Abstract
Objective
Vitamin D has been linked to anti-Müllerian hormone levels, suggesting a possible association with greater ovarian reserve, but large population-based studies are lacking. Our objective was to explore the association between vitamin D and FSH in premenopausal women.
Methods
The Uterine Fibroid Study (1996 – 1999) enrolled randomly-selected 30 – 49 year-old members of a Washington D. C. health plan (N=1430). Women provided a blood and urine sample in addition to questionnaire data. The vitamin D metabolite 25-hydroxyvitamin D (25(OH)D) was measured in stored plasma samples. Urinary FSH (mIU/mg creatinine) was measured with an immunofluorometric assay. To obtain baseline measures, this investigation was limited to urine samples collected in the first 5 days of the menstrual cycle or 5 days prior to menses onset. Additionally, post-menopausal women and women using oral contraceptives were excluded, leaving 527 women in our analysis. FSH was creatinine-adjusted, normalized by log-transformation, and then modeled with multivariable linear regression.
Results
The median 25(OH)D level was 12 ng/mL, with approximately 75% of participants below the recommended level of 20 ng/mL. FSH and 25(OH)D were inversely related. For an increase of 10 ng/mL in 25(OH)D, urinary FSH decreased 14% (95% Confidence Interval: −23%, −5%), p=0.003.
Conclusions
Vitamin D is inversely related to FSH. This is consistent with literature relating low vitamin D with lower anti-Müllerian hormone. Prospective studies should investigate whether low levels of vitamin D contribute to decreased ovarian reserve.
Keywords: menopause, FSH, AMH, fecundability, fertility
Introduction
Vitamin D is known for its role in bone health1, but its role in reproduction is an active area of investigation2–4. Vitamin D receptors are expressed in the ovary, placenta, and the uterus2–4. Lower Vitamin D has been related to premenstrual syndrome, uterine fibroids5, dysmenorrhea and early menarche4.
Vitamin D deficiency has been associated with dramatically reduced fertility in both rats and mice when diet interventions reduce levels2,6. Female knock-out mice with no functional vitamin D receptor showed hypergonadotropic hypogonadism, a condition characterized by high levels of follicle stimulating hormone (FSH) and low levels of estrogen. Low levels of 25(OH)D have also been associated with primary ovarian insufficiency in humans7. Data from prostate cancer cells show that the AMH promoter region contains a vitamin D response element8.
Given these studies, we hypothesized that vitamin D may be important for maintaining the health of primordial follicles, or limiting excess recruitment from the primordial follicle pool, and thus, depletion of ovarian reserve. Early follicular-phase follicle stimulating hormone (FSH), which can be measured in blood or urine, is a biomarker of ovarian reserve which rises across the late reproductive lifespan and is inversely related to AMH9. We theorized that vitamin D, measured as serum 25-hydroxyvitamin D (25(OH)D), would be inversely correlated with FSH in late reproductive-age women.
