Abstract
Background
Advanced motorized prosthetic devices are currently controlled by EMG signals generated by residual muscles and recorded by surface electrodes on the skin. These surface recordings are often inconsistent and unreliable, leading to high prosthetic abandonment rates for individuals with upper limb amputation. Surface electrodes are limited because of poor skin contact, socket rotation, residual limb sweating, and their ability to only record signals from superficial muscles, whose function frequently does not relate to the intended prosthetic function. More sophisticated prosthetic devices require a stable and reliable interface between the user and robotic hand to improve upper limb prosthetic function.
New Method
Implantable Myoelectric Sensors (IMES®) are small electrodes intended to detect and wirelessly transmit EMG signals to an electromechanical prosthetic hand via an electromagnetic coil built into the prosthetic socket. This system is designed to simultaneously capture EMG signals from multiple residual limb muscles, allowing the natural control of multiple degrees of freedom simultaneously.
Results
We report the status of the first FDA-approved clinical trial of the IMES® System. This study is currently in progress, limiting reporting to only preliminary results.
Comparison with Existing Methods
Our first subject has reported the ability to accomplish a greater variety and complexity of tasks in his everyday life compared to what could be achieved with his previous myoelectric prosthesis.
Conclusion
The interim results of this study indicate the feasibility of utilizing IMES® technology to reliably sense and wirelessly transmit EMG signals from residual muscles to intuitively control a three degree-of-freedom prosthetic arm.
Keywords: Implantable devices, myoelectric, prosthesis, IMES®, implantable electrodes
1. Introduction
Current estimates place the prevalence of limb loss in the United States at 1.6 million as of 2005 and project upwards of 2.2 million individuals by 2020. (Ziegler-Graham et al, 2008). In addition, over the past decade, a significant number of U.S. service members have sustained injuries resulting in the loss of one or more limbs. While there have been significant advances in lower limb prosthetics over the past two decades, there have only been modest improvements in clinically available upper limb prosthetics. A study by McFarland and colleagues looking at prosthetic device use and satisfaction among U.S. service members with combat-related, unilateral upper limb amputation reported that 30% of soldiers from the Vietnam War and 22% of soldiers from Operations Enduring Freedom and Iraqi Freedom abandoned prosthesis use altogether, complaining of weight, discomfort, pain, lack of functionality, and poor fit (McFarland et al, 2010). Even the most advanced myoelectric upper limb prosthetics have high rejection rates. A survey by Biddis and Chau reported a mean rejection rate of 23% for adult myoelectric users (Biddis & Chau, 2007), nearly 50 years after the first myoelectric devices were clinically implemented (McLean, 2004). While the negative functional implications of abandoning an upper limb prosthesis are obvious, individuals who rely on only one arm/hand for daily use are also at a much higher risk of developing overuse injuries and arthritis of their neck, upper back and remaining limbs, negatively influencing long-term morbidity and quality of life (Jones & Davidson, 1999).
While much attention has been directed towards enhancing the dexterity of prosthetic arms/hands to replicate near human-like movements, a significant gap continues to remain in improving the user’s ability to control a robotic hand in a more intuitive and reliable manner. Current strategies to control myoelectric prosthetic devices rely primarily on using surface electrodes placed on the skin of the residual limb to record underlying superficial arm muscles. The signals will then be used to control the prosthetic limb (e.g. open/ close hand or pronate/supinate wrist). While these surface myoelectric signals have proven to be sufficient for controlling the movement of a powered prosthesis, they have significant limitations (Schultz & Kuiken, 2011). First, the surface of the skin itself presents fundamental challenges to recording EMG signals from the underlying musculature. These limitations include: 1) susceptibility to electrical noise generated by the environment, 2) recording of electrical activity from other muscles adjacent to the electrode, thereby triggering unintended actions, 3) movement of the surface electrodes on the skin, especially with socket rotation, and 4) perspiration of the skin changing the electrical impedance (Weir et al, 2009). In addition, surface electrodes do not allow the simultaneous capture of multiple individual superficial and deep muscles of the forearm to control multiple degrees of freedom simultaneously. Current myoelectric devices require the user to control a prosthesis using unnatural muscle contractions, e.g. contracting the wrist extensor muscles to signal the prosthetic wrist to supinate and then using that same contraction to signal the hand to open. Furthermore they are limited in their ability to only control one degree of freedom at a time, so the user has to choose between wrist rotation and hand opening and is unable to do these activities simultaneously, resulting in a non-intuitive, sequential prosthetic joint manipulation rather than intuitive, simultaneous control. Limited dexterity of control is often cited as the primary reason for abandonment of myoelectric prostheses (Atkins et al, 1996). For these reasons, many amputees in the United States prefer to use a “body-powered” prostheses controlled by a harness and cables – a technology that was first developed in the 1860s.
The Implantable Myoelectric Sensor (IMES®) System, developed by the Alfred Mann Foundation, is a potential alternative to surface EMG for prosthetic control. IMES® are small, cylindrical electrodes (16mm long and 2.5mm in diameter) capable of detecting and wirelessly transmitting EMG data (Fig. 1A). The ability to place these electrodes directly within residual limb muscles, rather than on the surface of the skin, may provide numerous advantages, including stronger and more reliable signals that do not change with arm positioning, socket rotation or sweating. Equally important, these electrodes present the possibility of recording from individual superficial and deep muscles simultaneously, permitting more intuitive control of a multiple-degree-of-freedom prosthesis by providing more signals from muscles that were responsible for hand and wrist movement prior to the amputation and coupling them to the same prosthetic functions (e.g. an electrode in the supinator muscle is used to control prosthetic wrist supination).
