Abstract
Models of reading must explain how orthographic input activates a phonological representation, and elicits the retrieval of word meaning from semantic memory. Comparisons between tasks that theoretically differ with respect to the degree to which they rely on connections between orthographic, phonological and semantic systems during reading can thus provide valuable insight into models of reading, but such direct comparisons are not well-represented in the literature. An ALE meta-analysis explored lexicality effects directly contrasting words and pseudowords using the lexical decision task and overt or covert naming, which we assume rely most on the semantic and phonological systems, respectively. Interactions between task and lexicality effects demonstrate that different demands of the lexical decision and naming tasks lead to different manifestations of lexicality effects.
1 Introduction
Reading entails the decoding of visual orthographic representations into a phonological representation. The ease with which skilled readers map between these very different representational systems is the product of a great deal of explicit and implicit learning. In alphabetic languages, on which we focus here, a fluent reader will have spent considerable time undertaking explicit instruction in the rules for mapping letters and letter combinations to existing verbal representations (i.e., the alphabetic principle). Models of reading development and disorders agree that phonologically decoding a particular string of letters depends on whether or not those letters map to a word with which an individual is familiar. Lexicality manipulations are consequently an important tool for investigating reading processes. Lexicality refers to whether a letter string represents a word with an associated meaning (e.g., TRAY). Letter strings that do not represent words can be either pseudowords (e.g., TAYR), which are pronounceable strings of letters sharing characteristics of legal words but without an associated meaning, or non-words (e.g., RTYA), which have no associated meaning and additionally violate the spelling rules for a language. Lexicality presumably influences many aspects of language processing and may consequently be investigated using any number of experimental tasks. Of these, however, the lexical decision task (LDT) and naming (overt or covert) dominate the neuroimaging literature (Katz et al., 2012).
1.1 LDT and Naming Task Characteristics
In the context of orthographic processing, the LDT requires participants to indicate whether a given letter string is associated with a real word. Participants are not expected to retrieve or even possess robust semantic representations for these words, but must merely be aware that some such representation exists, and this task has consequently been described as a signal detection process (Jacobs, Graf, & Kinder, 2003). Not all models of reading agree on the degree to which the LDT relies on semantic knowledge. For example, in the dual route cascaded (DRC) model of reading aloud (Coltheart, Rastle, Perry, Langdon, & Ziegler, 2001), lexicality decisions are based on the outcome of a lookup process in the orthographic lexicon, and may proceed even if the semantic system is removed entirely (Coltheart, Saunders, & Tree, 2010). A contrasting perspective, taken by parallel distributed processing (PDP) models, such as the triangle model (Seidenberg & McClelland, 1989) is that there are no lexicons (Dilkina, McClelland, & Plaut, 2010). Rather, reading in these models is the product of the dynamic interaction of orthographic, phonological and semantic processing systems (Harm & Seidenberg, 2004). The centrality of these interactions to the triangle model of reading, which assumes that skilled reading is the dynamic product of interactions between these systems, suggests this model as a framework for their interpretation. Unfortunately, only one study to date (Harm & Seidenberg, 2004) has fully implemented the triangle model (i.e., containing semantic, orthographic and phonological representational units), and this study did not explore the interaction between task and lexicality. Within the triangle model, the presence or absence of associations between a particular orthographic/phonological pattern and a semantic representation determine the lexicality status of a token. We take the position that the LDT is, by definition, tied to semantic memory, as even in the DRC model, lexical entries exists only for a letter strings with underlying semantic representations. This position is supported behaviorally, as LDT appears to automatically activate semantic representations, if available, though this activation may decay quickly without active maintenance (Neely, O’Connor, & Calabrese, 2010). Moreover, compared to naming, LDT performance appears to be more dependent on semantic properties of words (Balota, Cortese, Sergent-Marshall, Spieler, & Yap, 2004; Yap & Balota, 2009). We reiterate for clarity, however, that different models make different assumptions regarding the nature and degree of support that semantic knowledge provides. Within the DRC, for example, the semantic system may provide input into the phonological and orthographic lexicons, providing a basis for semantic priming effects in LDT and naming tasks (Blazely, Coltheart, & Casey, 2005), but it is not strictly required for either task. Moreover, simulations of semantic processing in these tasks within the DRC do not exist. Thus, it is unclear whether the DRC predicts that the LDT should be particularly sensitive to semantic input.
Naming, whether overt or covert, requires participants to transform a given letter string into the corresponding phonological representation, and in the case of overt naming, or “reading aloud”, additionally generate the articulatory motor sequences required to verbalize that representation. Because the spelling-to-sound mappings for pseudowords are unfamiliar, reading aloud should be more difficult for these items. The triangle model assumes that naming taps semantic representations, and the neuroimaging literature supports this argument (Binder, Medler, Desai, Conant, & Liebenthal, 2005). However, we assume that naming task performance is more tightly bound to processing within the phono-articulatory system, and this too is borne out behaviorally: Balota and colleagues carried out hierarchical regression analyses of naming and LDT latencies for monosyllabic (Balota et al., 2004) and multisyllabic words (Yap & Balota, 2009). These studies, which examined the influences of phonological (e.g., onset phoneme characteristics), lexical (e.g., orthographic neighborhood size) and semantic (e.g., imageability) features show that phonological features and word length (both characteristics relevant to pronunciation) are more predictive of naming performance, whereas semantic variables were more predictive of LDT performance.
Because only words have associated semantic content, we predict increased activation for words relative to pseudowords in regions implicated in semantic processing, most pronounced for the LDT. Conversely, we predict increased pseudoword activation in phono-articulatory areas, reflecting the increased difficulty in making spelling-to-sound mapping for these items, and this should most pronounced in naming.
To our knowledge, only Carreiras, Mechelli, Estevez, and Price (2007) have explored task by lexicality interactions, finding some evidence that lexicality effects are modulated by task. Naming was associated with greater left precentral gyrus activation than the LDT for the [Pseudowords > Words] contrast, which the authors argued reflects non-semantic phonological retrieval for pseudowords, supporting the argument that naming more strongly taps phonological processes and that these activations should be stronger for pseudowords. However, the LDT was associated with greater right inferior frontal gyrus activation (IFG) for words, which they argued reflected response inhibition for pseudowords, rather than semantic activation for words. Because processes related to response selection and attention have not been modeled within the triangle model, we will not speculate on this result. Carreiras et al. did, however, find greater activity for words than for pseudowords in a middle temporal region implicated in semantic processing (Binder, Desai, Graves, & Conant, 2009) that was numerically greater for LDT. This leaves open the possibility of a subtle task by lexicality interaction within this region, or that the items used in this particular experiment were not ideally suited for eliciting robust semantic activation. A meta-analytic review of task and lexicality effects may thus reveal semantic-processing related interactions between lexicality and task in middle temporal regions.
1.2 Previous Meta-Analyses of Lexicality Effects
Reading in alphabetic languages involves the coordination of a network of brain regions that, broadly speaking, play specialized roles in supporting orthographic, phonological and semantic processing. The role of individual or networks of brain regions underlying these processes has been studied in great deal. Orthographic processing is attributed to bilateral occipitotemporal cortex and left mid-fusiform gyrus. Phonological processing is attributed to left superior posterior temporal cortex and the temporoparietal junction and inferior frontal gyrus extending to premotor cortex. Finally, semantic processing is attributed to anterior fusiform and inferior and middle temporal gyrus and the anterior inferior frontal sulcus. Though a thorough summary of the literature supporting these functional assignments is beyond the scope of the present article, they fall from meta-analyses of the neuroimaging literature (Taylor, Rastle, & Davis, 2013), and are also consistent with a large body of patient studies (e.g., Damasio, 1992; Schwartz et al., 2009; Turkeltaub et al., 2013).
As argued earlier, lexicality effects provide insight into the effect of word knowledge on reading, and experimental manipulations involving words and pseudowords are commonly used. Three previous meta-analyses have examined the patterns of word and pseudoword activations across multiple tasks, including naming, lexical decision, phonological decision and semantic tasks. Jobard, Crivello, and Tzourio-Mazoyer (2003) and Cattinelli, Borghese, Gallucci, and Paulesu (2013) used anatomical label as a clustering mechanism, in contrast with the ALE approach used by Taylor, Rastle and Davis (2013), and in the present study, which assesses inter-study concordance by measuring co-activations within gaussian fields. There are many ways in which words and nonwords differ, and lexicality effects can consequently be used to provide insight into many aspects of reading. The Cattinelli study aimed to further qualify the subnetworks that support different aspects of reading, and the authors argued that word and pseudoword reading depends on distinct subnetworks involved in lexical/semantic processing and in phonological/orthographic processing, respectively. Because models often make different assumptions about how lexicality influences reading, lexicality effects are often used to support or challenge these models. The Jobard and Taylor meta-analyses examined many such studies to assess whether the neuroimaging literature generally supports the DRC (Jobard et al., 2003), and test several predictions made by the DRC, connectionist dual-process (CDP+) and triangle models (Taylor et al., 2013). Though Cattinelli et al. (2013) separately examined the effects of lexicality, task and difficulty (which may also be task-dependent), none of the previous meta-analyses have examined interactions between lexicality and task.