Methods
Study sample
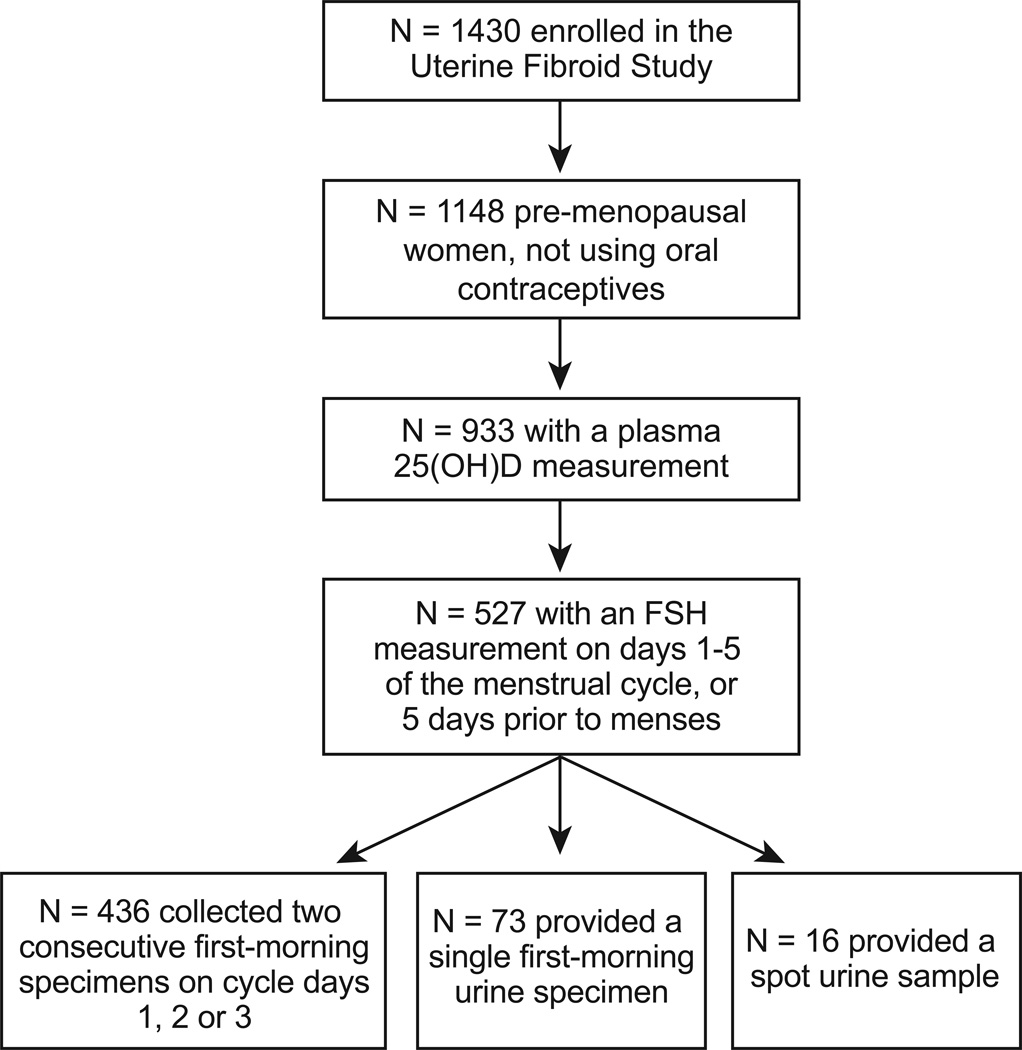
The National Institute of Environmental Health Sciences (NIEHS) Uterine Fibroid Study, 1996 – 1999, enrolled participants identified from a large health plan in Washington, DC5,10,11. In brief, randomly selected health plan members between the ages of 30 and 49 were contacted and 80% of those eligible participated (N=1430). For this analysis, only women who were pre-menopausal and not currently using oral contraceptives were included (N=1148) (Figure 1). Women were invited to the primary care site for an in-person study visit that included a blood draw. Blood samples were processed and stored at −80C.
Figure 1.
Flowchart showing the number of women in the Uterine Fibroid Study included in the analysis of 25(OH)D and urinary FSH level during the early follicular or late luteal phase of the menstrual cycle.
Vitamin D measurement
Vitamin D status was ascertained through the measurement of the circulating metabolite 25-hydroxyvitamin D (25(OH)D) in stored plasma samples. 25(OH)D is a widely accepted biomarker for vitamin D12. 25(OH)D was measured by radioimmunoassay13 at a laboratory that has been certified by the international Vitamin D External Quality Assessment Scheme for the past 12 years (intra- and interassay coefficients of variation were 7.6 and 10.6%5). The antibody was co-specific for both 25(OH)D2 and 25(OH)D3. One hundred and fifty-five women were missing a 25(OH)D measurement, most of whom had no available blood sample. This left 993 available for analysis of vitamin D.