Figure 1. The IMES® System.
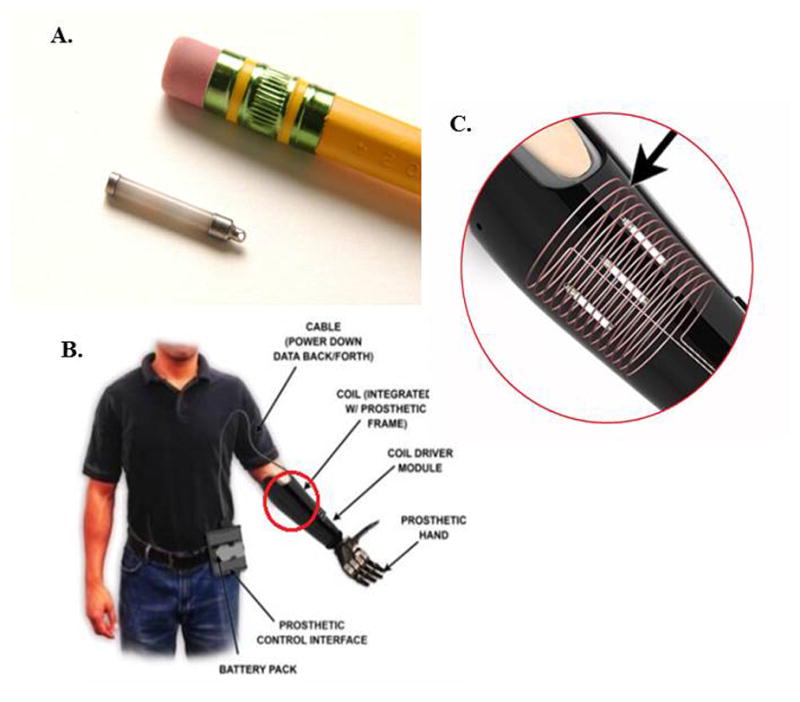
A) Size example of an IMES® electrode. B) The IMES® System C) The magnetic coil built into the prosthetic frame that powers IMES® and receives EMG information.
Researchers at the Uniformed Services University of the Health Sciences, Walter Reed National Military Medical Center (WRNMMC) and the Alfred Mann Foundation, have recently initiated the first FDA-approved human feasibility trial of the IMES® System, utilizing IMES® for patients with transradial upper limb amputation. The primary goal of the study is to determine if the IMES® System can be used safely to provide reliable control of a multiple-degree-of-freedom electromechanical prosthetic wrist and hand. Our secondary goal is to assess whether the IMES® system improves function based on already validated assessment tools and whether those measurement tools are reasonably sensitive metrics to assess the efficacy of the system. The study is currently being conducted at WRNMMC under an Institutional Review Board (IRB) approved protocol, with full human use ethics review and patient informed consent.
2. Materials and Methods
2.1 Materials
The IMES® System is a group of components that function together as an integrated prosthetic control system (Fig. 1B). The System registers and transmits the electrical impulses generated during muscle contraction and then processes this information to affect motors that move the joints of a myoelectric prosthesis.
The IMES® System currently allows for simultaneous control of up to three distinct movements, or degrees of freedom (DOF). These include wrist pronation/supination, hand open/close, and thumb abduction/adduction. Two IMES® are required to control each DOF, one for each of the opposing motions, such that six IMES® are used. Each IMES® device acts as a wireless, independent, intramuscular differential amplifier consisting of custom electronics housed within a biocompatible, hermetically sealed ceramic cylinder with metal end caps. They amplify, filter, rectify and integrate the detected signal using parameters set up during initialization. The signal is then digitized and transmitted on a configurable frame format that allows the interspersing of data from multiple devices.
The IMES® operate on a bi-directional, half-duplex communication scheme through modulation of a magnetic field generated by a coil that is laminated within the wall of the prosthetic frame (Fig. 1C). Reverse telemetry is used to transfer the data from the implanted sensor while forward telemetry is used to transmit the power and configuration settings to the sensors. The IMES interlaly rectifies and integrates the EMG signal in a pass band between 4.4 and 2200Hz. The integration window length is 13.5 milliseconds and is determined by the sampling rate, which, in the 3 DOF configuration, is 74Hz. Each sample is converted to an 8 bit digital representation inside the implant before being transmitted to the Prosthetic Control Interface (PCI). While we have not specifically investigated the specificity of the IMES recordings, the pickup area is localized and we expect minimal cross-talk from nearby muscles to influence the measurements. The pickup area was determined by a theoretical compute model as described in a paper by Lowery et al. It was determined to be an ellipsoid of 5mm radius when the implant is oriented along the muscle fibers (Lowery et al, 2006).
The PCI, a “Walkman”-sized electronic box worn on the user’s belt, receives this data stream, separates out the samples from each individual IMES device, and performs additional filtering. Each analog signal is then routed to a selected motor control input of the electromechanical prosthesis to affect the desired action. This interface was chosen such that the IMES® System mimics the characteristics of the surface myoelectric sensors that are commercially available for prosthetic control. This enables us to use commercially available prosthetic components without modification.
