0.626 | |||
| Estimated PASP | 0.544 | 0.725 | 0.626 | ||||
| Model 1 | 0.967 | 0.824 | |||||
| Model 2 | 0.850 |
PASP: Pulmonary artery systolic pressure
PVR: Pulmonary vascular resistance
RVOT-VTI: Right ventricular outflow tract velocity time integral
TTFV: Trans-tricuspid flow velocity
Discussion
We describe derivation and validation of an equation to estimate PVR using clinically available echocardiographic parameters. The model includes the ratio of estimated PASP (PASPDoppler= 8+4xTTFV2) to RVOT-VTI, with addition of a constant (+3) for patients with RVOT Doppler flow envelope mid-systolic notching. The equation is simple and easily integrated into clinical practice. In addition, it is as applicable to patients with normal PVR as it is to those with markedly elevated PVR.
Prior investigators have demonstrated that an echocardiographic estimate based on a ratio of estimated PA pressure to flow can approximate PVR. Initial models included TTFV as the numerator. While there is a direct correlation between TTFV/VTI and PVR at any level of PVR,24 absolute agreement between this ratio and PVR is not robust. TTFV/VTI underestimates PVR at higher levels, as it does not account for the quadratic relationship between velocity and pressure. Other models, such as reported by Kouzu, were derived in populations with elevated PVR to address this limitation. They use estimates of transtricuspid pressure gradient or PASP (4xTTFV2±RA pressure estimate) in the numerator. Because of universally high PVR in derivation samples, however, the equation includes a large constant, resulting in overestimation of PVR in the normal range.9 The current model provides good agreement with true PVR throughout the range of PVR, addressing a limitation of previously proposed models.
All the models correlate reasonably well with catheter-derived PVR (r≥0.7). While Model 3 correlated best with PVR the small difference makes ease of use and interpretation important considerations in choosing between these options. Most clinical echocardiographic laboratories report PASPDoppler or transtricuspid gradient. Units for PASPDoppler or transtricuspid gradient are consistent (mmHg), while different options exist for TTFV (cm/s or m/s), so consideration must be given to units in the calculation of Model 1. Including notching may seem to add complexity, but it results in a coefficient of 1 for PASP/VTI (vs. 1.2 in Model 2). Finally, while a given cutoff of Model 1 has similar test characteristics to PVR=3 for Model 3, Model 3's superior agreement between absolute values of estimated PVR makes it a more reliable predictor of actual numerical PVR in an individual patient and interpretation is thereby simplified. Based on these considerations, we believe Model 3 is preferable.
Inclusion of mid-systolic notching is useful from a clinical perspective, and is consistent with prior data that almost all patients with such notching have elevated PVR.20 It improves sensitivity for high PVR since it provides a second mechanism for the equation to produce a result with elevated PVR; even if the VTI and TTFV are acquired or measured incorrectly, MSN highlights the likelihood of PVR>3WU.
The derived equations include an estimate of pressure in the numerator and flow in the denominator. Both terms are simplifications of the physiology captured by catheterization. First, PVR is calculated with transpulmonary gradient, the difference between mean PAP and PAWP (or LA pressure), as the numerator. The proposed equations use PASPDoppler as a surrogate. PASP is not equivalent to mPAP, though highly correlated (r=0.95 in this sample). While mPAP can be estimated using echocardiography, this is more time consuming and not commonly applied in practice. Likewise, PAWP can be estimated by echocardiography but including such estimates did not improve model performance. This relates to imprecision of PASP and PAWP estimates, and limited relative contribution of PAWP to the absolute PVR value, compared with the greater influence of PAP and CO. Second, the equations use RVOT-VTI as a marker of flow. VTI reflects the average distance traveled by the blood column, but only indirectly suggests stroke volume in the absence of data on area. RVOT-VTI varies inversely with the cross sectional area of the RVOT, and presumably indirectly with PVR.24 LVOT area may be less susceptible to such and some have suggested LVOT VTI would provide a better marker of stroke volume. One study reported no benefit to using LVOT over RVOT-VTI.10 In addition, CO is the product of stroke volume and heart rate. Integrating heart rate into the equation did not improve estimates. In patients with elevated PVR, we would expect larger RVOT area and higher heart rate, both of which would result in VTI underestimating cardiac output in patients with elevated PVR and would bias the model to overestimate PVR at high values. Not only do we not observe that in our cohort, such a phenomenon would actually increase the likelihood that patients with high PVR are identified. The absolute value in a patient with very high PVR is less important than that the clinician is alerted to presence of high PVR.
