Abstract
Arginine-vasotocin(AVT)/arginine vasopressin (AVP) are members of the AVP/oxytocin (OT) superfamily of peptides that are involved in the regulation of social behavior, social cognition and emotion. Comparative studies have revealed that AVT/AVP and their receptors are found throughout the “Social Behavior Neural Network” and display the properties expected from a signaling system that controls social behavior (i.e., species, sex and individual differences and modulation by gonadal hormones and social factors). Neurochemical signaling within the SBNN likely involves a complex combination of synaptic mechanisms that co-release multiple chemical signals (e.g., classical neurotransmitters and AVT/AVP as well as other peptides) and non-synaptic mechanisms (i.e., volume transmission). Crosstalk between AVP/OT peptides and receptors within the SBNN is likely. A better understanding of the functional properties of neurochemical signaling in the SBNN will allow for a more refined examination of the relationships between this peptide system and species, sex and individual differences in sociality.
Keywords: oxytocin, sociality, aggression, affiliation, pair bonding, communication, testosterone, estradiol, V1a, V1b
1. Introduction
The arginine-vasotocin (AVT)/ arginine-vasopressin (AVP) family of peptides regulates a variety of behavioral processes in a wide range of species. While the importance of AVT/AVP in reproductive behaviors was first discovered over 70 years ago, the role of these peptides in controlling non-reproductive social behavior is a more recent development (i.e., approximately 30 years ago)(Albers, 2012). AVT, AVP and a number of other structurally related peptides are part of a larger superfamily that includes various forms of oxytocin (OT) (for a review see (Caldwell and Young III, 2006)). Because of the critical importance of these peptide systems in regulating social behavior, social cognition and emotion they have become a focus in the investigation of the basic mechanisms underlying a variety of psychiatric disorders. While this is a developing research area of great importance, the focus of the current review will be on the basic mechanisms underlying the role of AVT/AVP in sociality. The reader interested in the role of these peptides in translational and clinical research is referred to the large number of excellent recent reviews(Caldwell and others, 2008a; Insel, 2010; Rotzinger and others, 2010; Harony and Wagner, 2010; Neumann and Landgraf, 2012; Burkett and Young, 2012; Lukas and Neumann, 2013).
The AVP/OT peptide superfamily evolved more than 600 million years ago from an ancestral form of AVT through gene duplication(Acher and Chauvet, 1995). These peptides are often called nonapeptides because they are composed of nine amino acid residues. They have a highly conserved structure across vertebrates. For example, AVP and OT share seven of nine amino acid sequences, differing only in the third and eighth positions. AVT is found in fish, amphibians, reptiles and birds while AVP or AVP-like peptides (e.g., lysinevasopressin) occur in mammals. There are a number of different OT-like peptides found in vertebrates; fish produce isotocin (IT) and amphibians, reptiles and birds produce mesotocin (MT). Even in mammals not all forms of OT are identical in structure(Lee and others, 2011). Although amino acid sequence differences exist across members of this peptide family, the structure of these peptides has been largely conserved during vertebrate evolution. The AVP/OT family of peptides is also found in a large number of invertebrate species such as mollusks, nematodes and arthropods(Gruber, 2014).
In mammals, four nonapeptide receptors have been identified: V1a, V1b, V2 and OT(Barberis and Tribollet, 1996; Caldwell and Young III, 2006; Hasunuma and others, 2013). These receptors belong to the G protein-coupled receptor superfamily that have seven putative transmembrane domains and appear to be evolutionarily ancient. Interestingly, recent studies indicate that the original expression site of AVP/OT receptors may have been in the central nervous system and not peripheral tissues, as many have previously assumed(Yamashita and Kitano, 2013). V1a and OT receptors are robustly expressed in many regions of the mammalian brain. V1b receptors appear have a much more restricted distribution in the brain, although they are expressed prominently in the hippocampus and at lower levels in the hypothalamus and amygdala(Young and others, 2006). V2 receptors have also been reported in the adult and developing mammalian brain, but these findings are controversial and it seems unlikely that they play a significant role in regulating sociality(Hirasawa and others, 1994; Kato and others, 1995; Foletta and others, 2002; Vargas and others, 2009). The V1a-like receptor is the most widely distributed AVT/AVP receptor in the brains of vertebrates, and plays a critical role in the control of social behavior(e.g., Albers and others, 1986; Ferris and others, 1996). There is increasing evidence, however, that V1b receptors also play a role in the regulation of social behavior (see (Stevenson and Caldwell, 2012) for a review). Finally, it is possible that at least some of the effects of AVP- and OT-like peptides on social behavior might be the result of crosstalk between the canonical receptors.
Less is known about nonapeptide receptors in non-mammalian species, although receptors with similarity to mammalian nonapeptide receptors have been identified in all major vertebrate groups except reptiles (see Table 1). In fish, there are two V1a receptors, V1a1 and V1a2, as well as a V2 receptor and an IT receptor(Lema, 2010; Kline and others, 2011; Ocampo and others, 2012; Yamaguchi and others, 2012; Lema and others, 2012). V1a1, V1a2 and IT receptors are found in brain but V2 receptors are not(Lema, 2010). In newts (i.e., C. pyrrhogaster), three types of AVT receptors have been cloned and based on their structure they have been designated as V1a, V2 and V3/V1b receptors corresponding to the mammalian V1a, V2 and V1b receptors, respectively(Hasunuma and others, 2007). MT receptors have also been characterized and sequenced in amphibians(Akhundova and others, 1996; Kohno and others, 2003; Acharjee and others, 2004; Searcy and others, 2011). In birds, there are four nonapeptide receptors VT1-VT4(Tan and others, 2000; Gubrij and others, 2005; Leung and others, 2011). The VT4, VT2, VT1, and VT3 avian receptors appear to be homologues of the mammalian V1a, V1b, V2 and OT receptors, respectively. Interestingly however, the V1b-like VT2 receptor has not been detected in avian brain, but the V2-like VT1 receptors is found in avian brain as well as the periphery. In summary, the nomenclature of vertebrate AVT/AVP receptors has become increasingly complex, perhaps unnecessarily. Despite some species differences, it would be useful to simplify this receptor nomenclature by adopting the mammalian designation for these receptors in birds. The situation, in amphibia and fish, however, appears to be more complex.
Table 1.
Nomenclature of vertebrate AVP/OT family of peptide receptors
| Mammals | V1a | V1b | V2 | OT |
|---|---|---|---|---|
| Birds | VT4 | VT2 | VT1 | VT3 |
| Reptiles | - | - | - | - |
| Amphibia | V1a | V3/V1b | V2 | MT |
| Fish | V1a1/ | - | V2 | IT |
| V1a2 |
The focus of this review will be to examine the relationships between sociality and species, sex and individual differences in AVT/AVP systems. The plasticity in the expression of this signaling system, and in particular, how gonadal hormones and social experience can modulate its signaling capacity will be reviewed. Finally, how the complex interplay between AVP-like peptides and their receptors might control sociality by their actions in the “social behavior neural network” will be discussed.
2. The “Social Behavior Neural Network”
There is a substantial amount of evidence that manipulation of peptides in the AVP/OT family can have dramatic and powerful effects on social behavior by acting within specific CNS sites. A full understanding of the neurobiology of social behavior, however, will require an understanding of the action of these peptides and their receptors across a complex neural network. The concept of a “social behavior neural network” (SBNN) has emerged relatively recently. Over the last 30 years, it has become clear that there is a large degree of overlap in the neural circuitry controlling different social behaviors. As originally proposed by Newman(Newman, 1999), the SBNN is composed of neural groups or “nodes” including, but not limited to, the extended amygdala, LS, PAG, MPOA, VMH, and AH. Each node within the SBNN fulfills several criteria. They are reciprocally connected, all contain neurons with gonadal hormone receptors, and each has been identified as an important site of regulation or activation of more than one social behavior. Further, there is a substantial body of evidence that this network is involved in controlling a wide range of social behaviors including both offensive and defensive aggression, social recognition/memory, sex behavior, parental behavior and social communication(Albers and others, 2002; Caldwell and others, 2008a; Adkins-Regan, 2009; Albers, 2012; Bosch and Neumann, 2012; Goodson and Kingsbury, 2013). There is now evidence that social behavior neural networks also exist in non-mammalian vertebrates(Crews, 2003; Goodson, 2005; O'Connell and Hofmann, 2011). In fact, a comparative analysis across mammals, birds, reptiles, amphibians and teleost fish provides support for the proposition that there may be homologous nodes in the SBNN of these major vertebrate lineages and that these mechanisms are evolutionarily quite old. However, whether these non-mammalian networks are composed of a series of nodes that are homologous to those in mammals is controversial (for a review see(Goodson and Kingsbury, 2013)). The overarching hypothesis is that the diversity and complexity of different social behaviors across a wide range of species and individuals can be accounted for by variations in the functional interactions within and across the nodes of this highly conserved network. As such, social behaviors emerge from the entire network and not from its individual elements.
Given the diversity of the animals in which these networks are found, it seems almost certain that the specific nodes contained within the SBNN as well as their functional activity will vary across species. For example, studies in birds have revealed far more steroid hormone-induced modulation across nodes of the SBNN than that seen in mammals(Maney and others, 2008). It also seems likely that the criteria for establishing a structure as a node in the network will be reconsidered (e.g., must all nodes have steroid receptors?). Certainly, when one considers the relative importance of different forms of sensory information in the expression of social behavior in different species, it is not at all surprising that there are going to be substantial differences in the structures providing sensory input. Because rodents rely heavily on olfactory information to guide their social behavior, the olfactory system is a key node in their SBNN. In contrast, in species such as birds and primates where visual cues guide social behavior, the structures mediating visual information will likely play a similar key role.
Nevertheless, the construct of the SBNN is a transformative way of looking at the neural mechanisms controlling social behavior when compared to previous approaches of studying the role of single structures in regulating single behaviors. It is important to point out, however, that without decades of studies employing the single structure/single behavior approach, it would not have been possible to pull this large body of data together and to recognize that the same structures control such a large number of different social behaviors. The next order of questions to be addressed will focus on how social behavior emerges from these complex neural networks. While the concept that specific social behaviors are produced by a pattern of activity across the network is attractive, the challenge is finding ways to critically test this hypothesis. Experimental approaches that can explore the signaling across multiple nodes in the SBNN such as large scale ensemble recording techniques, fMRI and transcriptomics will be essential to understanding how social behavior emerges from this network. The success of this approach will also require new analytic techniques in order to analyze the large data sets that define the dynamics of neural activity across such large networks (e.g.,(Lin and others, 2006)).
As discussed below, AVT/AVP and their receptors are found throughout the nodes of the SBNN and display the properties that would be expected from a signaling system that plays a critical role in controlling social behavior by its actions throughout this network (i.e., species, sex and individual differences as well as modulation by gonadal hormones and social factors). However, future progress in understanding the neurobiology of sociality will require a far better understanding of the signals produced by these peptides and how they interact across the network.
3. Sociality and comparative studies of the AVT/AVP system
The investigation of the role of nonapeptides in social behavior has benefited greatly from the large number of species that have been, and continue be, studied(Phelps and others, 2010). One of the great advantages of the comparative approach is that it is possible to examine the relationships between the extraordinary diversity in the types of social systems that animals display and the characteristics of their nonapeptide systems. This approach has the potential to address the relationship between the evolution of social behavior and its linkage to nonapeptide systems and to ask overarching theoretical questions such as, ‘Do species differences in nonapeptide systems underlie species differences in sociality?”. We are, however, at a very early stage because, with a very few exceptions, the existing data examining whether species differences in sociality are the result of species differences in nonapeptide systems have been correlational. Specifically, sociality has been correlated with species differences in the distribution of AVT/AVP or their receptors. As discussed below, there are many other species differences in the AVT/AVP system beyond its “wiring diagram” that may prove useful in understanding the relationships between this system and sociality.
Studies to investigate this relationship also raise the complex question of what constitutes “sociality” and how do we characterize it so that we can investigate its relationship with nonapeptide systems(Ophir, 2011). As discussed by Goodson(Goodson, 2013), the concept of sociality has historically scientific roots but has now become used much more broadly to encompass a wide range of behavioral processes from pair bonding to aggression. In the present discussion sociality will be defined broadly to include social behavior, social cognition and emotion. The comparative approach provides the opportunity to use the evolution of nonapeptide systems and sociality as a guide to understand basic underlying mechanisms and their potential translation to clinical application. It must be acknowledged, however, that increasing taxonomic diversity in evolutionary studies of sociality is not without its own set of complexities; for example, what constitutes anatomical homology?. Nevertheless, developing simple categories of sociality across or even within species can be useful for a first order analysis of the relationship between sociality and nonapeptides systems(Caldwell, 2012). One of the best examples of the success of using dichotomous categories of sociality across species is the comparative analysis of monogamous and non-monogamous vole species (discussed in detail below). The initial studies using this approach were purely correlational; for example, the amount of V1a receptor binding in the ventral pallidum was found to predict whether closely related species were monogamous or non-monogamous. The hypothesis that the number of V1a receptors in the ventral pallidum determined whether a species is monogamous was then tested by examining whether the induction of V1a receptors within the ventral palladum could induce pair bonding in a non-monogamous species of vole. Other dichotomous categories of species differences in sociality may not be as clear-cut as monogamy versus nonmonogamy, but they are applicable to a much larger groups of animals given that monogamy occurs in only 3% of mammalian species(Kleiman, 1977). One approach that has been useful has been to compare nonapeptide systems in species that are categorized simply as “asocial” or “social” (e.g., gregarious)(Goodson and Wang, 2006). For example, in studies of several related species of birds there are neuronal patterns of activation in the medial bed nucleus of the stria terminalis (BNST) in response to social stimuli that are very different in asocial and social species. In summary, the use of dichotomous categories of sociality (e.g., monogamous versus non-monogamous) have proven to be useful in the analysis of the relationship between species differences in sociality and nonapeptides. This approach remains necessary because of the lack of a comprehensive understanding of sociality that can only be achieved with extensive field studies as well as detailed knowledge of the anatomical characteristics of nonapeptide systems in a broad representation of species. Of course, as we expand our knowledge about sociality and nonapeptides across diverse groups of species the power to define these relationships will be enhanced substantially.
3.1. Sociality and comparative studies of AVT/AVP: Methodological considerations
There are a number of issues that are important to consider when investigating how AVT/AVP and their receptors are distributed throughout the CNS, particularly when comparing across species, sexes and physiological states. The predominant approaches to determining the location of AVP/AVT within the brain have been immunohistochemistry and in situ hybridization. Immunohistochemistry is useful for identifying neuronal cell bodies and processes (e.g., fibers) that contain AVT/AVP peptide, while in situ hybridization identifies the cell bodies that transcribe the peptide gene. The distribution of immunoreactive product can be influenced by a variety of methodological and physiological variables. For example, one method used to examine the location of peptide-producing cells involves the administration of colchicine. Colchicine inhibits the transport of newly synthesized peptide thereby concentrating the peptide within the cell body and making it easier to visualize. While this can be a very useful approach to identify peptide-producing cell bodies, it can also create complications in comparing the distribution of a peptide generated in studies using colchicine with those not using colchicine. Physiological changes in peptide synthesis, degradation and release can also complicate the comparison of the distribution of peptides. For example, high levels of peptide release have the potential to reduce or eliminate the ability of immunohistochemistry to identify peptide-producing neurons.
Studies of the distribution of peptide receptors have also used immunohistochemistry, in situ hybridization, as well as receptor autoradiography. Unfortunately, immunohistochemistry for the AVP/OT family of receptors has not proven to be feasible because of the inability to generate antisera that recognize these receptors. In situ hybridization has proven to be a useful technique to localize the cells synthesizing receptors, but provides little information on where the receptors are localized. One of the most common approaches to localizing peptide receptors is receptor autoradiography. This approach uses radiolabeled ligands to localize receptor binding sites. This is a powerful approach, but it is dependent upon the specificity and potency of the ligands used. Because of the prevalence of species differences in selectivity of the ligands employed in receptor binding studies, caution should be used in the interpretation of these studies.
4. Comparative studies of AVT/AVP and their receptors
4.1. Species differences in AVT/AVP
AVT/AVP containing neurons are distributed widely in the brains of all vertebrates studied so far (Moore and Lowry, 1998; De Vries and Panzica, 2006). There are excellent reviews of the neuroanatomy of AVT/AVP in fish(Godwin and Thompson, 2012; Thompson and Walton, 2013), amphibians(Woolley and others, 2004; Wilczynski and others, 2005; Boyd, 2013), reptiles(Woolley and others, 2004), birds(Goodson and Bass, 2001; Goodson and others, 2012) and mammals(Rood and De Vries, 2011; Ragen and Bales, 2013). The patterns with which AVT/AVP cell bodies and fibers are distributed in the brain are fairly consistent across vertebrate species(Moore and Lowry, 1998; Goodson and Bass, 2001; Godwin and Thompson, 2012). A very common feature of AVT/AVP systems across vertebrates is that both parvocellular and magnocellular AVT/AVP containing neurons can be found in the preoptic area, as well as in a number of hypothalamic regions, and that these cell bodies project to other hypothalamic and extrahypothalamic regions including the midbrain tegmentum, medulla and spinal cord. Comparative studies of AVT/AVP synthesizing neurons have found that they cluster in identifiable groups based on their anatomical characteristics and that these may represent homologous structures across species despite the fact that these neurons do not always fall within classical neuroanatomical borders. For example, scattered AVP-positive magnocellular neurons found in the hypothalamus have been called the accessory nuclei and occur in a variety of mammals, including humans(Krisch, 1976; Castel and Morris, 1988; Mahoney and others, 1990; Ishunina and Swaab, 1999; Rood and De Vries, 2011). Homologs of these accessory neurons may also exist in nonmammalian species. Many vertebrate species have AVT/AVP producing cell bodies in the extended amygdala that project to other forebrain structures such as the lateral septum (LS)(De Vries and Panzica, 2006). Projections from the paraventricular nucleus (PVN) and supraoptic nucleus (SON) to the posterior pituitary are the source of systemically released AVP and OT. These nuclei, however, are also the source of AVP-containing fibers that project to other brain regions(Buijs, 1978; Sawchenko and Swanson, 1982; De Vries and Buijs, 1983; Alonso and others, 1986). For example, in Syrian hamsters it has been estimated that 30% of AVP-containing magnocellular neurons in the lateral and medial SON project to other hypothalamic and extra-hypothalamic sites and not to the pituitary(Mahoney and others, 1990; Ferris and others, 1992).
Despite the many similarities in the pattern of AVT/AVP expression, there are also potentially important differences in their distribution both within and across all major groups of vertebrates. In teleost fish, for example, in addition to magnocellular neurons, populations of hypothalamic gigantocellular AVT neurons can also be found(Godwin and Thompson, 2012). In amphibians, AVT cell bodies are found in the medial amygdala (Me) of some species but not others(Smeets and Gonzalez, 2001) and in birds, there are species differences in the number of AVT producing neurons in the medial BNST (Goodson and Wang, 2006; Goodson J.L., 2008). In many mammalian species, AVP producing neurons in the Me and BNST that project to the LS have been implicated in both social and emotional behavior(Wang and others, 1994a; Ring, 2005; Beiderbeck and others, 2007; Kelly and others, 2011; Neumann and Landgraf, 2012). There can be, however, considerable variations in AVP expression across species with no obvious correlation to differences in sociality. In Syrian hamsters, a relatively “asocial’ species, there is a nearly complete lack of AVP containing cell bodies in the extended amygdala as well as an absence of AVP fibers in the LS (Dubois-Dauphin and others, 1990; Albers and others, 1991; Ferris and others, 1995). On the other hand, in naked mole rats, a highly “social” species, AVP producing cells are also nearly absent in the BNST and Me and no AVP-containing fibers are seen in the LS(Rosen and others, 2007). In guinea pigs, also a relatively “social” species(Sachser, 1998), there are unusually high levels of AVP innervation in the septum(Dubois-Dauphin and others, 1989). In both human and non-human primates, AVP containing cell bodies in the amygdala are absent or substantially fewer in number than that seen in many rodents, and AVP containing fibers are absent in the LS(Fliers and others, 1986; Caffe and others, 1989; Wang and others, 1997a; Wang and others, 1997b). As such, the existing data do not suggest any simple relationships between the distribution of AVT/AVP and “social” or “asocial” patterns of sociality. A few studies have examined more specific hypotheses on the distribution of AVP and sociality. For example, it was hypothesized that the amount of AVP immunoreactivity in the BNST and Me would be negatively correlated with the amount of parental behavior. This hypothesis was based on data collected in voles where AVP immunoreactivity was greater in the BNST and Me of species with higher levels of parental behavior(Wang, 1995). Studies in two species of mice revealed, however, that parental behavior did not predict differences in the patterns of AVP immunoreactivity(Bester-Meredith and others, 1999).
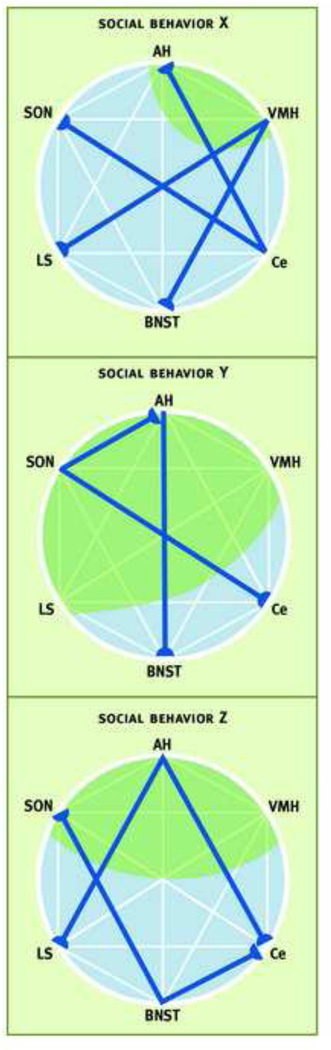
In summary, AVT/AVP expression occurs throughout the SBNN across vertebrate species. While consistent relationships between the distribution of AVT/AVP and sociality have yet to be defined, the investigation of the covariation of social behavior and AVT systems in fish, amphibians and reptiles is still at an early stage. Similarly, in mammals striking relationships between AVP and sociality have yet to be identified. One approach that has proven useful is the examination of the relationships between the functional activity of AVT/AVP neurons (e.g., neuronal activation as measured by immediate-early gene expression) and social behavior (Figure 1), e.g.,(Delville and others, 2000; Gobrogge and others, 2007). The approach has been applied productively to comparative studies examining whether the neuronal activation of AVT neurons in five species of birds that are “social” or “asocial” (Goodson and Wang, 2006). Using this approach a relationship between the neuronal activation of AVT containing neurons and sociality was identifed. More specifically, the social species displayed more neuronal activation in AVT-containing neurons in the mBNST in response to same sex social stimuli than did the asocial species.
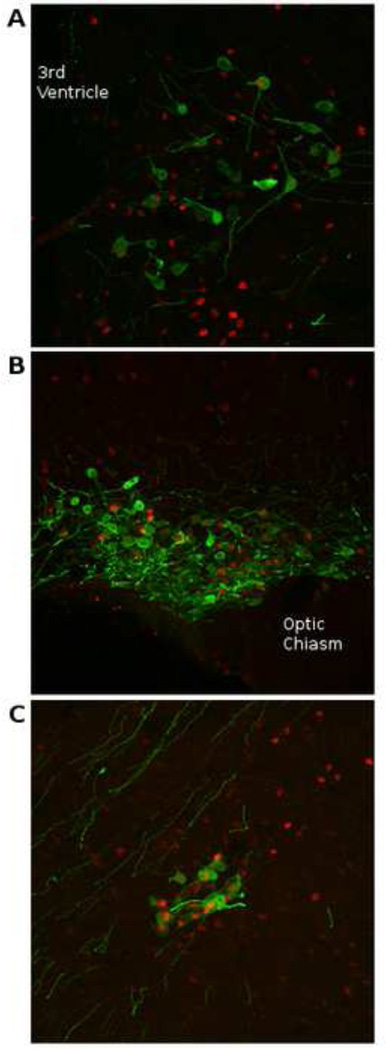
Figure 1.