The PCI applies additional processing to the EMG signals received from the IMES® implants to make the signals more appropriate as control signals for the prosthetic wrist and hand. Furthermore, the user can select settings for three different levels of signal smoothing, i.e. quick, medium and smooth. The first stage of processing is a low pass filter which is either a 2nd order 5Hz filter for the quick setting or a 4th order 3Hz filter for the medium and smooth settings. Following the low pass filter is a baseline offset removal algorithm that removes a potentially variable baseline due to some of the internal circuitry in the IMES® implant. The baseline removal is adaptive and is calculated based on a data buffer containing several seconds of EMG samples. To further increase the amplitude of EMG signals that differ from resting activity, a non-linear amplification stage is added. The final stage of processing is a median filter that adds extra smoothing to the data, depending on user selection. For the quick setting the median filter has a depth of 3 samples, while it is 7 samples for the medium setting and 19 samples for the smooth setting. Figure 2 below illustrates the signal processing pathway from IMES® sensing electrodes to the analog output provided to the prosthetic controller.
Fig. 2. Signal processing pathway from IMES® to PCI.
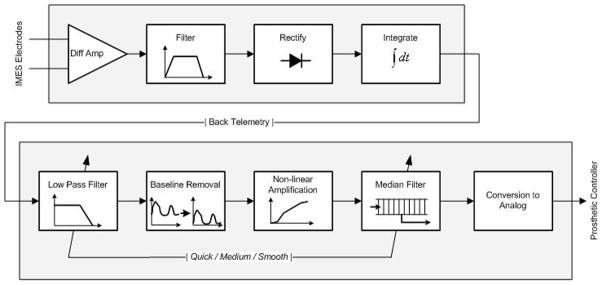
Before signals are received by the PCI, they undergo extensive filtering, rectification, integration, and amplification to prepare them for conversion to analog, a more appropriate control signal for the prosthetic wrist and hand.
Each transmission from the IMES implants to the PCI includes a 4 bit Hamming code as a means for error detection. The system will tolerate up to 5% overall error in the last 100 transmissions (1.35 sec), or up to 3 consecutive transmission erros (40 msec) per IMES before marking an IMES as inactive and giving a warning to the user. When individual transmission data samples are detected as erroneous, they are ignored and the filter data buffer and the PCI output for that specific channel are not updated.
The IMES® System socket and frame are custom fit to each subject and serve as the connection between the subject’s residual limb and the prosthetic wrist and hand. There is a flexible inner socket that fits around the residual limb and is surrounded by a rigid frame. The frame contains a collar for attachment of an electromechanical wrist and hand. At present, the IMES® System has been designed to interface with a Motion Control Wrist Rotator (Motion Control Inc., Salt Lake City, UT) and the i-Limb Ultra prosthetic hand (Touch Bionics Inc., Livingston, UK). It is also compatible with a Motion Control Electric Split Hook (Motion Control Inc., Salt Lake City, UT) or an Ottobock Greifer (Ottobock GmbH, Duderstadt, Germany).
The frame contains a charging port, a battery that supplies power to the wrist and hand, and a port to connect the wrist to a computer in order to program the settings of the prosthesis. Programming is an iterative process that involves adjusting a set of signal processing parameters (gain, degree of signal smoothing, and threshold) such that the resulting electromechanical wrist and hand movements are consistent with user intent.
The programming is accomplished by first optimizing the signal detected by the IMES® electrodes. The settings are then fine-tuned with Touch Bionics software integrated with the modified i-Limb Ultra multi-articulating prosthetic hand and the Motion Control Wrist Rotator.
2.2 Methods
In our ongoing clinical trial, potential study subjects are first screened to determine their eligibility. The major eligibility criteria include:
Patients must:
Be at least 18 years old
Have suffered transradial amputation with one-third or greater residual forearm length
Have residual forearm anatomy (based on number and size of residual muscles) that will support the implantation and control of at least six IMES®
Have experience using a surface myoelectric prosthesis
Have no metal fragments or metal implants in the amputated arm
Have no active implants (pacemaker, drug infusion device, etc)
Be willing to come to the hospital for prosthetic training and assessment testing
Be willing to wear the IMES® prosthesis at home
These criteria are intended to ensure patient safety and allow for full evaluation of the prosthetic controls. Once a potential subject has been identified, they must complete a baseline assessment. This includes a Box and Blocks Test (BBT), the Southampton Hand Assessment Procedure (SHAP), and an Assessment for Capacity of Myoelectric Control (ACMC) using their current, surface EMG-controlled myoelectric prosthesis. The BBT measures unilateral gross manual dexterity by asking the subject to move, one by one, the maximum number of 1″×1″ square wooden blocks from one compartment of a box to another of equal size, within 60 seconds. The SHAP is a clinically validated hand function test developed by Light et al, at the University of Southampton. Participants must manipulate sixteen abstract objects (eight distinct shapes with both heavy and light variants) and complete fourteen simulated Activities of Daily Living (ADL). A score is calculated based on how long participants take to complete each action. The ACMC is a standardized clinical assessment designed to assess prosthetic control in myoelectric prosthesis users (Lindner et al, 2009). The subject’s ability to perform a selected everyday task is rated by the occupational therapist on 22 items representing different aspects of control that are classified as gripping, holding, releasing, and coordinating between hands.