1.3 Summary of Predictions
Analyses of lexicality by task interactions would provide valuable insight into how semantic and phonological knowledge interact with the orthographic system during reading. Because these interactions have not been formally modeled in a fully-implemented simulation of the triangle model, our predictions are inferred from properties of the model discovered through related simulations, and those that are generally true of this class of connectionist models. The present meta-analysis explores task-driven interactions between semantic, phonological, and orthographic systems in the context of the triangle model of reading. There is a rich body of neuroimaging literature exploring the neural substrates of these systems. Understanding how these systems interact during reading and help constrain models of reading. We predict that task effects will emerge in brain regions implicated in semantic and phonological processing between the LDT and Naming tasks, which we assume to depend differently on semantic and phonological processing. Moreover, because words may have directly associated semantic representations, but pseudowords do not, and pseudowords should be more difficult to decode, we similarly predict that lexicality effects favoring words or pseudowords should be apparent in brain regions implicated in semantic and phonological processing, respectively. Finally, we predict that task and lexicality effects will interact additively, such that activation for naming relative to LDT will be strongest for pseudowords, and that activation for LDT relative to naming will be strongest for words.
2 Material and Methods
2.1 ALE Dataset
Searches for candidate reading studies were conducted in the PubMed and Google Scholar databases for fMRI and PET studies investigating reading that employed either the LDT or overt or covert naming tasks where the terms “fMRI” or “PET” and “Lexical Decision Task” or “Naming” or “Covert Reading” or “Overt Reading” and “Pseudoword” appeared in the title or abstract. Iterative searches within the citations among candidate studies located additional candidate studies with the intention of creating a comprehensive list of studies examining naming or LDT tasks. Studies cited in recent meta-analyses looking at these tasks (Cattinelli et al., 2013; Taylor et al., 2013) were reviewed to further assure completeness of the pool of candidate studies. We subsequently filtered candidate studies to include only those that met additional criteria critical to our research question. First, we retained only those studies that examined unimpaired adults reading in their native, alphabetic, language. We excluded studies that explicitly investigated reading in multilinguals (e.g., Nosarti, Mechelli, Green, & Price, 2010). A number of retained studies failed to report whether their participants were monolingual, however in all cases the authors of these studies made claims about reading in general, rather than in multilingual populations. Thus, we assumed that the sample compositions for these studies represented normal monolingual readers. Second, all retained studies reported whole-brain direct contrasts between words and pseudowords; we excluded those that failed to directly contrast these lexicality conditions, or did so only in the context of region of interest analyses. By including only direct contrasts between words and pseudowords, the spatial distributions associated with processing each type of item are less likely to be obscured by contrasts versus (heterogeneous) baselines. Some studies reported activation foci for contrasts at multiple significance thresholds. For example, Carreiras et al. (2007) investigated interactions between task (LDT vs reading aloud) and lexicality. The authors reported activation foci and Z-statistics for both tasks where the lexicality contrast was significant for either or both tasks, when corrected for multiple comparisons. We included coordinates only for significant contrasts between orthographically comparable words and pseudowords (i.e. non-pseudohomophones). In Carreiras et al. (2007), coordinates were reported for a right inferior frontal activation that was associated with a significant Z-score for LDT, but not naming. Thus, this activation focus was associated only with the LDT task in our analysis. The resulting dataset included 33 studies published between 1997 and 2012, of which 16 used the LDT and 17 used a naming task. 1 LDT study and 3 naming studies used PET. The ratio of PET to fMRI studies used did not differ between tasks, χ2(1, N=33) =1.28, p>.25. These studies are summarized in Table 1.
Table 1.
Studies used in the ALE meta-analysis.
| DOI | First Author |
Year | Langu age |
Meth od |
Task |
|---|---|---|---|---|---|
| 10.1162/089892903321593108 | Binder | 2003 | EN | fMRI | LDT |
| 10.1162/0898929054021102 | Binder | 2005 | EN | fMRI | LDT |
| 10.1162/jocn.2007.19.3.433 | Carreiras | 2007 | SP | fMRI | LDT |
| 10.1162/jocn.2007.19.11.1768 | Diaz | 2007 | EN | fMRI | LDT |
| 10.1162/089892902317205285 | Fiebach | 2002 | GE | fMRI | LDT |
| 10.1523/JNEUROSCI.4107-04.2005 | Fiebach | 2005 | GE | fMRI | LDT |
| 10.1016/j.neuroimage.2007.04.004 | Fiebach | 2007 | GE | fMRI | LDT |
| 10.1006/nimg.2001.0940 | Henson | 2002 | EN | fMRI | LDT |
| 10.1016/j.eplepsyres.2010.12.003 | Jensen | 2011 | EN | fMRI | LDT |
| 10.1162/jocn.2007.19.10.1584 | Kronbichler | 2007 | EN | fMRI | LDT (Phonological) |
| 10.1016/j.neuroimage.2005.06.050 | Kuchinke | 2005 | GE | fMRI | LDT |
| 10.1093/brain/122.12.2337 | Perani | 1999 | IT | PET | LDT |
| 10.1016/j.brainres.2008.03.045 | Sachs | 2008 | GE | fMRI | LDT |
| 10.1016/j.neuroimage.2009.10.082 | Schurz | 2010 | GE | fMRI | LDT (Phonological) |
| 10.1162/jocn.2007.19.11.1753 | Thompson | 2007 | EN | fMRI | LDT |
| 10.1162/jocn.2010.21502 | Woollams | 2011 | EN | fMRI | LDT |
| 10.1016/j.neuroimage.2005.04.029 | Binder | 2005 | EN | fMRI | NAM |
| 10.1162/jocn.2007.19.3.433 | Carreiras | 2007 | SP | fMRI | NAM |
| 10.1002/hbm.20122 | Dietz | 2005 | EN | fMRI | NAM |
| 10.1162/089892999563490 | Hagoort | 1999 | GE | PET | NAM |
| 10.1016/j.bandl.2011.12.005 | Heim | 2012 | GE | fMRI | NAM |
| 10.1111/1467-9450.00229 | Henson | 2001 | EN | fMRI | NAM (Covert) |
| 10.1002/(SICI)1097-0193(1997)5:2<84::AID- | |||||
| HBM2>3.0.CO;2-I | Herbster | 1997 | EN | PET | NAM |
| 10.1016/S0093-934X(03)00403-6 | Joubert | 2004 | EN | fMRI | NAM (Covert) |
| 10.1016/j.neuroimage.2003.10.021 | Kronbichler | 2004 | EN | fMRI | NAM (Covert) |
| 10.1016/j.neuroimage.2008.08.008 | Levy | 2008 | EN | fMRI | NAM (Covert) |
| 10.1162/089892905774589190 | Mechelli | 2005 | EN | fMRI | NAM (Covert) |
| 10.1162/089892903321208196 | Mechelli | 2003 | EN | fMRI | NAM (Covert) |
| 10.1162/089892900564000 | Mechelli | 2006 | EN | fMRI | NAM (Covert) |
| 10.1016/j.neuroimage.2010.09.049 | Osipowicz | 2011 | EN | fMRI | NAM (Covert) |
| 10.1038/71163 | Paulesu | 2000 | EN/IT | PET | NAM |
| 10.1523/JNEUROSCI.3113-10.2011 | Vartiainen | 2011 | FI | fMRI | NAM (Covert) |
| 10.1016/j.neuroimage.2012.02.009 | Wilson | 2012 | EN | fMRI | NAM |
Note: DOI, Digital Object Identifier accession number; EN, English; FI, Finnish; FR, French; GE, German; IT, Italian; SP; Spanish; LDT, Lexical Decision Task; NAM, Naming or Reading Task.
2.2 ALE Analysis
[Words>Pseudowords] and [Pseudowords>Words] activation foci reported across the neuroimaging literature were analyzed using a widely used activation likelihood estimate (ALE) meta-analytic approach (Eickhoff, Bzdok, Laird, Kurth, & Fox, 2012). Analyses were carried out in Montreal Neurological Institute (MNI) standard space. Activation foci that were reported in Talairach standard space (Talairach & Tournoux, 1988) were transformed into MNI space using the tal2icbm transformation (Lancaster et al., 2007). Analyses were performed using GingerALE 2.3 (http://brainmap.org/ale/), and were performed as follows: As a first step, ALE maps were created within each task for [Words>Pseudowords] and for [Pseudowords>Words] using a Monte Carlo nonparametric test of significance with a false-detection rate (FDR) corrected significance level of pN=.05, with an additional cluster level significance threshold constraint of p=.05 over 1,000 iterations. In other words, clusters, of which at most 5% of their constituent voxels would be expected to be activated by chance, were retained in each map if they were at least as large as the top 5th percentile of clusters drawn from a random distribution of voxels with a density identical to the ALE data. The second step statistically compared these simple main effect ALE maps between tasks using a Monte Carlo nonparametric test of significance using a FDR=0.05 over 10,000 iterations. These contrasts identified significant interactions between task and lexicality effects in studies of normal reading, and were central to our primary goal of assessing task differences among lexicality effects. We additionally created ALE maps for the main effect of lexicality (collapsing across task) and the main effect of task (collapsing across lexicality), using a FDR corrected significance threshold of pN=.05 and cluster size threshold of p = .05, matching that used for the simple main effect maps. Approximate anatomical regions and Brodmann areas for ALE clusters were determined by locating the weighted cluster centroids within the Automated Anatomical Labeling (AAL) atlas (Tzourio-Mazoyer et al., 2002) and Brodmann Atlas, respectively, using the MRIcron software package. Though only a single region is reported for each cluster, note that larger clusters may extend into adjacent anatomical regions.
3 Results
In this section we highlight task and lexicality effects in regions that have been extensively implicated in reading including prefrontal cortex (Inferior and Middle Frontal Gyri), inferior parietal cortex (Supramarginal and Angular Gyri), lateral temporal cortex (Superior and Middle Temporal Gyri) and ventral temporal cortex (Fusiform and Inferior Temporal Gyri). All peaks are indicated in the tables and most peaks are illustrated in the figures.