Confocal images of (A) medial paraventricular nucleus (mPVN), (B) medial supraoptic nucleus (mSON), and (C) nucleus circularis (NC) in a male Syrian hamster one hour after an agonistic encounter with another male. Green staining indicates arginine-vasopressin (AVP) and red staining indicates c-Fos. The agonistic encounter significantly increased AVP and c-Fos colocalization in the mSON and NC (p < 0.05) but not the mPVN (p > 0.05) as compared to controls (data not shown). From Terranova and Albers, unpublished data.
4.2. Species differences in AVT/AVP receptors
The possibility that species differences in AVT/AVP receptor distribution might underlie species differences in sociality has been a matter of active investigation for over 20 years (Insel and others, 1991; Witt and others, 1991; Insel and others, 1993). There appear to be more substantial species differences in the distribution of AVP/OT receptors than for the receptors of other neurochemical signals(Insel and Shapiro, 1992). Species differences in AVT/AVP receptors could influence social behavior in a number of different ways. It is possible that species differences exist in the number of AVT/AVP receptors found within specific brain regions, in the coding sequence or promoter regions of the receptor gene and/or in how the receptors respond to receptor activation. As discussed below, the vast majority of research that has looked at species differences in V1a receptors has focused on differences in receptor distribution as revealed by the density of receptor binding or the pattern of V1a receptor mRNA. There has also been a considerable amount of work investigating whether species differences in the distribution of V1a receptors might be the result of differences in DNA sequences (i.e., microsatellites) located in the 5’ flanking region of the V1a receptor gene. There are surprisingly large species differences in both the coding region of the V1a receptor and in the amino acid sequence of the receptor, itself(Fink and others, 2007; Turner and others, 2010). While these species differences are interesting and potentially important for understanding species differences in social behavior, the diversity in the amino acid sequences does not appear to alter the signaling capacity of the receptor. There are also data to suggest that activation of V1a receptors can result in very different behavioral responses. For example, injection of AVP into the AH stimulates aggression in male Syrian hamsters but inhibits it in females(Ferris and Potegal, 1988; Potegal and Ferris, 1989; Gutzler and others, 2010; Ferris and others, 2013). These opposite effects of AVP on aggression appears to be mediated by the V1a receptor because injection of selective V1a receptor antagonists inhibits aggression in males and stimulates aggression in females. More recently, a similar sex difference has been observed in the effects of centrally administered AVP and a V1a receptor antagonist (i.e., intracerebrventricular injections (ICV)) on play behavior in rats(Veenema and others, 2013). It will be interesting to determine if these sex differences in the behavioral response to activation of V1a receptors are the result of sex differences in intracellular signaling pathways or whether they are the result of sex differences in the local networks and/or efferent pathways that coordinate these behaviors.
The distribution of AVT receptors has been studied in fish(Moons and others, 1989; Kline and others, 2011; Lema and others, 2012; Huffman and others, 2012) and amphibians(Tripp and Moore, 1988; Boyd and Moore, 1991; Acharjee and others, 2004; Lewis and others, 2005; Hasunuma and others, 2010; Hasunuma and others, 2013) and birds (Voorhuis and others, 1988; Voorhuis and others, 1990; Goodson and others, 2006; Leung and others, 2009; Leung and others, 2011), but not yet in reptiles. The vast majority of data on the distribution of nonapeptide receptors, however, comes from mammals. Even in relatively closely related vertebrates, there can be significant species differences in the distribution of AVT/AVP receptors, although the data remain quite sparse in many groups. Despite these species differences in the distribution of AVT/AVP receptors, there are also some consistent features in their patterning across species. For example, V1a receptor binding has been found quite consistently in the LS, even though the magnitude of receptor binding in the LS can differ among species(Insel and others, 1994; Turner and others, 2010).
One very productive line of research has been the investigation of the relationship between the distribution of V1a receptors and whether a species is monogamous or non-monogamous. Non-monogamous male rodents have different patterns of V1a receptor binding than monogamous males(Insel and others, 1991; Insel and others, 1994; Young and others, 1997b; Bester-Meredith and others, 1999). Interestingly, comparison of V1a binding in a variety of mammalian species revealed that higher densities of V1a receptor binding are found in the ventral pallidum in monogamous species than in non-monogamous species(Insel and others, 1994; Young, 1999; Bester-Meredith and others, 1999b; Young and others, 1999b). Because of this difference in V1a receptor binding and because the ventral pallidum is involved in reward systems, it was hypothesized that V1a receptors facilitate pair-bonding(Winslow and others, 1993; Young and others, 1997a; Pitkow and others, 2001; Lim and others, 2004; Young and Wang, 2004). In a series of elegant experiments employing monogamous and non-monogamous vole species, strong support for this hypothesis has been provided. For example, induction of V1a receptors in the ventral pallidum of non-monogamous voles by viral vector gene transfer results in pair bonding similar to that seen in monogamous voles. Transgenic mice containing the prairie vole receptor gene were found to have a distribution of V1a binding similar to prairie voles and to increase their affiliative behavior in response to the injection of AVP(Young, 1999; Young and others, 1999a). Although the prairie vole-like pattern of V1a receptor binding distribution in the transgenic mouse was associated with increased affiliation in response to AVP it did not result in the ability to form pair bonds. Nevertheless, this work has demonstrated that the change in the expression of a single gene can have powerful effects on social behavior.
In a related line of research, recent studies have investigated whether polymorphisms in microsatellite sequences in the V1a receptor gene might produce different patterns of V1a receptors and thereby determine whether a species is monogamous or non-monogamous. In support of this hypothesis, comparative analysis of the V1a receptor gene in several species of voles found the microsatellite region to be significantly longer in monogamous species compared to non-monogamous species (Young and others, 1999a; Hammock and Young, 2002; Hammock and Young, 2004; Hammock and Young, 2005). Another approach used to test this hypothesis was to determine whether the production of transgenic mice that contained the V1a receptor gene including the microsatellite, exons, introns and some downstream elements from the monogamous vole would produce mice that pair bond(Young and others, 1999a). In one of the four independently derived transgenic mouse lines an increase in the affiliative behavior did occur, although pair bonding was not observed. The fact that only one of the four lines of mice carrying the randomly inserted but identical transgene had a prairie vole-like pattern suggests that the insertion site within the genome has a significant impact on the expression pattern and that the V1a transgene might not be responsible for species differences(Donaldson and Young, 2013).
More recent studies employing knock–in mice found that insertion of the microsatellite region from different species of voles did not produce distributions of V1a receptor binding typical of the donor species, although there were changes in binding that moved in the direction normally displayed by that species(Donaldson and Young, 2013). The effects, however, were primarily observed in brain regions not known to influence social behavior. More extensive studies have now been conducted in a variety of mammalian species, and they have revealed that monogamy is not the result of a single polymorphism in the 5’ regulatory microsatellite in the V1a receptor gene. In fact, long microsatellites are common elements of the V1a receptor gene in both monogamous and non-monogamous species(Fink and others, 2006; Turner and others, 2010) (Figure 2). Studies in primates have also revealed a substantial amount of variability in the 5’ region of the V1a receptor gene, e.g. (Donaldson and others, 2008; Rosso and others, 2008; Babb and others, 2010), however there is currently no definitive evidence that these differences are responsible for species differences in V1a receptor distribution or in species differences in social behavior. While it is clear that microsatellites can, in some cases, influence the distribution of V1a receptors and that the distribution V1a receptors can influence social behavior, it now seems unlikely that the length of the microsatellite region of the V1a receptor is the primary driver of species differences in social behavior. In summary, despite the clear evidence that the induction of V1a receptors in non-monogamous voles can induce pair-bonding behavior, the distribution of V1a receptors is not a universal determinate of whether a species is monogamous(Bester-Meredith and others, 1999; Turner and others, 2010; Phelps, 2010).
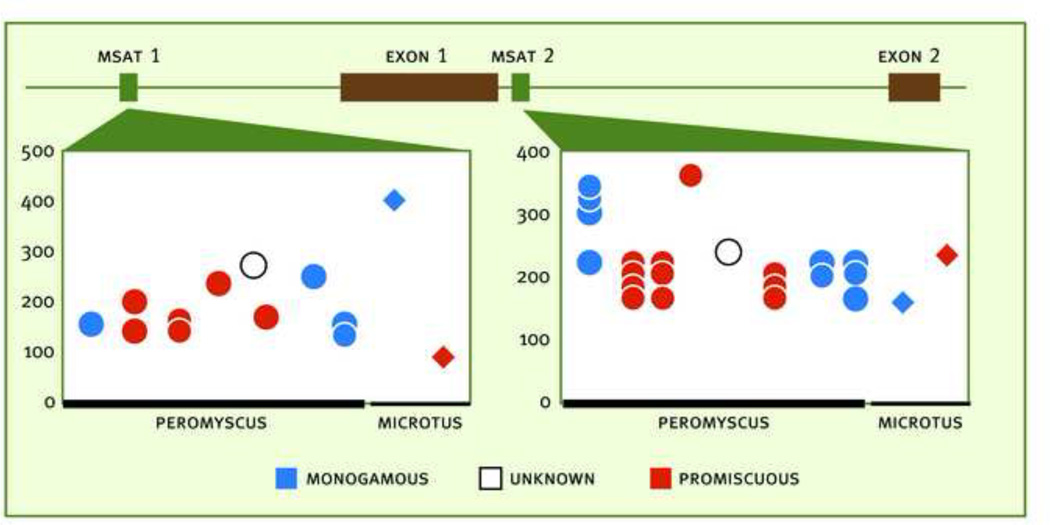
Figure 2.
Variability in the microsatellite length of the V1a receptor gene in monogamous and promiscuous species of Peromyscus and Microtus. Top: Structure of the V1a gene: Green boxes represent microsatellites (MSAT 1 and MSAT 2) and brown regions represent Exons 1 and 2 (microsatellite 3 is not shown). Bottom: Allele sizes (basepairs) in eight species of Peromyscus (circles) and two species of Microtus (diamonds). Size of the homologous repeats were inferred from alignment of primers to GenBanK sequences (AF069304, AF070010) for comparison. Monogogamous species appear in blue and promiscuous appear in blue. Modified from (Turner and others, 2010).
There have also been some interesting comparative studies examining the possible relationships between the distribution of AVT/AVP receptors and aspects of sociality other than monogamy. In birds, where there are substantial species differences in the distribution of AVT receptors(Leung and others, 2009; Leung and others, 2011), the distribution of these receptors can differ in species that display different patterns of sociality. Studies employing five species of birds that differ along the “social-asocial” continuum have significantly different amounts of V1a receptor binding in the LS; the more “social” the species, the greater magnitude of V1a receptor binding(Goodson and others, 2006). In contrast, in two closely related polygamous species of South American rodents, no relationship between V1a receptor binding in the LS and sociality was observed(Beery and others, 2008). Among the substantial differences in the pattern of V1a receptor binding observed in these species, however, significantly lower levels of V1a receptor binding in the ventral pallidum was found in the social species compared to the asocial species. The distribution of V1a receptors has also been compared in two species of “singing” mice from Central American that have highly developed forms of vocal social communication(Campbell and others, 2009). While both species are social and non-monogamous, they live in different habitats and have different social structures. Given the important role of V1a receptors in the AH, BNST and PAG in the control of social communication(Ferris and others, 1984; Irvin and others, 1990; Hennessey and others, 1992; Albers and Cooper, 1995; Goodson and Bass, 2000), it is interesting that there were substantial species differences in the magnitude of V1a receptor binding in these structures. Other interesting relationships between the pattern of V1a receptor distribution and sociality have also been suggested, such as a relationship between V1a receptor binding and social spacing(Goodson and Bass, 2001). While this hypothesis is yet to be fully explored, it is clear that there is not always a correlation between social spacing and the pattern of V1a receptor distribution(Turner and others, 2010).
In primates, the distribution of V1a receptors has been studied in marmosets, coppery titi monkeys, rhesus monkeys and humans, however the data remain quite limited. In marmosets, heavy to moderate V1a receptor binding occurs in several limbic regions including the ventromedial hypothalamus (VMH), LS and BNST. Interestingly, V1a receptor binding in the SON, present in most other species so far examined, could not be confirmed(Wang and others, 1997b; Kozorovitskiy and others, 2006; Schorscher-Petcu and others, 2009). In coppery titi monkeys V1a receptor binding is remarkably diffuse throughout the brain(Freeman and others, 2014). Of particular interest is the widespread distribution of V1a receptor binding in the cortex particularly in areas involved in the processing of visual information, the control of attention and emotion. As such, it has been suggested that V1a receptors might provide a link between visual stimuli important in social communication and the emotional processing of this information. In rhesus monkeys, V1a receptor binding in subcortical structures is similar to that seen in other species and there is a high density of V1a receptor binding in the cortex(Young and others, 1999b). In humans, V1a receptor binding in the forebrain also appears to be limited, however intense V1a receptor binding is found in the LS. Only weak binding is seen in the BNST(Loup and others, 1991). The lack of more comprehensive data on the distribution, characteristics and functions of AVP receptors in primate brain and in particular human brain is a substantial gap in our knowledge and a significant impediment to more translational research.
In summary, like AVT/AVP, the receptors of these peptides occur extensively throughout the SBNN. While AVT/AVP and their receptors are often found in the same anatomical structures there are cases where there are anatomical “mismatchs” in the location of peptide and receptors. Species differences in the distribution of V1a receptors can result in dramatic species differences in social behavior, however in most cases clear relationships between sociality and the distribution of AVT/AVP expression or their receptors have yet to be defined. More refined analyses of these relationships will become possible when we consider more than just the anatomical distribution of AVT/AVP and AVT/AVP receptors (see discussion in section 10).
5. Sex differences in AVT/AVP and their receptors
5.1. Sex differences in AVP/AVT
The existence of sex differences in the distribution of AVT/AVP has been a matter of extensive investigation for more than 30 years. The first demonstration of sex differences in the distribution of AVP came from the serendipitous discovery of sex differences in AVP projections in rat brain(De Vries and others, 1981). More AVP-containing neurons and projections were found in males compared to females. Subsequent lesion, tracing and immunocytochemical studies in rats went on to demonstrate that the BNST and Me are the primary sources of sexually dimorphic AVP innervation(De Vries and Buijs, 1983; De Vries and others, 1985; Caffe and others, 1987). While studies in a variety of species have found sex differences in AVP immunoreactivity in the extended amygdala, detailed anatomical analysis of the origin of these sexually dimorphic projections has been restricted almost exclusively to rats (however, see (Absil and others, 2002)). Interestingly, while gonadal hormones during the prenatal period of development have a major “organizational” role in inducing sex differences in the AVT/AVP system, there can be important differences in which steroid triggers sexual differentiation of this system. For example, estradiol masculinizes the AVP system in rats but feminizes this system in Japanese quail(Panzica and others, 1998; Han and De Vries, 2003).
Because sex differences in AVT/AVP systems have been reviewed extensively elsewhere, they will be discussed only briefly here(De Vries and Panzica, 2006; De Vries, 2008). Sex differences in AVT/AVP expression in the extended amygdala or homologous structures have been observed in amphibians, reptiles, birds as well as mammals. There are exceptions, however, to this pattern of sex differences in AVT/AVP in both mammals and birds (e.g., (Voorhuis and De Kloet, 1992; Dubois-Dauphin and others, 1994b). While the sex differences in the AVT/AVP system across species are generally similar to those observed in rats, there are cases where sex differences in AVT/AVP take different forms. For example, sex differences have been identified in AVT neurons in non-mammalian species that do not appear to be homologous to those seen in the mammalian species(Moore and others, 2000; Panzica and others, 2001). There are also mammalian species where sex differences in AVP expression in the BNST, MeA and LS have not been observed. For example, Syrian hamsters have few AVP neurons in the extended amygdala(Albers and others, 1991; Ferris and others, 1995; Miller and others, 1999), and no sex differences in AVP content within this region have been reported (Hennessey and others, 1994). Similarly, naked mole-rats and hyenas have substantially fewer AVP positive neurons in the extended amygdala and do not display a sex difference in AVP content within these areas(Rosen and others, 2006; Rosen and others, 2007). Interestingly, however, sex differences in AVP concentrations in magnocellular neurons in the SON have been reported in Syrian hamsters, rats and humans(Delville and others, 1994; Madeira and others, 1993; Taylor and others, 2012; Ishunina and Swaab). The limited data available in primates have not identified sex differences in AVP distribution in the extended amygdala(Fliers and others, 1986; Ishunina and others, 1999).
In summary, in the majority of species so far examined significant sex differences in AVT/AVP within the extended amygdala are observed, although exceptions to this pattern occur both within and across vertebrate groups. It is also interesting that sex differences in AVP concentrations are found in the SON in several mammalian species. How these sex differences in the distribution of AVT/AVP translate into sex differences in the patterns of AVT/AVP release will depend on the modes of peptide release that occur in these nodes of the SBNN (see section 9).
5.2. Sex differences in AVT/AVP receptors
In contrast to the intensive investigation of sex differences in AVT/AVP content, comparatively little attention has been paid to the possibility of sex differences in the distribution of AVT/AVP receptors. V1a receptor binding has been examined in both males and females in a number of studies where sex differences were not identified but also were not the focus of investigation. Not infrequently, such studies will note that no “obvious” sex differences in V1a receptor binding were observed. As such, reports of the absence of sex differences should be taken cautiously because there have been cases where sex differences were reported in studies more focused on their examination.
As discussed above, comparatively little is known about the distribution of AVT receptors in non-mammalian species and even less about the possibility of sex differences in their distribution. In an African cichlid fish, no sex differences were observed in the distribution of AVT receptors, although it remains possible that the numbers of receptors within neural regions might differ between males and females(Huffman and others, 2012). In one species of sex changing fish no obvious sex differences in AVT receptor distribution were identified(Kline and others, 2011), however in another species of sex changing fish, sex differences were observed in the number of AVT receptors(Lema and others, 2012). In terminal phase males (i.e., with functional testes) higher levels of V1a2 receptors were found in whole brain and in hypothalamus than in initial phase males or females. Although the possibility of sex differences in AVT binding has been examined in several bird species, the only sex difference so far identified was in zebra finches where males have significantly higher levels of V1a receptor binding in the septohippocampal septum than do females(Goodson and others, 2006). At present, there is not sufficient data available to fully evaluate the extent to which sex differences exist in AVT receptors in non-mammalian species.
In several rodent species sex differences in V1a receptor binding have been observed in a number of hypothalamic regions. In Siberian hamsters, the density of V1a binding is lower in females than in males in the VMH and ventrolateral (VLH) (i.e., medial tuberal nucleus) and in the premammillary nuclei of the hypothalamus(Dubois-Dauphin and others, 1991; Dubois-Dauphin and others, 1994). In Syrian hamsters, the density of V1a receptor binding is also found to be lower in the VLH in females than in males(Delville and Ferris, 1995; Delville and others, 1996). More recently, we have found there are sex differences in V1a receptor binding in the AH and MPOA in Syrian hamsters, with males displaying a significantly higher density than females(Ross, Song and Albers unpub). Interestingly, sex differences in V1a receptor binding have also been identified in hypothalamic regions in Mus musculus, but with a pattern opposite to that seen in hamsters. In the MPOA and AH, significant V1a binding was found in females (diestrus) but this binding was absent in males. In addition, sex differences were also observed in V1a receptor binding in the mammillary nuclei with significantly higher levels in females compared to males(Dubois-Dauphin and others, 1996). These same sex differences were not observed in two other species of mice, however sex differences in V1a binding were observed in the thalamic corticomedial nucleus in P. maniculatus(Insel and others, 1991). Taken together, the existing data suggests that sex differences within several hypothalamic regions can be observed in hamsters and mice but that the direction of these sex differences are not always consistent.
In voles, initial studies of the distribution of V1a receptor binding in males and females did not report sex differences in receptor distribution in either monogamous or non-monogamous species(Insel and others, 1994; Young and others, 1997a; Phelps and Young, 2003), More recently, however, studies focused on the prefrontal cortex have identified significantly more V1a receptor binding in the prefrontal cortex in males compared to females in both monogamous and non-monogamous vole species(Smeltzer and others, 2006). Other recent data suggests that the distribution of V1a receptors in male and female voles may be labile. Substantial sex differences in V1a receptor binding in several regions linked to social behavior can be induced in voles by neonatal manipulation of OT(Bales and others, 2007). Neonatal OT administration produces dramatic sex differences in the adult pattern of V1a receptor binding in the MPOA, VP, LS and cingulate cortex by increasing binding in males and decreasing binding in females.
In rats, initial surveys of the distribution of V1a mRNA and receptor binding in males and females did not identify sex differences in their distribution(Tribollet and others, 1990; Szot and others, 1994). More recent quantitative studies focused on the LS have found that there are significantly higher levels of V1a receptor binding in females than in males(Veenema and others, 2013). In contrast, female rats have significantly less V1a receptor binding than do males in the spinal cord, although the sex differences were limited to the nuclei that innervate the external genitalia (i.e., pudental nuclei)(Tribollet and others, 1997). V1a receptor binding in the pudental nuclei is also reduced by castration in male rats. Surveys of V1a receptor binding in several other species of rodents that display different levels of sociality and that have different social organizations have not revealed sex differences in V1a distribution(Beery and others, 2008; Campbell and others, 2009). In the very limited amount of V1a receptor binding data available in humans and non-human primates, no sex differences in the distribution of V1a receptor binding has been noted(Loup and others, 1991; Young and others, 1999b).
In conclusion, sex differences in the distribution of V1a receptors have been found in primarily in the hypothalamus or prefrontal cortex in a number of species. Whether this variability is due to the limited amount of data available, a large degree of species variability or some other factor is not known. An interesting possibility is suggested by comparison of sex differences in AVP and V1a distribution in hamsters and rats. There are substantial sex differences in AVP immunoreactivity in rats, but few sex differences in V1a receptor binding. In contrast, in Siberian and Syrian hamsters there are few sex differences in AVP immunoreactivity, but more sex differences in V1a receptor binding. Perhaps sex differences in the functioning of the AVP system is biased toward sex differences in AVP content in some species and toward V1a receptor number in other species.
6. Plasticity in AVT/AVP and their receptors: Gonadal hormones
6.1. Effects of Gonadal Hormones on AVT/AVP
As mentioned above, gonadal hormones play a significant “organizational” role during the perinatal period in the development of sex differences in the AVT/AVP system. In the majority of species examined so far, gonadal hormones also have “activational” effects on the expression of AVT/AVP in select groups of AVT/AVP neurons in adults (Mayes and others, 1988; De Vries, 1990; Dubois-Dauphin and others, 1994), and these activational effects account for some but not all of the sex differences observed in the AVT/AVP system(De Vries and al Shamma, 1990; De Vries and others, 1994). There are also examples where sex differences in the AVP system are absent even though gonadal hormones can regulate AVP expression in a subset of AVP-containing neurons(Dubois-Dauphin and others, 1994). In amphibians, birds and mammals castration is generally found to reduce AVT/AVP in subpopulations of cell bodies (e.g., BNST) and projections (e.g., LS) and replacement therapy with gonadal hormones to restore pre-castration levels of AVP/AVP(De Vries and Panzica, 2006). Both estrogens and androgens are important for maintaining AVP expression in these sites perhaps by their direct action on AVP-containing cells(De Vries and others, 1984; de Vries, 2008; Dhakar and others, 2013).
In contrast, this pattern of sensitivity of AVT/AVP neurons to gonadal hormones is not universal. Recent work in hyenas have found that, like in rats, AVP expression is reduced in the LS in gonadectomized females, but in males AVP expression in the LS appears to be negatively correlated with circulating levels of testosterone(Rosen and others, 2006). In Syrian hamsters, gonadal hormones do not appear to alter AVP expression in either males or females(Albers and others, 1991; Huhman and Albers, 1993). Similarly, studies in zebra finches have not revealed any effects of testosterone on AVT expression(Voorhuis and De Kloet, 1992). In summary, the effects of gonadal hormones on AVT/AVP expression are restricted to subpopulations of AVT/AVP expressing neurons, and these subpopulations can differ across vertebrate groups. In mammals, the most commonly observed effects of gonadal hormones are on AVT/AVP cell bodies in the Me and BNST and their projections.