FSH measurement
We measured FSH in urine. Urinary FSH is highly correlated with serum FSH (r=0.9, p<0.0114) and it is much easier to obtain urine samples timed to the menstrual cycle than timed blood samples because women can collect their own urine at home. The first approximately 600 pre-menopausal women enrolled in the study were asked to collect first-morning urine samples on the 2nd and 3rd days of their menstrual cycle. Samples were refrigerated and then shipped overnight in a cold-storage kit with freezer packs (provided by the study) to the study site in North Carolina. Upon arrival, equal aliquots from each day were pooled. Women who were not asked to collect menstrually-timed urine samples instead collected a first-morning sample on the day of their study clinic visit. Women who attended the clinic visit without bringing a first-morning urine sample were asked to provide a spot sample. Glycerol was added to all urine samples, which were then stored at −80C. Menstrual cycle day was reported at the clinic visit and in menstrual diary data collected during the menstrual cycle following the visit.
Urinary FSH (mIU/mg creatinine) was measured in duplicate using a noncompetitive, time-resolved immunofluorometric assay15. Creatinine was measured spectrophotometrically. Within- and between-assay percent coefficients of variation were 3.1% and 1.1% for FSH and 2.2% and 4.2% for creatinine. To avoid the mid-cycle peak of FSH we used FSH information for women whose urine sample was collected during the first 5 days of the menstrual cycle or 5 days prior to the onset of the subsequent menses (N=527) (Figure 1). Of these 527 women, 436 women used the urine collection kit on days 2 and 3 of the menstrual cycle to collect two first-morning urine samples that were subsequently pooled. Of the remaining women, single first-morning urine specimens were provided by 73 women while 16 provided a spot urine sample at their study visit. Prior to statistical analysis creatinine-adjusted FSH was log-transformed to achieve normality.
Covariates
Data on covariates were collected through a self-administered questionnaire and a telephone interview. Body weight was measured at the clinic visit. Potential confounders considered included: age, education, race, body mass index (BMI), alcohol intake, cigarette smoking, physical activity, age at menarche, gravidity, mother’s age at menopause, menstrual cycle day of urine collection and season of blood draw (winter vs. other).
Statistical analysis
The association between 25(OH)D and the natural log of FSH was analyzed through multivariable linear regression. Regression coefficients from this model were first multiplied by 10 corresponding to a 10 ng/mL increase in 25(OH)D, exponentiated, and then multiplied by 100 and are presented as the percent change in FSH.
The analysis of FSH was adjusted at first for only age, race, and cycle day of urine collection, “Model 1”. A second, fully-adjusted model, “Model 2,” included the previous variables and BMI, current smoking, and season of blood draw. Adjustment for education, alcohol intake, gravidity, and participant’s mother’s age at menopause did not alter the results and were excluded from the final model. Sensitivity analyses were performed to compare the association among women who collected urine only on days two or three of the menstrual cycle (N=436) with the sample as a whole (N=527) (Figure 1). Of the 527 women in the complete sample, 7 women reported having had one ovary removed (one woman was missing this information) and 11 self-reported a diagnosis of polycystic ovary syndrome (PCOS). In a sensitivity analysis, results were unchanged after excluding these women.