3.1 Interactions between Task and Lexicality
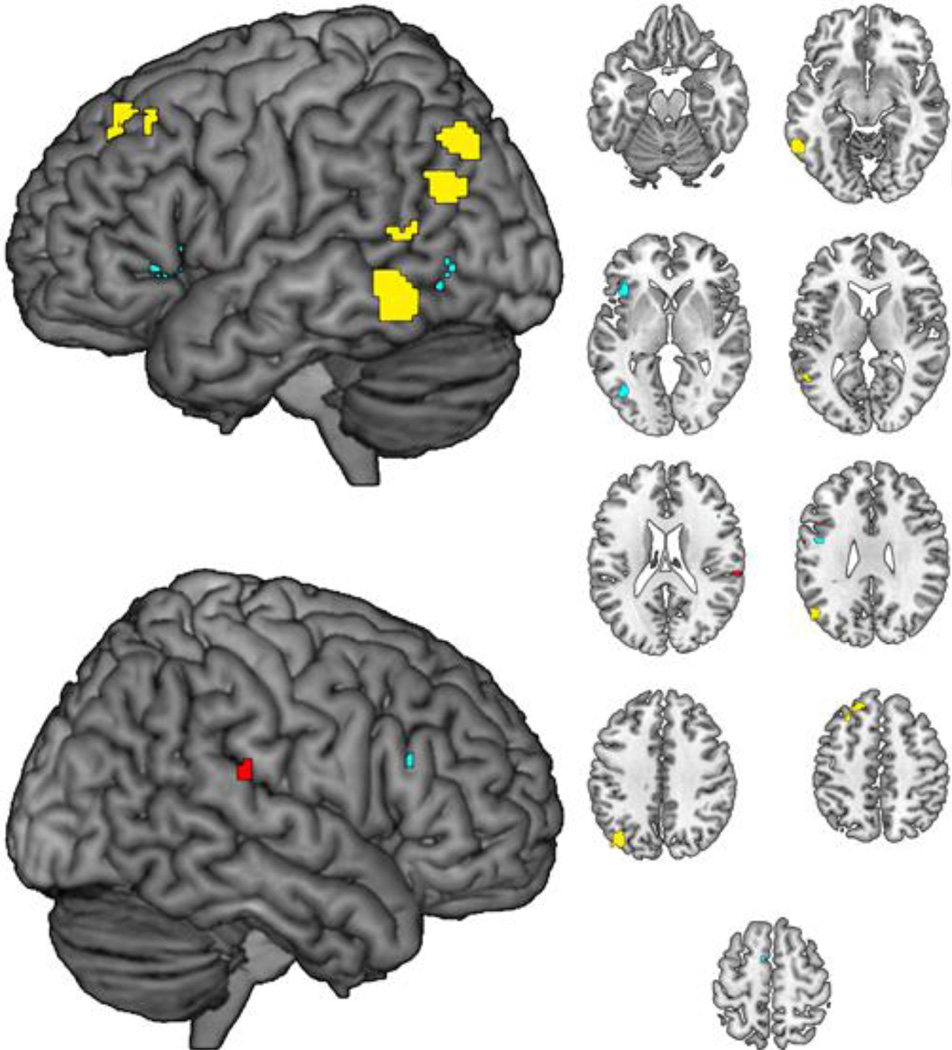
As outlined earlier, the nature of task-by-lexicality interactions remains unclear, and our primary goal was to assess whether lexicality effects (i.e. words versus pseudowords) depended on task (i.e. lexical decision versus naming). Task-related differences for the [Words>Pseudowords] and [Pseudowords>Words] contrasts are presented in Table 2 and in Figure 1. When contrasting words versus pseudowords, LDT was more likely to recruit left middle temporal gyrus, and a number of left temporoparietal regions, extending posteriorly from posterior middle temporal gyrus to angular gyrus and inferior parietal lobule, whereas naming was more likely to recruit right posterior superior temporal gyrus. When contrasting pseudowords versus words, LDT was more likely to recruit bilateral inferior frontal gyrus (Pars Triangularis) and a left-hemisphere cluster extending ventrally from middle occipital gyrus into inferior occipital gyrus. Naming was not more likely than LDT to recruit any region when contrasting pseudowords versus words.
Table 2.
ALE foci for task-dependent lexicality effects.
| Contrast | Region | BA | Volume | x | y | z | Max ALE | |
|---|---|---|---|---|---|---|---|---|
| Words > Pseudowords | l Inferior Temporal Lobe | 37 | 1552 | −61 | −52 | −9 | 3.432 | |
| LDT > Naming | l Middle Occipital Gyrus | 19 | 912 | −39 | −73 | 41 | 2.077 | |
| l Angular Gyrus | 39 | 688 | −50 | −68 | 26 | 2.251 | ||
| l Posterior Cingulate Gyrus | 23 | 680 | −4 | −35 | 33 | 1.999 | ||
| l Superior Frontal Gyrus | 9 | 472 | −20 | 36 | 48 | 2.536 | ||
| l Middle Frontal Gyrus | 9 | 232 | −30 | 26 | 48 | 2.409 | ||
| l Middle Temporal Gyrus | 21 | 104 | −56 | −54 | 11 | 2.506 | ||
| Naming > LDT | r Superior Temporal Gyrus | 42 | 64 | 63 | −29 | 20 | 1.700 | |
| Pseudowords > Words | l Inferior Occipital Gyrus | 37 | 464 | −40 | −66 | −1 | 2.106 | |
| LDT > Naming | l IFG (Pars Triangularis) | 45 | 440 | −41 | 22 | 2 | 2.423 | |
| l Precentral Gyrus | 6 | 96 | −47 | −3 | 28 | 1.920 | ||
| r IFG (Pars Triangularis) | 45 | 64 | 46 | 22 | 23 | 1.730 | ||
| l Supplemental Motor Area | 6 | 64 | −6 | 3 | 62 | 1.899 | ||
| Naming > LDT | No significant clusters | |||||||
Note: l=left; r=right; BA=Brodmann Area; LDT=Lexical Decision Task; Volume= cluster volume in mm3; IFG=Inferior Frontal Gyrus; coordinates given in MNI stereotaxic space.
Figure 1.
Cluster-size significance corrected ALE clusters for task comparisons within Words > Pseudowords (A) showing LDT > Naming (yellow) and Naming > LDT (red) and within Pseudowords > Words (B) showing LDT > Naming (cyan). Axial slices span Z=−20 to Z=60 in 10 mm intervals.
3.2 Main Effects of Lexicality and Task
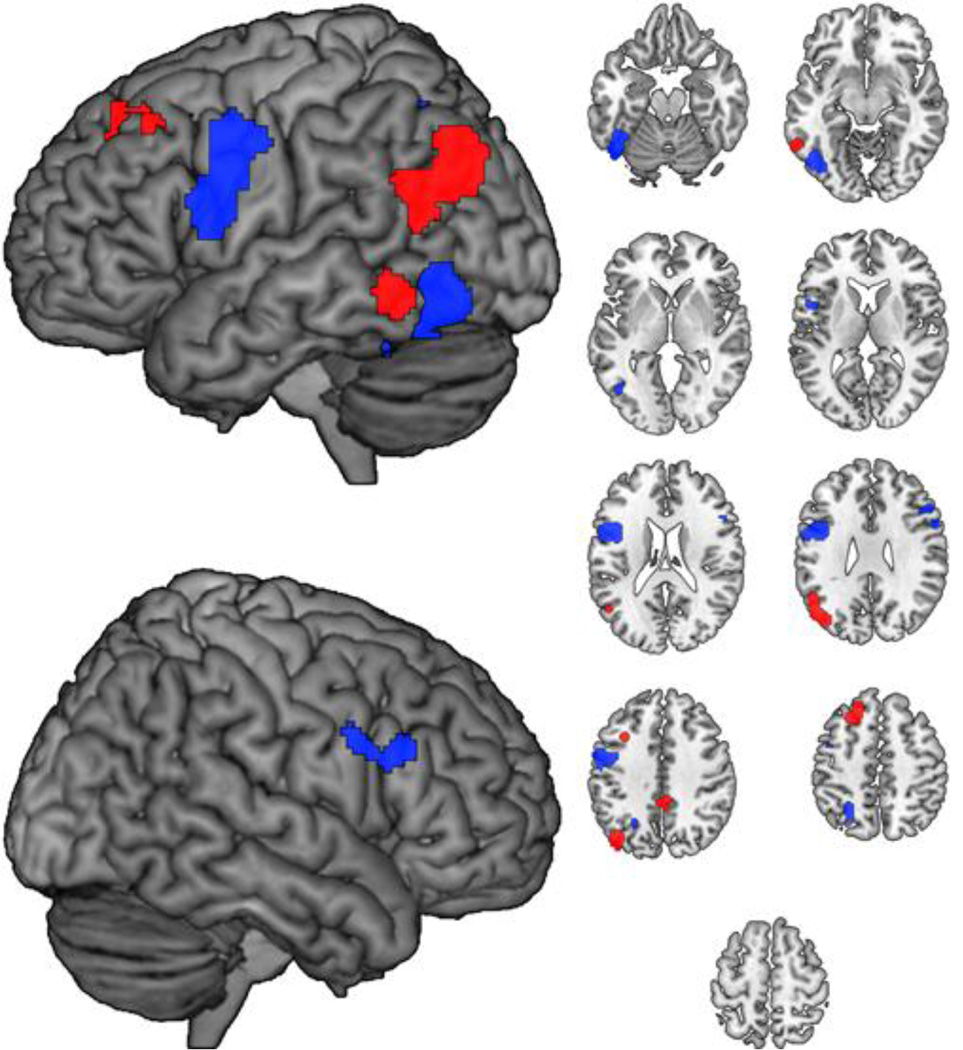
The significant interaction between task and lexicality effects indicates that one should interpret main effects of lexicality and task with caution. Nonetheless, we analyzed lexicality effects across tasks to replicate previous meta-analyses that included not only direct word versus pseudoword contrasts, but also contrasts versus baseline (Cattinelli et al., 2013; Jobard et al., 2003). Because the contrasts associated with our input activation foci are mutually exclusive, the corresponding ALE clusters across and within each task were spatially distinct. Cluster extents and foci for [Words > Pseudowords] and for [Pseudowords > Words], collapsed across all tasks, are presented in Table 3 and illustrated in Figure 2. Words were associated with reliably greater activation in left middle/inferior temporal gyrus, angular gyrus and left middle frontal gyrus. Pseudowords were associated with reliably greater activation in left fusiform and inferior occipital gyrus, superior parietal lobule and left inferior frontal gyrus (Pars Triangularis and Pars Opercularis).