There are also significant seasonal changes in AVT/AVP expression in a large number of species (e.g., (Fuminier and others, 1993; De Vries and Panzica, 2006; Maruska and others, 2007b; O'Bryant and Wilczynski, 2010)) It is not surprising that AVT/AVP expression declines during the non-breeding season when gonadal hormone levels decline in a number of regions where AVT/AVP are gonadal hormone-dependent(Buijs and others, 1986; Hermes and others, 1990; Bittman and others, 1996; Rasri and others, 2008). There are, however, also examples where AVP expression declines during the non-breeding season in structures that are not gonadal hormone-dependent(Lakhdar-Ghazal and others, 1995). For instance, significant seasonal rhythms in AVP have been documented in the SCN(Hofman and Swaab, 1993; Duncan, 1998). There are also cases where higher levels of AVT/AVP have been observed in the nonbreeding season(Maruska and others, 2007a; O'Bryant and Wilczynski, 2010). In summary, the reduced levels of AVT/AVP expression that occurs during the nonbreeding season appear to be, in most but not all cases, the result of the corresponding decline in circulating levels of gonadal hormones.
6.2. Effects of Gonadal Hormones on AVT/AVP Receptors
In addition to the well known effects of gonadal hormones on AVT/AVP, there is a limited but increasing body of evidence that gonadal hormones can modulate the expression of AVT/AVP receptors. Again, studies of AVT receptors in non-mammalian species have been quite limited in number. In birds, testosterone has been found to modulate AVT receptor mRNA levels and AVT receptor binding (Voorhuis and others, 1988; Grozhik and others, 2013). The effects of testosterone on VT4 (i.e., V1a) mRNA levels was striking in several limbic regions including the MPOA, BNST and VMH following its administration to non-breeding males.
Studies in mammals demonstrating that gonadal hormones regulate the number of V1a receptors have been limited to hamsters. In male Siberian hamsters, castration reduces V1a receptor binding in the VMH and tuberal nuclei and testosterone restores pre-castration levels of V1a receptor binding(Dubois-Dauphin and others, 1994). In Syrian hamsters, castration reduces V1a mRNA within the medial preoptic nucleus (MPN) and receptor binding in and around the MPOA-AH, VMH and BNST(Johnson and others, 1995; Young and others, 2000). Comparison of V1a receptor binding and mRNA in intact, castrated and castrated-testosterone treated hamsters has revealed that V1a receptors in the MPN are regulated by testosterone. The upregulation of V1a receptor gene expression occurs prominently in a cluster of neurons concentrated in the ventromedial part of the MPN. Interestingly, V1a receptor mRNA is anatomically more restricted in several areas compared to the pattern of receptor binding, suggesting that there is a significant spread of receptor protein along neuronal processes. In other studies in Syrian hamsters, testosterone-dependent V1a receptor binding has been found in the VLH of males and females(Johnson and others, 1995; Delville and Ferris, 1995; Delville and others, 1996; Young and others, 2000). Finally, the presence of gonadal hormones during adolescence can have an organizational effect on the number of V1a receptors found within the LS(Schulz and others, 2006). Taken together, these data indicate that gonadal hormones can have selective but significant effects on the pattern of V1a binding in at least two vertebrate groups. At present, the most pronounced effects of gonadal hormones have been on hypothalamic V1a receptor binding. Interestingly, studies in rats have yet to reveal any evidence that gonadal hormones can influence V1a receptor binding in this species (Tribollet and others, 1990; Gao and others, 1994). The evidence to date suggests that gonadal hormones can modulate AVP signaling by acting on the expression of AVP, but not V1a receptors in some species (e.g., rats), by acting on V1a receptors but not AVT/AVP expression in other species (e.g., Syrian hamsters) or by acting on both AVP and V1a receptors in still other species (e.g., Siberian hamsters).
Another less direct indication that gonadal hormones modulate AVT/AVP receptors comes from studies of seasonal changes in AVT/AVP receptor binding. In goldfish, there are higher levels of AVT receptor expression within the hindbrain during the breeding season than at other times of year(Walton and others, 2010). In Siberian hamsters there are also seasonal variations in the density of V1a receptor binding in which V1a receptor binding is lower in the nonbreeding season (i.e., following exposure to short “winter” like photoperiods) in males and females than in those housed in long “summer-like” photoperiods(Dubois-Dauphin and others, 1991). Specifically, V1a receptor binding was lower in VMH and VLH, but not in the premammillary nuclei in short photoperiod exposed hamsters. Significant seasonal changes in V1a receptor binding are also found in both male and female Syrian hamsters. In male hamsters, exposure to short photoperiod results in significantly lower levels of V1a receptor binding in the MPN and the MPOA when compared to males exposed to long photoperiods(Caldwell and Albers, 2003; Caldwell and others, 2008b). In short photoperiod exposed males the levels of V1a receptor binding in the MPN and MPOA are similar to those observed in castrated males exposed to long photoperiods. In female hamsters, short photoperiod exposure also significantly reduces V1a receptor binding in the MPN and MPOA as well as in several other limbic structures not affected by short photoperiod in males (e.g., BNST, Ce)(Caldwell and Albers, 2004b). Taken together, the present data are consistent with the possibility that the reduced levels of V1a receptor binding observed during the non-breeding season are the result of the decline in gonadal hormones.
Much remains to be learned about the functional significance of the effects of gonadal hormones on V1a receptors. One of the most robust effects of gonadal hormones on AVP-induced social behavior is seen on a form of social communication in Syrian hamsters called flank marking(Johnston, 1975; Albers and others, 1992). Gonadectomy dramatically reduces the ability of AVP to induce flank marking following its injection into the MPOA-AH(Albers and others, 1988; Huhman and Albers, 1993; Delville and others, 1996; Albers and others, 1996; Albers and Bamshad, 1998; Ferris and others, 2013). The finding that the V1a receptor binding in the MPOA is also dependent on gonadal hormones strongly supports the hypothesis that the effects of gonadal hormones on flank marking are mediated by their effects on the biosynthesis of V1a receptors. Exposure to a short “winter-like” photoperiod significantly reduces circulating levels of gonadal hormones and reduces V1a receptor binding in the same regions as does following castration. Interestingly, however, short photoperiod exposure does not reduce the amount of flanking making induced in response to injection of AVP(Caldwell and Albers, 2003). The reasons for this discrepancy in the relationship between V1a receptor binding and the ability of AVP to induce flank marking are not known(Gutzler and others, 2011), but they do illustrate the importance of cautiously interpreting apparently simple relationships between nonapeptide receptor binding and the behavioral effects of these peptides within specific CNS sites.
7. Plasticity in AVT/AVP and their receptors: Social Factors
One of the most interesting new areas of investigation of nonapeptides and sociality is the study of how social factors can modulate the activity of this system. This work has examined the effect of the immediate social environment, or “social context” as well as the effects of longer-term exposure to different social environments or “social experience”. Only a very limited amount of data is available on how social context can regulate the behavioral responses to nonapeptides. Nevertheless, these findings are dramatic because they demonstrate that social context can determine whether social behavior can be induced in response to the presence of a peptide within a specific brain site(e.g., Harmon and others, 2002b; Kabelik and others, 2009)). Although the effects of social experience on nonapeptides and their receptors have received more attention, the existing data remains limited.
7.1. Social experience and AVT/AVP
There is evidence in prairie voles that male and female cohabitation can modulate AVP expression(Bamshad and others, 1993; Bamshad and others, 1994; Wang and others, 1994b). Following the initiation of male/ female cohabitation, the majority of pairs mate within three days and deliver a litter of pups around 21 days later(Bamshad and others, 1994). Cohabitation for three days reduces AVP fibers in the LS and lateral habenular nucleus (LHN) but increases AVP mRNA within the BNST in males (but not females). Additional studies have confirmed that the expression of AVP can be altered by cohabitation in a testosterone-independent manner in male prairie voles(Bamshad and others, 1994). Studies of male/female cohabitation from its initial onset to after the birth of the first litter of pups found a dramatic reduction in AVP fibers in the LS and LHN and then a gradual increase in AVP immunoreactivity throughout gestation. A second drop in AVP immunoreactivity was observed after the first litter was born. All of these changes in AVP immunoreactivity occurred despite the absence of any significant changes in circulating testosterone. Additional studies in male voles strongly suggest that many different types of social interactions may alter AVP expression(Bamshad and others, 1994). When two males are placed together and cohabitate in a novel social environment for as little as three days AVP immunoreactivity within the LS and LBN is significantly higher than in males housed in their home cage with a male sibling. These data provide strong support for the possibility that different types of social experience can produce different patterns of AVP expression within brain regions important in the control of social behavior, and that the changes in AVP expression can be mediated by mechanisms other than gonadal hormones.
Another example where social interaction appears to alter AVP expression comes from studies of territoriality and dominance in males. In naked mole rats, the volume and number of AVP containing neurons is higher in dominant breeding males and females than in subordinates(Rosen and others, 2007). Recent studies in male and female mandarin voles have also found significant differences in the distribution of AVP immunoreactivity in dominant versus subordinate animals(Qiao and others, 2014). Dominant males have significantly higher levels of AVP immunoreactivity in the PVN, SON, AH and lateral hypothalamus (LH) than subordinate males. Dominant females have significantly higher levels of AVP immunoreactivity in the AH and LH than subordinate females. The role of gonadal hormones, if any, in the effects of dominance on AVP immunoreactivity in mole rats and mandarin voles is not known.
Dominance and territoriality can alter AVP expression in the absence of changes in circulating levels of gonadal hormones. In Syrian hamsters, dominant male hamsters display significantly higher levels of AVP immunoreactivity in the AH than do their subordinate partners or socially isolated controls administered testosterone(Ferris and others, 1989). Similarly, in green anole lizards dominant males have more AVT immunoreactive cells in the POA compared to subordinates and subordinates had lower numbers of AVT immunoreactive cells than control males housed alone(Hattori and Wilczynski, 2009). In fish, there are some very interesting but complex relationships between AVT expression, territoriality and dominant/subordinate behaviors(Greenwood and others, 2008). For example, in territorial African cichlid males AVT expression is higher in gigantocellular neurons of the posterior preoptic area than in non-territorial males. In contrast, in the anterior preoptic area AVT expression is lower in territorial males than in non-territorial males. AVT also appears to mediate other important behavioral changes induced by social stimuli in fish. In the sex changing bluehead wrasse social stimuli that induce sex changes in behavior are mediated by the AVT system and these effects are gonadal hormone-independent(Godwin and others, 1996; Godwin and others, 2000; Semsar and others, 2001; Semsar and Godwin, 2003; Semsar and Godwin, 2004). Taken together, these data indicate that AVP expression can be altered significantly and in complex ways by different types of social experience and that at least some of these effects are testosterone-independent.
7.2. Social experience and AVT/AVP receptors
There is also evidence that social experience can alter the number of V1a receptors within specific brain regions. In Syrian hamsters, social experience can modulate the amount of V1a receptor binding in a manner that is independent of changes in circulating testosterone. Dominant male hamsters have higher levels of V1a receptor binding in the VLH and AH than do their subordinate partners or controls even though no differences were observed in circulating levels of testosterone(Cooper and others, 2005). Remarkably brief periods of social interaction can alter the number of V1a receptors(Albers and others, 2006). The density of V1a receptor binding is modulated in several limbic structures in male hamsters allowed to interact with other males for as little 90 minutes distributed over a three week period. For example, socially isolated males exhibit significantly higher levels of V1a receptor binding in the AH than do males allowed to socially interact. These differences were observed despite the absence of any differences in testosterone levels. Other forms of social experience also appear to modulate the ability of AVP to stimulate aggression in males by its action in the AH. AVP injected into the AH significantly increases aggression in hamsters that had previously been trained to fight but not in hamsters housed in stable social groups(Ferris and others, 1997; Huhman and others, 1998). Interestingly, although social isolation also increases aggression in both males and females(Brain, 1972), social isolation increases V1a receptor binding in males but not females (Ross, Song and Albers, unpub.). This sex difference in the effects of social experience on V1a receptor binding may not be surprising given the opposite effects of AVP injected into the AH on aggression in males and females(Ferris and others, 1997; Caldwell and Albers, 2004a; Gutzler and others, 2010).
Social experience can also regulate V1a receptor binding in prairie voles. Pair bonded male voles have significantly higher levels of V1a receptor binding within the AH and MPOA, but not other brain regions when compared to sexually naïve male voles(Winslow and others, 1993; Gobrogge and others, 2009). The increased number of V1a receptors in the AH may contribute to the higher levels of aggression observed in pair bonded males compared to sexually naïve prairie voles. In support of this possibility are the findings that AVP injected into the AH increases aggression and that increasing the number of V1a receptors in the AH with viral vector gene transfer significantly increases aggression.
In marmosets, where males provide substantial levels of paternal care, fathers have significantly higher levels of V1a receptor binding in the prefrontal cortex as compared to males that were not fathers(Kozorovitskiy and others, 2006). It is not known, however, whether these effects might be mediated by higher levels of testosterone that may be associated with fatherhood.
In summary, there is clear evidence that different types of social experience can produce different patterns of V1a binding within limbic structures and that these effects may modulate social behaviors such as aggression. To date, the effects of social experience on V1a receptors have only been reported in males, most likely because of the paucity of studies conducted with females. It is important to note that sex differences can emerge in AVP expression and V1a receptor number following certain types of social experience.
7.3. Early social experience and AVT/AVP and AVT/AVP receptors
Early developmental events can also modulate the AVP system in adults. In mice, changes in early social experience produced by cross fostering can alter AVP immunoreactivity in several sites including the BNST and SON(Bester-Meredith and Marler, 2001). Other studies in mice have shown that the amount of paternal grooming behavior experienced by male pups alters the distribution of their adult expression of AVP(Frazier and others, 2006). Early social experience can also alter the distribution of V1a receptors. Maternal licking and grooming increases V1a receptor binding within the amygdala in adult male but not female rats (Francis and others, 2002). Interestingly, manipulation of OT during the perinatal period can produce profound effects on the distribution of V1a receptors in prairie voles(Bales and others, 2007a). While there were no sex differences in V1a receptor binding in adult controls, in voles administered OT neonatally V1a receptor binding increased in males and decreased in females in several regions including the LS. Taken together, these data indicate that social and hormonal experience during early development can serve to sculpt the AVP system in both sexes. In at least some cases early experience induces sex differences in the AVP system in adults.
8. Individual Differences in AVT/AVP and their receptors
Given the plasticity of the AVT/AVP system, it not surprising that there are often significant inter-individual differences in AVT/AVP and their receptors, e.g.,(Insel and others, 1994; Campbell and others, 2009; Leung and others, 2011). One likely source of these inter-individual differences is the individual differences that can occur in circulating gonadal hormone levels. It is well known, for example, that a variety of different types of social factors can significantly change the levels of circulating testosterone(Macrides and others, 1974; Harding, 1981; Wingfield and others, 1987; Wingfield and others, 1990; Pfeiffer and Johnston, 1992). Because gonadal hormones can modulate AVT/AVP and their receptors within specific CNS sites, this seems one likely mechanism underlying individual differences. Another likely source of individual differences are the individual differences that occur in social experience. As discussed above, exposure to different types of social stimuli can produce distinctly different patterns of AVP expression and V1a receptor binding in the SBNN.
To examine how individual differences in AVT/AVP and their receptors may influence social behavior a number of studies have been conducted using naturalistic conditions(Phelps and Young, 2003; Ophir and others, 2008; Zheng and others, 2013). For example, individual differences in the pattern of V1a receptor binding was examined in over 30 male and female prairie voles captured in the field. This study identified significant intra-specific co-variation in V1a receptor binding across brain regions that was on the order of differences that can be observed across species. Some brain areas exhibited little interindividual variability (e.g., ventral pallidum and Me) while others had considerable inter-individual differences (e.g., LS). Interestingly, the inter-individual differences seen in prairie voles may not be due to differences in gonadal hormones because the existing data suggests that gonadal hormones do not alter V1a receptor binding in this species. It is tempting to speculate that the brain regions that exhibit the least inter-individual variation in V1a receptors are those that mediate the more fundamental mechanisms underlying the social behaviors of that particular species. In contrast, brain regions that contain the most pronounced inter-individual variations are those that control the more variable aspects of these behaviors and that these mechanisms are more labile in response to social experience.
Another possible mechanism underlying inter-individual variability could lie in the structure of the V1a gene. Studies of the 5’ regulatory microsatellite region within the prairie vole found that there were significant differences in its length across individuals(Hammock and Young, 2002; Hammock and others, 2005). In contrast, in montane voles, where the microsatellite sequence is small with few inter-individual differences, there are few individual differences in the distribution of V1a receptor binding. These and other studies in voles led to the hypothesis that polymorphisms in the microsatellite are responsible for individual differences in how V1a receptors are distributed in brain(Hammock and others, 2005; Hammock and Young, 2005). Additional studies in voles provided only limited support for this hypothesis in that correlations were found between microsatellite length and V1a receptor levels in some brain regions (e.g., amygdala), but not in others (e.g., VP)(Donaldson and Young, 2013).
Studies in other species also report large individual differences in microsatellites. In primates, there is a substantial amount of individual variability in the microsatellite region of the V1a receptor gene(Donaldson and others, 2008; Rosso and others, 2008; Hopkins and others, 2012). There is one report that individual differences in the promoter region are associated with individual differences in the quantity of V1a receptor mRNA in at least some regions, i.e., hippocampus(Knafo and others, 2008). There is also an association between individual differences in microsatellites and characteristics that might relate to human social behavior(Kim and others, 2002; Bachner-Melman and others, 2005; Yirmiya and others, 2006; Walum and others, 2008; Meyer-Lindenberg and others, 2009). For example, relationships have been identified between microsatellite sequences and creativity in dance as well as in marital status and perceived marital problems.
Interestingly, there is not a simple relationship between microsatellite length and V1a receptor levels throughout the brain. In fact, there is evidence that the effects of microsatellite length on gene expression depends on the phenotype of the cell in which it is expressed(Hammock and Young, 2004). Therefore, polymorphisms in the microsatellite could increase or decrease V1a receptor number in different brain regions depending on the phenotype of the neurons expressing the gene thereby inducing different patterns of V1a receptor distribution across individuals (Hammock and Young, 2004). Microsatellites also provide a very interesting way of producing individual differences because they mutate at higher rates than do non-repetitive regions of DNA(Li and others, 2004). The inherent instability of microsatellite sequences is thought to be involved in individual variability in gene expression and in the evolution of the patterns of gene expression(Hammock and Young, 2002).
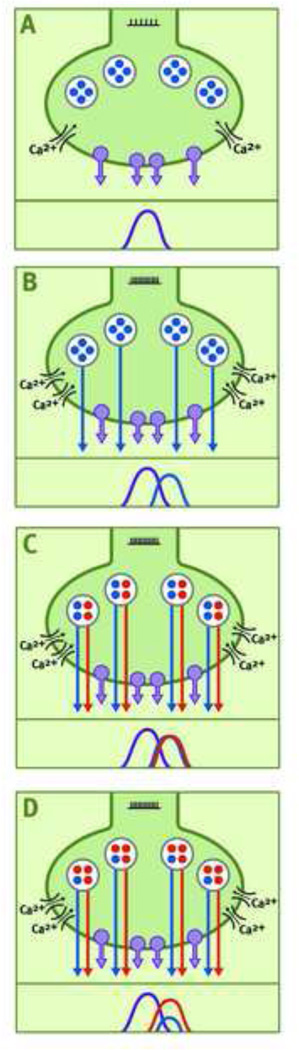
9. Neurochemical signaling in the SBNN
Over the last several decades it has become clear that peptides can be released in a variety of patterns. On the one hand, they can act in a highly localized manner as occurs following synaptic release, a mechanism consistent with our conceptualization of classic neurotransmitter action. On the other hand, these peptides can also be released in a much more diffuse manner, potentially impacting large numbers of neurons in multiple sites(Engelmann and others, 2000; Landgraf and Neumann, 2004; Ludwig and Leng, 2006). This diversity of action has long been recognized but not always emphasized in the literature examining the role of nonapeptides in the control of social behavior. Peptides like AVT and AVP are usually packaged in large dense-core vesicles (LDCV) that can be found in all areas of neurons including pre-synaptic regions(van Leeuwen and others, 1978; Buijs and Swaab, 1979; Jakab and others, 1991). Because of this distribution throughout the cell, these peptides can act locally within in the synapse or much more broadly through their release from a wide range of nonsynaptic regions (e.g., dendrites) in what has been called volume transmission.
9.1.1 Non-synapatic signaling in the SBNN: Volume transmission
Volume transmission was initially suggested by early demonstrations that peptides can diffuse over long distances to reach their receptors(Jan and Jan, 1982). These findings combined with the observation that there can be substantial anatomical “mismatches” between the site where a neurochemical signal is released and the location of its receptors, suggest that volume transmission might have a significant functional role in CNS signaling(Agnati and others, 1986; Herkenham, 1987). Volume transmission is now recognized as a widespread non-synaptic mode of intercellular communication for a variety of molecules (e.g., monamines, amino acids) (Vizi and others, 2010; Trueta and De Miguel, 2012).
Much of what we know about volume transmission comes from studies of AVP and OT release from the magnocellular neurons of the SON and PVN(Pow and Morris, 1989; Morris and Pow, 1991; Ludwig and Leng, 2006). These neurons are useful for the study of non-synaptic release because many of their axons project to the posterior pituitary while their dendrites form a thick plexus at the ventral aspect of the brain, which makes it possible to distinguish AVP or OT released synaptically from that released non-synaptically. Release of OT is mediated by Ca2+ release from intracellular stores, while the release of AVP may require the combined effects of extracellular Ca2+ (through voltage gated channels) as well as intracellular stores(Lambert and others, 1994; Dayanithi and others, 1996). As such, OT and possibly AVP can be released non-synaptically without an increase in electrical activity thus making synaptic and non-synaptic release potentially independent of each other(Mens and others, 1983; Stark and others, 1989). Another interesting feature of how these mechanisms function in magnocellular neurons is that OT releasing neurons have OT receptors(Freund-Mercier and others, 1994) and AVP neurons have AVP receptors(Hurbin and others, 2002). Unlike the typical view that such autoreceptors would be involved in a negative feedback loop, activation of these OT and AVP receptors can induce further peptide release without altering electrical activity(Moos and others, 1989). Once non-synaptic release of OT and AVP is initiated, the positive feedback effects of these peptides on their own release produces substantial new, long-lasting release of more peptide. These autoregulatory events can result in sustained peptide release and, when combined with the relatively long half life of these peptides in brain (around 20 minutes)(Mens and others, 1983), have the potential to produce a potent and sustained peptidergic signal. Peptide release can be further enhanced by a phenomenon called “priming”. Priming moves peptide-containing vesicles closer to the membrane in response to stimuli that release intracellular stores of Ca2+ thereby increasing their probability of release. Priming substantially increases the magnitude of peptide release, which can persist for relatively long time intervals (e.g., 90 minutes). In magnocellular neurons, stimuli that mobilize intracellular Ca2+ stores (e.g., thapsigargin) produce priming effects for both OT and AVP, however OT appears to be more effective in inducing priming of its own vesicles (Ludwig and others, 2002; Ludwig and others, 2005).
Much has been learned about non-synaptic release of AVP and OT from studies of magnocellular neurons. Many factors affect the profiles of peptide release such as the size of the neurons from which the peptide is released, the spread of peptide after release and the timing and intensity of its degradation by peptidases. While the spatial and temporal profiles of the peptides released via volume transmission are not well understood(Leng and Ludwig, 2008), estimates suggest that they may travel as far as 4–5 mm from their site of release(Engelmann and others, 2000). Magnocellular neurons in the hypothalamus represent the largest pool of nonapeptides in the brain and there is evidence that they are activated by a variety of stimuli related to social behavior (e.g., Figure 1(Delville and others, 2000)). As a result, it seems likely that volume transmission of nonapeptides from these neurons play an important role in regulating social behavior by acting on nonapeptide receptors throughout the SBNN.
