Abstract
Objective
To evaluate endothelial function and vascular stiffness in large, medium, small and microcirculatory blood vessels in very early diffuse systemic sclerosis (SSc).
Methods
We studied consecutive early diffuse SSc patients, defined as < 2 years from first SSc symptom who did not have a prior cardiovascular event. Age, gender and race-matched controls were recruited. All underwent assessment of aortic pulse wave velocity (PWV), carotid intima-media thickness (IMT) brachial flow-mediated dilation (FMD), digital peripheral artery tonometer (EndoPAT) assessment and laser speckle contrast imaging (LSCI).
Results
15 early diffuse SSc and controls were evaluated. The average age was 49 years, 63% were female and 93% were Caucasian. There were no differences in body mass index, hypertension, diabetes or hyperlipidemia between controls and SSc patients. Mean SSc disease duration was 1.3 years. In the large central vessels, there was no difference in aortic PWV (p=0.71) or carotid IMT (p=0.92) between SSc patients and controls. Similarly, there was no difference in endothelial dysfunction with brachial artery FMD after ischemia (p=0.55) and nitroglycerin administration (p=0.74). There were significantly lower values for digital EndoPAT measures (p=0.0001) in SSc patients. LSCI revealed a distinct pattern of microcirculatory abnormalities in response to ischemia in SSc patients compared to controls. Imaging demonstrated a blunted microcirculatory hyperemia of the hand with greater subsequent response to nitroglycerin.
Conclusions
These findings suggest that earliest endothelial changes occur in smaller arterioles and microvascular beds, but not in medium or macrovascular beds, in early diffuse SSc.
Key Indexing Terms: scleroderma, systemic, scleroderma, diffuse, endothelium, vascular, laser speckle contrast analysis
Introduction
Systemic sclerosis (SSc) is an autoimmune disease characterized by vascular abnormalities, immune system activation and fibrosis. Current theories postulate that endothelial injury is an early, inciting event in SSc pathogenesis, with microvascular dysfunction as a hallmark of the disease (i.e. Raynaud phenomenon). Several cross-sectional studies have noted macrovascular abnormalities in SSc consisting of endothelial dysfunction as measured by brachial flow-mediated dilation (FMD) when compared to healthy controls (1–7). However, these studies have two limitations. First, they use prevalent populations with longstanding disease. Second, they have most often combined patients with limited and diffuse cutaneous SSc subsets, despite that these two subsets have a different natural history of disease.
A few reports have shown a modest correlation of disease duration with the extent of arterial stiffness, suggesting that the macrovascular changes progress over the disease course (8, 9), while others conclude that macrovascular dysfunction appears in early disease (7, 10). Only two studies specifically examined patients with diffuse scleroderma, finding that all of them had evidence of macrovascular changes with increased arterial stiffness and endothelial dysfunction as measured by brachial FMD, but disease duration was not reported (1, 11). Digital (small blood vessel) endothelial dysfunction by EndoPAT has only been examined in a very small cohort of SSc patients (12) with no difference in microvascular endothelial function compared to controls, but abnormal in those SSc patients with coexistent pulmonary hypertension. This is counterintuitive to the current hypotheses that microvascular dysfunction is one of the earliest vascular abnormalities in SSc.
Laser speckle contrast imaging is a newer, functional imaging technique that assesses cutaneous microcirculation and has been proposed as a marker for microvascular endothelial function (13, 14). When used with human skin, the LSCI has better reproducibility than laser Doppler flow (15–17)) and importantly in SSc patients, is less dependent on capillary density. Laser imaging techniques have been shown to respond to exercise (16) and drug therapy (18, 19), suggesting modification of endothelial function with these modalities and sensitivity to change of the technique. Only one published manuscript has reported LSCI findings suggesting that primary Raynaud phenomenon and SSc-Raynaud patients have markedly different patterns of microcirculatory flow response to ischemia challenge (20). Greater reactive hyperemia was noted in very early diffuse patients, suggesting that microvascular changes may be progressive over disease course. This contrasts with results reported for EndoPAT, raising the question of whether microcirculatory abnormalities are omnipresent in SSc, and if the time course to development is similar among patients.
We hypothesized that microcirculatory and microvascular changes are present in very early diffuse SSc, with the macrovascular changes occurring during an intermediate to late phase of the disease. The objective of this pilot study was to evaluate endothelial dysfunction in medium, small and microcirculatory vessels using an inception cohort of patients with very early diffuse SSc.
Materials and Methods
Patient Selection
We included consecutive early diffuse cutaneous SSc patients seen at the University of Pittsburgh Scleroderma Clinic from April 2011 to June 2012. Patients were eligible if they were evaluated and enrolled within two years of the first symptom attributable to SSc and had diffuse cutaneous involvement, defined as skin thickening proximal to the elbows or knees at the time of the first visit. All patients were > 18 years of age and able to sign an informed consent. Patients were excluded if they had pre-existing cardiovascular events or a serum creatinine> 2.0 mg/dL. For every SSc case, one age- (± 2 years), gender- and race-matched control subject without diabetes or known cardiovascular disease or events was studied.