Results
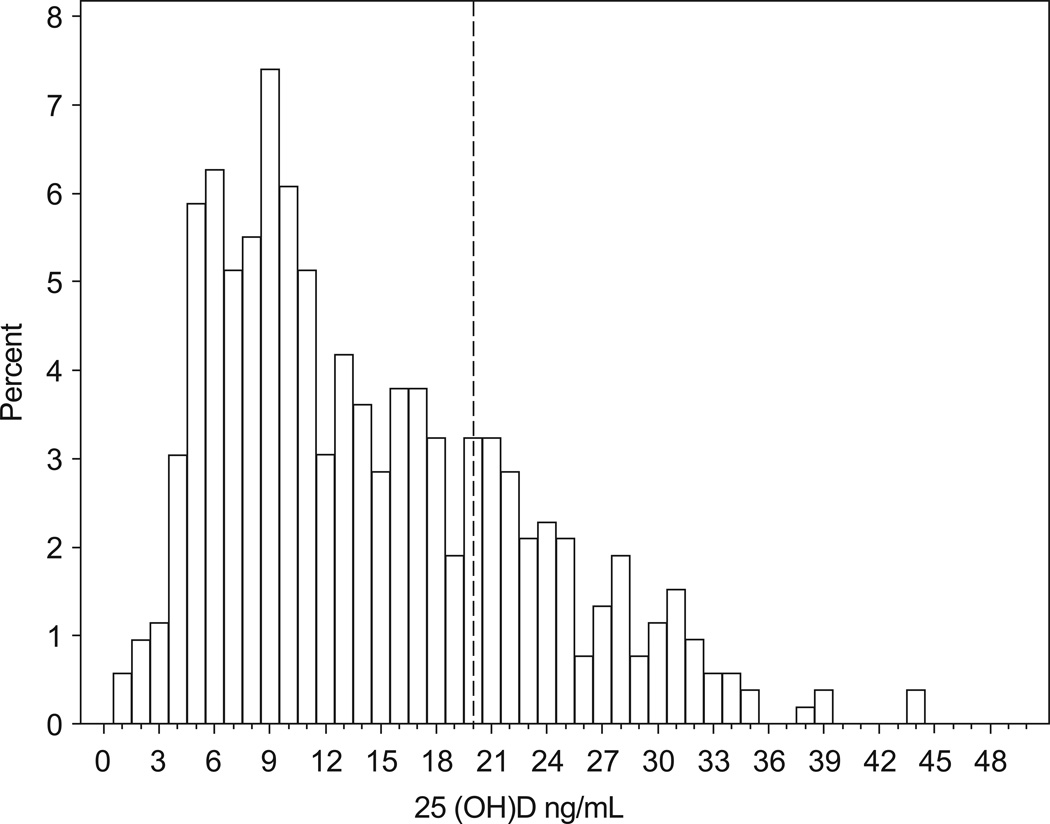
Participants were between the ages 35 and 51 at the time of the blood draw, with a mean and median age of 42 years (Table 1). The majority of women were of black race, a little over half had at least a college degree, and almost 80% had been pregnant at least once. Approximately 60% of the women were overweight or obese, about 80% of the women were non-smokers and about half of the women drank no more than one drink per week on average. The mean 25(OH)D level was 14.3 ng/mL (standard error: 0.36), with a median of 12.3 ng/mL (interquartile range: 7.8, 20.1 ng/mL) (Figure 2). Approximately 75% of the women had a 25(OH)D level below the IOM recommendation of 20 ng/mL for “adequate” vitamin D.
Table 1.
Characteristics of participants in the Uterine Fibroid Study who were included in the analysis of 25(OH)D and FSH (N = 527), Washington D.C. (1996 – 1999).
| Mean (SE) | |
|---|---|
| FSH, mIU/mg CRT (mean (SE)) | 11.5 (0.36) |
| N (%) | |
| Age | |
| 35 – 39 | 160 (30) |
| 40 – 44 | 199 (38) |
| 45 – 51 | 168 (32) |
| Race | |
| White | 197 (37) |
| Black | 298 (57) |
| Other | 32 (6) |
| Education | |
| High school graduate or less | 63 (12) |
| Some college or technical school | 176 (33) |
| College degree or some graduate school | 141 (27) |
| Post-graduate degree | 145 (28) |
| Missing | 2 (0.4) |
| Gravidity | |
| 0 | 109 (21) |
| 1, 2 | 173 (33) |
| ≥ 3 | 245 (46) |
| Body mass index | |
| < 25 kg/m2 | 214 (41) |
| 25 – < 30 | 144 (27) |
| 30 – <40 | 129 (24) |
| ≥40 | 40 (8) |
| Current Smoker | 103 (20) |
| Alcohol (drinks per week) | |
| 0 | 106 (20) |
| > 0 – 1 | 157 (30) |
| > 1 – 7 | 172 (33) |
| > 7 | 65 (12) |
| Missing | 27 (5) |
| Age at menarche | |
| <11 years | 45 (9) |
| 11 – 14 | 428 (81) |
| >14 years | 53 (10) |
| Missing | 1 (0.2) |
| Mother’s age at menopause | |
| 19 – 49 | 150 (28) |
| 50 – 65 | 127 (24) |
| Missing | 193 (37) |
Figure 2.