The baseline functional tests serve to describe the subject’s current level of myoelectric prosthetic control and function. Following the baseline assessment, subjects undergo an ultrasound and needle electromyography (EMG) examination of the muscles in the residual forearm. This is done to identify target residual muscles in the forearm and ensure that the subject can volitionally elicit contraction in a sufficient number of these muscles to control the three DOFs offered by the IMES® prosthesis. The exam is also used to rule out any other neuromuscular conditions that could exclude the subject from participation. This ultrasound and EMG evaluation helps to also serve as a map for eventual IMES® placement.
Each subject within this study is implanted with up to eight IMES®; six will be activated to control the three DOF the current IMES® System offers. The additional two IMES® are implanted as a potential backup for replacement of any IMES® that may not provide suitable control signals. These additional IMES® could potentially also be activated to control a four DOF system, if one becomes available in the future. To ensure accurate placement of each sensor, surgical placement is performed by a team of Board Certified Hand Surgeons, allowing each targeted muscle to be identified through a cut down dissection. Local anesthesia is used so that each subject can volitionally activate each targeted muscle during the operation. Electrode placement is conducted by a small incision (about 5 mm), made in the epimysium and then a probe is inserted into the belly of the muscle. A cannulated dilator nested inside a sheath is then advanced over the probe to reach the target implant site. The dilator and probe are then removed, leaving the sheath in place. A piece of non-absorbable suture is then threaded through an eyelet on one end of the IMES®. The IMES® is then inserted down the sheath and is deployed into the muscle tissue using an ejector tool. The sheath is withdrawn, leaving the suture extending from the muscle and available for use to retrieve the device in the case of later explant. This process is repeated for each muscle target.
Following a two-week recovery from the implantation surgery, subjects engage in seven months of training with the IMES® System. Subjects first undergo “pre-prosthetic training” for approximately a month using a universal prosthetic device that is supported on a stand that sits atop a table. This device has an overly large forearm socket and allows training while the subject’s forearm is still swollen and potentially tender from the surgical procedure. During this time, subjects are orientated to the various functions and features of the IMES® prosthesis and the study prosthetist will begin the programming process of determining the gain, filtering and threshold levels so that subjects can start learning to use specific residual limb musculature to activate basic functions of the electromechanical wrist and hand.
Once the residual limb swelling subsides, each subject is fit with a customized, take-home 3 DOF prosthesis, engineered to be controlled by the IMES® signals. Subjects then continue occupational therapy training 4–5 times per week and are encouraged to utilize their prosthesis outside the clinic based on the clinical team’s recommendations and the subject’s comfort level. The pace of training, level of difficulty, and the amount of time allotted to master each skill is tailored to the individual, depending on their motor learning ability. Subjects begin by practicing rote tasks that involve approaching, grasping, positioning and releasing objects and control of tension. They then gradually advance toward performing functional, two-handed tasks like using a cell phone to make a call, opening a pill bottle, and using a knife and fork to cut. In-clinic training is reduced to once every other week for another five months, during which time subjects are encouraged to increase the amount and variety of their at-home usage. Their at-home usage is tracked throughout the study through self-reporting.
Beginning with an Initial Assessment, subjects undergo monthly functional assessments in order to track their progress with the System. This consists of the BBT, SHAP, ACMC, and an Accuracy Test, which is an in-house evaluation of the subject’s ability to execute a series of different hand movements that test independent and simultaneous control over the three DOF offered. During the Accuracy Test, a signal splitter is inserted between the coil driver and the PCI so that the IMES® signals can be viewed and recorded. This allows comparison between user intent and the signaling result. These are the same evaluations completed during the Baseline Assessment, with the addition of the Accuracy Test.
After the seventh month of training (approximately 6 months after receiving their “take-home” prosthesis), subjects are provided with the option of continuing with the study (visiting the clinic every six months and undergoing an assessment at Year 1 and Year 2 post implant) or exiting the study at that time. Upon exiting the study, the subjects will have the option of leaving the IMES implanted or having them explanted. The subjects are required to return their IMES prosthesis upon study exit. They will have the option of returning to use of a standard myoelectric prosthetic system using surface electrodes following this clinical trial.
3. Results
At present, two subjects have been enrolled in the study, with both having undergone IMES®. As the second subject has only recently completed his surgical intervention, we herein only reports the results generated by the first implanted subject. Additionally, as this study is currently in progress under an FDA-approved protocol, we are limited to reporting only descriptive preliminary results at this time. Our first subject is a Caucasian male who was 30-years old at the time of surgery. He had minimal health issues prior to his amputation with the only chronic issue being recurrent cases of sinusitis and nasal polyps, which had been addressed through surgical removal years prior. He sustained a right transradial amputation secondary to trauma caused by an Improvised Explosive Device (IED) blast injury while serving as a member of the armed forces in 2012. This blast also caused hand bone fractures in both hands, fractures in the bones of his right arm, and damage to the left eye that has left him permanently blind in that eye. The fractures were all well-healed by the time of enrollment in this study. Additionally, the subject was diagnosed with mild Traumatic Brain Injury, short-term memory loss, phantom limb pain, tinnitus, and hearing loss in the months following this injury, during his recovery process. These secondary diagnoses were largely resolved or reduced through therapeutic intervention during the subject’s initial recovery process, prior to his enrollment in this study. He has had no issues with neuroma formation.