We found lower correlation between predicted and actual PVR than most prior reports. This may in part be due to use of clinical echocardiograms or time between studies. Our sample size and PVR distribution also differ. Most of the 44 subjects used to derive Model 1 had normal PVR; only 6 had PVR>3WU and 3 had PVR>4WU.8 In small studies, outliers have undue influence and inflate correlation.
We see several potential uses of the derived equation. First, it can clinically assess whether a patient has importantly elevated PVR. An estimate significantly <3WU argues against elevated PVR. Patients with suspected PH or elevated PASP in whom it would be unclear whether PH is due to elevated PVR, flow (e.g., cirrhosis) or LA pressure (e.g., left heart disease) would be a relevant population. Second, it may be used to assess treatment response to vasodilators. This would not replace RHC, but could supplement information since RHC is invasive and expensive. Finally, large epidemiologic studies have shown elevated estimated PASP is a predictor of heart failure and poor outcomes in patients with normal and reduced ejection fraction.25-27 Being able to estimate PVR on a population scale would help distinguish whether this reflects the adverse prognostic import of elevated LA pressure or an independent vascular remodeling as suggested by one study in patients with coronary disease.16
These data are derived from clinical echocardiograms and there is a time delay between echocardiogram and catheterization. While empirically we found the model performed equally well whether performed close to the time of catheterization or with many months in between tests, such a delay could in theory increase non-differential error and bias results towards the null. Non-differential error would be expected to be more of an issue for PASPDoppler than for TTFV, since PASP estimation involves squaring any error. MSN can vary with respiration and sample volume placement. Based on these considerations, we would expect the test characteristics of Models 2 and 3 to be more susceptible to random error than Model 1. Inclusion of notching improves sensitivity for elevated PVR as outlined above; even if VTI and TTFV underestimate PVR, MSN will highlight the likelihood of PVR>3WU.
PASP estimation using TTFV requires addition of RA pressure to transtricuspid gradient. Some suggest adding a constant value (e.g. 8 or 14mmg), while others use a clinical estimate from assessment of the jugular venous pressure or make a gross estimate based on features of the IVC. In choosing a constant, we opted for a middle value (8mmHg). Average VTI in the sample where PVR was <5WU (the subset likely to affected by small differences in the numerator) was ∼15cm, omitting RA pressure altogether might be expected to underestimate PVR, on average, by ∼0.5WU; adding 14mmHg might overestimate by ∼0.4WU. The relevance of such small differences is questionable when considered in light of the intrinsic variability of echocardiographic variables and catheter PVR.
Acknowledgments
This work was supported by National Institutes of Health 5-T32-HL07604-25 (ARO) and the Dunlevie Fund (ARO/MJL).
Funding sources: ARO supported in part by NIH 5 T32 HL07604-25, NIH UL1 RR 025758 and the Dunlevie Fund.
Footnotes
Disclosure: None.