Distribution of plasma 25-hydroxyvitamin D status among women with an early follicular or late luteal measure of urinary FSH (n=527). A vertical line is drawn at 20 ng/mL, the IOM recommendation for adequate 25(OH)D.
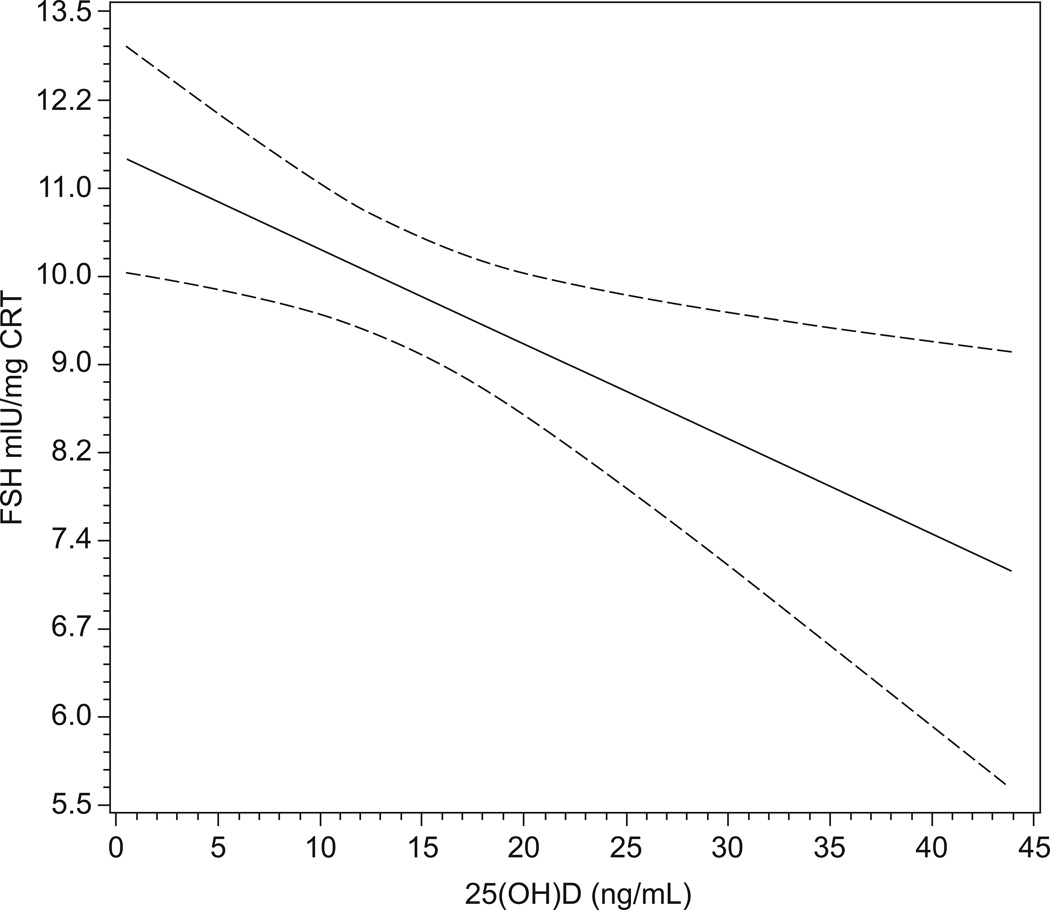
In Model 1, there was a highly significant inverse association between 25(OH)D and FSH levels, with a 10 ng/mL decrease in 25(OH)D associated with an approximate 10% increase in FSH (Table 2, Figure 3). Adjustment for all covariates increased the estimate to 14% (Table 2). Limiting the sample to women who collected pooled urine samples only on days 1, 2, or 3 of the menstrual cycle did not substantially alter these associations (Table 2). Excluding women with diabetes (N=17) also did not alter these associations (data not shown). The adjusted association was slightly stronger for women less than 40 years of age (Percent change (CI): −19% (−33, −6), p=0.007) compared with women at least 40 years of age (Percent change (CI): −12% (−24, −.1), p=0.05). Ninety percent of black women had a 25(OH)D below 20 ng/mL compared with 50% of the white women. The adjusted association of 25(OH)D and FSH was stronger among black women (Percent change (CI): −18% (−31, −4), p=0.01), compared with White women (Percent change (CI): −9% (−23, 4), p=0.18), although a test of the multiplicative interaction term between race and 25(OH)D in the model was not significant (p=0.30).