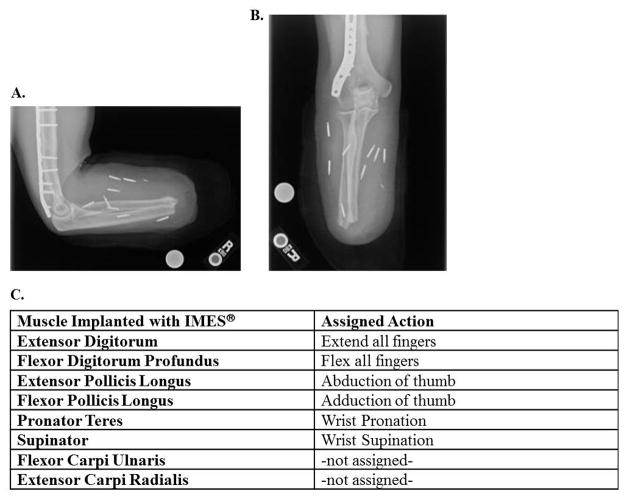
The first subject underwent the surgical procedure for this study at approximately 11 months post-amputation. The only medication he was taking at the time was over-the-counter anti-inflammatories as needed (approximately once a week). Five incisions were made to implant eight IMES® in eight individual residual forearm muscles (Fig. 3A–B). Each muscle was chosen based on its natural function and paired with an associated prosthetic function in an attempt to provide intuitive control for the user (Fig. 3C). While the IMES® system can only receive and transmit signals from as many as six electrodes at any given time, permission was granted by the hospital’s Investigational Review Board and the FDA to implant study subjects with as many as eight devices. The additional two devices are intended as back -up electrodes, in case other primary electrodes do not provide suitable control signals or cease functioning. The surgery was completed in less than 2.5 hours, with no complications. Following identification of the appropriate muscles, the subject was put to sleep with additional anesthesia for the remainder of the procedure. A communication test was conducted at the end of the procedure to determine if all IMES were functioning properly. This was completed with a surgical test coil: an open-ended coil designed to the same specifications as the prosthetic socket that has the ability to communicate with the IMES and connect with a PCI and programming laptop to confirm device function without constricting the arm to pressures of a prosthetic socket. The communication test was successful and all IMES functioned properly.
Figure 3. IMES® Locations in Subject Limb.
A, B) X-ray mapping of implanted IMES® locations in subject’s residual limb. C) Muscular locations and assigned action for all eight IMES® devices implanted.
During a post-operative X-ray the day after surgery, it was discovered that the subject had a metal hemoclip embedded in his residual limb that had previously gone unnoticed prior to surgery. A postoperative follow-up appointment scheduled for one week after the surgery was delayed until risk analysis of this metal could be performed. This analysis, completed by AMF and submitted to WRNMMC IRB, showed that the clip posed no interference to power transmission or communication capability. Additionally, AMF performed analyses of similar orthopedic hardware and vascular clips and concluded that the clip posed no risk to the subject’s safety. In the post-operative visit two weeks following implantation, the incisions appeared well-healed and another successful communication test was completed.
The subject experienced some expected increased edema post-surgery; however, this quickly resolved with the use of a compression garment and he reported no other adverse events over the course of the study. In order to prevent migration of the IMES®, the subject was asked to limit movement of the residual limb for the first two weeks following surgical placement to allow connective tissue encapsulation of the electrode. Three weeks after his surgery,, the subject began to train with the tabletop prosthesis. Because no surface electrodes were utilized, specific alignment within the socket was only necessary for comfort. Therefore, on the first day of training the subject was able to operate the prosthesis almost immediately; all prosthetic components functioned as intended and the subject demonstrated the ability to control, both individually and simultaneously, all three degrees of freedom offered by the electromechanical wrist and hand, each from the intended targeted forearm muscle. Despite this rapid progress, additional programming refinements and training with the device were necessary to help enhance the subject’s ability to differentiate between individual muscle contractions. After the initial programming refinements, the gain and filter parameters programmed into the IMES and PCI stabilized at gain levels of 170, 500, 800, 1500, or 2400 (different for each IMES) and the smooth signal smoothing filter on all IMES channels was used. The signal amplitude difference between activity and rest was easily discernible due to the baseline offset removal algorithm, resulting in a signal to noise ratio in the range of 30–52 decibels (dB).
The subject experienced unintentional activation of some prosthetic functions while trying to initiate another. This was especially true with differentiating between volitional wrist pronation and independent volitional thumb adduction; however, the more the subject practiced with the System, the better he was able to isolate each degree of freedom, decreasing the incidence of co-activation. Over the six months since the subject received his IMES, he continued to improve his isolated recruitment of motor units with reliable signals recorded and enhanced prosthetic function.
Early on during the training sessions, the subject encountered some issues with his prosthesis suddenly losing power. This was attributed to an overload of the battery protection circuit. Since the IMES® prosthesis offers simultaneous use of three DOF; the prosthesis was engineered to run all seven motors (four finger motors, two thumb motors, and one wrist motor) at the same time. This created a high current draw that overload the battery circuit and turned the prosthetic motors off. The subject is able to cycle power to reset the device when this occurs and the rate of incident has decreased over the course of the study.
After the first two months of training with the take-home prosthesis, a new socket needed to be fabricated to address issues of comfort and fit. Unlike traditional myoelectric prostheses, the IMES® system does not rely on the maintenance of tight electrode-to-skin surface contact from sensors embedded in the socket. Not facing such a restriction enabled the prosthetist to more easily fabricate a more comfortable socket for the subject than the original pre-surgical socket. Moreover, compared to using his previous myoelectric device, the subject reports much less fatigue during the day while using his prosthesis. This could be attributed to the fact that the additional sites of control allow the subject to only activate those muscles required for the specific prosthetic function he is desires to execute – he does not need to activate the same muscles for every prosthetic function, as he did with his prior myoelectric device. Furthermore, he reports no change decrease in ability to control his prosthesis with reaching above his head, below his waist (during both of which the limb becomes positioned differently within the socket), or during times of limb sweating.