A role for volume transmission in the control of social behavior may not be limited to nonapeptides released from magnocellular neurons. For example, there is evidence that non-synaptic release can occur in AVP producing parvocellular neurons in the SCN(Castel and others, 1996). It seems possible that parvocellular neurons can release substantial amounts of AVP as evidenced by the prominent circadian rhythms of AVP in cerebrospinal fluid produced by this relatively small number of parvocellular neurons(Schwartz and Reppert, 1985; Kalsbeek and others, 1995). It remains to be seen if these same mechanisms occur in other AVP and OT producing neurons throughout the brain. It will also be important to determine whether principles of volume transmission identified in studies of magnocellular hypothalamic neurons apply to other AVP/OT neurons. For example, are intracellular and extracellular sources of Ca2+ required for non-synaptic release in AVP neurons, does priming occur, etc?
9.1.2 Synaptic signaling in the SBNN
As discussed above, peptides including AVT and AVP are frequently found in LDCVs in the synaptic region of the axon terminals. Many regions of the brain contain AVP-immunoreactive fibers and have nonapeptide receptors in close proximity. There is also recent evidence that nonapeptide containing projections from hypothalamic neurons release peptide from their axonal terminals into synapses in several forebrain regions(Knobloch and others, 2012). Of course, axon terminals also contain low molecular weight neurotransmitters and/or neuromodulators or what are often called “classical neurotransmitters” (e.g., glutamate and GABA). In fact, peptides appear to be co-localized with classical neurotransmitters in most if not all neurons(Hokfelt and others, 1984; Hokfelt and others, 1986; Merighi, 2002; Salio and others, 2006). This evidence comes from studies of the distribution of the different types of vesicles in which classical neurotransmitters and peptides are stored. Typically, classical neurotransmitters are packaged in small synaptic vesicles (SSV) that are found in pre-synaptic regions (for a review see (Ludwig and Leng, 2006)). The exocytosis of both SSV and LDCVs in synaptic regions is tied to the electrical activity produced by Ca2+ through voltage gated ion channels resulting from depolarization of the terminal area (Figure 3). As a result, peptides can be released from synaptic regions along with classical neurotransmitters, although peptide release does not appear to be as localized because of the more distributed pattern of LDCVs. Because SSVs are resident in closer proximity to the membrane than are LDCVs, it takes less intense electrical activity for classical neurotransmitter to be released than for peptides to be released because additional Ca2+ is required for exocytosis of LDCVs. Therefore, synaptic peptide release lags with respect to classical neurotransmitter release and requires more intense electrical activity.
Figure 3.
Illustration of different patterns of synaptic release of ‘classical neurotransmitter’ (NT) and peptides. A: Moderate neuronal firing produces moderate levels of Ca2+ influx through voltage gated ion channels resulting in exocytosis of small synaptic vesicles (SSV; in purple) resulting in release of NT. B: High levels of neuronal firing produces high levels of Ca2+ influx through voltage gated ion channels resulting in exocytosis of both SSVs and large densecore vesicles (LDCV; in gray) resulting in release of NT and peptide. C: Two different peptides are packaged in LDCVs (red and blue) in a ratio of 1:1. High levels of neuronal firing produces high levels of Ca2+ influx through voltage gated ion channels resulting in exocytosis of SSVs and resulting in release of NT and exocytosis of LDCVs resulting the release of “cocktail” of peptides in a 1:1 ratio. D. Two different peptides are packaged in LDCVs (red and blue) in a ratio of 3:1. High levels of neuronal firing produces high levels of Ca2+ influx through voltage gated ion channels resulting in exocytosis of SSVs and resulting in release of NT and exocytosis of LDCVs resulting the release of “cocktail” of peptides in a 3:1 ratio.
Although the colocalization of classical neurotransmitters and peptides has been known for many years, there is surprisingly little data on their functional interactions in brain(Merighi, 2002; Merighi and others, 2011; van den Pol, 2012). Theoretically at least, co-released classical neurotransmitters and peptides could act more or less independently because their effects are transduced through very different types of receptors. Nevertheless, it seems far more likely that these coreleased signals do interact in multiple and complex ways. It is likely that peptides modulate the actions of neurotransmitters by both pre- and postsynaptic mechanisms likely affecting both excitatory and inhibitory transmission. Given the likely fundamental role of the co-release of these signals, there is surprisingly little understanding of this phenomenon. The data actually demonstrating the colocalizaton of AVP with different classical neurotransmitters is quite limited, e.g.(Buijs and others, 1995). There is also only a limited amount of data demonstrating that interactions between AVP and amino acid transmitters have functional consequences in the control of social behavior. One example is that glutamate antagonists block the ability of AVP injected into the hypothalamus to induce flank marking in Syrian hamsters(Bamshad and others, 1996). The functional consequences of these interactions between classical neurotransmitters and AVP in the regulation of social behavior are likely to be extremely important and should be a focus of future research.
9.1.3. Colocalization of peptides in the SBNN
Many peptides, including AVP, are colocalized with other peptides throughout the brain. Different peptides produced within the same neuron are generally thought to be packaged together in the same LCDVs and released together in a “cocktail” (Figure 3). Because many AVP neurons produce multiple peptides(Brownstein and Mezey, 1986), it seems likely that AVP would be frequently released with other peptides. One example of a peptide that is colocalized with AVP in a number of brain regions is galanin (GAL)(Gai and others, 1990; Miller and others, 1993; Planas and others, 1995a; Planas and others, 1995b). While the behavioral effects of the co-release of AVP and GAL have not been studied, it is clear that exogenously administered GAL can antagonize the behavioral effects of AVP. Co-injection of GAL and AVP into the AH inhibits AVP-induced flank marking in a dose-dependent manner(Ferris and others, 1999). These data suggest that different ratios of GAL to AVP should induce differing amounts of this behavior. Therefore, one factor that could regulate the expression of this and possibly other social behaviors would be the rate of GAL biosynthesis compared to the rate of AVP biosynthesis (e.g., Figure 3). Recent data also suggest another interesting possibility. There is evidence that GAL and AVP can be co-packaged in vesicles targeted for non-synaptic regions, while vesicles directed to the synaptic region contain only AVP(Landry and others, 2003). Such a mechanism could result in the co-release of GAL and AVP from non-synaptic regions in response to the release of Ca2+ from intracellular stores and the release of AVP, alone, from synaptic regions in response to voltage-dependent Ca2+ release.
Another peptide that appears to be colocalized with AVP is OT. While it was originally thought that AVP and OT were produced in mutually exclusive sets of neurons(Mohr and others, 1988), it has become clear that at least some magnocellular neurons can produce both AVP and OT(Baldino, Jr. and others, 1988; Kiyama and Emson, 1990; Mezey and Kiss, 1991; Xi and others, 1999; Gainer, 2012). However, no instances of colocalization have been reported in parvocellular neurons. How frequently AVP and OT are colocalized in magnocellular neurons is not known. It is clear, however, that there are brain regions where AVP and OT are not colocalized. Nevertheless, there are areas where AVP and OT are found in close proximity where colocalization could occur and could influence social behavior (e.g., nucleus circularis)(Hennessey and others, 1994; Delville and others, 1994; Whitman and Albers, 1998) (Figure 1). The possibility of the co-release of AVP and OT, even if it is restricted to a subpopulation of hypothalamic neurons, may have important functional consequences given the many effects of these peptides on social behavior within these regions. The limited existing data suggests that there may be large differences in the ratios of AVP/OT produced. There is also evidence that there may be dynamic changes in the relative amounts of OT and AVP produced in response to factors such as synaptic activity and hormones(Baldino and others, 1988; Telleria-Diaz and others, 2001). In summary, although it is clear that AVP/OT can be colocalized, is not known how frequently this may occur or if the release of different ratios of AVP/OT might have functional consequences in the control of social behavior.
9.1.4 Nonapeptide receptors in the SBNN
Although OT is considered to have only one canonical receptor and AVP to have three, the selectivity of AVP and OT for OT, V1a and V1b receptors has only been thoroughly examined in three species, humans, rats and mice (Table 2). It is important to consider that AVP and OT have similar affinities for all three receptors in these species(Manning and others, 2008; Manning and others, 2012). The only exception identified to date is seen in humans where OT is selective for the OT receptor. This overall lack of receptor selectivity is not surprising when one considers that these receptors have a high degree of structural homology (e.g., 85% between V1a and OT). In fact, given the lack of selectivity of these receptors in mice and rats, we should consider V1a, V1b and OT receptors as receptor subtypes for both AVP and OT in these species. Such a view is also supported by pharmacological data. Manning et al. (Manning and others, 2012) have concluded that there is a substantial lack of receptor selectivity in AVP/OT receptor agonists and antagonists and that it is likely that receptor selectivity of these drugs differs greatly across species. For example, the efficacy of the most commonly used V1a antagonist, Manning Compound, to discriminate between V1a and OT receptors can vary significantly across species. Thus, it is possible that at least some of the effects attributed to V1a receptors (or OT receptors) may have been mediated by OT receptors (or V1a receptors) or a combination of both OT and V1a receptors. It has been suggested that, in contrast to other signaling systems, receptor selectivity for AVP and OT is not achieved by the ligand-receptor interaction, itself, but rather by a number of other factors including receptor up- or down-regulation, release of specific ligand-degrading enzymes, local ligand production and receptor clustering(Koehbach and others, 2013). In summary, it will likely prove useful to consider V1a, V1b and OT receptors as different subtypes of “AVP receptors” in studies in mice and rats. It seems likely that the selectivity of OT for the OT receptor but not the V1a and V1b receptor in humans has significant functional importance. One might speculate that species differences in the selectivity of AVP and OT for V1a, V1b and OT receptors might underlie at least some of the species differences in sociality.
Table 2.
Selectivity of vasopressin (AVP) V1a, AVP V1b and oxytocin (OT) receptors to AVP and OT.
| AVP | OT | |||||
|---|---|---|---|---|---|---|
| Receptor | V1a | V1b | OT | V1a | V1b | OT |
| Humans | 1.1 | 0.7 | 1.7 | 120 | >1000 | 0.8* |
| Rats | 2.6 | 0.3 | 1.7 | 71 | 294 | 1.0 |
| Mice | 1.3 | 0.3 | 1.8 | 46.1 | 94 | 0.6 |
Affinity values in Ki (nm);
defined as selective as compared with the two other receptors of that species by the selectivity criteria of having a two orders of magnitude lower Ki; References:
Although structural and pharmacological data suggest the possibility that AVP and OT could potentially act through each other’s receptors, is there any evidence that OT can activate “AVP” receptors or that AVP can activate the “OT” receptor? In fact, two recent studies have provided evidence that exogenously administered OT can act on V1a receptors to exert its effects(Schorscher-Petcu and others, 2010; Sala and others, 2011). First, administration of OT has been found to produce analgesia in a number of different pain assays. Interestingly, OT administered to OT receptor knock-out mice produces analgesia at levels that are similar to its analgesic effects in wide-type mice, but the analgesic effects of OT are absent in V1a receptor knock-out mice. In addition, OT-induced analgesia is blocked following pretreatment with a V1a receptor antagonist but not with an OT receptor antagonist. In another study, exogenously administered OT has been found to influence social behaviors in OT receptor knock-out mice by acting on V1a receptors(188). Administration of OT or AVP to OT receptor knock-out mice significantly increased social recognition and exploration as well as reduced aggression. Administration of a V1a antagonist blocked these effects of OT and AVP. Taken together, these studies demonstrate that exogenous administered OT can influence behavior by acting through V1a receptors.
More recently, we have collected data that supports that possibility that endogenously released OT, as well as exogenously administered OT can act on V1a receptors to influence social behavior in wild-type animals (Song, McCann, McNeill, Larkin, Huhman and Albers, unpublished data). Central injection (ICV) of either AVP or OT induces flank marking behavior in Syrian hamsters in a dose-dependent manner, although AVP is approximately 100 fold more potent in inducing the behavior(Figure 4). Injection of highly selective agonists of the V1a receptor stimulate flank marking in a dose-dependent manner, but highly selective agonists for the OT receptor do not induce flank marking. In addition, injection of a V1a antagonist inhibits OT-induced flank marking but injection of an OT antagonist does not. We also examined whether endogenously released OT might act on V1a receptors to induce flank marking. Hamsters were injected with α-melanocyte stimulating hormone (α-MSH) ICV in order to induce release of OT but not AVP (Sabatier and others, 2003; Sabatier, 2006) and were then tested in a cage containing conspecific odors. α-MSH significantly increased flank marking compared to injection of saline and injection of a V1a antagonist significantly inhibited α-MSH induced flank marking. These data provide further support the possibility that OT can act through V1a receptors and, perhaps more importantly, suggest the possibility that the concentrations of OT released endogenously are sufficient to activate V1a receptors. These data also suggest that some of the effects ascribed to OT may in fact be mediated by the V1a receptor,(Albers and Ferris, 1985; Caldwell, 1992; Whitman and Albers, 1995; Harmon and others, 2002a).
Figure 4.
The induction of flank marking by intracerebroventricular injection of oxytocin (OT) is mediated by the vasopressin V1a receptor (V1aR). A. The amount of flank marking induced by the injection of the three concentrations of the selective V1aR agonist (Ag), [Phe2]OVT, OT or two concentrations of the selective OTR Ag, [Thr4,Gly7]OT, in 1 microliter of vehicle. (* and # indicate significant differences compared to the OT group) B. The amount of flank marking induced by the injection of 90 µM OT combined with vehicle or three concentrations of the selective V1a receptor antagonist (A), d(CH2)5[Tyr(Me)2]AVP (Manning Compound), or three concentrations of the selective OTR A, desGly-NH2-d(CH2)5[D-Tyr2,Thr4]OVT. (* indicates significant difference from the 90µM OT group). C. The amount of flank marking induced by the injection of various concentrations of OT and arginine-vasopressin (AVP) (* indicates significant difference between OT and AVP). From Song, McCann, McNeill, Larkin, Huhman and Albers, unpublished data.
While it seems possible that AVP and OT are endogenous ligands for all of the nonapeptide receptors, activation of each of these receptors by either ligand can result in very different cellular responses(for a review see(Stoop, 2012; Neumann and van den Burg, 2013)). The different cellular responses to these receptors are due to their coupling to different intracellular signaling cascades. V1a, V1b and OT receptors are coupled to Gq/11α GTP binding proteins but subsequently diverge onto different intracellular signaling pathways. Interestingly, it appears that even the same receptor can produce different cellular responses depending on the specific G proteins they activate(Gravati and others, 2010). For example, recent studies in brain have shown that activation of OT receptors can mediate current inhibition through a PTX-resistant G protein and that activation of other OT receptors can mediate current activation through coupling to a PTX-sensitive Gi/o. While much remains to be learned about the events that occur following activation of V1a, V1b or OT receptors, it is clear that they produce very different cellular responses a result of their coupling to a diversity of complex intracellular signaling cascades. It will be important to consider that different types of receptor coupling may occur in neurons of differing phenotypes and that these differences are likely to have functional consequences in the control of social behavior.
9.1.5 Local networks in the SBNN
Despite the fact that AVP and OT differ by only two amino acids, activate each other’s receptors, and typically increase neuronal excitability(Raggenbass, 2008), these peptides have been ascribed dramatically different physiological and behavioral functions over the years. In fact, some investigators have suggested that AVP and OT are the “ying-yang” of peptides in regard to at least some physiological functions and that AVP may be the “selfish” and OT the “altruistic” peptide when it comes to social behavior(Legros, 2001; Stoop, 2012). Other investigators have proposed that that AVP tends to have anxiogenic and depressive actions, while OT has anxiolytic and antidepressive actions(Neumann and Landgraf, 2012). How can this be?
Recent studies suggest that local networks within and across nodes of the SBNN may be responsible for the “opposite” effects of AVP and OT in number of cases(for a review see(Stoop, 2012)). For example, the opposite effects of AVP and OT on fear appear to be mediated by two non-overlapping populations of V1a and OT receptors in the Ce(Huber and others, 2005). In a series of elegant studies it was shown that activation of V1a receptors localized in the medial Ce potentiate efferent projections inducing the autonomic fear response, whereas activation of OT receptors in the lateral Ce inhibit these V1a-stimulated events through activation of GABAergic interneurons to the medial Ce (Figure 5). Such local networks of anatomically separable populations of V1a and OT receptors may be involved in mediating other examples where AVP and OT appear to have opposite effects because V1a and OT receptors are found throughout the extended amygdala but rarely overlap(Veinante and Freund-Mercier, 1997). It seems that the ability of these local networks to produce their opposite effects on behavior requires the activation of small anatomically separable populations of receptors by the local release of peptide from synaptic sources. It is interesting to consider the possibility that such local networks could be “turned off” if large amounts of AVP or OT released by volume transmission served to activate all the receptors within a region despite their anatomical separation. Such a mechanism could provide a way to dramatically alter the communication within and across nodes of the SBNN.
Figure 5.
Local network underlying the opposite effects of oxytocin (OT) and arginine-vasopressin (AVP) on fear. In the central amygdala (Ce) there are two adjacent but not overlapping populations of OT receptors (in the Ce lateral: CeL) and V1a receptors (in the Ce medial: CeM). In the CeL OT excites GABA containing inhibitory interneurons that synapse on neurons in the CeM that are excited by AVP and project to the brainstem. As a result, the effects of OT and AVP on fear are strikingly opposite. Modified from (Viviani and Stoop, 2008)
10. Conclusions
Although much progress has been made in our understanding of the diversity and complexity of AVT/AVP systems, the extent to which species differences in the AVT/AVP system are responsible for species differences in sociality remains to be fully determined. There are, however, convincing examples illustrating how species differences in the AVP system can underlie species differences in social organization indicating that this is a fruitful avenue for research. One of the most striking examples is the data illustrating that induction of the expression of a single gene, the V1a receptor gene, within specific regions of the brain can transform a species’ mating strategy from nonmonogamous to monogamous. These findings, along with other recent data, have stimulated research examining the relationship between sociality and the AVT/AVP family of peptides using the comparative approach. An increasingly diverse group of species that display a wide range of social behaviors and social organizations are being examined. One significant problem is that it has been difficult to characterize the inherent complexity of species differences in sociality in ways that are useful to investigate the hypothesis that species differences in nonapeptide systems underlie species differences in sociality. While the use of dichotomous categories (e.g., monogamous versus non-monogamous or social versus asocial) has been a reasonable first order approach, it is clearly too simplistic to explore the relationships that would be required to fully test this hypothesis. Another problem has been that the comparison of species differences in sociality and AVT/AVP systems has been limited primarily to the examination of species differences in the anatomical distribution of AVT/AVP and their receptors. As has been discussed above the factors governing signaling by the AVT/AVP system are complex and likely to differ across species. One can imagine how species differences in a variety of features of this system such as receptor selectivity or the extent of non-synaptic peptide release could contribute to species differences in how this system influences social behavior. As the species differences in the signaling properties of the AVT/AVP system become better understood and this information is combined with the species differences in the “wiring diagrams” within the SBNN (i.e., the distribution of AVT/AVP and their receptors) we will have a much clearer understanding of the mechanism underlying species differences in sociality.
Another approach to the investigation of the role of the AVT/AVP system in sociality has been to examine the relationships between these peptides and sociality within species. There is an increasing body of evidence that the sex differences across the SBNN regulate sex differences in social behavior in animals(Paul and others, 2014) and humans(Rilling and others, 2014). The AVT/AVP system has also been implicated in the modulation of mood and anxiety(Neumann and Landgraf, 2012) and a better understanding of the functional consequences of sex differences in the AVP/OT system could provide insight into the substantial sex differences in many psychiatric disorders. Recent studies support this possibility(Marshall, 2013; Miller and others, 2013). Another interesting development that will likely stimulate more research is the hypothesis that sex differences in the AVT/AVP system may not only be responsible for sex differences in some behaviors, it might also serve to prevent sex differences in other behaviors through compensatory mechanisms(De Vries and Boyle, 1998; De Vries, 2004).
Studies demonstrating that gonadal hormones can modulate the expression of AVP were the first to demonstrate plasticity in the AVT/AVP system. Increasingly, however, it has become clear that social factors are powerful modulators of the AVT/AVP system as well. While some of the effects of social experience on this system may be mediated by gonadal hormones, others are clearly independent of these hormones. Therefore, the social and hormonal environment is likely a major factor in determining individual differences in social behavior along with individual differences in genetic background (e.g., the V1a receptor gene). Taken together, the existing data indicate that there are many species, sex and individual differences in the patterns of expression of AVT/AVP and their receptors over the nodes of the SBNN. It also appears likely that these mechanisms are highly plastic and that the structure and function of the SBNN is continuously changing in response to changes in the social and hormonal environment.
Neurochemical signaling within the SBNN probably involves a complex combination of synaptic and non-synaptic mechanisms. It is also likely that AVT/ AVP containing neurons fall into many categories based on the number and type of neurochemical signals (i.e., classical neurotransmitters and other peptides) they release and whether they release peptides by non-synaptic and/or synaptic mechanisms. The signals produced by these neurons can communicate even more information when one considers that the ratio of the different peptides produced and released by these neurons can potentially be regulated by many factors, including synaptic input and hormones. Whether both non-synaptic and synaptic release occurs throughout the SBNN is not known. It has been suggested that non-synaptic release of nonapeptides may occur more commonly in hypothalamic neurons, while synaptic release may occur more frequently in other limbic structures(Engelmann and others, 2000). It would seem, however, that non-synaptic release might play an important role in at least some regions outside of the hypothalamus where V1a receptors are found in the absence of AVT/AVP, e.g., the LS in the Syrian hamster. A fuller definition of the different classes of AVT/AVP containing neurons will also be critical for a more refined examination of the relationships between sociality, sex and individual differences and the neurons that contain these peptides and their receptors.
A striking feature of the AVP/OT peptide family is the structural similarity in both peptides and receptors. These similarities in structure have the potential to produce substantial crosstalk. As a result, it will be important to more fully understand the properties of AVP/OT receptors as well as the extent to which there are species differences in the selectivity of the different receptor subtypes. Although data is only available in a few species, it seems likely that the central effects of AVP- and OT-like peptides are mediated through V1a, V1b and OT receptors in many vertebrate groups. It will also be important to more fully understand the efficacy of the many AVP/OT agonists and antagonists across species because they represent such powerful tools for comparative studies. Despite the apparent lack of selectivity of AVP/OT receptors for naturally produced AVP- and OT-like peptides, there appears to be significant differences in the cellular response to the activation of each of these receptors. Therefore, the phenotype of the cells containing these different receptors and the second messenger pathways activated by the ligands may be far more important in determining the functional response to receptor activation than whether the receptor is activated by an AVP- or an OT-like peptide. Of course, another consideration is whether the peptides are released locally via synaptic mechanisms or released more broadly via non-synaptic (volume transmission) mechanisms. There is already evidence that the activation of anatomically segregated V1a and OT receptors in local networks can produce opposite effects. Perhaps the activation of AVP/OT receptors in local networks by synaptic processes combined with the activation of larger pools of receptors across nodes of the SBNN could underlie the emergence of social behavior from this network (Figure 6). If so, different patterns of synaptic and non-synaptic activation across the SBNN could be responsible for the expression of different social behaviors.
Figure 6.
Hypothetical examples of how different combinations of synaptic and non-synaptic release of AVP-like peptides might regulate the expression of different social behaviors (i.e., X, Y or Z) by their action in the social behavior neural network. Blue lines represent neuronal activity in projections resulting in local synaptic release and green regions represent peptide released by volume transmission. See text for abbreviations.
Highlights.
Neurochemical signaling in the social behavior neural network
Species, sex and individual differences in AVT/AVP and sociality
Plasticity in the AVT/AVP system: gonadal hormones and social factors
Synaptic, non-synaptic and volume transmission; nonapeptide receptors; local networks
Acknowledgements
The original research discussed in this paper was supported by NIH and NSF grants including NSF IOS-092330. I would also like to thank Drs. Heather Caldwell, Geert de Vries, Kim Huhman and James Walton for their comments on the manuscript and Drs. Ron Stoop and Leslie Turner for the permission to adapt figures.