Table 2.
Results of the adjusted linear regression models estimating the association between plasma 25(OH)D with FSH, Uterine Fibroid Study, Washington D.C. (1996 – 1999).
| Percent change (95% CI) per 10 ng/mL increase in 25(OH)D | |||||
|---|---|---|---|---|---|
| N | Model 1a | p-value | Mode 2b | p-value | |
| FSH | 527 | −10 (−19, −2) | 0.02 | −14 (−23, −5) | 0.003 |
| FSHc | 436 | −9 (−19, 0.1) | 0.05 | −13 (−23, −3) | 0.01 |
Adjusted for age, race and cycle day of urine collection only
Adjusted for: age, race, cycle day of urine collection, BMI, current smoking, and season of blood draw.
Among women who collected pooled urine specimens on days 2 or 3 of the menstrual cycle
Figure 3.
Results of the unadjusted linear regression model of 25(OH)D predicting the natural log of FSH (solid line) (and predicted 95% confidence bands (dotted lines)) among 436 women who collected urine on cycle days 2 and 3 of the menstrual cycle. The y-axis tick marks are spaced on the natural log scale, but labeled on the absolute scale, for ease of interpretation.
Discussion
Lower plasma 25(OH)D was associated with higher FSH levels, a biomarker of ovarian reserve, which suggests a decrease in primordial follicles and possible acceleration towards menopause. Previous studies examining the relationship between Vitamin D and ovarian reserve have measured AMH levels. AMH, which is produced by the granulosa cells of preantral and early antral follicles, appears to be an important regulator of follicle development16. Serum levels of AMH have also been shown to be an indicator of ovarian reserve9,17. Human studies have reported a positive correlation between serum 25-hydroxyvitamin D (25(OH)D) and AMH18,19. One of these studies involved a unique cohort of women participating in an HIV-infection study and reported that the association was limited to women over 40 years of age19. The other publication describes an intervention study in New Zealand that included only 33 women18. The authors report that AMH varied across seasons and that supplementation with cholecalciferol (vitamin D3) prevented seasonal AMH changes18. This would suggest that vitamin D may affect the preantral or antral follicle pool, which can fluctuate in size, but not the primordial follicles.
Age of menopause has been associated with sun exposure in two studies from Turkish populations20,21 and in a study of Italian nuns22. In all three studies, women whose lifelong sun exposure was estimated to be low (due to sun avoidance, covered dressing, or a lack of outdoor activity) had an earlier age at menopause. It is possible that vitamin D is a mechanism underlying this association.
The ability to produce vitamin D in the skin is dependent upon skin pigmentation,23 and pigmentation varies with race/ethnicity. In the United States, vitamin D levels are correspondingly lower among African-Americans compared with non-Hispanic Whites24. However, ethnic groups differ on many factors, and the data on age at menopause and race/ethnicity are mixed25–30.