Vascular Studies
All vascular studies were performed in the University of Pittsburgh Vascular Clinical &Translational Research Center (VCTRC). Subjects held all vasodilator drugs for 24 hours before the noninvasive vascular imaging studies. We selected tests shown to be reproducible and reliable to evaluate different vascular bed sizes in SSc patients. Prior to beginning vascular testing, all patients rested for 15 minutes in a quiet, dark and temperature-controlled room. At baseline visits, patients initially underwent aortic and carotid scanning first. Following this, they were allowed to rest prior to baseline measures being obtained for brachial flow mediated dilation (FMD), EndoPAT™ and LSCI. Measurements of brachial FMD, EndoPAT™ and LSCI were done simultaneously. Early diffuse SSc patients and controls underwent brachial FMD and EndoPAT testing at baseline and at one year of follow-up. Laser speckle contrast imaging was performed at only one time point in SSc patients and controls.
Carotid intima media thickness (IMT)
Carotid IMT was assessed through B-mode ultrasound imaging on both right and left common carotid arteries. Scanning was performed from the far walls of the distal common carotid arteries of each side to the far walls of the carotid bulbs using a GE 9L linear array transducer with frequency of 3–10 MHz (9L-GE, Healthcare Japan Corporation, Hino-shi, Tokyo, Japan) and GE Vivid 7 Dimension ultrasound (GE Vingmed Ultrasound A/S, Horten, Norway). The lumen-intima interface was measured electronically across a 1 cm segment. An average IMT score was obtained.
Aortic pulse wave velocity (PWV)
Arterial stiffness was assessed by measuring carotid–femoral pulse wave velocity (PWV) (with higher numbers signifying greater stiffness) using SphygmoCor CPVH (AtCor, version 9; AtCor Medical, Sydney, Australia). A hand-held high-fidelity SphygmoCor tonometer (AtCor, version 9; AtCor Medical, Sydney, Australia) was placed over the carotid and then the femoral arteries to record pressure waves simultaneously along with ECG tracings. The length of the descending aorta was approximated by subtracting the manubrium–carotid artery distance from the manubrium–femoral artery distance using a caliper. PWV in m/s was calculated in an automated fashion by proprietary software (AtCor, version 9; AtCor Medical, Sydney, Australia).
Brachial artery flow-mediated dilation (FMD)
As previously published (21), brachial artery FMD was used to assess endothelial function using two validated methods: 1) assessment of endothelial-dependent FMD (reactive hyperemia) by GE Vivid 7 Dimension ultrasound (GE Vingmed Ultrasound A/S, Horten, Norway) before and after vaso-occlusion of the medium-sized brachial artery induced by pneumatic cuff at a pressure of 280 mmHg or 60 mmHg higher than systolic blood pressure (whichever was the highest), for 5 minutes using GE 9L linear array transducer with frequency of 3–10 MHz (9L-GE, Healthcare Japan Corporation, Hino-shi, Tokyo, Japan) and 2) endothelial-independent vasodilation of the brachial artery before and after 0.4 mg of sublingual nitroglycerin (NTG) was administered as a liquid spray into the mouth. Brachial artery diameter was measured and calculated at baseline, 1, 2 and 3 minutes after reactive hyperemia induced by 5-minute cuff occlusion on the forearm and at 3, 5 and 10 minutes after NTG administration by means of averaging 3 measurements. FMD was calculated as the percent change (%FMD) in brachial diameter from the resting state (100*[hyperemic diameter at selected time resting diameter]/resting diameter) for reactive hyperemia. Similarly, vasodilator response to NTG was expressed as percentage change (NTG%) in diameter between baseline and post-NTG administration.
Digital pulse amplitude tonometry (Endo-PAT™)
Digital Pulse amplitude was measured with a Peripheral Arterial Tonometry (PAT) device by placing the probes on the tips of both index fingers (Endo-PAT 2000, version 3.3.2, Hamar Medical, Caesavea, Israel) as previously described (21). PAT signal measurement was performed with the digital probe inflation pressure set at 10mmHg below the diastolic pressure or 70 mmHg (whichever was the lowest) as previously described in the Framingham study (22). Briefly, baseline pulse amplitude was recorded bilaterally on tips of the index fingers for 5 minutes. This was followed by vaso-occlusion on the right side (the study finger) as described above for brachial FMD. After 5 minutes, the cuff was rapidly deflated and the PAT signal measurement was recorded for an additional 5 minutes. As the control, measurement of non-endothelial-dependent systemic changes occurring during the study was done on the contralateral finger. Mean PAT amplitudes were measured 90 seconds after the occlusion for a duration of 60 seconds. Finally, the ratio of the post-to-pre occlusion PAT amplitude of the tested arm, divided by the post-to-pre occlusion ratio of the control arm, was calculated as the Reactive Hyperemia Index (RHI). All PAT amplitudes and RHI are automatically calculated by the EndoPAT™ with an RHI of <1.67 previously validated as the cut off to define endothelial dysfunction.