Table 3.
ALE foci for lexicality effects collapsed across task
| Contrast | Region | BA | Volume | x | y | z | Max ALE |
|---|---|---|---|---|---|---|---|
| Words > Pseudowords | l Angular Gyrus | 39 | 5304 | −45 | −69 | 30 | 3.719 |
| l Middle Frontal Gyrus | 8 | 2800 | −25 | 25 | 48 | 3.062 | |
| l Middle Cingulate Gyrus | 23 | 1456 | −1 | −38 | 37 | 3.891 | |
| l Inferior Temporal Gyrus | 37 | 1064 | −62 | −52 | −9 | 2.512 | |
| Pseudowords > Words | l Precentral Gyrus | 6 | 9216 | −49 | 3 | 28 | 3.891 |
| l Fusiform Gyrus | 19 | 5728 | −44 | −64 | −13 | 3.891 | |
| l Superior Parietal Lobule | 7 | 1504 | −26 | −58 | 47 | 2.807 | |
| r IFG (Pars Opercularus) | 44 | 1144 | 52 | 16 | 27 | 2.382 |
Note: l=left; r=right; BA=Brodmann Area; Volume= cluster volume in mm3; IFG=Inferior Frontal Gyrus; coordinates given in MNI stereotaxic space.
Figure 2.
Cluster-size significance corrected ALE clusters for Words > Pseudowords (red) and Pseudowords > Words (blue) contrasts collapsed across lexical decision and naming tasks. Axial slices span Z=−20 to Z=60 in 10 mm intervals.
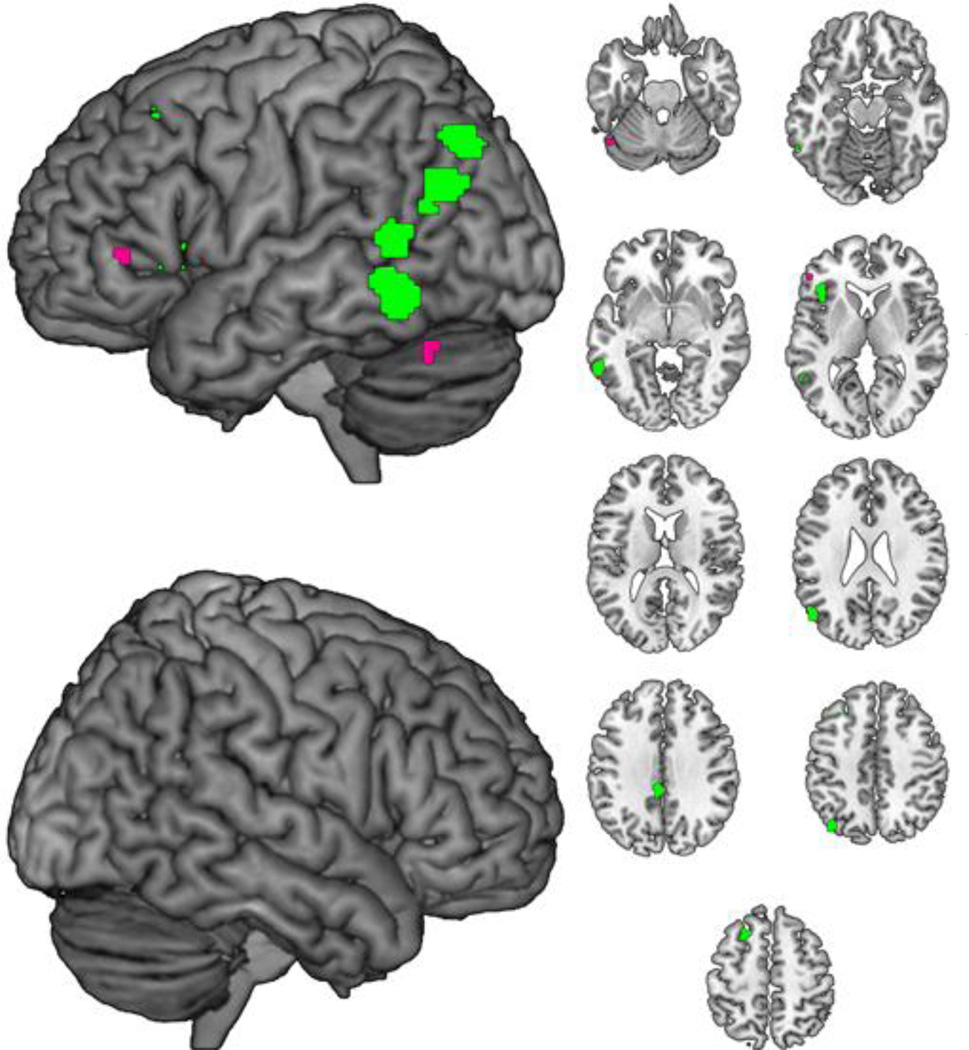
Cluster extents and foci for [LDT > Naming] and for [Naming > LDT] task effects, collapsed across lexicality, are presented in Table 4 and illustrated in Figure 3. Overall task contrasts revealed several clusters along a belt of cortex following the posterior middle temporal gyrus to angular gyrus and a cluster overlapping left inferior frontal gyrus (Pars Triangularis) and insula where LDT showed more reliable activations than naming. The reverse contrast revealed small clusters in left inferior frontal gyrus (Pars Triangularis) and left cerebellum where naming showed more reliable activations than LDT.
Table 4.
ALE foci for task effects, collapsed across lexicality
| Contrast | Region | BA | Volume | x | y | z | Max ALE |
|---|---|---|---|---|---|---|---|
| LDT > Naming | l Middle Temporal Gyrus | 21 | 1256 | −62 | −52 | −8 | 3.353 |
| l Middle Occipital Gyrus | 19 | 856 | −39 | −73 | 40 | 2.241 | |
| l Angular Gyrus | 39 | 712 | −50 | −67 | 25 | 2.437 | |
| l Posterior Cingulate Gyrus | 23 | 560 | −4 | −35 | 34 | 1.988 | |
| l Inferior Frontal Gyrus | 47 | 552 | −40 | 23 | 3 | 2.549 | |
| l Middle Temporal Gyrus | 21 | 448 | −57 | −50 | 9 | 2.484 | |
| l Middle Frontal Gyrus | 8 | 376 | −24 | 21 | 52 | 2.254 | |
| l Middle Occipital Gyrus | 19 | 280 | −40 | −67 | −1 | 2.183 | |
| Naming > LDT | l Cerebellum | 72 | −46 | −62 | −27 | 1.674 | |
| l IFG (Pars Triangularis) | 45 | 48 | −51 | 37 | 3 | 1.825 |
Note: l=left; r=right; BA=Brodmann Area; Volume= cluster volume in mm3; IFG=Inferior Frontal Gyrus; coordinates given in MNI stereotaxic space.
Figure 3.
Cluster-size significance corrected ALE clusters for LDT>Naming (green) and Naming>LDT (violet) contrasts, collapsed across lexicality. Axial slices span Z=−25 to Z=55 in 10 mm intervals.
4 Discussion
Our ALE meta-analysis examined the neuroimaging literature investigating lexicality effects using LDT and naming – two tasks that are widely used in reading research. This approach quantifies concordance of reported activations within neuroimaging data, showing which brain regions are reliably activated in contrasts between words and pseudowords when participants are engaged in either of these tasks. Our primary goal, however, was to explore how task demands modulate lexicality effects, which in turn can be used to inform experimental task selection and guide the interpretation of the existing literature. Our major finding was that lexicality effects are task-dependent, and we will thus devote the next section to the discussion of these interactions.
4.1 Lexicality by Task Interactions
Employing multiple tasks in a single experiment increases the complexity and duration of the study. Consequently, few investigators have explored how task demands interact with neural processes in reading (Carreiras et al., 2007; Carreiras, Mechelli, & Price, 2006; Valdois et al., 2006). Our between-task comparisons therefore provide important insight into these interactions. As we argued earlier, there are clear theoretical ties between the LDT and semantic processing, and between naming and phonological processing, and that satisfactory performance on these tasks consequently places different loads on the semantic and phonological systems. Without exception, all reported behavioral data among the studies we examined indicated that pseudowords were associated with slower lexical decision and production latencies. The right inferior frontal activation for the [pseudowords > words] contrast may be attributable to response inhibition for pseudowords (Carreiras et al., 2007). However, we found a number of additional regions not identified by Carreiras and colleagues that also showed a greater effect for pseudowords than for words in the LDT. Lexicality decisions entail a decision component, whereas naming does not. Thus, the inferior frontal activations associated with LDT may reflect decision-related, rather than phonological processes. The Multiple Demand network, described by Duncan (2010) overlaps with the phonological network, and is argued to play a critical role in managing cognitive demands. Because decisions on pseudowords are assumed to be more demanding – they are associated with longer RTs – the IFG activations may correspond to the increased burden placed on this region during lexicality decisions on pseudowords, rather than from phonological processes, though the present results do not strongly support one explanation over the other.