Abbreviations
- AVP
Arginine-vasopressin
- AVT
Arginine-vasotocin
- GABA
Gamma-aminobutyric acid
- GAL
Galanin
- IT
Isotocin
- LH
Lateral Hypothalamus
- LDCV
large dense-core vesicles
- LHN
lateral habenular nucleus
- LS
Lateral Septum
- Me
Medial amygdala
- MPN
Medial preoptic nucleus
- MPOA
Medial preoptic area
- MT
Mesotocin
- OT
Oxytocin
- PAG
Periaquaductal gray
- SCN
Suprachiasmatic nucleus
- SON
Supraoptic nucleus
- SSV
small synaptic vesicles
- VLH
Ventrolateral hypothalamus
- VMH
Ventromedial hypothalamus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Absil P, Papello M, Viglietti-Panzica C, Balthazart J, Panzica G. The medial preoptic nucleus receives vasotocinergic inputs in male quail: a tract-tracing and immunocytochemical study. J Chem Neuroanat. 2002;24(1):27–39. doi: 10.1016/s0891-0618(02)00017-0. [DOI] [PubMed] [Google Scholar]
- Acharjee S, Do-Rego JL, Oh DY, Moon JS, Ahn RS, Lee K, Bai DG, Vaudry H, Kwon HB, Seong JY. Molecular cloning, pharmacological characterization, and histochemical distribution of frog vasotocin and mesotocin receptors. J Mol Endocrinol. 2004;33(1):293–313. doi: 10.1677/jme.0.0330293. [DOI] [PubMed] [Google Scholar]
- Acher R, Chauvet J. The neurohypophyseal endocrine regulatory cascade: Precursors, mediators, receptors, and effectors. Front Neuroendocrinol. 1995;16:237–289. doi: 10.1006/frne.1995.1009. [DOI] [PubMed] [Google Scholar]
- Adkins-Regan E. Neuroendocrinology of social behavior. ILAR J. 2009;50(1):5–14. doi: 10.1093/ilar.50.1.5. [DOI] [PubMed] [Google Scholar]
- Agnati LF, Fuxe K, Zoli M, Ozini I, Toffano G, Ferraguti F. A correlation analysis of the regional distribution of central enkephalin and beta-endorphin immunoreactive terminals and of opiate receptors in adult and old male rats. Evidence for the existence of two main types of communication in the central nervous system: the volume transmission and the wiring transmission. Acta Physiol Scand. 1986;128(2):201–207. doi: 10.1111/j.1748-1716.1986.tb07967.x. [DOI] [PubMed] [Google Scholar]
- Akhundova A, Getmanova E, Gorbulev V, Carnazzi E, Eggena P, Fahrenholz F. Cloning and functional characterization of the amphibian mesotocin receptor, a member of the oxytocin/vasopressin receptor superfamily. Eur J Biochem. 1996;237(3):759–767. doi: 10.1111/j.1432-1033.1996.0759p.x. [DOI] [PubMed] [Google Scholar]
- Albers HE. The regulation of social recognition, social communication and aggression: vasopressin in the social behavior neural network. Horm Behav. 2012;61(3):283–292. doi: 10.1016/j.yhbeh.2011.10.007. [DOI] [PubMed] [Google Scholar]
- Albers HE, Bamshad M. Role of vasopressin and oxytocin in the control of social behavior in Syrian hamsters (Mesocricetus auratus) Prog Brain Res. 1998;119:395–408. doi: 10.1016/s0079-6123(08)61583-6. [DOI] [PubMed] [Google Scholar]
- Albers HE, Cooper TT. Effects of testosterone on the behavioral response to arginine vasopressin microinjected into the central gray and septum. Peptides. 1995;16(2):269–273. doi: 10.1016/0196-9781(94)00188-x. [DOI] [PubMed] [Google Scholar]
- Albers HE, Dean A, Karom MC, Smith D, Huhman KL. Role of V1a vasopressin receptors in the control of aggression in Syrian hamsters. Brain Res. 2006;1073–1074:425–430. doi: 10.1016/j.brainres.2005.12.081. [DOI] [PubMed] [Google Scholar]
- Albers HE, Ferris CF. Behavioral effects of vasopressin and oxytocin within the medial preoptic area of the golden hamster. Regul Peptides. 1985;12:257–260. doi: 10.1016/0167-0115(85)90067-9. [DOI] [PubMed] [Google Scholar]
- Albers HE, Hennessey AC, Whitman DC. Vasopressin and the regulation of hamster social behavior. Ann NY Acad Sci. 1992;652:227–242. doi: 10.1111/j.1749-6632.1992.tb34358.x. [DOI] [PubMed] [Google Scholar]
- Albers HE, Huhman KL, Meisel RL. Hormonal Basis of Social Conflict and Communication. In: Pfaff D, Arnold AP, Etgen A, Fahrbach SE, Rubin RT, editors. Hormones, Brain and Behavior. Amsterdam: Academic Press; 2002. pp. 393–433. [Google Scholar]
- Albers HE, Karom M, Whitman DC. Ovarian hormones alter the behavioral response of the medial preoptic anterior hypothalamus to arginine-vasopressin. Peptides. 1996;17:1359–1363. doi: 10.1016/s0196-9781(96)00194-5. [DOI] [PubMed] [Google Scholar]
- Albers HE, Liou SY, Ferris CF. Testosterone alters the behavioral response of the medial preoptic-anterior hypothalamus to microinjection of arginine vasopressin in the hamster. Brain Res. 1988;456:382–386. doi: 10.1016/0006-8993(88)90244-2. [DOI] [PubMed] [Google Scholar]
- Albers HE, Pollock J, Simmons WH, Ferris CF. A V1-like receptor mediates vasopressin-induced flank marking behavior in hamster hypothalamus. J Neurosci. 1986;6(7):2085–2089. doi: 10.1523/JNEUROSCI.06-07-02085.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albers HE, Rowland CM, Ferris CF. Arginine-vasopressin immunoreactivity is not altered by photoperiod or gonadal hormones in the Syrian hamster (Mesocricetus auratus) Brain Res. 1991;539:137–142. doi: 10.1016/0006-8993(91)90696-s. [DOI] [PubMed] [Google Scholar]
- Alonso G, Szafarczyk A, Assenmacher I. Radioautographic evidence that axons from the area of supraoptic nuclei in the rat project to extrahypothalamic brain regions. Neurosci Lett. 1986;66(3):251–256. doi: 10.1016/0304-3940(86)90027-3. [DOI] [PubMed] [Google Scholar]
- Babb PL, Fernandez-Duque E, Schurr TG. AVPR1A sequence variation in monogamous owl monkeys (Aotus azarai) and its implications for the evolution of platyrrhine social behavior. J Mol Evol. 2010;71(4):279–297. doi: 10.1007/s00239-010-9383-6. [DOI] [PubMed] [Google Scholar]
- Bachner-Melman R, Dina C, Zohar AH, Constantini N, Lerer E, Hoch S, Sella S, Nemanov L, Gritsenko I, Lichtenberg P, Granot R, Ebstein RP. AVPR1a and SLC6A4 gene polymorphisms are associated with creative dance performance. PLoS Genet. 2005;1(3):e42. doi: 10.1371/journal.pgen.0010042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldino F, Jr, O'Kane TM, Fitzpatrick-McElligott S, Wolfson B. Coordinate hormonal and synaptic regulation of vasopressin messenger RNA. Science. 1988;241(4868):978–981. doi: 10.1126/science.3406747. [DOI] [PubMed] [Google Scholar]
- Bales KL, Plotsky PM, Young LJ, Lim MM, Grotte N, Ferrer E, Carter CS. Neonatal oxytocin manipulations have long-lasting, sexually dimorphic effects on vasopressin receptors. Neuroscience. 2007a;144(1):38–45. doi: 10.1016/j.neuroscience.2006.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bales KL, Plotsky PM, Young LJ, Lim MM, Grotte N, Ferrer E, Carter CS. Neonatal oxytocin manipulations have long-lasting, sexually dimorphic effects on vasopressin receptors. Neuroscience. 2007b;144(1):38–45. doi: 10.1016/j.neuroscience.2006.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamshad M, Cooper TT, Karom M, Albers HE. Glutamate and vasopressin interact to control scent marking in Syrian hamsters (Mesocricetus auratus) Brain Res. 1996 doi: 10.1016/0006-8993(96)00670-1. [DOI] [PubMed] [Google Scholar]
- Bamshad M, Novak MA, De Vries GJ. Sex and species differences in the vasopressin innervation of sexually naive and parental prairie voles, Microtus ochrogaster and meadow voles, Microtus pennsylvanicus. J Neuroendocrinol. 1993;5:247–255. doi: 10.1111/j.1365-2826.1993.tb00480.x. [DOI] [PubMed] [Google Scholar]
- Bamshad M, Novak MA, De Vries GJ. Cohabitation alters vasopressin innervation and paternal behavior in prairie voles (Microtus ochrogaster) Physiol Behav. 1994;56(4):751–758. doi: 10.1016/0031-9384(94)90238-0. [DOI] [PubMed] [Google Scholar]
- Barberis C, Tribollet E. Vasopressin and oxytocin receptors in the central nervous system. Crit Rev Neurobiol. 1996;10(1):119–154. doi: 10.1615/critrevneurobiol.v10.i1.60. [DOI] [PubMed] [Google Scholar]
- Beery AK, Lacey EA, Francis DD. Oxytocin and vasopressin receptor distributions in a solitary and a social species of tuco-tuco (Ctenomys haigi and Ctenomys sociabilis) J Comp Neurol. 2008;507(6):1847–1859. doi: 10.1002/cne.21638. [DOI] [PubMed] [Google Scholar]
- Beiderbeck DI, Neumann ID, Veenema AH. Differences in intermale aggression are accompanied by opposite vasopressin release patterns within the septum in rats bred for low and high anxiety. Eur J Neurosci. 2007;26(12):3597–3605. doi: 10.1111/j.1460-9568.2007.05974.x. [DOI] [PubMed] [Google Scholar]
- Bester-Meredith JK, Marler CA. Vasopressin and aggression in cross-fostered California mice (Peromyscus californicus) and white-footed mice (Peromyscus leucopus) Horm Behav. 2001;40(1):51–64. doi: 10.1006/hbeh.2001.1666. [DOI] [PubMed] [Google Scholar]
- Bester-Meredith JK, Young LJ, Marler CA. Species differences in paternal behavior and aggression in peromyscus and their associations with vasopressin immunoreactivity and receptors. Horm Behav. 1999;36(1):25–38. doi: 10.1006/hbeh.1999.1522. [DOI] [PubMed] [Google Scholar]
- Bittman EL, Jetton AE, Villalba C, DeVries GJ. Effects of photoperiod and androgen on pituitary function and neuropeptide staining in Siberian hamsters. Am J Physiol. 1996;271(1 Pt 2):R64–R72. doi: 10.1152/ajpregu.1996.271.1.R64. [DOI] [PubMed] [Google Scholar]
- Bosch OJ, Neumann ID. Both oxytocin and vasopressin are mediators of maternal care and aggression in rodents: from central release to sites of action. Horm Behav. 2012;61(3):293–303. doi: 10.1016/j.yhbeh.2011.11.002. [DOI] [PubMed] [Google Scholar]
- Boyd SK. Vasotocin modulation of social behaviorsin amphibians. In: Choleris E, Pfaff DW, Kavaliers M, editors. Oxytocin, Vasopressin and Related Peptides in the Regulation of Behavior. Cambridge: Cambride University Press; 2013. pp. 97–109. [Google Scholar]
- Boyd SK, Moore FL. Gonadectomy reduces the concentrations of putative receptors for arginine vasotocin in the brain of an amphibian. Brain Res. 1991;541(2):193–197. doi: 10.1016/0006-8993(91)91018-v. [DOI] [PubMed] [Google Scholar]
- Brain PF. Effects of isolation/grouping on endocrine function and fighting behavior in male and female golden hamsters (Mesocricetus auratus Waterhouse) Behav Biol. 1972;7:349–357. doi: 10.1016/s0091-6773(72)80106-8. [DOI] [PubMed] [Google Scholar]
- Brownstein MJ, Mezey E. Multiple chemical messengers in hypothalamic magnocellular neurons. Prog Brain Res. 1986;68:161–168. doi: 10.1016/s0079-6123(08)60237-x. [DOI] [PubMed] [Google Scholar]
- Buijs RM. Intra- and extrahypothalamic vasopressin and oxytocin pathways in the rat. Pathways to the limbic system, medulla oblongata and spinal cord. Cell Tissue Res. 1978;192(3):423–435. doi: 10.1007/BF00212323. [DOI] [PubMed] [Google Scholar]
- Buijs RM, Pevet P, Masson-Pevet M, Pool CW, DeVries GJ, Canguilhem B, Vivien-Roels B. Seasonal variation in vasopressin innervation in the brain of the European hamster (Cricetus cricetus) Brain Res. 1986;371:193–196. doi: 10.1016/0006-8993(86)90829-2. [DOI] [PubMed] [Google Scholar]
- Buijs RM, Swaab DF. Immuno-electron microscopical demonstration of vasopressin and oxytocin synapses in the limbic system of the rat. Cell Tiss Res. 1979;204:355–365. doi: 10.1007/BF00233648. [DOI] [PubMed] [Google Scholar]
- Buijs RM, Wortel J, Hou YX. Colocalization of gamma-aminobutyric acid with vasopressin, vasoactive intestinal peptide, and somatostatin in the rat suprachiasmatic nucleus. J Comp Neurol. 1995;358(3):343–352. doi: 10.1002/cne.903580304. [DOI] [PubMed] [Google Scholar]
- Burkett JP, Young LJ. The behavioral, anatomical and pharmacological parallels between social attachment, love and addiction. Psychopharmacology (Berl) 2012;224(1):1–26. doi: 10.1007/s00213-012-2794-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caffe AR, van Leeuwen FW, Luiten PG. Vasopressin cells in the medial amygdala of the rat project to the lateral septum and ventral hippocampus. J Comp Neurol. 1987;261(2):237–252. doi: 10.1002/cne.902610206. [DOI] [PubMed] [Google Scholar]
- Caffe AR, Van Ryen PC, Van der Woude TP, van Leeuwen FW. Vasopressin and oxytocin systems in the brain and upper spinal cord of Macaca fascicularis. J Comp Neurol. 1989;287(3):302–325. doi: 10.1002/cne.902870304. [DOI] [PubMed] [Google Scholar]
- Caldwell HK. Neurobiology of Sociability. In: Lopez-Larrea C, editor. Sensing in Nature. US: Springer; 2012. pp. 187–205. [Google Scholar]
- Caldwell HK, Albers HE. Short-photoperiod exposure reduces vasopressin (V-1a) receptor binding but not arginine-vasopressin-induced flank marking in male Syrian hamsters. J Neuroendocrinol. 2003;15(10):971–977. doi: 10.1046/j.1365-2826.2003.01086.x. [DOI] [PubMed] [Google Scholar]
- Caldwell HK, Albers HE. Effect of photoperiod on vasopressin-induced aggression in Syrian hamsters. Horm Behav. 2004a;46(4):444–449. doi: 10.1016/j.yhbeh.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Caldwell HK, Albers HE. Photoperiodic regulation of vasopressin receptor binding in female Syrian hamsters. Brain Res. 2004b;1002(1–2):136–141. doi: 10.1016/j.brainres.2003.12.025. [DOI] [PubMed] [Google Scholar]
- Caldwell HK, Lee HJ, Macbeth AH, Young WS., III Vasopressin: behavioral roles of an "original" neuropeptide. Prog Neurobiol. 2008a;84(1):1–24. doi: 10.1016/j.pneurobio.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell HK, Smith DA, Albers HE. Photoperiodic mechanisms controlling scent marking: interactions of vasopressin and gonadal steroids. Eur J Neurosci. 2008b;27(5):1189–1196. doi: 10.1111/j.1460-9568.2008.06071.x. [DOI] [PubMed] [Google Scholar]
- Caldwell HK, Young WS., III . Oxytocin and Vasopressin: Genetics and behavioral implications. In: Lim R, editor. Neuroactive Proteins and Peptides. New York: Springer; 2006. pp. 573–607. [Google Scholar]
- Caldwell JD. Central oxytocin and female sexual behavior. Ann NY Acad Sci. 1992;652:166–179. doi: 10.1111/j.1749-6632.1992.tb34353.x. [DOI] [PubMed] [Google Scholar]
- Campbell P, Ophir AG, Phelps SM. Central vasopressin and oxytocin receptor distributions in two species of singing mice. J Comp Neurol. 2009;516(4):321–333. doi: 10.1002/cne.22116. [DOI] [PubMed] [Google Scholar]
- Castel M, Morris J, Belenky M. Non-synaptic and dendritic exocytosis from dense-cored vesicles in the suprachiasmatic nucleus. NeuroReport. 1996;7(2):543–547. doi: 10.1097/00001756-199601310-00040. [DOI] [PubMed] [Google Scholar]
- Castel M, Morris JF. The neurophysin-containing innervation of the forebrain of the mouse. Neuroscience. 1988;24(3):937–966. doi: 10.1016/0306-4522(88)90078-4. [DOI] [PubMed] [Google Scholar]
- Chini B, Mouillac B, Ala Y, Balestre MN, Trumpp-Kallmeyer S, Hoflack J, Elands J, Hibert M, Manning M, Jard S. Tyr115 is the key residue for determining agonist selectivity in the V1a vasopressin receptor. EMBO J. 1995;14(10):2176–2182. doi: 10.1002/j.1460-2075.1995.tb07211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper MA, Karom M, Huhman KL, Albers HE. Repeated agonistic encounters in hamsters modulate AVP V1a receptor binding. Horm Behav. 2005;48(5):545–551. doi: 10.1016/j.yhbeh.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Crews D. The development of phenotypic plasticity: where biology and psychology meet. Dev Psychobiol. 2003;43(1):1–10. doi: 10.1002/dev.10115. [DOI] [PubMed] [Google Scholar]
- Dayanithi G, Widmer H, Richard P. Vasopressin-induced intracellular Ca2+ increase in isolated rat supraoptic cells. J Physiol. 1996;490(Pt 3):713–727. doi: 10.1113/jphysiol.1996.sp021180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vries GJ. Sex differences in neurotransmitter systems. J Neuroendocrinol. 1990;2(1):1–13. doi: 10.1111/j.1365-2826.1990.tb00385.x. [DOI] [PubMed] [Google Scholar]
- De Vries GJ. Minireview: Sex differences in adult and developing brains: compensation, compensation, compensation. Endocrinol. 2004;145(3):1063–1068. doi: 10.1210/en.2003-1504. [DOI] [PubMed] [Google Scholar]
- De Vries GJ. Sex differences in vasopressin and oxytocin innervation of the brain. Prog Brain Res. 2008;170:17–27. doi: 10.1016/S0079-6123(08)00402-0. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, al Shamma HA. Sex differences in hormonal responses of vasopressin pathways in the rat brain. J Neurobiol. 1990;21(5):686–693. doi: 10.1002/neu.480210503. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Boyle PA. Double duty for sex differences in the brain. Behav Brain Res. 1998;92(2):205–213. doi: 10.1016/s0166-4328(97)00192-7. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Buijs RM. The origin of the vasopressinergic and oxytocinergic innervation of the rat brain with special reference to the lateral septum. Brain Res. 1983;273(2):307–317. doi: 10.1016/0006-8993(83)90855-7. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Buijs RM, Sluiter AA. Gonadal hormone actions on the morphology of the vasopressinergic innervation of the adult rat brain. Brain Res. 1984;298:141–145. doi: 10.1016/0006-8993(84)91157-0. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Buijs RM, Swaab DF. Ontogeny of the vasopressinergic neurons of the suprachiasmatic nucleus and their extrahypothalamic projections in the rat brain--presence of a sex difference in the lateral septum. Brain Res. 1981;218(1–2):67–78. doi: 10.1016/0006-8993(81)90989-6. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Buijs RM, van Leeuwen FW, Caffe AR, Swaab DF. The vasopressinergic innervation of the brain in normal and castrated rats. J Comp Neurol. 1985;233:236–254. doi: 10.1002/cne.902330206. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Panzica GC. Sexual differentiation of central vasopressin and vasotocin systems in vertebrates: different mechanisms, similar endpoints. Neuroscience. 2006;138(3):947–955. doi: 10.1016/j.neuroscience.2005.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vries GJ, Wang Z, Bullock NA, Numan S. Sex differences in the effects of testosterone and its metabolites on vasopressin messenger RNA levels in the bed nucleus of the stria terminalis of rats. J Neurosci. 1994;14(3 Pt 2):1789–1794. doi: 10.1523/JNEUROSCI.14-03-01789.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delville Y, De Vries GJ, Ferris CF. Neural connections of the anterior hypothalamus and agonistic behavior in golden hamster. Brain Behav Evol. 2000;55(2):53–76. doi: 10.1159/000006642. [DOI] [PubMed] [Google Scholar]
- Delville Y, Ferris CF. Sexual differences in vasopressin receptor binding within the ventrolateral hypothalamus in golden hamsters. Brain Res. 1995;681(1–2):91–96. doi: 10.1016/0006-8993(95)00291-w. [DOI] [PubMed] [Google Scholar]
- Delville Y, Koh ET, Ferris CF. Sexual differences in the magnocellular vasopressinergic system in golden hamsters. Brain Res Bull. 1994;33(5):535–540. doi: 10.1016/0361-9230(94)90080-9. [DOI] [PubMed] [Google Scholar]
- Delville Y, Mansour KM, Ferris CF. Testosterone facilitates aggression by modulating vasopressin receptors in the hypothalamus. Physiol & Behav. 1996;60(1):25–29. doi: 10.1016/0031-9384(95)02246-5. [DOI] [PubMed] [Google Scholar]
- Derick S, Cheng LL, Voirol MJ, Stoev S, Giacomini M, Wo NC, Szeto HH, Ben Mimoun M, Andres M, Gaillard RC, Guillon G, Manning M. [1-deamino-4-cyclohexylalanine] arginine vasopressin: a potent and specific agonist for vasopressin V1b receptors. Endocrinol. 2002;143(12):4655–4664. doi: 10.1210/en.2002-220363. [DOI] [PubMed] [Google Scholar]
- Dhakar MB, Stevenson EL, Caldwell HK. Oxytocin, vasopressin, and their interplay with gonadal hormones. In: Choleris E, Pfaff DW, Kavaliers M, editors. Oxytocin, vasopressin and related peptides in the regulation of behavior. Cambridge: Cambridge: 2013. pp. 3–26. [Google Scholar]
- Donaldson ZR, Kondrashov FA, Putnam A, Bai Y, Stoinski TL, Hammock EA, Young LJ. Evolution of a behavior-linked microsatellite-containing element in the 5' flanking region of the primate AVPR1A gene. BMC Evol Biol. 2008;8:180. doi: 10.1186/1471-2148-8-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson ZR, Young LJ. The relative contribution of proximal 5' flanking sequence and microsatellite variation on brain vasopressin 1a receptor (Avpr1a) gene expression and behavior. PLoS Genet. 2013;9(8):e1003729. doi: 10.1371/journal.pgen.1003729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois-Dauphin M, Barberis C, de Bilbao F. Vasopressin receptors in the mouse (Mus musculus) brain: sex-related expression in the medial preoptic area and hypothalamus. Brain Res. 1996;743(1–2):32–39. doi: 10.1016/s0006-8993(96)01019-0. [DOI] [PubMed] [Google Scholar]
- Dubois-Dauphin M, Pevet P, Tribollet E, Dreifuss JJ. Vasopressin in the brain of the golden hamster: The distribution of vasopressin binding sites and of immunoreactivity to the vasopressin-related glycopeptide. J Comp Neurol. 1990;300:535–548. doi: 10.1002/cne.903000408. [DOI] [PubMed] [Google Scholar]
- Dubois-Dauphin M, Theler JM, Ouarour A, Pevet P, Barberis C, Dreifuss JJ. Regional differences in testosterone effects on vasopressin receptors and on vasopressin immunoreactivity in intact and castrated Siberian hamsters. Brain Res. 1994;638(1–2):267–276. doi: 10.1016/0006-8993(94)90659-9. [DOI] [PubMed] [Google Scholar]