Laser Speckle Contrast Imaging
The LSCI system produces a near infrared (780nm) laser light. The backscattered light forms a random interference (speckle) pattern. Movement, as produced by red blood cells, causes the speckle pattern intensity to change, thus allowing for quantification of flow (Ruaro 2013). For this study the PeriCam PSI system (PerimedAB, Jarfalla, Sweden) camera was placed 20 cm above the SSc patient hand, which was immobilized by a fluid support. Baseline LSCI measurements were obtained continuously beginning five minutes prior to vaso-occlusion until 5 minutes post-NTG administration. We selected a region of interest on the 2nd proximal metacarpophalangeal joint and obtained blood flow measures there at baseline (prior to occlusion), during ischemia, immediately post-ischemia (reactive hyperemia; RH), and the maximal value 3–5 minutes post-NTG administration. Since LSCI provides relative blood flow measures only all values were normalized to the patient’s baseline flow and expressed as a percentage.
Statistical Analysis
Descriptive statistics were used for baseline data. Differences between SSc patients and controls were assed for brachial artery FMD%, NTG% and fingertip. EndoPAT RHI was assessed by paired t-tests or Wilcoxon tests where appropriate at baseline and follow-up. Changes in FMD%, NTG% and RHI over one year between subjects were assessed by repeated measures analysis of variance. LSCI values measured including peak RH blood flow, time to peak RH, reperfusion nadir, post-NTG blood flow peak and NTG effect (the difference between pre- and post-NTG dose) were compared between SSc and control subjects using Mann-Whitney U test due to skewed distributions. Having concluded that SSc patients have endothelial dysfunction, we examined the ability of peak RH and reperfusion nadir blood flow numbers to predict endothelial dysfunction (ie SSc) using the area under the receiver operator curve.
Results
Fifteen individuals met inclusion criteria for early diffuse SSc, and 15 age-, gender- and race-matched controls were identified and consented. Baseline characteristics of the patients and controls are shown in Table 1. There were no significant difference in age, gender, race or cardiovascular risk factors between cases and controls. The average age was 49.5 years in cases, and 47.5 years in controls (p=0.80). Sixty-two percent of participants were female and 93% Caucasian. SSc patients were very early in their disease, with an average disease duration of 1.3 ± 0.5 years from first SSc symptom. From a vascular standpoint, nearly all (85%) patients had Raynaud phenomenon, but only 36% had digital tip pitting scars as the result of digital ischemia. Forty-three percent of patients had interstitial lung disease and gastrointestinal involvement, but none had cardiac or renal involvement. The majority (94%) of early diffuse SSc patients were taking SSc-directed therapy (mycophenolate mofetil, cyclophosphamide, methotrexate or d-penicillamine) at the time of the study, but no SSc patients or controls were taking a corticosteroid preparation. Forty percent of SSc patients were taking calcium channel blockers and 13% ACE-inhibitors, compared to 0% and 7% in controls, respectively. 13% of the patients and controls reported hyperlipidemia, and there was no difference in statin use between the groups. All SSc patients were felt to have active cutaneous disease at the time of the baseline study. Nine sets of matched cases and controls completed the one year follow-up study.
Table 1.
Baseline demographic, cardiovascular and disease characteristics in early diffuse systemic sclerosis (SSc) patients compared to controls
| Early diffuse SSc n=15 |
Healthy Controls n=15 |
p-value | |
|---|---|---|---|
| Demographic | |||
| Mean age | 49.5 ± 14.3 | 47.5 ± 13.7 | 0.80 |
| Female (%) | 62% | 62% | -- |
| Caucasian (%) | 93% | 93% | -- |
| Cardiovascular Risk Factors | |||
| Mean body mass index | 27.4 ± 6.2 | 27.5 ± 5.6 | 0.71 |
| Hypertension | 13% | 27% | 0.65 |
| Diabetes Mellitus | 0% | 0% | 1.00 |
| Hyperlipidemia | 47% | 53% | 0.99 |
| Tobacco use | 13% | 0% | 0.48 |
| Family history of cardiovascular disease | 20% | 27% | 0.92 |
| SSc disease characteristics | |||
| Mean disease duration ± SD | 1.3 ± 0.5 years | ||
| Mean modified Rodnan skin score ± SD | 20.2 ± 8.7 | ||
| Active tendon friction rubs | 47% | ||
| Raynaud phenomena | 86% | ||
| Digital pitting scars | 36% | ||
| Digital ulcers | 29% | ||
| Telangiectasias | 20% | ||
| Interstitial lung disease | 43% | ||
| Gastrointestinal manifestations | 43% | ||
| Cardiac manifestations | 0% | ||
| Scleroderma renal crisis | 0% | ||
| Medication use | |||
| Mycophenolate mofetil | 40% | - | |
| D-penicillamine | 40% | - | |
| Methotrexate | 7% | - | |
| Cyclophosphamide | 7% | - | |
| Calcium channel blockers | 40% | 0% | 0.02 |
| Angiotensin receptor blockers | 0% | 0% | 1.0 |
| Ace-Inhibitors | 13% | 7% | 0.99 |
| Statin use | 27% | 20% | 0.99 |
Aortic PWV and Carotid IMT
At baseline, assessment of the large central vessels revealed no difference in carotid IMT between cases and controls (p=0.92) with a mean carotid IMT of 0.70 ± 0.12 in early diffuse SSc and 0.69 ± 0.13 in controls. Similarly, there was no evidence of increased aortic PWV in patients with early diffuse SSc (6.60 ± 1.43) compared to controls (6.43 ± 1.10; p=0.71). When the augmentation index was calculated there was also no difference (p=0.35) between cases and controls.