Compared to naming, lexical decisions for the [words > pseudowords] contrast were more likely to produce activations within the left-hemisphere general semantic regions described in reviews by Binder et al. (2009) and Noonan, Jefferies, Visser, and Lambon Ralph (2013). The left middle frontal gyrus (Brodmann Area 9) activation, falls within a region that has been argued to participate in the frontoparietal control network (Noonan et al., 2013) and thus may reflect goal directed semantic retrieval for words. Activations fell within the ventrolateral region of the angular gyrus, which Seghier, Fagan, and Price (2010) argue plays a critical role in conceptual identification of visual stimuli. The posterior left middle temporal activations fall within a region often implicated in semantic processing (Binder et al., 2009), and Noonan et al. (2013) argue that this region is not a semantic repository, but instead involved in the strategic retrieval of semantic information, presumably represented elsewhere. In models employing distributed semantic representations, posterior middle temporal gyrus would thus act as a hub or convergence zone (McNorgan, Reid, & McRae, 2011; Patterson, Nestor, & Rogers, 2007), potentially integrating information from multiple representational sources. Under this interpretation, the initiation of semantic retrieval would appear to be obligatory for known words, even during lexicality decisions, when such information is not strictly necessary for the task. Lexical decisions on pseudowords, in contrast, were more likely to activate left inferior occipital and fusiform gyrus, associated with orthographic processing (McCandliss, Cohen, & Dehaene, 2003), and the frontal phonological network (Vigneau et al., 2006). This pattern of activation for the [pseudoword > word] contrast suggests that lexicality decisions on pseudowords more strongly tax the orthographic and phonological processing systems. This would suggest that lexicality decisions do not rely solely on detecting a semantic representation, but also on input from the orthographic and phonological systems. In conjunction with the overall task effects described below, these results are consistent with the argument that lexical decisions more strongly rely on the semantic system than naming, and that words more strongly activate this system than do pseudowords because only they have semantic content. This does not imply, however, that all words should activate the semantic system equally, as not all words are associated with robust semantic knowledge (Pexman, Hargreaves, Siakaluk, Bodner, & Pope, 2008). Rather, it follows from the fact that words collectively have more associated semantic content than pseudowords.
Compared to lexical decision tasks, naming elicited reliably more activity only when contrasting words versus pseudowords, and only in the right superior temporal gyrus. Phonemic-level processing during comprehension and production is typically associated with left, but not right superior temporal gyrus (Buchsbaum, Hickok, & Humphries, 2001), and thus this right-lateralization was not predicted. We predicted that naming would more strongly tap phonological processes, and because pseudowords should be more difficult to process, we expected that pseudoword naming would show the greatest activation in phonological processing areas, as found by Carreiras et al. (2007). However, assuming that this right superior temporal gyrus activity is an index of phonological processing difficulty, our results do not support this prediction. Only one study, Hagoort et al. (1999), contributed directly to this cluster. Using both overt and covert naming of words and pseudowords, the authors reported left superior temporal activation for pseudowords, but right superior temporal activation for words, collapsing across naming task. Though the left superior temporal activation is consistent with our predictions, the ALE cluster to which it contributed did not reach significance in our analysis. Hagoort and colleagues do not, however, provide an explanation for the right superior temporal activation for words, making it difficult to speculate what this activation represents.
The results were inconsistent with our prediction that regions implicated in phonological processing should show the strongest effects for pseudowords during naming tasks. The lexicality effects described below suggest pseudowords are associated with an increase in phonological processing difficulty. The lack of an effect for pseudowords in the naming task was suprising, given that we had hypothesized that phonological processing should be most directly tapped during pseudoword naming. One interpretation of the pattern of interactions is that increases in phonological processing difficulty for pseudowords are similar for the two tasks. However, as noted below in our discussion of task effects, the large proportion of covert naming studies may have decreased the sensitivity of the analysis to phonological effects associated with naming.
4.2 Overall Lexicality Differences
The overall lexicality effects we found are consistent with recent meta-analyses by Taylor et al. (2013), and Cattinelli et al. (2013). As in these studies, greater activations for words were most reliably found in left middle temporal gyrus, angular gyrus, and inferior temporal gyrus, which are thought to be core regions of the semantic processing network (Binder et al., 2009; Taylor et al., 2013). As with the Taylor et al. (2013) study, we found greater activation for pseudowords in the frontal phonological network (Vigneau et al., 2006) and in left superior parietal cortex, which Taylor et al. argue is involved in spelling to sound mapping. These authors suggest that these pseudoword activations reflect of the prolonged effort required to carry out spelling-to-sound mapping and compute phonological output for unfamiliar pseudowords. Unlike the Taylor study, greater activations for pseudowords were not observed in left superior temporal gyrus, which, as noted earlier, is traditionally associated with phonological processing (Buchsbaum et al., 2001), however, this discrepancy may be attributable to slight differences between the studies included in each meta-analysis. For example, because Vigneau, Jobard, Mazoyer, and Tzourio-Mazoyer (2005) examined passive reading of nonwords (rather than pseudowords), it was excluded from our study, though it was included in Taylor et al. (2013). Similarly, Taylor et al. (2013) included studies such as Tagamets, Novick, Chalmers, and Friedman (2000), and (Xu et al., 2001), which we excluded because they used neither lexical decision nor naming tasks.
4.3 Overall Task Differences
As predicted, LDT was more likely than naming to recruit regions implicated in lexical semantic processing. Behavioral studies have shown that, though semantic variables appear to influence both LDT and naming performance, LDT behavioral performance appears to be more strongly related to semantics (Balota et al., 2004), which our findings support. Interestingly, the significant clusters for LDT appeared to be a subset of those comprising the network derived from all semantic contrasts in the ALE analysis by Binder et al. (2009). However, the distribution of these clusters is also quite similar to those in the task by lexicality interaction, where the most reliable activation for words in the LDT falls within the middle temporal/angular gyrus region. This suggests that the overall task differences in the semantic network are primarily driven by lexicality decisions for words, and not equally by both lexicality decisions.
Naming was more likely than LDT to be associated with significant activations in two clusters located in left inferior frontal gyrus and left cerebellum. Because these regions are implicated in phono-articulatory planning and motor execution, the results for the contrast of naming versus LDT is consistent with the behavioral literature showing a reliance of naming on articulatory variables (Balota et al., 2004; Ferrand et al., 2011). Overall, however, the activations associated with naming were weak. Mapping between orthographic and phonological representations should entail similar processes for overt and covert naming, and thus recruit many of the same brain regions. However, direct contrasts between the two response modalities by Palmer et al. (2001) showed that, though overt and covert responses have a similar spatial distributions, covert responses were associated with weaker response magnitudes. One interpretation of the overall task differences might be that the LDT is more cognitively demanding, however, this pattern may also reflect that a large proportion of the naming studies in our analysis employed covert naming, and thus would have shown weaker effects.
4.4 Implications for Cross-Linguistic Differences
Our analyses looked exclusively at studies involving alphabetic languages, in which there exist mappings between orthographic and phonological word forms, the regularity of which depends on orthographic depth (Bentin & Frost, 1987). Among all such languages, the relationship between word form and semantic meaning is far less regular (ignoring for a moment the important cues that morphemic information may provide). That is, in languages with transparent orthographies, the printed form of a word is a perfect cue to its pronunciation (and vice versa), and even in languages with opaque orthographies that contain many exception words, there is nonetheless a great deal of consistency among letter-sound correspondences. However, among transparent and opaque languages alike, one cannot infer from the meaning of, for example, CAT the meaning of a word with similar orthography and phonology that does not share the same morphemic root, such as SAT. Among the studies we reviewed, a task by lexicality interaction emerged, showing LDT for words tapped the semantic system most strongly. We argue from the pattern of main effects for task and lexicality that this interaction is the result of the additive effects of task sensitivity and lexicality dependency on semantic knowledge. That is, though task demands and lexicality are individually sufficient to dictate the extent of semantic processing (as indicated by the main effects), these factors may contribute additively towards semantic processing, such that they have a greater influence on semantic processing in combination than either of them have in isolation (as suggested by the interaction). In logographic languages, such as Chinese, however, the orthographic forms of many words cue their meanings, and in such languages, a different relationship may exist.
When parafoveal information about an upcoming word is available, reading time for that word is facilitated when it is the next fixation target (Rayner, 1975). In alphabetic languages, this preview benefit does not extend to semantic processing. That is, a semantic relationship between the foveated and parafoveal word does not influence initial fixation duration when the parafoveal word becomes foveated. Rayner, White, Kambe, Miller, and Liversedge (2003) take this lack of preview benefit in alphabetic languages to suggest that semantic activation in these languages comes after orthographic processing. Using these same eyetracking measures, Yan, Richter, Shu, and Kliegl (2009) found Chinese, but not English, readers enjoyed a semantic preview benefit (i.e., shorter fixation times) for parafoveal words. This suggests orthographic information more quickly and directly activates semantic knowledge in logographic languages than in alphabetic languages, without the need for first phonologically decoding the word.