References
- 1.McLaughlin VV, Presberg KW, Doyle RL, Abman SH, McCrory DC, Fortin T, Ahearn G. Prognosis of pulmonary arterial hypertension: ACCP evidence-based clinical practice guidelines. Chest. 2004;126:78S–92S. doi: 10.1378/chest.126.1_suppl.78S. [DOI] [PubMed] [Google Scholar]
- 2.Galie N, Hoeper MM, Humbert M, Torbicki A, Vachiery JL, Barbera JA, Beghetti M, Corris P, Gaine S, Gibbs JS, Gomez-Sanchez MA, Jondeau G, Klepetko W, Opitz C, Peacock A, Rubin L, Zellweger M, Simonneau G. Guidelines for the diagnosis and treatment of pulmonary hypertension: the Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT) Eur Heart J. 2009;30:2493–2537. doi: 10.1093/eurheartj/ehp297. [DOI] [PubMed] [Google Scholar]
- 3.Habib G, Torbicki A. The role of echocardiography in the diagnosis and management of patients with pulmonary hypertension. Eur Respir Rev. 2010;19:288–299. doi: 10.1183/09059180.00008110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fisher MR, Forfia PR, Chamera E, Housten-Harris T, Champion HC, Girgis RE, Corretti MC, Hassoun PM. Accuracy of Doppler echocardiography in the hemodynamic assessment of pulmonary hypertension. Am J Respir Crit Care Med. 2009;179:615–621. doi: 10.1164/rccm.200811-1691OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rich JD, Shah SJ, Swamy RS, Kamp A, Rich S. Inaccuracy of Doppler echocardiographic estimates of pulmonary artery pressures in patients with pulmonary hypertension: implications for clinical practice. Chest. 2011;139:988–993. doi: 10.1378/chest.10-1269. [DOI] [PubMed] [Google Scholar]
- 6.Janda S, Shahidi N, Gin K, Swiston J. Diagnostic accuracy of echocardiography for pulmonary hypertension: a systematic review and meta-analysis. Heart. 2011;97:612–622. doi: 10.1136/hrt.2010.212084. [DOI] [PubMed] [Google Scholar]
- 7.Hirschfeld S, Meyer R, Schwartz DC, Kofhagen J, Kaplan S. The echocardiographic assessment of pulmonary artery pressure and pulmonary vascular resistance. Circulation. 1975;52:642–650. doi: 10.1161/01.cir.52.4.642. [DOI] [PubMed] [Google Scholar]
- 8.Abbas AE, Fortuin FD, Schiller NB, Appleton CP, Moreno CA, Lester SJ. A simple method for noninvasive estimation of pulmonary vascular resistance. J Am Coll Cardiol. 2003;41:1021–1027. doi: 10.1016/s0735-1097(02)02973-x. [DOI] [PubMed] [Google Scholar]
- 9.Kouzu H, Nakatani S, Kyotani S, Kanzaki H, Nakanishi N, Kitakaze M. Noninvasive estimation of pulmonary vascular resistance by Doppler echocardiography in patients with pulmonary arterial hypertension. Am J Cardiol. 2009;103:872–876. doi: 10.1016/j.amjcard.2008.11.039. [DOI] [PubMed] [Google Scholar]
- 10.Roule V, Labombarda F, Pellissier A, Sabatier R, Lognone T, Gomes S, Bergot E, Milliez P, Grollier G, Saloux E. Echocardiographic assessment of pulmonary vascular resistance in pulmonary arterial hypertension. Cardiovasc Ultrasound. 2010;8:21. doi: 10.1186/1476-7120-8-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Selimovic N, Rundqvist B, Bergh CH, Andersson B, Petersson S, Johansson L, Bech-Hanssen O. Assessment of pulmonary vascular resistance by Doppler echocardiography in patients with pulmonary arterial hypertension. J Heart Lung Transplant. 2007;26:927–934. doi: 10.1016/j.healun.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 12.Scapellato F, Temporelli PL, Eleuteri E, Corra U, Imparato A, Giannuzzi P. Accurate noninvasive estimation of pulmonary vascular resistance by Doppler echocardiography in patients with chronic failure heart failure. J Am Coll Cardiol. 2001;37:1813–1819. doi: 10.1016/s0735-1097(01)01271-2. [DOI] [PubMed] [Google Scholar]
- 13.Dahiya A, Vollbon W, Jellis C, Prior D, Wahi S, Marwick T. Echocardiographic assessment of raised pulmonary vascular resistance: application to diagnosis and follow-up of pulmonary hypertension. Heart. 2010;96:2005–2009. doi: 10.1136/hrt.2010.204834. [DOI] [PubMed] [Google Scholar]