Brachial FMD
At baseline, there was no difference in baseline brachial artery diameter between SSc patients (0.32 ± 0.05) and controls (0.33 ± 0.07; p=0.77). During RH there was no difference in the FMD% between SSc (4.3%±5.2) and controls (3.2%±4.2; p=0.55). After administration of NTG, the response of SSc (18.1 ± 10.3%) and controls (19.2 ± 9.4%) were not significantly different from one another (p=0.74).
At one year of follow-up, there again remained no difference in brachial artery size prior to occlusion and no change in FMD% between cases (median 3.6% (IQR 2.3, 5.9)) and controls (3.7% (2.4, 8.0); p=0.75). Similar results were found after NTG challenge, with SSc patients having a mean change of 20.7% ±0.9, compared to controls at 15.5% ± 7.5 (p=0.52). The presence of digital pitting scars or history of ulcerations did no change the results (p=0.72). Similarly, there was no difference in the change in FMD% over one year between baseline and controls. When the results were examined within subjects and between groups at baseline and follow-up, the change in vasodilator response to ischemia over one year was not different between the SSc patients and controls (p=0.66)
Digital pulse amplitude tonometry (Endo-PAT™)
There was a significant difference in baseline EndoPAT results between cases and controls with a mean RHI of 1.11 ±0.57 for SSc patients compared to control subject RHI of 2.05 ± 0.51 (p= 0.001). Overall, using standard cut-offs (<1.67 defining presence of endothelial dysfunction (ref)), 85% of SSc patients had an abnormal RHI suggesting endothelial dysfunction at baseline, compared to 23% of controls (p=0.002). At one year of follow-up, all of the returning SSc patients had endothelial dysfunction by RHI, compared to 15% of controls re-studied (p=0.001). There was no difference in the pattern of results between patients with digital pitting scars or history of digital tip ulcerations compared to those without these vascular manifestations.
Laser speckle contrast imaging
LSCI revealed a distinct pattern of microcirculatory abnormalities in response to ischemia in SSc patients compared to controls, that is summarized in Figure 1. SSc patients had lower median RH peak blood flow compared to controls (median [IQR]: 174.4% [120.3, 204] vs. 315.3% [243, 433]; p=0.002), and a longer median time to peak RH (35 sec [9.5, 47] vs.10 sec [7, 15.5]; p=0.019). SSc patients also demonstrated a lower median nadir blood flow after ischemia compared to controls (84.5% [78.7, 90.5] vs. 95.4% [93.3, 100.3]; p=−001) and greater median response to NTG (117% [111.5, 137.5] vs. 104% [97.5, 111]; p=0.007). The area under the ROC for diagnosing SSc compared to control was highly significant using criteria of peak RH (AUC=0.85; p=0.003) and reperfusion nadir (AUC=0.86; p=−.002). The reperfusion peak had an area under the curve (AUC) = 0.80 (p=0.01) for predicting response to NTG in SSc patients.
Figure 1. Changes in microvascular blood flow using laser speckle contrast imaging (LSCI).