Despite the potential cross-linguistic differences in the directness with which orthography is mapped to semantics, reading in logographic and alphabetic languages appear to otherwise place similar demands on the reading system. Chee et al. (2000) found that bilingual English/Mandarin readers recruited left middle temporal/fusiform gyrus and left prefrontal gyrus (Pars Opercularis) when making semantic relatedness decisions to either Mandarin characters or English words. The authors concluded that processing written Mandarin otherwise resembles reading in alphabetic languages more than it does identifying pictures. Similarly, Chinese readers familiar with Pinyin (a writing system for transcribing Mandarin phonemes into the Latin alphabet) engage comparable networks when making lexicality decisions to items presented in Mandarin or Pinyin (Chen, Fu, Iversen, Smith, & Matthews, 2002). Functional MRI investigating the neural substrates of word naming shows that English and Chinese show word regularity effects in a similar network of regions (Tan et al., 2001).
Wu, Ho, and Chen (2012) provides an overall picture of semantic and phonological processing in Chinese in their recent meta-analysis of fMRI studies. The authors separately analyzed studies using semantic and phonological tasks, respectively (four of 11 phonological tasks were naming tasks), and concluded that the network recruited for Chinese character processing was generally comparable to that typically recruited for alphabetic language processing. One notable exception was that semantic, phonological and orthographic processing in Chinese tended to recruit bilateral fusiform gyrus. Though these activations were left-hemisphere dominant and thus left-lateralized, reading in English most reliably activates only left fusiform (Wu et al., 2012), though laterality in English is likely a matter of degree, as several authors have found bilateral fusiform activation in English readers (e.g., Seghier & Price, 2011; Taylor, Rastle, & Davis, 2014)
To summarize, reading in alphabetic and logographic languages appears to rely on similar neural processes. Though our analyses were restricted to alphabetic languages, it is reasonable to expect that these results apply to logographic languages, however early automatic semantic activation in such languages may moderate potential task differences in semantic activation.
4.5 Implications for Distributed Models of Reading
One challenge for distributed models is that they must explain how the same learning process that leads to increased semantic activation for familiar items (i.e., words > pseudowords), but decreased phonological and orthographic activation for the same items, as seen in the significant [Pseudowords > Words] contrast effects in left fusiform, precentral and inferior frontal gyri. Increased pseudoword activation in the phonological and orthographic system is predicted by models with attractor dynamics, such as those used in implementations of the triangle model by Harm and Seidenberg (1999, 2004). In these models, experience with regular patterns leads to the development of attractor basins, which are points in multidimensional (e.g., phonological or semantic) network state space to which nearby points are drawn (Plaut & Shallice, 1993). Attractor basins inhibit activations of unfamiliar pattern elements (e.g., incompatible phonemic combinations, but also combinations that are not frequently encountered) and excite those for familiar pattern elements. This predicts that words should show less activation than pseudowords in orthographic and phonological systems. However, as other models also predict greater phonologically-related activation for pseudowords than words (see, for example, Taylor et al., 2013), our results do not therefore support the triangle model of reading over other models.
As indicated earlier, only Harm and Seidenberg (2004) have fully implemented the triangle model to date. They used this model to investigate the individual and joint contributions of the orthography-phonology-semantic and orthography-semantic pathways in a number of reading phenomena, including interactions between word frequency and regularity, and main effects of imageability and homophone and pseudohomophone reading. Though they simulated pseudoword reading (Simulation 4), demonstrating that the model was capable of inferring phonological representations for novel orthographic patterns, this simulation did not contrast pseudoword and word reading. Moreover, lexicality decisions were not simulated in the model, precluding any examination of task-by-lexicality interactions. Explorations of reading within a distributed framework would thus benefit greatly from models that permit simulations of interactions between task and lexicality among orthographic, phonological and semantic representations. Our results suggest phenomena for which neurologically plausible distributed computational models of reading should account.
Though there are many orthographic, phonological and semantic representational schemes from which one must choose for a computational model, many are relatively straightforward to implement. For example, both the Harm and Seidenberg (2004) implementation of the triangle model and the DRC model maintain letter-level orthographic representational units, allowing words to be composed of combinations of single-letter activations. Similarly, (Cree, McNorgan, & McRae, 2006) implemented semantic representations of concrete objects (e.g., ROBIN) as combinations of features (e.g., <has wings>, <eats worms>) derived from feature production norms (McRae, Cree, Seidenberg, & McNorgan, 2005). The implementation of a cognitive task in these models, however, is much less straightforward. Task simulations entail considerations such as computational tractability, interpretability, and the complexity of orchestrating the many sub-processes entailed in even the simplest cognitive tasks. To simulate a task computationally, a researcher may have to choose among multiple possible implementations, and make simplifying assumptions about task characteristics and computational parameters. Though there may be disagreement about the chosen parameters, one advantage of formal models is that they make these decisions explicit, fostering further discussion and research regarding the validity of these assumptions (Hintzman, 1991). Because lexicality-by-task interactions have not yet been investigated in distributed models, it is unclear how this pattern of interactions would be explained within that paradigm.
4.6 Conclusion
We presented a meta-analysis of the neuroimaging literature examining the effects of lexicality among studies using lexical decision and naming tasks. We found that processing pseudowords is more strongly associated with activations in regions associated with phonological and orthographic processing, and that this lexicality effect is greatest during lexical decision tasks. We found that processing words is more strongly associated with activations in regions associated with semantic processing, and that this lexicality effect is greatest during lexical decision tasks. Understanding interactions among orthographic, phonological and semantic systems has important methodological and theoretical implications: Neuroimaging experiments investigating reading do not typically use both LDT and naming tasks. The dependency of lexicality effects on task would imply that task selection should align with the hypothesis to be tested, and that interpreting lexicality effects should account for task.
Highlights.
Explored word vs. pseudoword lexicality effects in lexical decision and naming tasks
Identified interactions between lexicality effects and task effects
Words and lexicality decisions activated semantic network
Pseudowords activated phono-articulatory and orthographic network
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Balota DA, Cortese MJ, Sergent-Marshall SD, Spieler DH, Yap MJ. Visual Word Recognition of Single-Syllable Words. Journal of Experimental Psychology: General. 2004;133:283–316. doi: 10.1037/0096-3445.133.2.283. [DOI] [PubMed] [Google Scholar]
- Bentin S, Frost R. Processing lexical ambiguity and visual word recognition in a deep orthography. Memory and Cognition. 1987;15:13–23. doi: 10.3758/bf03197708. [DOI] [PubMed] [Google Scholar]
- Binder JR, Desai RH, Graves WW, Conant LL. Where Is the Semantic System? A Critical Review and Meta-Analysis of 120 Functional Neuroimaging Studies. Cerebral Cortex. 2009;19:2767–2796. doi: 10.1093/cercor/bhp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, McKiernan KA, Parsons ME, Westbury CF, Possing ET, Kaufman JN, Buchanan L. Neural correlates of lexical access during visual word recognition. Journal of Cognitive Neuroscience. 2003;15:372–393. doi: 10.1162/089892903321593108. [DOI] [PubMed] [Google Scholar]
- Binder JR, Medler DA, Desai R, Conant LL, Liebenthal E. Some neurophysiological constraints on models of word naming. Neuroimage. 2005;27:677–693. doi: 10.1016/j.neuroimage.2005.04.029. [DOI] [PubMed] [Google Scholar]
- Blazely AM, Coltheart M, Casey BJ. Semantic impairment with and without surface dyslexia: Implications for models of reading. Cognitive Neuropsychology. 2005;22:695–717. doi: 10.1080/02643290442000257. [DOI] [PubMed] [Google Scholar]
- Buchsbaum BR, Hickok G, Humphries C. Role of left posterior superior temporal gyrus in phonological processing for speech perception and production. Cognitive Science. 2001;25:663–678. [Google Scholar]