- Dubois-Dauphin M, Theler JM, Zaganidis N, Dominik W, Tribollet E, Pevet P, Charpak G, Dreifuss JJ. Expression of vasopressin receptors in hamster hypothalamus is sexually dimorphic and dependent upon photoperiod. Proc Natl Sci. 1991;88:11163–11167. doi: 10.1073/pnas.88.24.11163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois-Dauphin M, Tribollet E, Dreifuss JJ. Distribution of neurohypophysial peptides in the guinea pig brain. I. An immunocytochemical study of the vasopressin-related glycopeptide. Brain Res. 1989;496(1–2):45–65. doi: 10.1016/0006-8993(89)91051-2. [DOI] [PubMed] [Google Scholar]
- Duncan MJ. Photoperiodic regulation of hypothalamic neuropeptide messenger RNA expression: effect of pinealectomy and neuroanatomical location. Brain Res Mol Brain Res. 1998;57(1):142–148. doi: 10.1016/s0169-328x(98)00084-9. [DOI] [PubMed] [Google Scholar]
- Elands J, Barberis C, Jard S. [3H]-[Thr4,Gly7]OT: a highly selective ligand for central and peripheral OT receptors. Am J Physiol. 1988;254(1 Pt 1):E31–E38. doi: 10.1152/ajpendo.1988.254.1.E31. [DOI] [PubMed] [Google Scholar]
- Engelmann M, Wotjak CT, Ebner K, Landgraf R. Behavioural impact of intraseptally released vasopressin and oxytocin in rats. Exp Physiol. 2000;85 doi: 10.1111/j.1469-445x.2000.tb00015.x. Spec No:125S-30S. [DOI] [PubMed] [Google Scholar]
- Ferris CF, Melloni RH, Albers HE. Role of vasopressin in flank marking and aggression. In: Choleris E, Pfaff DW, Kavaliers M, editors. Oxytocin, Vasopressin and Related Peptides in the Regulation of Behavior. Cambridge: Cambridge University Press; 2013. pp. 213–231. [Google Scholar]
- Ferris CF, Albers HE, Wesolowski SM, Goldman BD, Leeman SE. Vasopressin injected into the hypothalamus triggers a complex stereotypic behavior in Golden hamsters. Science. 1984;224:521–523. doi: 10.1126/science.6538700. [DOI] [PubMed] [Google Scholar]
- Ferris CF, Axelson JF, Martin M, Robrege LF. Vasopressin immunoreactivity in the anterior hypothalamus is altered during the establishment of dominant/subordinate relationships between hamsters. Neuroscience. 1989;29:675–683. doi: 10.1016/0306-4522(89)90140-1. [DOI] [PubMed] [Google Scholar]
- Ferris CF, Delville Y, Bonigut S, Miller MA. Galanin antagonizes vasopressin-stimulated flank marking in male golden hamsters. Brain Res. 1999;832(1–2):1–6. doi: 10.1016/s0006-8993(99)01432-8. [DOI] [PubMed] [Google Scholar]
- Ferris CF, Delville Y, Miller MA, Dorsa DM, DeVries GJ. Distribution of small vasopressinergic neurons in golden hamsters. J Comp Neuro. 1995;360:589–598. doi: 10.1002/cne.903600404. [DOI] [PubMed] [Google Scholar]
- Ferris CF, Melloni RH, Jr, Koppel G, Perry KW, Fuller RW, Delville Y. Vasopressin/serotonin interactions in the anterior hypothalamus control aggressive behavior in golden hamsters. J Neurosci. 1997;17(11):4331–4340. doi: 10.1523/JNEUROSCI.17-11-04331.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris CF, Pilapil CG, Hayden-Hixson D, Wiley RG, Koh ET. Functionally and anatomically distinct populations of vasopressinergic magnocellular neurons in the female golden hamster. J Neuroendocrinol. 1992;4(2):193–205. doi: 10.1111/j.1365-2826.1992.tb00159.x. [DOI] [PubMed] [Google Scholar]
- Ferris CF, Potegal M. Vasopressin receptor blockade in the anterior hypothalamus suppresses aggression in hamsters. Physiol & Behav. 1988;44:235–239. doi: 10.1016/0031-9384(88)90144-8. [DOI] [PubMed] [Google Scholar]
- Ferris CF, Singer EA, Meenan DM, Albers HE. Inhibition of vasopressin-stimulated flank marking behavior by V1-receptor antagonists. Eur J Pharmacol. 1988;154(2):153–159. doi: 10.1016/0014-2999(88)90092-1. [DOI] [PubMed] [Google Scholar]
- Fink S, Excoffier L, Heckel G. Mammalian monogamy is not controlled by a single gene. Proc Natl Acad Sci U S A. 2006;103(29):10956–10960. doi: 10.1073/pnas.0602380103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink S, Excoffier L, Heckel G. High variability and non-neutral evolution of the mammalian avpr1a gene. BMC Evol Biol. 2007;7:176. doi: 10.1186/1471-2148-7-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliers E, Guldenaar SE, van de WN, Swaab DF. Extrahypothalamic vasopressin and oxytocin in the human brain; presence of vasopressin cells in the bed nucleus of the stria terminalis. Brain Res. 1986;375(2):363–367. doi: 10.1016/0006-8993(86)90759-6. [DOI] [PubMed] [Google Scholar]
- Foletta VC, Brown FD, Young WS., III Cloning of rat ARHGAP4/C1, a RhoGAP family member expressed in the nervous system that colocalizes with the Golgi complex and microtubules. Brain Res Mol Brain Res. 2002;107(1):65–79. doi: 10.1016/s0169-328x(02)00448-5. [DOI] [PubMed] [Google Scholar]
- Francis DD, Young LJ, Meaney MJ, Insel TR. Naturally occurring differences in maternal care are associated with the expression of oxytocin and vasopressin (V1a) receptors: gender differences. J Neuroendocrinol. 2002;14(5):349–353. doi: 10.1046/j.0007-1331.2002.00776.x. [DOI] [PubMed] [Google Scholar]
- Frazier CR, Trainor BC, Cravens CJ, Whitney TK, Marler CA. Paternal behavior influences development of aggression and vasopressin expression in male California mouse offspring. Horm Behav. 2006;50(5):699–707. doi: 10.1016/j.yhbeh.2006.06.035. [DOI] [PubMed] [Google Scholar]
- Freeman SM, Walum H, Inoue K, Smith AL, Goodman MM, Bales KL, Young LJ. Neuroanatomical distribution of oxytocin and vasopressin 1a receptors in the socially monogamous coppery titi monkey (Callicebus cupreus) Neurosci. 2014;273:12–23. doi: 10.1016/j.neuroscience.2014.04.055. Trueta. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund-Mercier MJ, Stoeckel ME, Klein MJ. Oxytocin receptors on oxytocin neurones: histoautoradiographic detection in the lactating rat. J Physiol. 1994;480(Pt 1):155–161. doi: 10.1113/jphysiol.1994.sp020349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuminier F, Sicard B, Boissin-Agasse L, Boissin J. Seasonal changes in the hypothalamic vasopressinergic system of a wild Sahelian rodent, Taterillus petteri. Cell Tissue Res. 1993;271(2):309–316. doi: 10.1007/BF00318617. [DOI] [PubMed] [Google Scholar]
- Gai WP, Geffen LB, Blessing WW. Galanin immunoreactive neurons in the human hypothalamus: colocalization with vasopressin-containing neurons. J Comp Neurol. 1990;298(3):265–280. doi: 10.1002/cne.902980302. [DOI] [PubMed] [Google Scholar]
- Gainer H. Cell-type specific expression of oxytocin and vasopressin genes: an experimental odyssey. J Neuroendocrinol. 2012;24(4):528–538. doi: 10.1111/j.1365-2826.2011.02236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X, Phillips P, Oldfield B, Trinder D, Risvanis J, Stephenson J, Johnston C. Androgen manipulation and vasopressin binding in the rat brain and peripheral organs. Eur J Endocrinol. 1994;130(3):291–296. doi: 10.1530/eje.0.1300291. [DOI] [PubMed] [Google Scholar]
- Gobrogge KL, Liu Y, Jia X, Wang Z. Anterior hypothalamic neural activation and neurochemical associations with aggression in pair-bonded male prairie voles. J Comp Neurol. 2007;502(6):1109–1122. doi: 10.1002/cne.21364. [DOI] [PubMed] [Google Scholar]
- Gobrogge KL, Liu Y, Young LJ, Wang Z. Anterior hypothalamic vasopressin regulates pair-bonding and drug-induced aggression in a monogamous rodent. Proc Natl Acad Sci U S A. 2009;106(45):19144–19149. doi: 10.1073/pnas.0908620106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godwin J, Crews D, Warner RR. Behavioural sex change in the absence of gonads in a coral reef fish. Proc Biol Sci. 1996;263(1377):1683–1688. doi: 10.1098/rspb.1996.0246. [DOI] [PubMed] [Google Scholar]
- Godwin J, Sawby R, Warner RR, Crews D, Grober MS. Hypothalamic arginine vasotocin mRNA abundance variation across sexes and with sex change in a coral reef fish. Brain Behav Evol. 2000;55(2):77–84. doi: 10.1159/000006643. [DOI] [PubMed] [Google Scholar]
- Godwin J, Thompson R. Nonapeptides and social behavior in fishes. Horm Behav. 2012;61(3):230–238. doi: 10.1016/j.yhbeh.2011.12.016. [DOI] [PubMed] [Google Scholar]
- Goodson JL. Nonapeptides and the evolutionary patterning of sociality. In: Neumann IDLR, editor. Progress in Brain Research. Elsevier; 2008. pp. 3–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL. The vertebrate social behavior network: evolutionary themes and variations. Horm Behav. 2005;48(1):11–22. doi: 10.1016/j.yhbeh.2005.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL. Deconstructing sociality, social evolution and relevant nonapeptide functions. Psychoneuroendocrinol. 2013;38(4):465–478. doi: 10.1016/j.psyneuen.2012.12.005. [DOI] [PubMed] [Google Scholar]
- Goodson JL, Bass AH. Vasotocin innervation and modulation of vocal-acoustic circuitry in the teleost Porichthys notatus. J Comp Neurol. 2000;422(3):363–379. doi: 10.1002/1096-9861(20000703)422:3<363::aid-cne4>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Goodson JL, Bass AH. Social behavior functions and related anatomical characteristics of vasotocin/vasopressin systems in vertebrates. Brain Res Brain Res Rev. 2001;35(3):246–265. doi: 10.1016/s0165-0173(01)00043-1. [DOI] [PubMed] [Google Scholar]
- Goodson JL, Evans AK, Wang Y. Neuropeptide binding reflects convergent and divergent evolution in species-typical group sizes. Horm Behav. 2006;50(2):223–236. doi: 10.1016/j.yhbeh.2006.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Kelly AM, Kingsbury MA. Evolving nonapeptide mechanisms of gregariousness and social diversity in birds. Horm Behav. 2012;61(3):239–250. doi: 10.1016/j.yhbeh.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Kingsbury MA. What's in a name? Considerations of homologies and nomenclature for vertebrate social behavior networks. Horm Behav. 2013;64(1):103–112. doi: 10.1016/j.yhbeh.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Wang Y. Valence-sensitive neurons exhibit divergent functional profiles in gregarious and asocial species. Proc Natl Acad Sci U S A. 2006;103(45):17013–17017. doi: 10.1073/pnas.0606278103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gravati M, Busnelli M, Bulgheroni E, Reversi A, Spaiardi P, Parenti M, Toselli M, Chini B. Dual modulation of inward rectifier potassium currents in olfactory neuronal cells by promiscuous G protein coupling of the oxytocin receptor. J Neurochem. 2010;114(5):1424–1435. doi: 10.1111/j.1471-4159.2010.06861.x. [DOI] [PubMed] [Google Scholar]
- Greenwood AK, Wark AR, Fernald RD, Hofmann HA. Expression of arginine vasotocin in distinct preoptic regions is associated with dominant and subordinate behaviour in an African cichlid fish. Proc Biol Sci. 2008;275(1649):2393–2402. doi: 10.1098/rspb.2008.0622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grozhik AV, Horoszko CP, Horton BM, Hu Y, Voisin DA, Maney DL. Hormonal regulation of vasotocin receptor mRNA in a seasonally breeding songbird. Horm Behav. 2013 doi: 10.1016/j.yhbeh.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber CW. Physiology of invertebrate oxytocin and vasopressin neuropeptides. Exp Physiol. 2014;99(1):55–61. doi: 10.1113/expphysiol.2013.072561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubrij KI, Chaturvedi CM, Ali N, Cornett LE, Kirby JD, Wilkerson J, Mikhailova M, Turner ML, Baeyens DA. Molecular cloning of an oxytocin-like receptor expressed in the chicken shell gland. Comp Biochem Physiol B Biochem Mol Biol. 2005;142(1):37–45. doi: 10.1016/j.cbpc.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Gutzler SJ, Karom M, Erwin WD, Albers HE. Arginine-vasopressin and the regulation of aggression in female Syrian hamsters (Mesocricetus auratus) Eur J Neurosci. 2010;31(9):1655–1663. doi: 10.1111/j.1460-9568.2010.07190.x. [DOI] [PubMed] [Google Scholar]
- Gutzler SJ, Karom M, Erwin WD, Albers HE. Seasonal regulation of social communication by photoperiod and testosterone: effects of arginine-vasopressin, serotonin and galanin in the medial preoptic area-anterior hypothalamus. Behav Brain Res. 2011;216(1):214–219. doi: 10.1016/j.bbr.2010.07.042. [DOI] [PubMed] [Google Scholar]
- Hammock EA, Lim MM, Nair HP, Young LJ. Association of vasopressin 1a receptor levels with a regulatory microsatellite and behavior. Genes Brain Behav. 2005;4(5):289–301. doi: 10.1111/j.1601-183X.2005.00119.x. [DOI] [PubMed] [Google Scholar]
- Hammock EA, Young LJ. Variation in the vasopressin V1a receptor promoter and expression: implications for inter- and intraspecific variation in social behaviour. Eur J Neurosci. 2002;16(3):399–402. doi: 10.1046/j.1460-9568.2002.02083.x. [DOI] [PubMed] [Google Scholar]
- Hammock EA, Young LJ. Functional microsatellite polymorphism associated with divergent social structure in vole species. Mol Biol Evol. 2004;21(6):1057–1063. doi: 10.1093/molbev/msh104. [DOI] [PubMed] [Google Scholar]
- Hammock EA, Young LJ. Microsatellite instability generates diversity in brain and sociobehavioral traits. Science. 2005;308(5728):1630–1634. doi: 10.1126/science.1111427. [DOI] [PubMed] [Google Scholar]
- Han TM, De Vries GJ. Organizational effects of testosterone, estradiol, and dihydrotestosterone on vasopressin mRNA expression in the bed nucleus of the stria terminalis. J Neurobiol. 2003;54(3):502–510. doi: 10.1002/neu.10157. [DOI] [PubMed] [Google Scholar]
- Harding CF. Social modulation of circulating hormones in the male. Am Zool. 1981;21:223–232. [Google Scholar]
- Harmon AC, Huhman KL, Moore TO, Albers HE. Oxytocin inhibits aggression in female Syrian hamsters 2. J Neuroendocrinol. 2002a;14(12):963–9. doi: 10.1046/j.1365-2826.2002.00863.x. [DOI] [PubMed] [Google Scholar]
- Harmon AC, Moore TO, Huhman KL, Albers HE. Social experience and social context alter the behavioral response to centrally administered oxytocin in female Syrian hamsters. Neuroscience. 2002b;109(4):767–772. doi: 10.1016/s0306-4522(01)00523-1. [DOI] [PubMed] [Google Scholar]
- Harony H, Wagner S. The contribution of oxytocin and vasopressin to mammalian social behavior: potential role in autism spectrum disorder. Neurosignals. 2010;18(2):82–97. doi: 10.1159/000321035. [DOI] [PubMed] [Google Scholar]
- Hasunuma I, Sakai T, Nakada T, Toyoda F, Namiki H, Kikuyama S. Molecular cloning of three types of arginine vasotocin receptor in the newt, Cynops pyrrhogaster. Gen Comp Endocrinol. 2007;151(3):252–258. doi: 10.1016/j.ygcen.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Hasunuma I, Toyoda F, Kadono Y, Yamamoto K, Namiki H, Kikuyama S. Localization of three types of arginine vasotocin receptors in the brain and pituitary of the newt Cynops pyrrhogaster. Cell Tissue Res. 2010;342(3):437–457. doi: 10.1007/s00441-010-1079-0. [DOI] [PubMed] [Google Scholar]
- Hasunuma I, Toyoda F, Okada R, Yamamoto K, Kadono Y, Kikuyama S. Roles of arginine vasotocin receptors in the brain and pituitary of submammalian vertebrates. Int Rev Cell Mol Biol. 2013;304:191–225. doi: 10.1016/B978-0-12-407696-9.00004-X. [DOI] [PubMed] [Google Scholar]
- Hattori T, Wilczynski W. Comparison of arginine vasotocin immunoreactivity differences in dominant and subordinate green anole lizards. Physiol Behav. 2009;96(1):104–107. doi: 10.1016/j.physbeh.2008.09.010. [DOI] [PubMed] [Google Scholar]
- Hennessey AC, Huhman KL, Albers HE. Vasopressin and sex differences in hamster flank marking. Physiol Behav. 1994;55:905–911. doi: 10.1016/0031-9384(94)90078-7. [DOI] [PubMed] [Google Scholar]
- Hennessey AC, Whitman DC, Albers HE. Microinjection of argininevasopressin into the periaqueductal gray stimulates flank marking in Syrian hamsters (Mesocricetus auratus) Brain Res. 1992;569:136–140. doi: 10.1016/0006-8993(92)90379-n. [DOI] [PubMed] [Google Scholar]
- Herkenham M. Mismatches between neurotransmitter and receptor localizations in brain: observations and implications. Neuroscience. 1987;23(1):1–38. doi: 10.1016/0306-4522(87)90268-5. [DOI] [PubMed] [Google Scholar]
- Hermes ML, Buijs RM, Masson-Pevet M, Pevet P. Seasonal changes in vasopressin in the brain of the garden dormouse (Eliomys quercinus L.) J Comp Neurol. 1990;293(3):340–346. doi: 10.1002/cne.902930303. [DOI] [PubMed] [Google Scholar]
- Hirasawa A, Hashimoto K, Tsujimoto G. Distribution and developmental change of vasopressin V1A and V2 receptor mRNA in rats. Eur J Pharmacol. 1994;267(1):71–75. doi: 10.1016/0922-4106(94)90226-7. [DOI] [PubMed] [Google Scholar]
- Hofman MA, Swaab DF. Diurnal and seasonal rhythms of neuronal activity in the suprachiasmatic nucleus of humans. J Biol Rhythms. 1993;8(4):283–295. doi: 10.1177/074873049300800402. [DOI] [PubMed] [Google Scholar]
- Hokfelt T, Holets VR, Staines W, Meister B, Melander T, Schalling M, Schultzberg M, Freedman J, Bjorklund H, Olson L, Lindh B, Elfvin L-G, Lundberg JM, Lindgren JA, Samuelsson B, Pernow B, Terenius L, Post C, Everitt B, Goldstein M. Coexistence of neuronal messengers - an overview. In: Hokfelt T, Fuxe K, Pernow B, editors. Progress in Brain Research. Amsterdam: Elsevier; 1986. pp. 33–70. [DOI] [PubMed] [Google Scholar]
- Hokfelt T, Johansson O, Goldstein M. Chemical anatomy of the brain. Science. 1984;225(4668):1326–1334. doi: 10.1126/science.6147896. [DOI] [PubMed] [Google Scholar]
- Hoorneman EMD, Buijs RM. Vasopressin fiber pathways in the rat brain following suprachiasmatic nucleus lesioning. Brain Res. 1982;243:235–241. doi: 10.1016/0006-8993(82)90246-3. [DOI] [PubMed] [Google Scholar]
- Hopkins WD, Donaldson ZR, Young LJ. A polymorphic indel containing the RS3 microsatellite in the 5' flanking region of the vasopressin V1a receptor gene is associated with chimpanzee (Pan troglodytes) personality. Genes Brain Behav. 2012;11(5):552–558. doi: 10.1111/j.1601-183X.2012.00799.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber D, Veinante P, Stoop R. Vasopressin and oxytocin excite distinct neuronal populations in the central amygdala. Science. 2005;308(5719):245–248. doi: 10.1126/science.1105636. [DOI] [PubMed] [Google Scholar]
- Huffman LS, O'Connell LA, Kenkel CD, Kline RJ, Khan IA, Hofmann HA. Distribution of nonapeptide systems in the forebrain of an African cichlid fish, Astatotilapia burtoni. J Chem Neuroanat. 2012;44(2):86–97. doi: 10.1016/j.jchemneu.2012.05.002. [DOI] [PubMed] [Google Scholar]
- Huhman KL, Albers HE. Estradiol increases the behavioral response to arginine vasopressin (AVP) in the medial preoptic-anterior hypothalamus. Peptides. 1993;14:1049–1054. doi: 10.1016/0196-9781(93)90085-u. [DOI] [PubMed] [Google Scholar]
- Huhman KL, Jasnow AM, Janicki MM, Mickley NC, Albers HE. Vasopressin (AVP) dose-dependently increases flank marking but not aggressive behavior in Syrian hamsters. Society for Neuroscience Abstracts. 1998 [Google Scholar]
- Hurbin A, Orcel H, Alonso G, Moos F, Rabie A. The vasopressin receptors colocalize with vasopressin in the magnocellular neurons of the rat supraoptic nucleus and are modulated by water balance. Endocrinology. 2002;143(2):456–466. doi: 10.1210/endo.143.2.8643. [DOI] [PubMed] [Google Scholar]
- Insel TR. The challenge of translation in social neuroscience: a review of oxytocin, vasopressin, and affiliative behavior. Neuron. 2010;65(6):768–779. doi: 10.1016/j.neuron.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR, Gelhard R, Shapiro LE. The comparative distribution of forebrain receptors for neurohypophyseal peptides in monogamous and polygamous mice. Neuroscience. 1991;43(2–3):623–630. doi: 10.1016/0306-4522(91)90321-e. [DOI] [PubMed] [Google Scholar]
- Insel TR, Shapiro LE. Oxytocin receptor distribution reflects social organization in monogamous and polygamous voles. Proc Natl Acad Sci U S A. 1992;89(13):5981–5985. doi: 10.1073/pnas.89.13.5981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR, Wang ZX, Ferris CF. Patterns of brain vasopressin receptor distribution associated with social organization in microtine rodents. J Neurosci. 1994;14(9):5381–5392. doi: 10.1523/JNEUROSCI.14-09-05381.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR, Winslow JT, Williams JR, Hastings N, Shapiro LE, Carter CS. The role of neurohypophyseal peptides in the central mediation of complex social processes--evidence from comparative studies. Regul Pept. 1993;45(1–2):127–131. doi: 10.1016/0167-0115(93)90194-d. [DOI] [PubMed] [Google Scholar]
- Irvin RW, Szot P, Dorsa DM, Potegal M, Ferris CF. Vasopressin in the septal area of the Golden hamster controls scent marking and grooming. Physiol Behav. 1990;48:693–699. doi: 10.1016/0031-9384(90)90213-n. [DOI] [PubMed] [Google Scholar]
- Ishunina TA, Salehi A, Hofman MA, Swaab DF. Activity of vasopressinergic neurones of the human supraoptic nucleus is age- and sex-dependent. J Neuroendocrinol. 1999;11(4):251–258. doi: 10.1046/j.1365-2826.1999.00318.x. [DOI] [PubMed] [Google Scholar]
- Ishunina TA, Swaab DF. Vasopressin and oxytocin neurons of the human supraoptic and paraventricular nucleus: size changes in relation to age and sex. J Clin Endocrinol Metab. 1999;84(12):4637–4644. doi: 10.1210/jcem.84.12.6187. [DOI] [PubMed] [Google Scholar]
- Jan LY, Jan YN. Peptidergic transmission in sympathetic ganglia of the frog. J Physiol. 1982;327:219–246. doi: 10.1113/jphysiol.1982.sp014228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakab RL, Naftolin F, Leranth C. Convergent vasopressinergic and hippocampal input onto somatospiny neurons of the rat lateral septal area. Neurosci. 1991;40(2):413–421. doi: 10.1016/0306-4522(91)90129-c. [DOI] [PubMed] [Google Scholar]