Throughout the first six months of training and at each monthly assessment to date, the subject has demonstrated continued improvement in his ability to accurately, fluidly, and quickly manipulate his prosthesis. While data is not yet available for the ACMC, the subject’s scores on the Accuracy Test, BBT, and SHAP steadily increased in each of the first five months that he was evaluated and these high marks were sustained at the six month evaluation. His scores on the SHAP and BBT have nearly doubled in this time period, and he is now able to perform activities on the Accuracy Test not previously possible with his conventional myoelectric prosthesis. This includes exercising the three degrees of freedom available to simultaneously pronate the wrist, close the fingers, and adduct the thumb, from a “palm-up” position, to create a pinch between the thumb and forefinger “palm-down”. Perhaps the most noticeable change in the subject’s early use of the prosthesis was his stamina. During initial testing periods and prosthetic training, the subject often complained of muscle fatigue and required frequent breaks to get through the assigned tasks. Within a few weeks, the subject was able to complete hour-long prosthetic training sessions with ease and, within a few months, was able to perform the roughly 6 hours of monthly assessments with little to no break.
The subject has reported an increased ability to naturally and intuitively control the IMES® System, in comparison to his previous myoelectric and other prosthetic devices. He has repeatedly expressed his satisfaction with the capabilities and reliability of the IMES® system. He has reported a greater desire to wear the prosthesis in comparison to his previous myoelectric device and the ability to accomplish a greater variety and greater complexity of tasks in his everyday life.
4. Discussion
Although the current clinical trial assessing the IMES® system is not yet complete, our first subject has demonstrated that the system can record voluntary individual muscle activity and transmit these signals wirelessly to control a three DOF electromechanical prosthesis that has been engineered to support independent and simultaneous control of wrist pronation/supination, thumb abduction/adduction, and finger flexion/extension. The consistency of the signals generated by each of the implantable electrodes over the first six months since surgical placement, coupled with the precision control of the three DOF prosthesis indicates that the IMES have remained stable and that none of the electrodes have migrated from their original locations. Furthermore, this system has provided several additional benefits for the subject; including the ability to more intuitively control multiple degrees of freedom simultaneously, the removal of variability in sensor pickup due to socket rotation or limb sweating, and a reduction in fatigue and more control accuracy (particularly when manipulating the prosthesis during reaching activities). Together, these findings offer great enthusiasm about this new technology.
While other upper limb control strategies are currently being developed, they have multiple limitations compared to the IMES® system. Pattern recognition is one of these novel control techniques. The strategy employs algorithms to analyze patterns of EMG muscle activity across one or more muscles of the residual limb and has demonstrated the capability to control multiple degrees of freedom with more intuitive prosthetic control (Asghari Oskoei & Hu, 2007). The system utilizes surface electrodes that are either built into the prosthetic socket or liner to detect muscle contraction and measure magnitude, frequency, and speed of movement. The systems then probabilistically determine which movement is intended based on extensive user training and programming. Moreover, these systems rely upon surface electrodes and their attendant limitations, including the negative effect of socket rotation, limb sweating, or operating a prosthesis with reaching activities (particularly overhead). Therefore, successful implementation of this technology is contingent upon establishing stable EMG signaling (Scheme & Englehart, 2011). Recently, the first commercially available pattern recognition system has reached the consumer market (Complete Control, 2014).
Targeted muscle reinnervation (TMR) is another method that is being pursued to expand myoelectric control capacity for individuals with above-elbow amputation. For very proximal amputations, any type of muscle-based signaling to power the prosthetic wrist and hand is difficult because the naturally-associated muscle groups are no longer present. TMR involves the surgical re-connection of transected peripheral nerves to remaining muscles within the residual limb (Kuiken et al, 2004). Following the procedure, the nerve endings evolve functioning synapses with their new muscle partners. The result is that patients can contract these newly innervated muscle regions by attempting natural intended volitional movement. The muscle contractions have been successfully detected by surface EMG electrodes, creating additional sites of control for a myoelectric prosthesis and allowing natural-feeling operation of prosthetic limbs (Kuiken et al, 2009). TMR has also been combined with pattern recognition technology to provide above-elbow amputees control of multiple prosthetic functions simultaneously. While TMR surgery has been performed successfully in numerous patients, difficulties related to accuracy and stability of control are still significant obstacles. Most notable, challenges include difficulty with separating surface EMG signals, especially with co-activation of muscles within the residual limb, leading to signal interference (Schultz & Kuiken, 2011).