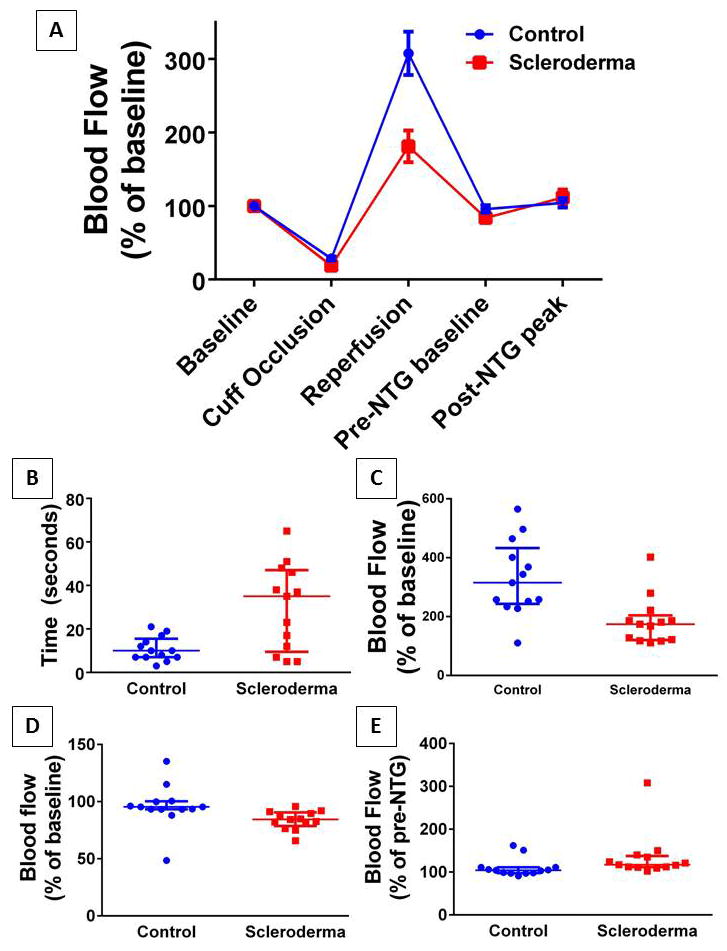
LSCI obtained continuous blood flow measurements in systemic scleroderma patients or matched controls during the course of the experimental protocol (summarized in A). All values were normalized to the mean baseline blood flow arbitrary units per group resulting in both groups beginning at 100% flow. Each dot represents a subject and the bars represent the median and interquartile range. Groups differed significantly in the median time required to reach peak post-occlusive reactive hyperemia (B; p=0.019) as well as the maximum blood flow reached (C; p=0.002). In addition scleroderma patients had a post-occlusive blood flow nadir which was less than baseline and significantly lower than controls (D; p=0.001) and a larger response to sublingual nitroglycerin (E; p=0.007).
Discussion
In this inception cohort of very early diffuse cutaneous SSc patients, we have demonstrated using EndoPAT and LSCI that there is endothelial dysfunction evident in the digital arterioles and capillaries when compared to age-, gender- and race-matched controls. However, there were no significant differences in endothelial function compared to controls in the larger muscular brachial artery. These results suggest that vascular dysfunction in early disease is limited to the microvascular arterioles and capillary microcirculation using these currently available methods of evaluation, and that macrovascular involvement may occur later in disease. This is the first description of the use of EndoPAT and LSCI in a homogenous group of patients with very early diffuse SSc.
Different results for micro and macrovascular endothelial function have been previously reported. In a recent report from the Framingham Heart Study, no relationship between PAT hyperemic response and FMD hyperemic flow was found (21), and separate risk factors for abnormal brachial FMD and digital vascular function (EndoPAT) were identified. While a lower brachial FMD was associated with age, gender, blood pressure and cardiovascular disease, abnormal digital PAT ratio was associated with gender and metabolic abnormalities (higher BMI, cholesterol, presence of diabetes, smoking and use of lipid-lowering medications). Interestingly, the prevalence of a low PAT hyperemic response did not vary with advancing age, suggesting preserved distal vessel hyperemic response as individuals age. Taken together, these findings support the possibility that vascular bed and vessel size determine sensitivity to early damage by specific cardiovascular risk factors and aging. Thus, it seems likely that disease-related factors in systemic sclerosis could impact distinct vascular beds differently.
There are only a few studies which have examined segmental vasoculopathy of different vessel size in SSc patients and controls. Although digital arteries were not examined (23), Liu et al. reported increased arterial PWV in the forearm (radial-brachial), as compared to no increased arterial stiffness in the carotid-brachial, carotid-femoral and femoral-ankle regions. The authors included patients with both limited and diffuse SSc of unreported disease duration, but did specify that no correlation between PWV parameters and disease duration was found. Anderson et al. examined 24 SSc patients with longstanding disease (mean 13.6 years) with both brachial artery FMD and radial artery pulse applanationtonometry (24). They found the radial artery to be stiffer in SSc patients, but this result did not correlate with disease duration. Similarly independent of disease duration, there was no difference in brachial FMD in response to ischemia or NTG administration. Only one study (1) specifically examined diffuse SSc patients and confirmed Anderson’s findings of abnormal radial artery stiffness by PWV. They also noted impaired brachial FMD response to ischemia in SSc patients, although response of the brachial artery to NTG was not different. Unfortunately, no specific information on disease duration was provided. These studies support the concept that there may be different abnormalities by the type of vasculature, although the relationship to disease subtype and duration remains unclear.
Only Peled et al. have previously assessed fingertip tomography using the EndoPAT 2000 device in SSc, comparing the RHI indices and augmentation index after hyperemia (12). They used SSc patients with and without pulmonary hypertension (PH), idiopathic PH and healthy controls. In their comparison, no difference was found in EndoPAT hyperemia results between healthy controls and SSc patients, but SSc-PH and idiopathic PH patients had lower indices than controls. The results were interpreted as suggesting that endothelial dysfunction is associated with PH regardless of etiology. Again, no information was provided on disease subtype or duration of SSc.