- Carreiras M, Mechelli A, Estevez A, Price CJ. Brain activation for lexical decision and reading aloud: Two sides of the same coin. Journal of Cognitive Neuroscience. 2007;19:433–444. doi: 10.1162/jocn.2007.19.3.433. [DOI] [PubMed] [Google Scholar]
- Carreiras M, Mechelli A, Price CJ. Effect of word and syllable frequency on activation during lexical decision and reading aloud. Human Brain Mapping. 2006;27:963–972. doi: 10.1002/hbm.20236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattinelli I, Borghese NA, Gallucci M, Paulesu E. Reading the reading brain: A new meta-analysis of functional imaging data on reading. Journal of Neurolinguistics. 2013;26:214–238. [Google Scholar]
- Chee MWL, Weekes B, Lee KM, Soon CS, Schreiber A, Hoon JJ, Chee M. Overlap and Dissociation of Semantic Processing of Chinese Characters, English Words, and Pictures: Evidence from fMRI. Neuroimage. 2000;12:392–403. doi: 10.1006/nimg.2000.0631. [DOI] [PubMed] [Google Scholar]
- Chen Y, Fu S, Iversen SD, Smith SM, Matthews PM. Testing for dual brain processing routes in reading: A direct contrast of Chinese character and pinyin reading using fMRI. Journal of Cognitive Neuroscience. 2002;14:1088–1098. doi: 10.1162/089892902320474535. [DOI] [PubMed] [Google Scholar]
- Coltheart M, Rastle K, Perry C, Langdon R, Ziegler J. DRC: A dual route cascaded model of visual word recognition and reading aloud. Psychological Review. 2001;108:204–256. doi: 10.1037/0033-295x.108.1.204. [DOI] [PubMed] [Google Scholar]
- Coltheart M, Saunders SJ, Tree JJ. Computational modelling of the effects of semantic dementia on visual word recognition. Cognitive Neuropsychology. 2010;27:101–114. doi: 10.1080/02643294.2010.502887. [DOI] [PubMed] [Google Scholar]
- Cree GS, McNorgan C, McRae K. Distinctive features hold a privileged status in the computation of word meaning: Implications for theories of semantic memory. Journal of Experimental Psychology. Learning, Memory, and Cognition. 2006;32:643–658. doi: 10.1037/0278-7393.32.4.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damasio AR. Aphasia. New England Journal of Medicine. 1992;326:531–539. doi: 10.1056/NEJM199202203260806. [DOI] [PubMed] [Google Scholar]
- Diaz MT, McCarthy G. Unconscious Word Processing Engages a Distributed Network of Brain Regions. Journal of Cognitive Neuroscience. 2007;19:1768–1775. doi: 10.1162/jocn.2007.19.11.1768. [DOI] [PubMed] [Google Scholar]
- Dietz NAE, Jones KM, Gareau L, Zeffiro TA, Eden GF. Phonological decoding involves left posterior fusiform gyrus. Human Brain Mapping. 2005;26:81–93. doi: 10.1002/hbm.20122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilkina K, McClelland JL, Plaut DC. Are there mental lexicons? The role of semantics in lexical decision. Brain Research. 2010;1365:66–81. doi: 10.1016/j.brainres.2010.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J. The multiple-demand (MD) system of the primate brain: mental programs for intelligent behaviour. Trends in Cognitive Sciences. 2010;14:172–179. doi: 10.1016/j.tics.2010.01.004. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Bzdok D, Laird AR, Kurth F, Fox PT. Activation likelihood estimation meta-analysis revisited. Neuroimage. 2012;59:2349–2361. doi: 10.1016/j.neuroimage.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrand L, Brysbaert M, Keuleers E, New B, Bonin P, Méot A, Augustinova M, Pallier C. Comparing word processing times in naming, lexical decision, and progressive demasking: Evidence from Chronolex. Frontiers in psychology. 2011:2. doi: 10.3389/fpsyg.2011.00306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiebach CJ, Friederici AD, Müller K, Cramon DYv. fMRI Evidence for Dual Routes to the Mental Lexicon in Visual Word Recognition. Journal of Cognitive Neuroscience. 2002;14:11–23. doi: 10.1162/089892902317205285. [DOI] [PubMed] [Google Scholar]
- Fiebach CJ, Gruber T, Supp GG. Neuronal Mechanisms of Repetition Priming in Occipitotemporal Cortex: Spatiotemporal Evidence from Functional Magnetic Resonance Imaging and Electroencephalography. The Journal of Neuroscience. 2005;25:3414–3422. doi: 10.1523/JNEUROSCI.4107-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiebach CJ, Ricker B, Friederici AD, Jacobs AM. Inhibition and facilitation in visual word recognition: Prefrontal contribution to the orthographic neighborhood size effect. Neuroimage. 2007;36:901–911. doi: 10.1016/j.neuroimage.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Hagoort P, Indefrey P, Brown C, Herzog H, Steinmetz H, Seitz RJ. The Neural Circuitry Involved in the Reading of German Words and Pseudowords: A PET Study. Journal of Cognitive Neuroscience. 1999;11:383–398. doi: 10.1162/089892999563490. [DOI] [PubMed] [Google Scholar]
- Harm MW, Seidenberg MS. Phonology, reading acquisition, and dyslexia: Insights from connectionist models. Psychological Review. 1999;106:491–528. doi: 10.1037/0033-295x.106.3.491. [DOI] [PubMed] [Google Scholar]
- Harm MW, Seidenberg MS. Computing the meanings of words in reading: cooperative division of labor between visual and phonological processes. Psychological Review. 2004;111:662. doi: 10.1037/0033-295X.111.3.662. [DOI] [PubMed] [Google Scholar]
- Heim S, Wehnelt A, Grande M, Huber W, Amunts K. Effects of lexicality and word frequency on brain activation in dyslexic readers. Brain and Language. 2013;125:194–202. doi: 10.1016/j.bandl.2011.12.005. [DOI] [PubMed] [Google Scholar]
- Henson RNA. Repetition effects for words and nonwords as indexed by event-related fMRI: A preliminary study. Scandinavian Journal of Psychology. 2001;42:179–186. doi: 10.1111/1467-9450.00229. [DOI] [PubMed] [Google Scholar]
- Henson RNA, Price CJ, Rugg MD, Turner R, Friston KJ. Detecting Latency Differences in Event-Related BOLD Responses: Application to Words versus Nonwords and Initial versus Repeated Face Presentations. Neuroimage. 2002;15:83–97. doi: 10.1006/nimg.2001.0940. [DOI] [PubMed] [Google Scholar]
- Herbster AN, Mintun MA, Nebes RD, Becker JT. Regional cerebral blood flow during word and nonword reading. Human Brain Mapping. 1997;5:84–92. doi: 10.1002/(sici)1097-0193(1997)5:2<84::aid-hbm2>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Hintzman DL. Why are formal models useful in psychology. Relating theory and data: Essays on human memory in honor of Bennet B. Murdock. 1991:39–56. [Google Scholar]
- Jacobs AM, Graf R, Kinder A. Receiver operating characteristics in the lexical decision task: Evidence for a simple signal-detection process simulated by the multiple read-out model. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2003;29:481. doi: 10.1037/0278-7393.29.3.481. [DOI] [PubMed] [Google Scholar]
- Jensen EJ, Hargreaves I, Bass A, Pexman P, Goodyear BG, Federico P. Cortical reorganization and reduced efficiency of visual word recognition in right temporal lobe epilepsy: A functional MRI study. Epilepsy Research. 2011;93:155–163. doi: 10.1016/j.eplepsyres.2010.12.003. [DOI] [PubMed] [Google Scholar]
- Jobard G, Crivello F, Tzourio-Mazoyer N. Evaluation of the dual route theory of reading: a metanalysis of 35 neuroimaging studies. Neuroimage. 2003;20:693–712. doi: 10.1016/S1053-8119(03)00343-4. [DOI] [PubMed] [Google Scholar]
- Joubert S, Beauregard M, Walter N, Bourgouin P, Beaudoin G, Leroux J-M, Karama S, Lecours AR. Neural correlates of lexical and sublexical processes in reading. Brain and Language. 2004;89:9–20. doi: 10.1016/S0093-934X(03)00403-6. [DOI] [PubMed] [Google Scholar]
- Katz L, Brancazio L, Irwin J, Katz S, Magnuson J, Whalen DH. What lexical decision and naming tell us about reading. Reading and Writing. 2012;25:1259–1282. doi: 10.1007/s11145-011-9316-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronbichler M, Bergmann J, Hutzler F, Staffen W, Mair A, Ladurner G, Wimmer H. Taxi vs. Taksi: On Orthographic Word Recognition in the Left Ventral Occipitotemporal Cortex. Journal of Cognitive Neuroscience. 2007;19:1584–1594. doi: 10.1162/jocn.2007.19.10.1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronbichler M, Hutzler F, Wimmer H, Mair A, Staffen W, Ladurner G. The visual word form area and the frequency with which words are encountered: evidence from a parametric fMRI study. Neuroimage. 2004;21:946–953. doi: 10.1016/j.neuroimage.2003.10.021. [DOI] [PubMed] [Google Scholar]
- Kuchinke L, Jacobs AM, Grubich C, Vo MLH, Conrad M, Herrmann M. Incidental effects of emotional valence in single word processing: An fMRI study. Neuroimage. 2005;28:1022–1032. doi: 10.1016/j.neuroimage.2005.06.050. [DOI] [PubMed] [Google Scholar]
- Lancaster JL, Tordesillas-Gutiérrez D, Martinez M, Salinas F, Evans A, Zilles K, Mazziotta JC, Fox PT. Bias between MNI and Talairach coordinates analyzed using the ICBM-152 brain template. Human Brain Mapping. 2007;28:1194–1205. doi: 10.1002/hbm.20345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy J, Pernet C, Treserras S, Boulanouar K, Berry I, Aubry F, Demonet J-F, Celsis P. Piecemeal recruitment of left-lateralized brain areas during reading: A spatio-functional account. Neuroimage. 2008;43:581–591. doi: 10.1016/j.neuroimage.2008.08.008. [DOI] [PubMed] [Google Scholar]
- McCandliss BD, Cohen L, Dehaene S. The visual word form area: expertise for reading in the fusiform gyrus. Trends Cogn Sci. 2003;7:293–299. doi: 10.1016/s1364-6613(03)00134-7. [DOI] [PubMed] [Google Scholar]
- McNorgan C, Reid J, McRae K. Integrating conceptual knowledge within and across representational modalities. Cognition. 2011;118:211–233. doi: 10.1016/j.cognition.2010.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRae K, Cree GS, Seidenberg MS, McNorgan C. Semantic feature production norms for a large set of living and nonliving things. Behav Res Methods. 2005;37:547–559. doi: 10.3758/bf03192726. [DOI] [PubMed] [Google Scholar]