Improving EMG signal recording has implications beyond just upper limb prosthetic control. Currently, the majority of lower limb prosthetic systems are considered “passive”, i.e. no active motors to propel an individual. Even advanced microprocessor controlled variable dampening prosthetic knees (such as the Genium, X3, and Rheo Knee) are “passive” systems that are unable to generate active power to propel an individual during walking, climbing stairs, or rising out of a chair.(Wolf et al, 2013) Therefore, individuals with above knee amputation must rely on the activity of more proximal muscles such as the hip, pelvis and lower back to generate power, which not only limits their function, but decreases intuitive control and biomechanical efficiency, likely contributing to long-term complications. (Gailey et al, 2008)
Currently, there are only two commercially available motorized lower limb prosthetics, the Ossur Power Knee and the Biom (powered foot/ankle). These prosthetic components utilize onboard sensors within the device rather than direct user control from myoelectric signals. As implantable sensors become more available, it is likely that they will also contribute to improving user control of lower limb prosthetics, particularly during transitions, such as sitting, standing, inclines and stairs, as well as negotiating obstacles and stumble recovery.
All of these innovations have a variety of disparate challenges to their successful, widespread implementation. Yet a core issue that they and any advancement in prosthetic systems must all address is how to extract biological signals that are stable and reliable over long periods of time. Implanted electrodes placed into residual musculature present a potential way by which to address this. Based on the preliminary results discussed in this paper, our group is exploring the integration of the IMES® system with a variety of prosthetic devices to provide intuitive and stable myoelectric control to individuals with other levels of amputation.
The development of a more specific, leaded implantable sensor (LIMES) is also underway, although it is not yet available for use in a clinical trial. The leaded configuration of the IMES® aims to allow for gathering the transmitter/receiver capsules at a central location where they can all be operated with a small, low-powered coil. If sensing sites are all in the same area with the exception of one or two outliers, leads can be used to bring the transmitter/receiver capsule from the outlying sensing sites to the region of the other IMES®, allowing for a smaller coil that can be more conveniently and comfortably integrated into the prosthetic system. LIMES could also be used in cases where the target muscle is too small or thin to accommodate both sensing ends of the IMES®, or in situations where sensing sites are very close together, in which case the span between the differential electrodes of the non-leaded IMES® is too great to differentiate between the two. For example, residual finger muscles in the forearm of a transradial amputee that are thin and may be atrophied can be accessed with a LIMES device.
5. Conclusion
This study stands only as an initial evaluation of the feasibility of the IMES® technology. Yet, the advances and improvements that the first subject has made in his control of the IMES® system to this point, along with the subject’s initial reports of more natural, intuitive control as compared to his previous myoelectric device, are encouraging. We therefore remain cautiously optimistic about the future of implantable myoelectric sensor technology and its ability to provide accurate, reliable, and dexterous control of myoelectric prostheses for all levels of amputation.
Supplementary Material
Figure 4. Subject Utilizing IMES® System in Clinic.
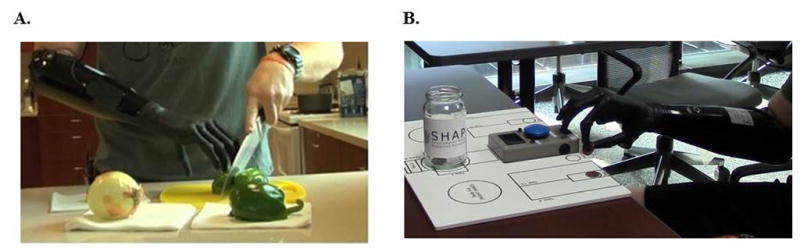
A) Subject manipulating and cutting vegetables during ACMC evaluation. B) Subject picking up and manipulating coins using IMES® System during SHAP.
Table 1.
Timeline of Clinical Trial
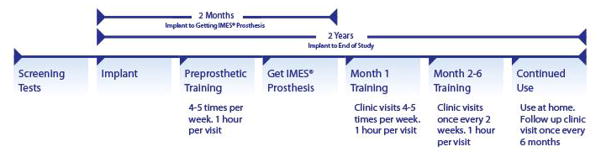
|
Highlights.
We implant transradial amputees with IMES® electrodes in residual muscles
We evaluate subject on ability to control prosthesis with IMES® System
First subject successfully operates IMES® System, reports more intuitive control
First subject shows continued improvement month to month
IMES® successfully sense and wirelessly transmit EMG from intramuscular positions
Acknowledgments
The authors would like to acknowledge Prof. Phil Troyk of Sigenics and the Illinois Institute of technology and Prof. Richard Weir, University of Colorado at Denver, for their contributions to the development of the Implantable Myoelectric Sensor (IMES) System. Our team would also like to acknowledge the Defense Advanced Research Projects Agency (DARPA), the Center for Rehabilitation Sciences Research (CRSR) at the Uniformed Services University of the Health Sciences, the Extremity Trauma and Amputee Center of Excellence (EACE), and the Office of Assistant Secretary of Defense for Health Affairs for their support.
This work is funded in part by the Alfred Mann Foundation, the U.S. Army Medical Research Material Command (USAMRMC) under Award No. W81XWH-14-2-0001m, and the National Institute of Health (NIH) under grant R01EB001672. Opinions, interpretations, conclusions and recommendations are those of the authors and are not necessarily endorsed by the Department of Defense or the NIH.”