- Johnson AE, Barberis C, Albers HE. Castration reduces vasopressin receptor binding in the hamster hypothalamus. Brain Res. 1995;674:153–158. doi: 10.1016/0006-8993(95)00010-n. [DOI] [PubMed] [Google Scholar]
- Johnston RE. Scent marking by male golden hamsters (Mesocricetus auratus). II. The role of the flank gland scent in the causation of marking. Z Tierpsychol. 1975;37(2):138–144. [PubMed] [Google Scholar]
- Kabelik D, Klatt JD, Dinsbury MA, Goodson Endogenous vasotocin exerts context-dependent behavioral effects in a semi-naturalistic colony environment. Horm Behav. 2009;56:101–107. doi: 10.1016/j.yhbeh.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalsbeek A, Buijs RM, Engelmann M, Wotjak CT, Landgraf R. In vivo measurement of a diurnal variation in vasopressin release in the rat suprachiasmatic nucleus. Brain Res. 1995;682(1–2):75–82. doi: 10.1016/0006-8993(95)00324-j. [DOI] [PubMed] [Google Scholar]
- Kato Y, Igarashi N, Hirasawa A, Tsujimoto G, Kobayashi M. Distribution and developmental changes in vasopressin V2 receptor mRNA in rat brain. Differentiation. 1995;59(3):163–169. doi: 10.1046/j.1432-0436.1995.5930163.x. [DOI] [PubMed] [Google Scholar]
- Kelly AM, Kingsbury MA, Hoffbuhr K, Schrock SE, Waxman B, Kabelik D, Thompson RR, Goodson JL. Vasotocin neurons and septal V1a-like receptors potently modulate songbird flocking and responses to novelty. Horm Behav. 2011;60(1):12–21. doi: 10.1016/j.yhbeh.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SJ, Young LJ, Gonen D, Veenstra-VanderWeele J, Courchesne R, Courchesne E, Lord C, Leventhal BL, Cook EH, Jr, Insel TR. Transmission disequilibrium testing of arginine vasopressin receptor 1A (AVPR1A) polymorphisms in autism. Mol Psychiatry. 2002;7(5):503–507. doi: 10.1038/sj.mp.4001125. [DOI] [PubMed] [Google Scholar]
- Kiyama H, Emson PC. Evidence for the co-expression of oxytocin and vasopressin messenger ribonucleic acids in magnocellular neurosecretory cells: simultaneous demonstration of two neurohypophysin messenger ribonucleic acids by hybridization histochemistry. J Neuroendocrinol. 1990;2(3):257–259. doi: 10.1111/j.1365-2826.1990.tb00401.x. [DOI] [PubMed] [Google Scholar]
- Kleiman DG. Monogamy in mammals. Q Rev Biol. 1977;52(1):39–69. doi: 10.1086/409721. [DOI] [PubMed] [Google Scholar]
- Kline RJ, O'Connell LA, Hofmann HA, Holt GJ, Khan IA. The distribution of an AVT V1a receptor in the brain of a sex changing fish, Epinephelus adscensionis. J Chem Neuroanat. 2011;42(1):72–88. doi: 10.1016/j.jchemneu.2011.06.005. [DOI] [PubMed] [Google Scholar]
- Knafo A, Israel S, Darvasi A, Bachner-Melman R, Uzefovsky F, Cohen L, Feldman E, Lerer E, Laiba E, Raz Y, Nemanov L, Gritsenko I, Dina C, Agam G, Dean B, Bornstein G, Ebstein RP. Individual differences in allocation of funds in the dictator game associated with length of the arginine vasopressin 1a receptor RS3 promoter region and correlation between RS3 length and hippocampal mRNA. Genes Brain Behav. 2008;7(3):266–275. doi: 10.1111/j.1601-183X.2007.00341.x. [DOI] [PubMed] [Google Scholar]
- Knobloch HS, Charlet A, Hoffmann LC, Eliava M, Khrulev S, Cetin AH, Osten P, Schwarz MK, Seeburg PH, Stoop R, Grinevich V. Evoked axonal oxytocin release in the central amygdala attenuates fear response. Neuron. 2012; 73(3):553–566. doi: 10.1016/j.neuron.2011.11.030. [DOI] [PubMed] [Google Scholar]
- Koehbach J, Stockner T, Bergmayr C, Muttenthaler M, Gruber CW. Insights into the molecular evolution of oxytocin receptor ligand binding. Biochem Soc Tran. 2013;41(1):197–204. doi: 10.1042/BST20120256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohno S, Kamishima Y, Iguchi T. Molecular cloning of an anuran V(2) type [Arg(8)] vasotocin receptor and mesotocin receptor: functional characterization and tissue expression in the Japanese tree frog (Hyla japonica) Gen Comp Endocrinol. 2003;132(3):485–498. doi: 10.1016/s0016-6480(03)00140-0. [DOI] [PubMed] [Google Scholar]
- Kozorovitskiy Y, Hughes M, Lee K, Gould E. Fatherhood affects dendritic spines and vasopressin V1a receptors in the primate prefrontal cortex. Nat Neurosci. 2006;9(9):1094–1095. doi: 10.1038/nn1753. [DOI] [PubMed] [Google Scholar]
- Krisch B. Immunohistochemical and electron microscopic study of the rat hypothalamic nuclei and cell clusters under various experimental conditions. Possible sites of hormone release. Cell Tissue Res. 1976;174(1):109–127. doi: 10.1007/BF00222154. [DOI] [PubMed] [Google Scholar]
- Lakhdar-Ghazal N, Dubois-Dauphin M, Hermes ML, Buijs RM, Bengelloun WA, Pevet P. Vasopressin in the brain of a desert hibernator, the jerboa (Jaculus orientalis): presence of sexual dimorphism and seasonal variation. J Comp Neurol. 1995;358(4):499–517. doi: 10.1002/cne.903580404. [DOI] [PubMed] [Google Scholar]
- Lambert RC, Dayanithi G, Moos FC, Richard P. A rise in the intracellular Ca2+ concentration of isolated rat supraoptic cells in response to oxytocin. J Physiol. 1994;478(Pt 2):275–287. doi: 10.1113/jphysiol.1994.sp020249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landgraf R, Neumann ID. Vasopressin and oxytocin release within the brain: a dynamic concept of multiple and variable modes of neuropeptide communication. Front Neuroendocrinol. 2004;25(3–4):150–176. doi: 10.1016/j.yfrne.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Landry M, Vila-Porcile E, Hokfelt T, Calas A. Differential routing of coexisting neuropeptides in vasopressin neurons. Eur J Neurosci. 2003;17(3):579–589. [PubMed] [Google Scholar]
- Lee AG, Cool DR, Grunwald WC, Jr, Neal DE, Buckmaster CL, Cheng MY, Hyde SA, Lyons DM, Parker KJ. A novel form of oxytocin in New World monkeys. Biol Lett. 2011;7(4):584–587. doi: 10.1098/rsbl.2011.0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legros JJ. Inhibitory effect of oxytocin on corticotrope function in humans: are vasopressin and oxytocin ying-yang neurohormones? Psychoneuroendocrinol. 2001;26(7):649–655. doi: 10.1016/s0306-4530(01)00018-x. [DOI] [PubMed] [Google Scholar]
- Lema SC. Identification of multiple vasotocin receptor cDNAs in teleost fish: sequences, phylogenetic analysis, sites of expression, and regulation in the hypothalamus and gill in response to hyperosmotic challenge. Mol Cell Endocrinol. 2010;321(2):215–230. doi: 10.1016/j.mce.2010.02.015. [DOI] [PubMed] [Google Scholar]
- Lema SC, Slane MA, Salvesen KE, Godwin J. Variation in gene transcript profiles of two V1a-type arginine vasotocin receptors among sexual phases of bluehead wrasse (Thalassoma bifasciatum) Gen Comp Endocrinol. 2012;179(3):451–464. doi: 10.1016/j.ygcen.2012.10.001. [DOI] [PubMed] [Google Scholar]
- Leng G, Ludwig M. Neurotransmitters and peptides: whispered secrets and public announcements. J Physiol. 2008;586(Pt 23):5625–5632. doi: 10.1113/jphysiol.2008.159103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung CH, Abebe DF, Earp SE, Goode CT, Grozhik AV, Mididoddi P, Maney DL. Neural distribution of vasotocin receptor mRNA in two species of songbird. Endocrinol. 2011;152(12):4865–4881. doi: 10.1210/en.2011-1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung CH, Goode CT, Young LJ, Maney DL. Neural distribution of nonapeptide binding sites in two species of songbird. J Comp Neurol. 2009;513(2):197–208. doi: 10.1002/cne.21947. [DOI] [PubMed] [Google Scholar]
- Lewis CM, Dolence EK, Hubbard CS, Rose JD. Identification of roughskin newt medullary vasotocin target neurons with a fluorescent vasotocin conjugate. J Comp Neurol. 2005;491(4):381–389. doi: 10.1002/cne.20701. [DOI] [PubMed] [Google Scholar]
- Li YC, Korol AB, Fahima T, Nevo E. Microsatellites within genes: structure, function, and evolution. Mol Biol Evol. 2004;21(6):991–1007. doi: 10.1093/molbev/msh073. [DOI] [PubMed] [Google Scholar]
- Lim MM, Wang Z, Olazabal DE, Ren X, Terwilliger EF, Young LJ. Enhanced partner preference in a promiscuous species by manipulating the expression of a single gene. Nature. 2004;429(6993):754–757. doi: 10.1038/nature02539. [DOI] [PubMed] [Google Scholar]
- Lin L, Osan R, Tsien JZ. Organizing principles of real-time memory encoding: neural clique assemblies and universal neural codes. Trends Neurosci. 2006;29(1):48–57. doi: 10.1016/j.tins.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Loup F, Tribollet E, Dubois-Dauphin M, Dreifuss JJ. Localization of highaffinity binding sites for oxytocin and vasopressin in the human brain. An autoradiographic study. Brain Res. 1991;555(2):220–232. doi: 10.1016/0006-8993(91)90345-v. [DOI] [PubMed] [Google Scholar]
- Ludwig M, Bull PM, Tobin VA, Sabatier N, Landgraf R, Dayanithi G, Leng G. Regulation of activity-dependent dendritic vasopressin release from rat supraoptic neurones. J Physiol. 2005;564(Pt 2):515–522. doi: 10.1113/jphysiol.2005.083931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig M, Leng G. Dendritic peptide release and peptide-dependent behaviours. Nat Rev Neurosci. 2006;7(2):126–136. doi: 10.1038/nrn1845. [DOI] [PubMed] [Google Scholar]
- Ludwig M, Sabatier N, Bull PM, Landgraf R, Dayanithi G, Leng G. Intracellular calcium stores regulate activity-dependent neuropeptide release from dendrites. Nature. 2002;418(6893):85–89. doi: 10.1038/nature00822. [DOI] [PubMed] [Google Scholar]
- Lukas M, Neumann ID. Oxytocin and vasopressin in rodent behaviors related to social dysfunctions in autism spectrum disorders. Behav Brain Res. 2013;251:85–94. doi: 10.1016/j.bbr.2012.08.011. [DOI] [PubMed] [Google Scholar]
- Macrides F, Bartke A, Fernandez F, D'Angelo W. Effects of exposure to vaginal odor and receptive females on plasma testosterone levels in the male hamster. Neuroendocrinol. 1974;15:355–364. doi: 10.1159/000122326. [DOI] [PubMed] [Google Scholar]
- Madeira MD, Sousa N, Cadete-Leite A, Lieberman AR, Paula-Barbosa MM. The supraoptic nucleus of the adult rat hypothalamus displays marked sexual dimorphism which is dependent on body weight. Neurosci. 52:497–513. doi: 10.1016/0306-4522(93)90402-2. [DOI] [PubMed] [Google Scholar]
- Mahoney PD, Koh ET, Irvin RW, Ferris CF. Computer-Aided Mapping of Vasopressin Neurons in the Hypothalamus of the Male Golden Hamster: Evidence of Magnocellular Neurons that do not Project to the Neurohypophysis. J Neuroendocrinol. 1990;2(2):113–122. doi: 10.1111/j.1365-2826.1990.tb00840.x. [DOI] [PubMed] [Google Scholar]
- Maney DL, Goode CT, Lange HS, Sanford SE, Solomon BL. Estradiol modulates neural responses to song in a seasonal songbird. J Comp Neurol. 2008;511(2):173–186. doi: 10.1002/cne.21830. [DOI] [PubMed] [Google Scholar]
- Manning M, Misicka A, Olma A, Bankowski K, Stoev S, Chini B, Durroux T, Mouillac B, Corbani M, Guillon G. Oxytocin and vasopressin agonists and antagonists as research tools and potential therapeutics. J Neuroendocrinol. 2012;24(4):609–628. doi: 10.1111/j.1365-2826.2012.02303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning M, Stoev S, Chini B, Durroux B, Mouillac B, Guillon G. Peptide and non-peptide agonists and antagonists for the vasopressin and oxytocin V1a, V1b, V2 and OT receptors: research tools and potential therapeutic agents. In: Neumann ID, Landgraf R, editors. Advances in Vasopressin and Oxytocin: From genes to behavior. Elsevier; 2008. pp. 473–512. [DOI] [PubMed] [Google Scholar]
- Marshall AD. Posttraumatic stress disorder and partner-specific social cognition: a pilot study of sex differences in the impact of arginine vasopressin. Biol Psychol. 2013;93(2):296–303. doi: 10.1016/j.biopsycho.2013.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruska KP, Mizobe MH, Tricas TC. Sex and seasonal co-variation of arginine vasotocin (AVT) and gonadotropin-releasing hormone (GnRH) neurons in the brain of the halfspotted goby. Comp Biochem Physiol A Mol Integr Physiol. 2007a;147(1):129–144. doi: 10.1016/j.cbpa.2006.12.019. [DOI] [PubMed] [Google Scholar]
- Maruska KP, Mizobe MH, Tricas TC. Sex and seasonal co-variation of arginine vasotocin (AVT) and gonadotropin-releasing hormone (GnRH) neurons in the brain of the halfspotted goby. Comp Biochem Physiol A Mol Integr Physiol. 2007b;147(1):129–144. doi: 10.1016/j.cbpa.2006.12.019. [DOI] [PubMed] [Google Scholar]
- Mayes CR, Watts AG, McQueen JK, Fink G, Charlton HM. Gonadal steroids influence neurophysin II distribution in the forebrain of normal and mutant mice. Neuroscience. 1988;25(3):1013–1022. doi: 10.1016/0306-4522(88)90054-1. [DOI] [PubMed] [Google Scholar]
- Mens WB, Witter A, Wimersma Greidanus TB. Penetration of neurohypophyseal hormones from plasma into cerebrospinal fluid (CSF): halftimes of disappearance of these neuropeptides from CSF. Brain Res. 1983;262(1):143–149. doi: 10.1016/0006-8993(83)90478-x. [DOI] [PubMed] [Google Scholar]
- Merighi A. Costorage and coexistence of neuropeptides in the mammalian CNS. Prog Neurobiol. 2002;66(3):161–190. doi: 10.1016/s0301-0082(01)00031-4. [DOI] [PubMed] [Google Scholar]
- Merighi A, Salio C, Ferrini F, Lossi L. Neuromodulatory function of neuropeptides in the normal CNS. J Chem Neuroanat. 2011;42(4):276–287. doi: 10.1016/j.jchemneu.2011.02.001. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Kolachana B, Gold B, Olsh A, Nicodemus KK, Mattay V, Dean M, Weinberger DR. Genetic variants in AVPR1A linked to autism predict amygdala activation and personality traits in healthy humans. Mol Psychiatry. 2009;14(10):968–975. doi: 10.1038/mp.2008.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezey E, Kiss JZ. Coexpression of vasopressin and oxytocin in hypothalamic supraoptic neurons of lactating rats. Endocrinol. 1991;129(4):1814–1820. doi: 10.1210/endo-129-4-1814. [DOI] [PubMed] [Google Scholar]
- Miller M, Bales KL, Taylor SL, Yoon J, Hostetler CM, Carter CS, Solomon M. Oxytocin and vasopressin in children and adolescents with autism spectrum disorders: sex differences and associations with symptoms. Autism Res. 2013;6(2):91–102. doi: 10.1002/aur.1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MA, Ferris CF, Kolb PE. Absence of vasopressin expression by galanin neurons in the golden hamster: Implications for species diferences in extrahypothalamus vasopressin pathways. Brain Res Mol Brain Res. 1999;67(1):28–35. doi: 10.1016/s0169-328x(99)00029-7. [DOI] [PubMed] [Google Scholar]
- Miller MA, Kolb PE, Raskind MA. Extra-hypothalamic vasopressin neurons coexpress galanin messenger RNA as shown by double in situ hybridization histochemistry. J Comp Neurol. 1993;329(3):378–384. doi: 10.1002/cne.903290308. [DOI] [PubMed] [Google Scholar]
- Mohr E, Bahnsen U, Kiessling C, Richter D. Expression of the vasopressin and oxytocin genes in rats occurs in mutually exclusive sets of hypothalamic neurons. FEBS Lett. 1988;242(1):144–148. doi: 10.1016/0014-5793(88)81003-2. [DOI] [PubMed] [Google Scholar]
- Moons L, Cambre M, Batten TF, Vandesande F. Autoradiographic localization of binding sites for vasotocin in the brain and pituitary of the sea bass (Dicentrarchus labrax) Neurosci Lett. 1989;100(1–3):11–16. doi: 10.1016/0304-3940(89)90652-6. [DOI] [PubMed] [Google Scholar]
- Moore FL, Lowry CA. Comparative neuroanatomy of vasotocin and vasopressin in amphibians and other vertebrates. Comparative Biochemistry and Physiology C. 1998;119:251–260. doi: 10.1016/s0742-8413(98)00014-0. [DOI] [PubMed] [Google Scholar]
- Moore FL, Richardson C, Lowry CA. Sexual dimorphism in numbers of vasotocin-immunoreactive neurons in brain areas associated with reproductive behaviors in the roughskin newt. Gen Comp Endocrinol. 2000;117(2):281–298. doi: 10.1006/gcen.1999.7424. [DOI] [PubMed] [Google Scholar]
- Moos F, Poulain DA, Rodriguez F, Guerne Y, Vincent JD, Richard P. Release of oxytocin within the supraoptic nucleus during the milk ejection reflex in rats. Exp Brain Res. 1989;76(3):593–602. doi: 10.1007/BF00248916. [DOI] [PubMed] [Google Scholar]
- Morris JF, Pow DV. Widespread release of peptides in the central nervous system: quantitation of tannic acid-captured exocytoses. Anat Rec. 1991;231(4):437–445. doi: 10.1002/ar.1092310406. [DOI] [PubMed] [Google Scholar]
- Mouillac B, Chini B, Balestre MN, Elands J, Trumpp-Kallmeyer S, Hoflack J, Hibert M, Jard S, Barberis C. The binding site of neuropeptide vasopressin V1a receptor. Evidence for a major localization within transmembrane regions. j Biol Chem. 1995;270(43):25771–25777. doi: 10.1074/jbc.270.43.25771. [DOI] [PubMed] [Google Scholar]
- Neumann ID, Landgraf R. Balance of brain oxytocin and vasopressin: implications for anxiety, depression, and social behaviors. Trends Neurosci. 2012;35(11):649–659. doi: 10.1016/j.tins.2012.08.004. [DOI] [PubMed] [Google Scholar]
- Neumann ID, van den Burg EH. Oxytocin and vasopressin and their receptor-mediated intracellular pathways that determine their behavioral effects. In: Choleris E, Pfaff DW, Kavaliers M, editors. Oxytocin, vasopressin and related peptides in the regulation of behavior. Cambridge: Cambridge Press; 2013. pp. 27–43. [Google Scholar]
- Newman SW. The medial extended amygdala in male reproductive behavior. A node in the mammalian social behavior network. Ann N Y Acad Sci. 1999;877:242–257. doi: 10.1111/j.1749-6632.1999.tb09271.x. [DOI] [PubMed] [Google Scholar]
- O'Bryant EL, Wilczynski W. Changes in plasma testosterone levels and brain AVT cell number during the breeding season in the green treefrog. Brain Behav Evol. 2010;75(4):271–281. doi: 10.1159/000316084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connell LA, Hofmann HA. The vertebrate mesolimbic reward system and social behavior network: a comparative synthesis. J Comp Neurol. 2011;519(18):3599–3639. doi: 10.1002/cne.22735. [DOI] [PubMed] [Google Scholar]
- Ocampo DD, Lewicka M, Larhammar D. The oxytocin/vasopressin receptor family has at least five members in the gnathostome lineage, inclucing two distinct V2 subtypes. Gen Comp Endocrinol. 2012;175(1):135–143. doi: 10.1016/j.ygcen.2011.10.011. [DOI] [PubMed] [Google Scholar]
- Ophir AG. Towards meeting Tinbergen's challenge. Horm Behav. 2011;60(1):22–27. doi: 10.1016/j.yhbeh.2011.03.012. [DOI] [PubMed] [Google Scholar]
- Ophir AG, Wolff JO, Phelps SM. Variation in neural V1aR predicts sexual fidelity and space use among male prairie voles in semi-natural settings. Proc Natl Acad Sci U S A. 2008;105(4):1249–1254. doi: 10.1073/pnas.0709116105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panzica GC, Aste N, Castagna C, Viglietti-Panzica C, Balthazart J. Steroid-induced plasticity in the sexually dimorphic vasotocinergic innervation of the avian brain: behavioral implications. Brain Res Brain Res Rev. 2001;37(1–3):178–200. doi: 10.1016/s0165-0173(01)00118-7. [DOI] [PubMed] [Google Scholar]
- Panzica GC, Castagna C, Viglietti-Panzica C, Russo C, Tlemcani O, Balthazart J. Organizational effects of estrogens on brain vasotocin and sexual behavior in quail. J Neurobiol. 1998;37(4):684–699. doi: 10.1002/(sici)1097-4695(199812)37:4<684::aid-neu15>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Paul MJ, Terranova JI, Probst CK, Murray EK, Ismail NI, De Vries GJ. Sexually dimorphic role for vasopressin in the development of social play. Front Behav Neurosci. 2014;8:58. doi: 10.3389/fnbeh.2014.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer CA, Johnston RE. Socially stimulated androgen surges in male hamsters: The roles of vaginal secretions, behavioral interactions, and housing conditions. Horm Behav. 1992;26:283–293. doi: 10.1016/0018-506x(92)90048-z. [DOI] [PubMed] [Google Scholar]
- Phelps SM. From endophenotypes to evolution: social attachment, sexual fidelity and the avpr1a locus. Curr Opin Neurobiol. 2010;20(6):795–802. doi: 10.1016/j.conb.2010.09.002. [DOI] [PubMed] [Google Scholar]
- Phelps SM, Campbell P, Zheng DJ, Ophir AG. Beating the boojum: comparative approaches to the neurobiology of social behavior. Neuropharm. 2010;58(1):17–28. doi: 10.1016/j.neuropharm.2009.06.043. [DOI] [PubMed] [Google Scholar]
- Phelps SM, Young LJ. Extraordinary diversity in vasopressin (V1a) receptor distributions among wild prairie voles (Microtus ochrogaster): patterns of variation and covariation. J Comp Neurol. 2003;466(4):564–576. doi: 10.1002/cne.10902. [DOI] [PubMed] [Google Scholar]
- Pitkow LJ, Sharer CA, Ren X, Insel TR, Terwilliger EF, Young LJ. Facilitation of affiliation and pair-bond formation by vasopressin receptor gene transfer into the ventral forebrain of a monogamous vole. J Neurosci. 2001;21(18):7392–7396. doi: 10.1523/JNEUROSCI.21-18-07392.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planas B, Kolb PE, Raskind MA, Miller MA. Sex difference in coexpression by galanin neurons accounts for sexual dimorphism of vasopressin in the bed nucleus of the stria terminalis. Endocrinol. 1995a;136(2):727–733. doi: 10.1210/endo.136.2.7530652. [DOI] [PubMed] [Google Scholar]
- Planas B, Kolb PE, Raskind MA, Miller MA. Vasopressin and galanin mRNAs coexist in the nucleus of the horizontal diagonal band: a novel site of vasopressin gene expression. J Comp Neurol. 1995b;361(1):48–56. doi: 10.1002/cne.903610105. [DOI] [PubMed] [Google Scholar]
- Potegal M, Ferris CF. Intraspecific aggression in male hamsters is inhibited by intrahypothalamic vasopressin-receptor antagonist. Aggressive Behav. 1989;15:311–320. [Google Scholar]