LSCI is a newer imaging modality to assess cutaneous blood perfusion, and has been found to be a marker of cutaneous endothelial dysfunction (25). Our data presented here with LSCI imaging demonstrates a unique pattern of blunted microcirculatory hyperemia of the hand post-ischemia with significantly lower reperfusion peak, longer time to perfusion peak and lower nadir blood flow in the early diffuse SSc patients, suggesting microcirculatory endothelial dysfunction. SSc patients then had an increased response to sublingual NTG compared to controls, suggesting that reduced endogenous NO availability may be the underlying mechanism for this observed microcirculatory endothelial dysfunction. There are few reports of LSCI in SSc patients (20, 26). These studies have used employed different techniques, specifying regions of interest in different locations of the hand, used single or combined hands, and exposed subjects to different conditions (ambient temperature, cold water challenge, post-ischemic hyperemia) making it difficult to compare directly to our study. However, in a study of 36 SSc patients, 20 Raynaud disease patients and 20 healthy controls, SSc patients had a slower time to recover post-occlusive hyperemia compared to controls or Raynaud disease patients, and peak flow was reduced in SSc patients compared to primary Raynaud patients. This is similar to our findings. In a subgroup of 8 very early SSc patients, there was higher peak flow and post-occlusive hyperemia compared to later SSc patients, suggesting a different vascular response in SSc patients based on disease duration, although they mixed diffuse and limited patients (20). Our findings in early diffuse SSc patients alone are somewhat contradictory to this finding as we noted lower peak RH. This difference may be related to our larger sample size, or to the restriction to the diffuse cutaneous subset, as they develop their internal organ complications in the early phases of disease compared to limited SSc patients. In a second study of 61 SSc patients of variable disease duration compared to age and gender-matched controls (26), LSCI showed significantly lower blood perfusion in all SSc patients. Blood perfusion correlated with degree of nailfold capillary abnormalities and diffuse SSc patients had lower average blood perfusion than limited SSc patients, although this was of borderline statistical significance. This same study found LSCI to have lower intra-operator variability compared to LDF, and to be reliable on repeated measures in SSc, supporting it as an easy to use and reliable method of assessing cutaneous circulation in SSc patients. Since LSCI does not provide absolute measure of blood flows even after subtraction of the biologic zero, we did not compare baselines between our patients rather their response to a similar stressor, namely vaso-occlusion.
Reports restricted to evaluation of diffuse SSc patients are few in number. Two studies compared brachial FMD during reactive hyperemia and found this to be low when compared to healthy controls (1, 4). Lekakis et al. reported on diffuse SSc patients with a mean duration of Raynaud symptoms > 8 years, suggesting later disease. Only Turiel et al. studied central vascular stiffness and carotid IMT in diffuse SSc patients, in this case with a mean disease duration of over 4 years after the first non-Raynaud symptom of SSc (11). When our results are considered in the context of these studies, it is likely that abnormalities in the medium sized conduits or larger arteries first occurs during the intermediate or later phase of these diseases, as opposed to early in the disease as suggested elsewhere (7, 10).
Our study has some limitations. The patient sample is small and from a single Scleroderma Center. One may expect that worse vascular complications may be related to more vascular abnormalities. We did perform a sensitivity analysis of those with evidence of microvascular damage (digital pitting scars or history of ulcerations) versus not, and there was no change in the results. However, it is conceivable that a difference may exist and be limited by our small numbers. Finally, we did not perform detailed nailfold capillaroscopy at the time of the exams, which may have provided additional data regarding microvascular damage. While our controls did not have cardiovascular disease or diabetes, several did have hyperlipidemia, which may have affected our comparison results for the brachial FMD. In order to address this, we did perform a sensitivity analysis for hyperlipidemia, and the results were unchanged.
In conclusion, patients with early diffuse cutaneous SSc have evidence of endothelial dysfunction in the microvasculature of the fingertips and hand, but no evidence of endothelial dysfunction in the medium-sized conduit arteries. This is the first study to report these findings together using these specific imaging techniques. These results suggest that the earliest endothelial abnormalities are microvascular, with macrovascular endothelial changes occurring later. Additional studies of early diffuse SSc are needed to further understand the progression of vascular changes and the disease-related and non-disease related factors which influence vascular changes.
Table 2.
Summary of Vascular Study Results at Baseline and One Year Follow-up
| Baseline SSc patients | Baseline Controls | p-value | One year SSc patients | One year Controls | p-value | |
|---|---|---|---|---|---|---|
|
| ||||||
| Aortic PWV (meter/second) | 6.60 ± 1.43 | 6.43 ± 1.10 | 0.71 | -- | -- | |
| Carotid IMT (mm) | 0.70 ± 0.12 | 0.69 ± 0.13 | 0.92 | -- | -- | |
| Brachial FMD (% change)* | 4.3 ± 5.2 | 3.2 ± 4.2 | 0.55 | 4.1 ± 5.7 | 5.7 ± 6.1 | 0.66 |
| Endo-PAT RHI | 1.11 ±0.57 | 2.05 ± 0.51 | 0.001 | 1.40 ± 0.52 | 2.37 ± 0.49 | 0.004 |
reactive hyperemia after forearm ischemia
Acknowledgments
This work was supported by a grant from the National Institutes of Health (NIAMS P60-AR054731). Dr. Domsic is supported by a grant from the National lnstitutes of Health (NIAMS K23AR057485). Dr. Dezfulian is supported by a grant from the National Institutes of Health (NINDS K08NS069817).