Footnotes
Conflict of Interest: The authors declare no competing financial interests. The coauthors David Hankin, Melissa Evangelist, Morten Hansen, and Joseph Lockhart of this publication are employees of the Alfred Mann Foundation, a not-for-profit corporation which has been developing the IMES devices integral to the operation of the prosthetic hand and the clinical study that is the subject of this reported research. None of these authors have any direct or indirect ownership in the IMES system or any financial interest in the work product described or any of the intellectual property resulting from it. Further, all co-authors employed by AMF have declared no direct or indirect conflicts of interest resulting from their employment, personal relationships, academic competition, or personal bias that could influence honest and open reporting. All clinical evaluations and procedures were conducted at the facilities of the WRNMMC and by the staff at those facilities, not by any employees of the Alfred Mann Foundation. A medical monitor outside of the study personnel has overseen the study to ensure patient safety and the absence of undue pressures on the study staff or the subjects.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Asghari Oskoei M, Hu H. Myoelectric control systems—A survey. Biomedical Signal Processing and Control. 2007;2(4):275–294. [Google Scholar]
- Atkins D, Heard D, Donovan W. Epidemiologic over-view of individuals with upper-limb loss and their reported research priorities. J Prosthet Orthot. 1996;8(1):2–11. http://www.oandp.org/jpo/library/1996_01_002.asp. [Google Scholar]
- Biddis EA, Chau TT. Upper limb prosthesis use and abandonment: a survey of the last 25 years. Prosthet Ortho tint. 2007;31(3):236–57. doi: 10.1080/03093640600994581. [DOI] [PubMed] [Google Scholar]
- Complete Control. Coapt. 2014 Jan 1; Retrieved July 1, 2014, from http://coaptengineering.com/complete-control.html.
- Gailey R, Allen K, Castles J, Kucharik J, Roeder M. Review of secondary physical conditions associated with lower-limb amputation and long-term prosthesis use. Journal of Rehabilitation Research & Development. 2008 Jan;45(1) doi: 10.1682/jrrd.2006.11.0147. [DOI] [PubMed] [Google Scholar]
- Jones LE, Davidson JH. Save that arm: a study of problems in the remaining arm of unilateral upper limb amputees. Prosthet Orthot Int. 1999;23(1):55–8. doi: 10.3109/03093649909071611. [DOI] [PubMed] [Google Scholar]
- Kuiken TA, Dumanian GA, Lipschutz RD, Miller LA, Stubblefield KA. The use of targeted muscle reinnervation for improved myoelectric prosthesis control in a bilateral shoulder disarticulation amputee. Prosthet Orthot Int. 2004;28(3):245–53. doi: 10.3109/03093640409167756. [DOI] [PubMed] [Google Scholar]
- Kuiken TA, Li G, Lock BA, et al. Targeted muscle reinnervation for real-time myoelectric control of multifunction artificial arms. JAMA. 2009;301(6):619–28. doi: 10.1001/jama.2009.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light CM, Chappell PH, Kyberd PJ. Establishing a standardized clinical assessment tool of pathologic and prosthetic hand function: Normative data, reliability, and validity. Archives of Physical Medicine and Rehabilitation. 2002;83(6):776–783. doi: 10.1053/apmr.2002.32737. [DOI] [PubMed] [Google Scholar]
- Lindner HY, Linacre JM, Norling hermansson LM. Assessment of capacity for myoelectric control: evaluation of construct and rating scale. J Rehabil Med. 2009;41(6):467–74. doi: 10.2340/16501977-0361. [DOI] [PubMed] [Google Scholar]
- Lowery MM, Weir RF, Kuiken TA. Simulation of intramuscular EMG signals detected using implantable myoelectric sensors (IMES) IEEE Trans Biomed Eng. 2006 Oct;53(10):1926–33. doi: 10.1109/TBME.2006.881774. [DOI] [PubMed] [Google Scholar]
- McFarland LV, Winkler SLH, Heinemann AW, Jones M, Esquenazi A. Unilateral upper-limb loss: Satisfaction and prosthetic-device use in veterans and servicemembers from Vietnam and OIF/OEF conflicts. JRRD. 2010;47(4):299–316. doi: 10.1682/jrrd.2009.03.0027. [DOI] [PubMed] [Google Scholar]
- McLean L. The Early History of Myoelectric Control of Prosthetic Limbs (1945–1970) In: Scott RN, Np, editors. Powered Upper Limb Prostheses. Springer; Berlin Heidelberg: 2004. pp. 1–15. Print. [Google Scholar]
- Scheme E, Englehart K. Electromyogram pattern recognition for control of powered upper-limb prostheses: State of the art and challenges for clinical use. JRRD. 2011;48(6):643. doi: 10.1682/jrrd.2010.09.0177. [DOI] [PubMed] [Google Scholar]
- Schultz AE, Kuiken TA. Neural interfaces for control of upper limb prostheses: the state of the art and future possibilities. PM R. 2011;3(1):55–67. doi: 10.1016/j.pmrj.2010.06.016. [DOI] [PubMed] [Google Scholar]
- Weir RF, Troyk PR, Demichele GA, Kerns DA, Schorsch JF, Maas H. Implantable myoelectric sensors (IMESs) for intramuscular electromyogram recording. IEEE Trans Biomed Eng. 2009;56(1):159–71. doi: 10.1109/TBME.2008.2005942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf EJ, Everding VQ, Linberg AA, Czerniecki JM, Gambel JM. Comparison of the Power Knee and C-Leg during step-up and sit-to-stand tasks. Gait Posture. 2013;38(3):397–402. doi: 10.1016/j.gaitpost.2013.01.007. [DOI] [PubMed] [Google Scholar]
- Ziegler-graham K, Mackenzie EJ, Ephraim PL, Travison TG, Brookmeyer R. Estimating the prevalence of limb loss in the United States: 2005 to 2050. Arch Phys Med Rehabil. 2008;89(3):422–9. doi: 10.1016/j.apmr.2007.11.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.