- Pow DV, Morris JF. Dendrites of hypothalamic magnocellular neurons release neurohypophysial peptides by exocytosis. Neuroscience. 1989;32(2):435–439. doi: 10.1016/0306-4522(89)90091-2. [DOI] [PubMed] [Google Scholar]
- Qiao X, Yan Y, Wu R, Tai F, Hao P, Cao Y, Wang J. Sociality and oxytocin and vasopressin in the brain of male and female dominant and subordinate mandarin voles. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2014;200(2):149–159. doi: 10.1007/s00359-013-0870-2. [DOI] [PubMed] [Google Scholar]
- Ragen BJ, Bales KL. Oxytocin and vasopressin in non-human primates. In: Choleris E, Pfaff DW, Kavaliers M, editors. Oxytocin, Vasopressin and Related Peptides in the Regulation of Behavior. Cambridge: Cambridge University Press; 2013. pp. 288–306. [Google Scholar]
- Raggenbass M. Overview of cellular electrophysiological actions of vasopressin. Eur J Pharmacol. 2008;583(2–3):243–254. doi: 10.1016/j.ejphar.2007.11.074. [DOI] [PubMed] [Google Scholar]
- Rasri K, Mason P, Govitrapong P, Pevet P, Klosen P. Testosterone-driven seasonal regulation of vasopressin and galanin in the bed nucleus of the stria terminalis of the Djungarian hamster (Phodopus sungorus) Neuroscience. 2008;157(1):174–187. doi: 10.1016/j.neuroscience.2008.08.058. [DOI] [PubMed] [Google Scholar]
- Rilling JK, DeMarco AC, Hackett PD, Chen X, Gautam P, Stair S, Haroon E, Thompson R, Ditzen B, Patel R, Pagnoni G. Sex differences in the neural and behavioral response to intranasal oxytocin and vasopressin during human social interaction. Psychoneuroendocrinol. 2014;39:237–248. doi: 10.1016/j.psyneuen.2013.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ring RH. The central vasopressinergic system: examining the opportunities for psychiatric drug development. Curr Pharm Des. 2005;11(2):205–225. doi: 10.2174/1381612053382241. [DOI] [PubMed] [Google Scholar]
- Rood BD, De Vries GJ. Vasopressin innervation of the mouse (Mus musculus) brain and spinal cord. J Comp Neurol. 2011;519(12):2434–2474. doi: 10.1002/cne.22635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen GJ, De Vries GJ, Goldman SL, Goldman BD, Forger NG. Distribution of vasopressin in the brain of the eusocial naked mole-rat. J Comp Neurol. 2007;500(6):1093–1105. doi: 10.1002/cne.21215. [DOI] [PubMed] [Google Scholar]
- Rosen GJ, De Vries GJ, Villalba C, Weldele ML, Place NJ, Coscia EM, Glickman SE, Forger NG. Distribution of vasopressin in the forebrain of spotted hyenas. J Comp Neurol. 2006;498(1):80–92. doi: 10.1002/cne.21032. [DOI] [PubMed] [Google Scholar]
- Rosso L, Keller L, Kaessmann H, Hammond RL. Mating system and avpr1a promoter variation in primates. Biol Lett. 2008;4(4):375–378. doi: 10.1098/rsbl.2008.0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotzinger S, Lovejoy DA, Tan LA. Behavioral effects of neuropeptides in rodent models of depression and anxiety. Peptides. 2010;31(4):736–756. doi: 10.1016/j.peptides.2009.12.015. [DOI] [PubMed] [Google Scholar]
- Sabatier N. alpha-Melanocyte-stimulating hormone and oxytocin: a peptide signalling cascade in the hypothalamus. J Neuroendocrinol. 2006;18(9):703–710. doi: 10.1111/j.1365-2826.2006.01464.x. [DOI] [PubMed] [Google Scholar]
- Sabatier N, Caquineau C, Dayanithi G, Bull P, Douglas AJ, Guan XM, Jiang M, Van der PL, Leng G. Alpha-melanocyte-stimulating hormone stimulates oxytocin release from the dendrites of hypothalamic neurons while inhibiting oxytocin release from their terminals in the neurohypophysis. J Neurosci. 2003;23(32):10351–10358. doi: 10.1523/JNEUROSCI.23-32-10351.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachser N. Of domestic and wild guinea pigs: studies in sociophysiology, domestication, and social evolution. Naturwissenschaften. 1998;85(7):307–317. doi: 10.1007/s001140050507. [DOI] [PubMed] [Google Scholar]
- Sala M, Braida D, Lentini D, Busnelli M, Bulgheroni E, Capurro V, Finardi A, Donzelli A, Pattini L, Rubino T, Parolaro D, Nishimori K, Parenti M, Chini B. Pharmacologic rescue of impaired cognitive flexibility, social deficits, increased aggression, and seizure susceptibility in oxytocin receptor null mice: a neurobehavioral model of autism. Biol Psychiatry. 2011;69(9):875–882. doi: 10.1016/j.biopsych.2010.12.022. [DOI] [PubMed] [Google Scholar]
- Salio C, Lossi L, Ferrini F, Merighi A. Neuropeptides as synaptic transmitters. Cell Tissue Res. 2006;326(2):583–598. doi: 10.1007/s00441-006-0268-3. [DOI] [PubMed] [Google Scholar]
- Sawchenko PE, Swanson LW. Immunohistochemical identification of neurons in the paraventricular nucleus of the hypothalamus that project to the medulla or to the spinal cord in the rat. J Comp Neurol. 1982;205(3):260–272. doi: 10.1002/cne.902050306. [DOI] [PubMed] [Google Scholar]
- Schorscher-Petcu A, Dupre A, Tribollet E. Distribution of vasopressin and oxytocin binding sites in the brain and upper spinal cord of the common marmoset. Neurosci Lett. 2009;461(3):217–222. doi: 10.1016/j.neulet.2009.06.016. [DOI] [PubMed] [Google Scholar]
- Schorscher-Petcu A, Sotocinal S, Ciura S, Dupre A, Ritchie J, Sorge RE, Crawley JN, Hu SB, Nishimori K, Young LJ, Tribollet E, Quirion R, Mogil JS. Oxytocin-induced analgesia and scratching are mediated by the vasopressin-1A receptor in the mouse. J Neurosci. 2010;30(24):8274–8284. doi: 10.1523/JNEUROSCI.1594-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz KM, Menard TA, Smith DA, Albers HE, Sisk CL. Testicular hormone exposure during adolescence organizes flank-marking behavior and vasopressin receptor binding in the lateral septum. Horm Behav. 2006;50(3):477–483. doi: 10.1016/j.yhbeh.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Schwartz WJ, Reppert SM. Neural regulation of the circadian vasopressin rhythm in cerebrospinal fluid: A pre-eminent role for the suprachiasmatic nuclei. J Neurosci. 1985;5:2771–2778. doi: 10.1523/JNEUROSCI.05-10-02771.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Searcy BT, Bradford CS, Thompson RR, Filtz TM, Moore FL. Identification and characterization of mesotocin and V1a-like vasotocin receptors in a urodele amphibian, Taricha granulosa. Gen Comp Endocrinol. 2011;170(1):131–143. doi: 10.1016/j.ygcen.2010.09.017. [DOI] [PubMed] [Google Scholar]
- Semsar K, Godwin J. Social influences on the arginine vasotocin system are independent of gonads in a sex-changing fish. J Neurosci. 2003;23(10):4386–4393. doi: 10.1523/JNEUROSCI.23-10-04386.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semsar K, Godwin J. Multiple mechanisms of phenotype development in the bluehead wrasse. Horm Behav. 2004;45(5):345–353. doi: 10.1016/j.yhbeh.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Semsar K, Kandel FL, Godwin J. Manipulations of the AVT system shift social status and related courtship and aggressive behavior in the bluehead wrasse. Horm Behav. 2001;40(1):21–31. doi: 10.1006/hbeh.2001.1663. [DOI] [PubMed] [Google Scholar]
- Serradeil-Le Gal C, Raufaste D, Derick S, Blankenstein J, Allen J, Pouzet B, Pascal M, Wagnon J, Ventura MA. Biological characterization of rodent and human vasopressin V1b receptors using SSR-149415, a nonpeptide V1b receptor ligand. Am J Physiol Regul Integr Comp Physiol. 2007;293(2):R938–R949. doi: 10.1152/ajpregu.00062.2007. [DOI] [PubMed] [Google Scholar]
- Smeets WJ, Gonzalez A. Vasotocin and mesotocin in the brains of amphibians: state of the art. Microsc Res Tech. 2001;54(3):125–136. doi: 10.1002/jemt.1128. [DOI] [PubMed] [Google Scholar]
- Smeltzer MD, Curtis JT, Aragona BJ, Wang Z. Dopamine, oxytocin, and vasopressin receptor binding in the medial prefrontal cortex of monogamous and promiscuous voles. Neurosci Lett. 2006;394(2):146–151. doi: 10.1016/j.neulet.2005.10.019. [DOI] [PubMed] [Google Scholar]
- Stark H, Burbach JP, Van der Kleij AA, De Wied D. In vivo conversion of vasopressin after microinjection into limbic brain areas of rats. Peptides. 1989;10(4):717–720. doi: 10.1016/0196-9781(89)90102-2. [DOI] [PubMed] [Google Scholar]
- Stevenson EL, Caldwell HK. The vasopressin 1b receptor and the neural regulation of social behavior. Horm Behav. 2012;61(3):277–282. doi: 10.1016/j.yhbeh.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoop R. Neuromodulation by oxytocin and vasopressin. Neuron. 2012;76(1):142–159. doi: 10.1016/j.neuron.2012.09.025. [DOI] [PubMed] [Google Scholar]
- Szot P, Bale TL, Dorsa DM. Distribution of messenger RNA for the vasopressin V1a receptor in the CNS of male and female rats. Brain Res Mol Brain Res. 1994;24(1–4):1–10. doi: 10.1016/0169-328x(94)90111-2. [DOI] [PubMed] [Google Scholar]
- Tan FL, Lolait SJ, Brownstein MJ, Saito N, MacLeod V, Baeyens DA, Mayeux PR, Jones SM, Cornett LE. Molecular cloning and functional characterization of a vasotocin receptor subtype that is expressed in the shell gland and brain of the domestic chicken. Biol Reprod. 2000;62(1):8–15. doi: 10.1095/biolreprod62.1.8. [DOI] [PubMed] [Google Scholar]
- Taylor PV, Veenema AH, Paul MJ, Bredewold R, Isaacs S, de Vries GJ. Sexually dimorphic effects of a prenatal immune challenge on social play and vasopressin expression in juvenile rats. Biol Sex Differ. 3(1):15. doi: 10.1186/2042-6410-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telleria-Diaz A, Grinevich VV, Jirikowski GF. Colocalization of vasopressin and oxytocin in hypothalamic magnocellular neurons in water-deprived rats. Neuropep. 2001;35(3–4):162–167. doi: 10.1054/npep.2001.0859. [DOI] [PubMed] [Google Scholar]
- Terrillon S, Cheng LL, Stoev S, Mouillac B, Barberis C, Manning M, Durroux T. Synthesis and characterization of fluorescent antagonists and agonists for human oxytocin and vasopressin V(1)(a) receptors. J Med Chem. 2002;45(12):2579–2588. doi: 10.1021/jm010526+. [DOI] [PubMed] [Google Scholar]
- Thibonnier M, Preston JA, Dulin N, Wilkins PL, Berti-Mattera LN, Mattera R. The human V3 pituitary vasopressin receptor: ligand binding profile and density-dependent signaling pathways. Endocrinol. 1997;138(10):4109–4122. doi: 10.1210/endo.138.10.5432. [DOI] [PubMed] [Google Scholar]
- Thompson RR, Walton JC. Social regulatory functions of vasotocin and isotocin in fish. In: Choleris E, Pfaff DW, Kavaliers M, editors. Oxytocin, Vasopressin and Related Peptides in the Regulation of Behavior. Cambridge: Cambridge University Press; 2013. pp. 75–96. [Google Scholar]
- Tribollet E, Audigier S, Dubois-Dauphin M, Dreifuss JJ. Gonadal steroids regulate oxytocin receptors but not vasopressin receptors in the brain of male and female rats. An autoradiographical study. Brain Res. 1990;511(1):129–140. doi: 10.1016/0006-8993(90)90232-z. [DOI] [PubMed] [Google Scholar]
- Tribollet E, Barberis C, Arsenijevic Y. Distribution of vasopressin and oxytocin receptors in the rat spinal cord: sex-related differences and effect of castration in pudendal motor nuclei. Neuroscience. 1997;78(2):499–509. doi: 10.1016/s0306-4522(96)00591-x. [DOI] [PubMed] [Google Scholar]
- Tripp SK, Moore FL. Autoradiographic characterization of binding sites labelled with vasopressin in the brain of a urodele amphibian. Neuroendocrinol. 1988;48(1):87–92. doi: 10.1159/000124994. [DOI] [PubMed] [Google Scholar]
- Trueta C, De Miguel FF. Extrasynaptic exocytosis and its mechanisms: a source of molecules mediating volume transmission in the nervous system. Front Physiol. 2012;3:319. doi: 10.3389/fphys.2012.00319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner LM, Young AR, Rompler H, Schoneberg T, Phelps SM, Hoekstra HE. Monogamy evolves through multiple mechanisms: evidence from V1aR in deer mice. Mol Biol Evol. 2010;27(6):1269–1278. doi: 10.1093/molbev/msq013. [DOI] [PubMed] [Google Scholar]
- van Leeuwen FW, Swaab DF, de Raay C. Immunoelectronmicroscopic localization of vasopressin in the rat suprachiasmatic nucleus. Cell Tiss Res. 1978;193:1–10. doi: 10.1007/BF00221596. [DOI] [PubMed] [Google Scholar]
- van den Pol AA. Neuropeptide transmission in brain circuits. Neuron. 76:98–115. doi: 10.1016/j.neuron.2012.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas KJ, Sarmiento JM, Ehrenfeld P, Anazco CC, Villanueva CI, Carmona PL, Brenet M, Navarro J, Muller-Esterl W, Gonzalez CB. Postnatal expression of V2 vasopressin receptor splice variants in the rat cerebellum. Differentiation. 2009;77(4):377–385. doi: 10.1016/j.diff.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenema AH, Bredewold R, De Vries GJ. Sex-specific modulation of juvenile social play by vasopressin. Psychoneuroendocrinol. 2013;38(11):2554–2561. doi: 10.1016/j.psyneuen.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veinante P, Freund-Mercier MJ. Distribution of oxytocin- and vasopressin-binding sites in the rat extended amygdala: a histoautoradiographic study. J Comp Neurol. 1997;383(3):305–325. [PubMed] [Google Scholar]
- Viviani D, Stoop R. Opposite effects of oxytocin and vasopressin on the emotional expression of the fear response. In: Neumann ID, Landgraf R, editors. Advances in Vasopressin and Oxytocin From Genes to Behaviour to Disease. Amsterdam: Elsevier; 2008. pp. 207–218. [DOI] [PubMed] [Google Scholar]
- Vizi ES, Fekete A, Karoly R, Mike A. Non-synaptic receptors and transporters involved in brain functions and targets of drug treatment. Brit J Pharmacol. 2010;160:785–809. doi: 10.1111/j.1476-5381.2009.00624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voorhuis TA, De Kloet ER. Immunoreactive vasotocin in the zebra finch brain (Taeniopygia guttata) Brain Res Dev Brain Res. 1992;69(1):1–10. doi: 10.1016/0165-3806(92)90116-e. [DOI] [PubMed] [Google Scholar]
- Voorhuis TA, De Kloet ER, De Wied D. The distribution and plasticity of [3H]vasopressin-labelled specific binding sites in the canary brain. Brain Res. 1988;457(1):148–153. doi: 10.1016/0006-8993(88)90067-4. [DOI] [PubMed] [Google Scholar]
- Voorhuis TA, Elands JP, Kloet ER. Vasotocin target sites in the capsular region surrounding the nucleus robustus archistriatalis of the canary brain. J Neuroendocrinol. 1990;2(5):653–657. doi: 10.1111/j.1365-2826.1990.tb00461.x. [DOI] [PubMed] [Google Scholar]
- Walton JC, Waxman B, Hoffbuhr K, Kennedy M, Beth E, Scangos J, Thompson RR. Behavioral effects of hindbrain vasotocin in goldfish are seasonally variable but not sexually dimorphic. Neuropharm. 2010;58(1):126–134. doi: 10.1016/j.neuropharm.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walum H, Westberg L, Henningsson S, Neiderhiser JM, Reiss D, Igl W, Ganiban JM, Spotts EL, Pedersen NL, Eriksson E, Lichtenstein P. Genetic variation in the vasopressin receptor 1a gene (AVPR1A) associates with pair-bonding behavior in humans. Proc Natl Acad Sci U S A. 2008;105(37):14153–14156. doi: 10.1073/pnas.0803081105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z. Species differences in the vasopressin-immunoreactive pathways in the bed nucleus of the stria terminalis and medial amygdaloid nucleus in prairie voles (Microtus ochrogaster) and meadow voles (Microtus pennsylvanicus) Behav Neurosci. 1995;109(2):305–311. doi: 10.1037//0735-7044.109.2.305. [DOI] [PubMed] [Google Scholar]
- Wang Z, Ferris CF, De Vries GJ. The role of septal vasopressin innervation in paternal behavior in prairie voles (Microtus ochrogaster) Proc Natl Sci. 1994a;91:400–404. doi: 10.1073/pnas.91.1.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Moody K, Newman JD, Insel TR. Vasopressin and oxytocin immunoreactive neurons and fibers in the forebrain of male and female common marmosets (Callithrix jacchus) Syn. 1997a;27(1):14–25. doi: 10.1002/(SICI)1098-2396(199709)27:1<14::AID-SYN2>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Wang Z, Smith W, Major DE, De Vries GJ. Sex and species differences in the effects of cohabitation on vasopressin messenger RNA expression in the bed nucleus of the stria terminalis in prairie voles (Microtus ochrogaster) and meadow voles (Microtus pennsylvanicus) Brain Res. 1994b;650(2):212–218. doi: 10.1016/0006-8993(94)91784-1. [DOI] [PubMed] [Google Scholar]
- Wang Z, Toloczko D, Young LJ, Moody K, Newman JD, Insel TR. Vasopressin in the forebrain of common marmosets (Callithrix jacchus): studies with in situ hybridization, immunocytochemistry and receptor autoradiography. Brain Res. 1997b;768(1–2):147–156. doi: 10.1016/s0006-8993(97)00636-7. [DOI] [PubMed] [Google Scholar]
- Whitman DC, Albers HE. Role of oxytocin in the hypothalamic regulation of sexual receptivity in hamsters. Brain Res. 1995;680:73–79. doi: 10.1016/0006-8993(95)00233-g. [DOI] [PubMed] [Google Scholar]
- Whitman DC, Albers HE. Oxytocin immunoreactivity in the hypothalamus of female hamsters. Cell Tissue Res. 1998;291(2):231–237. doi: 10.1007/s004410050993. [DOI] [PubMed] [Google Scholar]
- Wilczynski W, Lynch KS, O'Bryant EL. Current research in amphibians: studies integrating endocrinology, behavior, and neurobiology. Horm Behav. 2005;48(4):440–450. doi: 10.1016/j.yhbeh.2005.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingfield JC, Ball GF, Dufty AM, Hegner RE, Ramenofsky M. Testosterone and aggression in birds. American Scientist. 1987;75:602–608. [Google Scholar]
- Wingfield JC, Hegner RE, Dufty AMJ, Ball GF. The "Challenge Hypothesis": Theoretical implications for patterns of testosterone secretion, mating systems, and breeding strategies. The American Naturalist. 1990;136(6):829–846. [Google Scholar]
- Winslow JT, Hastings N, Carter CS, Harbaugh C, Insel TR. A role for central vasopressin in pair bonding in monogamous prairie voles. Nature. 1993;365:545–548. doi: 10.1038/365545a0. [DOI] [PubMed] [Google Scholar]
- Witt DM, Carter CS, Lnsel TR. Oxytocin receptor binding in female prairie voles: endogenous and exogenous oestradiol stimulation. J Neuroendocrinol. 1991;3(2):155–161. doi: 10.1111/j.1365-2826.1991.tb00258.x. [DOI] [PubMed] [Google Scholar]
- Woolley SC, Sakata JT, Crews D. Evolutionary insights into the regulation of courtship behavior in male amphibians and reptiles. Physiol Behav. 2004;83(2):347–360. doi: 10.1016/j.physbeh.2004.08.021. [DOI] [PubMed] [Google Scholar]
- Xi D, Kusano K, Gainer H. Quantitative analysis of oxytocin and vasopressin messenger ribonucleic acids in single magnocellular neurons isolated from supraoptic nucleus of rat hypothalamus. Endocrinol. 1999;140(10):4677–4682. doi: 10.1210/endo.140.10.7054. [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y, Kaiya H, Konno N, Iwata E, Miyazato M, Uchiyama M, Bell JD, Toop T, Donald JA, Brenner S, Venkatesh B, Hyodo S. The fifth neurohypophysial hormone receptor is structurally related to the V2-type receptor but functionally similar to V1-type receptors. Gen Comp Endocrinol. 2012;178(3):519–528. doi: 10.1016/j.ygcen.2012.07.008. [DOI] [PubMed] [Google Scholar]
- Yamashita K, Kitano T. Molecular evolution of the oxytocin-oxytocin receptor system in eutherians. Mol Phylogenet Evol. 2013;67(2):520–528. doi: 10.1016/j.ympev.2013.02.017. [DOI] [PubMed] [Google Scholar]
- Yirmiya N, Rosenberg C, Levi S, Salomon S, Shulman C, Nemanov L, Dina C, Ebstein RP. Association between the arginine vasopressin 1a receptor (AVPR1a) gene and autism in a family-based study: mediation by socialization skills. Mol Psychiatry. 2006;11(5):488–494. doi: 10.1038/sj.mp.4001812. [DOI] [PubMed] [Google Scholar]
- Young LJ. Frank A. Beach Award. Oxytocin and vasopressin receptors and species-typical social behaviors. Horm Behav. 1999;36(3):212–221. doi: 10.1006/hbeh.1999.1548. [DOI] [PubMed] [Google Scholar]
- Young LJ, Nilsen R, Waymire KG, MacGregor GR, Insel TR. Increased affiliative response to vasopressin in mice expressing the V1a receptor from a monogamous vole. Nature. 1999a;400:766–768. doi: 10.1038/23475. [DOI] [PubMed] [Google Scholar]
- Young LJ, Toloczko D, Insel TR, et al. Localization of vasopressin (V1a) receptor binding and mRNA in the rhesus monkey brain. J Neuroendocrinol. 1999b;11(4):291–297. doi: 10.1046/j.1365-2826.1999.00332.x. [DOI] [PubMed] [Google Scholar]
- Young LJ, Wang Z. The neurobiology of pair bonding. Nat Neurosci. 2004;7(10):1048–1054. doi: 10.1038/nn1327. [DOI] [PubMed] [Google Scholar]
- Young LJ, Wang Z, Cooper TT, Albers HE. Vasopressin receptor (V1a) in the hamster brain: synthesis, transport and transcriptional regulation by androgen. J Neuroendocrinol. 2000;12:1179–1185. doi: 10.1046/j.1365-2826.2000.00573.x. [DOI] [PubMed] [Google Scholar]
- Young LJ, Winslow JT, Nilsen R, Insel TR. Species differences in V1a receptor gene expression in monogamous and nonmonogamous voles: behavioral consequences. Behav Neurosci. 1997;111(3):599–605. doi: 10.1037//0735-7044.111.3.599. [DOI] [PubMed] [Google Scholar]
- Young WS, Li J, Wersinger SR, Palkovits M. The vasopressin 1b receptor is prominent in the hippocampal area CA2 where it is unaffected by restraint stress or adrenalectomy. Neuroscience. 2006;143(4):1031–1039. doi: 10.1016/j.neuroscience.2006.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng DJ, Larsson B, Phelps SM, Ophir AG. Female alternative mating tactics, reproductive success and nonapeptide receptor expression in the social decision-making network. Behav Brain Res. 2013;246:139–147. doi: 10.1016/j.bbr.2013.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]