The mechanism by which vitamin D may affect ovarian reserve is unknown. Ovarian reserve is susceptible to toxicants such as tobacco smoke31. The smoking effect on ovarian reserve seems to be most evident during the perimenopausal years, as evidenced by the fact that only current smoking during the late reproductive and peri-menopause appears to affect age at menopause32,33. In contrast, the current study found that the inverse relationship between FSH and vitamin D appeared to be somewhat stronger in the younger participants. This suggests the possibility that chronic low vitamin D levels might have a more continuous adverse effect on ovarian reserve.
This analysis incorporates a population-based sample and a larger sample size (N=527) compared with the two previous AMH studies (N=388 and 33). This study is also strengthened by its use of a plasma 25(OH)D measure performed by a well-recognized expert and developer of the assay12. The lab has participated in and been certified by the DEQAS survey for the past 12 years. The intra and inter assay variation is <10 % based on laboratory controls. Most of the women in this study had inadequate 25(OH)D levels based on the IOM standard. This improves the power of the study to detect an association between low vitamin D and FSH, although it may also limit the generalizability of our results to more vitamin D replete populations. Similarly, most of this population was of Black race and over 35 years of age which limits our generalizability to women of other races and younger women. This study included thorough collection of menstrual cycle information which minimizes misclassification of the FSH measure. FSH was measured in urine rather than in blood, which may limit its clinical interpretation. However, the correlation between blood and urine measurements is high14. AMH was not measured in this population, so we were unable to assess the association between 25(OH)D and AMH. While all the women in our analysis reported being premenopausal, some of them may have been perimenopausal, and levels of both 25(OH)D and FSH may change during menopause. However, when we limited our analysis to women under 40 the association between 25(OH)D and FSH was stronger, suggesting a minimal influence of perimenopause on our results.
Conclusions
Vitamin D was inversely related to urinary FSH levels in a population of older premenopausal women. This suggests vitamin D may influence ovarian reserve and as such, may have implications for fecundability in older women. The mechanism through which vitamin D may affect FSH is unknown, and warrants further investigation.
Acknowledgements
Financial support: This research was supported by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences, Z01ES0490003.
Footnotes
Conflict of interest: None
Contributor Information
Anne Marie Z. Jukic, Epidemiology Branch, National Institute of Environmental Health Sciences, PO Box 12233, Durham, NC 27703.
Anne Z. Steiner, Department of Obstetrics and Gynecology, University of North Carolina, Chapel Hill, NC.
Donna D. Baird, Epidemiology Branch, National Institute of Environmental Health Sciences, PO Box 12233, Durham, NC 27703.
References
- 1.Holick MF. Vitamin D deficiency. The New England journal of medicine. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 2.Lerchbaum E, Obermayer-Pietsch B. Vitamin D and fertility: a systematic review. European journal of endocrinology / European Federation of Endocrine Societies. 2012;166:765–778. doi: 10.1530/EJE-11-0984. [DOI] [PubMed] [Google Scholar]
- 3.Evans KN, Bulmer JN, Kilby MD, Hewison M. Vitamin D and placental-decidual function. Journal of the Society for Gynecologic Investigation. 2004;11:263–271. doi: 10.1016/j.jsgi.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 4.Luk J, Torrealday S, Neal Perry G, Pal L. Relevance of vitamin D in reproduction. Hum Reprod. 2012;27:3015–3027. doi: 10.1093/humrep/des248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baird DD, Hill MC, Schectman JM, Hollis BW. Vitamin d and the risk of uterine fibroids. Epidemiology. 2013;24:447–453. doi: 10.1097/EDE.0b013e31828acca0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun W, Xie H, Ji J, Zhou X, Goltzman D, Miao D. Defective female reproductive function in 1,25(OH)2D-deficient mice results from indirect effect mediated by extracellular calcium and/or phosphorus. American journal of physiology. 2010;299:E928–E935. doi: 10.1152/ajpendo.00378.2010. [DOI] [PubMed] [Google Scholar]