- 14.Haddad F, Zamanian R, Beraud AS, Schnittger I, Feinstein J, Peterson T, Yang P, Doyle R, Rosenthal D. A novel non-invasive method of estimating pulmonary vascular resistance in patients with pulmonary arterial hypertension. J Am Soc Echocardiogr. 2009;22:523–529. doi: 10.1016/j.echo.2009.01.021. [DOI] [PubMed] [Google Scholar]
- 15.Farzaneh-Far R, McKeown BH, Dang D, Roberts J, Schiller NB, Foster E. Accuracy of Doppler-estimated pulmonary vascular resistance in patients before liver transplantation. Am J Cardiol. 2008;101:259–262. doi: 10.1016/j.amjcard.2007.07.086. [DOI] [PubMed] [Google Scholar]
- 16.Farzaneh-Far R, Na B, Whooley MA, Schiller NB. Usefulness of noninvasive estimate of pulmonary vascular resistance to predict mortality, heart failure, and adverse cardiovascular events in Patients With stable coronary artery disease (from the Heart and Soul Study) Am J Cardiol. 2008;101:762–766. doi: 10.1016/j.amjcard.2007.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rajagopalan N, Simon MA, Suffoletto MS, Shah H, Edelman K, Mathier MA, Lopez-Candales A. Noninvasive estimation of pulmonary vascular resistance in pulmonary hypertension. Echocardiography. 2009;26:489–494. doi: 10.1111/j.1540-8175.2008.00837.x. [DOI] [PubMed] [Google Scholar]
- 18.Ajami GH, Cheriki S, Amoozgar H, Borzouee M, Soltani M. Accuracy of Doppler-derived estimation of pulmonary vascular resistance in congenital heart disease: an index of operability. Pediatr Cardiol. 2011;32:1168–1174. doi: 10.1007/s00246-011-0035-4. [DOI] [PubMed] [Google Scholar]
- 19.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ. Recommendations for chamber quantification. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 20.Arkles JS, Opotowsky AR, Ojeda J, Rogers F, Liu T, Prassana V, Marzec L, Palevsky HI, Ferrari VA, Forfia PR. Shape of the right ventricular Doppler envelope predicts hemodynamics and right heart function in pulmonary hypertension. Am J Respir Crit Care Med. 2011;183:268–276. doi: 10.1164/rccm.201004-0601OC. [DOI] [PubMed] [Google Scholar]
- 21.Nishimura RA, Tajik AJ. Evaluation of diastolic filling of left ventricle in health and disease: Doppler echocardiography is the clinician's Rosetta Stone. J Am Coll Cardiol. 1997;30:8–18. doi: 10.1016/s0735-1097(97)00144-7. [DOI] [PubMed] [Google Scholar]
- 22.Kircher BJ, Himelman RB, Schiller NB. Noninvasive estimation of right atrial pressure from the inspiratory collapse of the inferior vena cava. Am J Cardiol. 1990;66:493–496. doi: 10.1016/0002-9149(90)90711-9. [DOI] [PubMed] [Google Scholar]
- 23.Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, Solomon SD, Louie EK, Schiller NB. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23:685–713. doi: 10.1016/j.echo.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 24.Vlahos AP, Feinstein JA, Schiller NB, Silverman NH. Extension of Doppler-derived echocardiographic measures of pulmonary vascular resistance to patients with moderate or severe pulmonary vascular disease. J Am Soc Echocardiogr. 2008;21:711–714. doi: 10.1016/j.echo.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 25.Lam CS, Roger VL, Rodeheffer RJ, Borlaug BA, Enders FT, Redfield MM. Pulmonary hypertension in heart failure with preserved ejection fraction: a community-based study. J Am Coll Cardiol. 2009;53:1119–1126. doi: 10.1016/j.jacc.2008.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ristow B, Ali S, Ren X, Whooley MA, Schiller NB. Elevated pulmonary artery pressure by Doppler echocardiography predicts hospitalization for heart failure and mortality in ambulatory stable coronary artery disease: the Heart and Soul Study. J Am Coll Cardiol. 2007;49:43–49. doi: 10.1016/j.jacc.2006.04.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bursi F, McNallan SM, Redfield MM, Nkomo VT, Lam CS, Weston SA, Jiang R, Roger VL. Pulmonary pressures and death in heart failure: a community study. J Am Coll Cardiol. 2012;59:222–231. doi: 10.1016/j.jacc.2011.06.076. [DOI] [PMC free article] [PubMed] [Google Scholar]