References
- 1.Cypiene A, Laucevicius A, Venalis A, Dadoniene J, Ryliskyte L, Petrulioniene Z, Kovaite M, Gintautas J. The impact of systemic sclerosis on arterial wall stiffness parameters and endothelial function. Clinical rheumatology. 2008;27(12):1517–22. doi: 10.1007/s10067-008-0958-1. [DOI] [PubMed] [Google Scholar]
- 2.Fernandes TM, Bica BEG, Villela NR, Salles EF, de Azevedo MNL, Papi JAD, Martins RAG. Evaluation of endothelial function in patients with limited systemic sclerosis by use of brachial artery Doppler ultrasound. Revista Brasileira De Reumatologia. 2012;52(4):554–68. [PubMed] [Google Scholar]
- 3.Hofstee HM, Voskuyl AE, Vonk Noordegraaf A, Smulders YM, Postmus PE, Dijkmans BA, Serne EH. Pulmonary arterial hypertension in systemic sclerosis is associated with profound impairment of microvascular endothelium-dependent vasodilatation. The Journal of rheumatology. 2012;39(1):100–5. doi: 10.3899/jrheum.110397. [DOI] [PubMed] [Google Scholar]
- 4.Lekakis J, Papamichael C, Mavrikakis M, Voutsas A, Stamatelopoulos S. Effect of long-term estrogen therapy on brachial arterial endothelium-dependent vasodilation in women with Raynaud’s phenomenon secondary to systemic sclerosis. The American journal of cardiology. 1998;82(12):1555–7. A8. doi: 10.1016/s0002-9149(98)00708-5. [DOI] [PubMed] [Google Scholar]
- 5.Mavrikakis ME, Lekakis JP, Papamichael CM, Stamatelopoulos KS, Kostopoulos ChC, Stamatelopoulos SF. Ascorbic acid does not improve endothelium-dependent flow-mediated dilatation of the brachial artery in patients with Raynaud’s phenomenon secondary to systemic sclerosis. International journal for vitamin and nutrition research Internationale Zeitschrift fur Vitamin- und Ernahrungsforschung Journal international de vitaminologie et de nutrition. 2003;73(1):3–7. doi: 10.1024/0300-9831.73.1.3. [DOI] [PubMed] [Google Scholar]
- 6.Rossi P, Granel B, Marziale D, Le Mee F, Frances Y. Endothelial function and hemodynamics in systemic sclerosis. Clinical physiology and functional imaging. 2010;30(6):453–9. doi: 10.1111/j.1475-097X.2010.00965.x. [DOI] [PubMed] [Google Scholar]
- 7.Szucs G, Timar O, Szekanecz Z, Der H, Kerekes G, Szamosi S, Shoenfeld Y, Szegedi G, Soltesz P. Endothelial dysfunction precedes atherosclerosis in systemic sclerosis--relevance for prevention of vascular complications. Rheumatology. 2007;46(5):759–62. doi: 10.1093/rheumatology/kel426. [DOI] [PubMed] [Google Scholar]
- 8.Colac M, Giuggioli D, Mangredi A, Sebastiani M, Coppi F, Rossi R. Aortic pulse wave velocity measurement in systemic sclerosis patients. Reumatismo. 2012;64:360–7. doi: 10.4081/reumatismo.2012.360. [DOI] [PubMed] [Google Scholar]
- 9.Timar O, Soltesz P, Szamosi S, Der H, Szanto S, Szekanecz Z, Szucs G. Increased arterial stiffness as the marker of vascular involvement in systemic sclerosis. The Journal of rheumatology. 2008;35(7):1329–33. [PubMed] [Google Scholar]
- 10.Gosse P, Taillard J, Constans J, Investigators ES. Evolution of ambulatory measurement of blood pressure and parameters of arterial stiffness over a 1-year period in patients with systemic sclerosis: ERAMS study. Journal of human hypertension. 2002;16(9):627–30. doi: 10.1038/sj.jhh.1001466. [DOI] [PubMed] [Google Scholar]
- 11.Turiel M, Gianturco L, Ricci C, Sarzi-Puttini P, Tomasoni L, de Colonna VG, Ferrario P, Epis O, Atzeni F. Silent cardiovascular involvement in patients with diffuse systemic sclerosis: a controlled cross-sectional study. Arthritis care & research. 2013;65(2):274–80. doi: 10.1002/acr.21819. [DOI] [PubMed] [Google Scholar]
- 12.Peled N, Shitrit D, Fox BD, Shlomi D, Amital A, Bendayan D, Kramer MR. Peripheral arterial stiffness and endothelial dysfunction in idiopathic and scleroderma associated pulmonary arterial hypertension. The Journal of rheumatology. 2009;36(5):970–5. doi: 10.3899/jrheum.081088. [DOI] [PubMed] [Google Scholar]