- Mechelli A, Crinion JT, Long S, Friston KJ, Ralph MAL, Patterson K, McClelland JL, Price CJ. Dissociating Reading Processes on the Basis of Neuronal Interactions. Journal of Cognitive Neuroscience. 2005;17:1753–1765. doi: 10.1162/089892905774589190. [DOI] [PubMed] [Google Scholar]
- Mechelli A, Gorno-Tempini ML, Price CJ. Neuroimaging Studies of Word and Pseudoword Reading: Consistencies, Inconsistencies, and Limitations. Journal of Cognitive Neuroscience. 2003;15:260–271. doi: 10.1162/089892903321208196. [DOI] [PubMed] [Google Scholar]
- Neely JH, O’Connor PA, Calabrese G. Fast trial pacing in a lexical decision task reveals a decay of automatic semantic activation. Acta Psychologica. 2010;133:127–136. doi: 10.1016/j.actpsy.2009.11.001. [DOI] [PubMed] [Google Scholar]
- Noonan KA, Jefferies E, Visser M, Lambon Ralph MA. Going beyond Inferior Prefrontal Involvement in Semantic Control: Evidence for the Additional Contribution of Dorsal Angular Gyrus and Posterior Middle Temporal Cortex. Journal of Cognitive Neuroscience. 2013;25:1824–1850. doi: 10.1162/jocn_a_00442. [DOI] [PubMed] [Google Scholar]
- Nosarti C, Mechelli A, Green DW, Price CJ. The Impact of Second Language Learning on Semantic and Nonsemantic First Language Reading. Cerebral Cortex. 2010;20:315–327. doi: 10.1093/cercor/bhp101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osipowicz K, Rickards T, Shah A, Sharan A, Sperling M, Kahn W, Tracy J. A test of the role of the medial temporal lobe in single-word decoding. Neuroimage. 2011;54:1455–1464. doi: 10.1016/j.neuroimage.2010.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer ED, Rosen HJ, Ojemann JG, Buckner RL, Kelley WM, Petersen SE. An Event-Related fMRI Study of Overt and Covert Word Stem Completion. Neuroimage. 2001;14:182–193. doi: 10.1006/nimg.2001.0779. [DOI] [PubMed] [Google Scholar]
- Patterson K, Nestor PJ, Rogers TT. Where do you know what you know? The representation of semantic knowledge in the human brain. Nat Rev Neurosci. 2007;8:976–987. doi: 10.1038/nrn2277. [DOI] [PubMed] [Google Scholar]
- Paulesu E, McCrory E, Fazio F, Menoncello L, Brunswick N, Cappa SF, Cotelli M, Cossu G, Corte F, Lorusso M. A cultural effect on brain function. Nature Neuroscience. 2000;3:91–96. doi: 10.1038/71163. [DOI] [PubMed] [Google Scholar]
- Perani D, Cappa SF, Schnur T, Tettamanti M, Collina S, Rosa MM, Fazio F. The neural correlates of verb and noun processing. A PET study. Brain. 1999;122:2337–2344. doi: 10.1093/brain/122.12.2337. [DOI] [PubMed] [Google Scholar]
- Pexman PM, Hargreaves IS, Siakaluk PD, Bodner GE, Pope J. There are many ways to be rich: Effects of three measures of semantic richness on visual word recognition. Psychonomic Bulletin & Review. 2008;15:161–167. doi: 10.3758/pbr.15.1.161. [DOI] [PubMed] [Google Scholar]
- Plaut DC, Shallice T. Deep dyslexia: A case study of connectionist neuropsychology. Cognitive Neuropsychology. 1993;10:377–500. [Google Scholar]
- Rayner K. The perceptual span and peripheral cues in reading. Cognitive Psychology. 1975;7:65–81. [Google Scholar]
- Rayner K, White SJ, Kambe G, Miller B, Liversedge SP. On the processing of meaning from parafoveal vision during eye fixations in reading. Amsterdam: Elsevier; 2003. [Google Scholar]
- Sachs O, Weis S, Zellagui N, Huber W, Zvyagintsev M, Mathiak K, Kircher T. Automatic processing of semantic relations in fMRI: Neural activation during semantic priming of taxonomic and thematic categories. Brain Research. 2008;1218:194–205. doi: 10.1016/j.brainres.2008.03.045. [DOI] [PubMed] [Google Scholar]
- Schurz M, Sturm D, Richlan F, Kronbichler M, Ladurner G, Wimmer H. A dual-route perspective on brain activation in response to visual words: evidence for a length by lexicality interaction in the visual word form area (VWFA) Neuroimage. 2010;49:2649–2661. doi: 10.1016/j.neuroimage.2009.10.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MF, Kimberg DY, Walker GM, Faseyitan O, Brecher A, Dell GS, Coslett HB. Brain. Anterior temporal involvement in semantic word retrieval: voxel-based lesion-symptom mapping evidence from aphasia doi: 10.1093/brain/awp284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seghier ML, Fagan E, Price CJ. Functional Subdivisions in the Left Angular Gyrus Where the Semantic System Meets and Diverges from the Default Network. The Journal of Neuroscience. 2010;30:16809–16817. doi: 10.1523/JNEUROSCI.3377-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seghier ML, Price CJ. Explaining left lateralization for words in the ventral occipitotemporal cortex. The Journal of Neuroscience. 2011;31:14745–14753. doi: 10.1523/JNEUROSCI.2238-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidenberg MS, McClelland JL. A distributed, developmental model of word recognition and naming. Psychological Review. 1989;96:523. doi: 10.1037/0033-295x.96.4.523. [DOI] [PubMed] [Google Scholar]
- Tagamets M, Novick J, Chalmers M, Friedman R. A parametric approach to orthographic processing in the brain: an fMRI study. Cognitive Neuroscience, Journal of. 2000;12:281–297. doi: 10.1162/089892900562101. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. 3-Dimensional proportional system: an approach to cerebral imaging. 1988 [Google Scholar]
- Tan LH, Lui HL, Perfetti CA, Spinks JA, Fox PT, Gao JH. The neural system underlying Chinese logograph reading. Neuroimage. 2001;13:836–846. doi: 10.1006/nimg.2001.0749. [DOI] [PubMed] [Google Scholar]
- Taylor JSH, Rastle K, Davis MH. Can cognitive models explain brain activation during word and pseudoword reading? A meta-analysis of 36 neuroimaging studies. Psychological Bulletin. 2013;139:766–791. doi: 10.1037/a0030266. [DOI] [PubMed] [Google Scholar]
- Taylor JSH, Rastle K, Davis MH. Interpreting response time effects in functional imaging studies. Neuroimage. 2014;99:419–433. doi: 10.1016/j.neuroimage.2014.05.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson CK, Bonakdarpour B, Fix SC, Blumenfeld HK, Parrish TB, Gitelman DR, Mesulam MM. Neural correlates of verb argument structure processing. Journal of Cognitive Neuroscience. 2007;19:1753–1767. doi: 10.1162/jocn.2007.19.11.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkeltaub PE, Goldberg EM, Postman-Caucheteux WA, Palovcak M, Quinn C, Cantor C, Coslett HB. Alexia due to ischemic stroke of the visual word form area. Neurocase. 2013;20:230–235. doi: 10.1080/13554794.2013.770873. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated Anatomical Labeling of Activations in SPM Using a Macroscopic Anatomical Parcellation of the MNI MRI Single-Subject Brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Valdois S, Carbonnel S, Juphard A, Baciu M, Ans B, Peyrin C, Segebarth C. Polysyllabic pseudo-word processing in reading and lexical decision: Converging evidence from behavioral data, connectionist simulations and functional MRI. Brain Research. 2006;1085:149–162. doi: 10.1016/j.brainres.2006.02.049. [DOI] [PubMed] [Google Scholar]
- Vartiainen J, Liljeström M, Koskinen M, Renvall H, Salmelin R. Functional Magnetic Resonance Imaging Blood Oxygenation Level-Dependent Signal and Magnetoencephalography Evoked Responses Yield Different Neural Functionality in Reading. The Journal of Neuroscience. 2011;31:1048–1058. doi: 10.1523/JNEUROSCI.3113-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigneau M, Beaucousin V, Hervé PY, Duffau H, Crivello F, Houdé O, Mazoyer B, Tzourio-Mazoyer N. Meta-analyzing left hemisphere language areas: Phonology, semantics, and sentence processing. Neuroimage. 2006;30:1414–1432. doi: 10.1016/j.neuroimage.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Vigneau M, Jobard G, Mazoyer B, Tzourio-Mazoyer N. Word and non-word reading: what role for the visual word form area? Neuroimage. 2005;27:694–705. doi: 10.1016/j.neuroimage.2005.04.038. [DOI] [PubMed] [Google Scholar]
- Wilson MA, Joubert S, Ferré P, Belleville S, Ansaldo AI, Joanette Y, Rouleau I, Brambati SM. The role of the left anterior temporal lobe in exception word reading: Reconciling patient and neuroimaging findings. Neuroimage. 2012;60:2000–2007. doi: 10.1016/j.neuroimage.2012.02.009. [DOI] [PubMed] [Google Scholar]
- Woollams AM, Silani G, Okada K, Patterson K, Price CJ. Word or Word-like? Dissociating Orthographic Typicality from Lexicality in the Left Occipito-temporal Cortex. Journal of Cognitive Neuroscience. 2010;23:992–1002. doi: 10.1162/jocn.2010.21502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C-Y, Ho M-HR, Chen S-HA. A meta-analysis of fMRI studies on Chinese orthographic, phonological, and semantic processing. Neuroimage. 2012;63:381–391. doi: 10.1016/j.neuroimage.2012.06.047. [DOI] [PubMed] [Google Scholar]
- Xu B, Grafman J, Gaillard WD, Ishii K, Vega-Bermudez F, Pietrini P, Reeves-Tyer P, DiCamillo P, Theodore W. Conjoint and extended neural networks for the computation of speech codes: the neural basis of selective impairment in reading words and pseudowords. Cerebral Cortex. 2001;11:267–277. doi: 10.1093/cercor/11.3.267. [DOI] [PubMed] [Google Scholar]
- Yan M, Richter EM, Shu H, Kliegl R. Readers of Chinese extract semantic information from parafoveal words. Psychonomic Bulletin & Review. 2009;16:561–566. doi: 10.3758/PBR.16.3.561. [DOI] [PubMed] [Google Scholar]
- Yap MJ, Balota DA. Visual word recognition of multisyllabic words. Journal of Memory and Language. 2009;60:502–529. [Google Scholar]