- 13.Humeau-Heurtier A, Guerreschi E, Abraham P, Mahe G. Relevance of laser Doppler and laser speckle techniques for assessing vascular function: state of the art and future trends. IEEE Trans Biomed Eng. 2013;60(3):659–66. doi: 10.1109/TBME.2013.2243449. [DOI] [PubMed] [Google Scholar]
- 14.Senarathna J, Rege A, Li N, Thakor NV. Laser Speckle Contrast Imaging: theory, instrumentation and applications. IEEE Rev Biomed Eng. 2013;6:99–110. doi: 10.1109/RBME.2013.2243140. [DOI] [PubMed] [Google Scholar]
- 15.Puissant C, Abraham P, Durand S, Humeau-Heurtier A, Faure S, Leftheriotis G, Rousseau P, Mahe G. Reproducibility of non-invasive assessment of skin endothelial function using laser Doppler flowmetry and laser speckle contrast imaging. PLoS One. 2013;8(4):e61320. doi: 10.1371/journal.pone.0061320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tew GA, George KP, Cable NT, Hodges GJ. Endurance exercise training enhances cutaneous microvascular reactivity in post-menopausal women. Microvascular research. 2012;83(2):223–8. doi: 10.1016/j.mvr.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 17.Roustit M, Millet C, Blaise S, Dufournet B, Cracowski JL. Excellent reproducibility of laser speckle contrast imaging to assess skin microvascular reactivity. Microvascular research. 2010;80(3):505–11. doi: 10.1016/j.mvr.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 18.Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34) UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352(9131):854–65. [PubMed] [Google Scholar]
- 19.Jadhav S, Ferrell W, Greer IA, Petrie JR, Cobbe SM, Sattar N. Effects of metformin on microvascular function and exercise tolerance in women with angina and normal coronary arteries: a randomized, double-blind, placebo-controlled study. Journal of the American College of Cardiology. 2006;48(5):956–63. doi: 10.1016/j.jacc.2006.04.088. [DOI] [PubMed] [Google Scholar]
- 20.DellaRossa A, Cazzato M, Bencivelli W, Ascanio A, Mosca M, Bonbardieri S. Laser speckle contrast imaging may help in the differential diagnosis of Raynaud’s phenomenon (abstract) Arthritis and rheumatism. 2012:S636–7. [Google Scholar]
- 21.Hamburg NM, Palmisano J, Larson MG, Sullivan LM, Lehman BT, Vasan RS, Levy D, Mitchell GF, Vita JA, Benjamin EJ. Relation of brachial and digital measures of vascular function in the community: the Framingham heart study. Hypertension. 2011;57(3):390–6. doi: 10.1161/HYPERTENSIONAHA.110.160812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mulukutla SR, Venkitachalam L, Bambs C, Kip KE, Aiyer A, Marroquin OC, Reis SE. Black race is associated with digital artery endothelial dysfunction: results from the Heart SCORE study. European heart journal. 2010;31(22):2808–15. doi: 10.1093/eurheartj/ehq295. [DOI] [PubMed] [Google Scholar]
- 23.Liu J, Zhang Y, Cao TS, Duan YY, Yuan LJ, Yang YL, Li Y, Yao L. Preferential macrovasculopathy in systemic sclerosis detected by regional pulse wave velocity from wave intensity analysis: comparisons of local and regional arterial stiffness parameters in cases and controls. Arthritis care & research. 2011;63(4):579–87. doi: 10.1002/acr.20306. [DOI] [PubMed] [Google Scholar]
- 24.Anderson TJ, Elstein E, Haber H, Charbonneau F. Comparative study of ACE-inhibition, angiotensin II antagonism, and calcium channel blockade on flow-mediated vasodilation in patients with coronary disease (BANFF study) Journal of the American College of Cardiology. 2000;35(1):60–6. doi: 10.1016/s0735-1097(99)00537-9. [DOI] [PubMed] [Google Scholar]
- 25.Mahe G, Durand S, Humeau-Heurtier A, Leftheriotis G, Abraham P. Impact of experimental conditions on noncontact laser recordings in microvascular studies. Microcirculation. 2012;19(8):669–75. doi: 10.1111/j.1549-8719.2012.00205.x. [DOI] [PubMed] [Google Scholar]
- 26.Ruaro B, Sulli A, Alessandri E, Pizzorni C, Ferrari G, Cutolo M. Laser speckle contrast analysis: a new method to evaluate peripheral blood perfusion in systemic sclerosis patients. Annals of the rheumatic diseases. 2013 doi: 10.1136/annrheumdis-2013-203514. [Epub] [DOI] [PubMed] [Google Scholar]