- 7.Kebapcilar AG, Kulaksizoglu M, Kebapcilar L, et al. Is there a link between premature ovarian failure and serum concentrations of vitamin D, zinc, and copper? Menopause (New York, NY. 2013;20:94–99. doi: 10.1097/gme.0b013e31826015ca. [DOI] [PubMed] [Google Scholar]
- 8.Malloy PJ, Peng L, Wang J, Feldman D. Interaction of the vitamin D receptor with a vitamin D response element in the Mullerian-inhibiting substance (MIS) promoter: regulation of MIS expression by calcitriol in prostate cancer cells. Endocrinology. 2009;150:1580–1587. doi: 10.1210/en.2008-1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steiner AZ, Herring AH, Kesner JS, et al. Antimullerian hormone as a predictor of natural fecundability in women aged 30–42 years. Obstet Gynecol. 2011;117:798–804. doi: 10.1097/AOG.0b013e3182116bc8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baird DD, Dunson DB, Hill MC, Cousins D, Schectman JM. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188:100–107. doi: 10.1067/mob.2003.99. [DOI] [PubMed] [Google Scholar]
- 11.Baird DD, Dunson DB, Hill MC, Cousins D, Schectman JM. Association of physical activity with development of uterine leiomyoma. Am J Epidemiol. 2007;165:157–163. doi: 10.1093/aje/kwj363. [DOI] [PubMed] [Google Scholar]
- 12.Hollis BW. Editorial: The determination of circulating 25-hydroxyvitamin D: no easy task. The Journal of clinical endocrinology and metabolism. 2004;89:3149–3151. doi: 10.1210/jc.2004-0682. [DOI] [PubMed] [Google Scholar]
- 13.Hollis BW, Kamerud JQ, Selvaag SR, Lorenz JD, Napoli JL. Determination of vitamin D status by radioimmunoassay with an 125I-labeled tracer. Clinical chemistry. 1993;39:529–533. [PubMed] [Google Scholar]
- 14.Oosterhuis GJ, Lambalk CB, Michgelsen HW, De Koning CH, Vermes I, Schoemaker J. Follicle-stimulating hormone measured in unextracted urine: a reliable tool for easy assessment of ovarian capacity. Fertil Steril. 1998;70:544–548. doi: 10.1016/s0015-0282(98)00201-5. [DOI] [PubMed] [Google Scholar]
- 15.Kesner JS, Knecht EA, Krieg EF. Time-Resolved Immunofluorometric Assays for Urinary Luteinizing-Hormone and Follicle-Stimulating-Hormone. Anal Chim Acta. 1994;285:13–22. [Google Scholar]
- 16.Visser JA, de Jong FH, Laven JS, Themmen AP. Anti-Mullerian hormone: a new marker for ovarian function. Reproduction (Cambridge, England) 2006;131:1–9. doi: 10.1530/rep.1.00529. [DOI] [PubMed] [Google Scholar]
- 17.Baird DD, Steiner AZ. Anti-Mullerian hormone: a potential new tool in epidemiologic studies of female fecundability. Am J Epidemiol. 2012;175:245–249. doi: 10.1093/aje/kwr439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dennis NA, Houghton LA, Jones GT, van Rij AM, Morgan K, McLennan IS. The level of serum anti-Mullerian hormone correlates with vitamin D status in men and women but not in boys. The Journal of clinical endocrinology and metabolism. 2012;97:2450–2455. doi: 10.1210/jc.2012-1213. [DOI] [PubMed] [Google Scholar]
- 19.Merhi ZO, Seifer DB, Weedon J, et al. Circulating vitamin D correlates with serum antimullerian hormone levels in late-reproductive-aged women: Women's Interagency HIV Study. Fertil Steril. 2012;98:228–234. doi: 10.1016/j.fertnstert.2012.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aydin ZD, Erbas B, Karakus N, Aydin O, S KO. Sun exposure and age at natural menopause: a cross-sectional study in Turkish women. Maturitas. 2005;52:235–248. doi: 10.1016/j.maturitas.2005.02.023. [DOI] [PubMed] [Google Scholar]
- 21.Discigil G, Gemalmaz A, Tekin N, Basak O. Profile of menopausal women in west Anatolian rural region sample. Maturitas. 2006;55:247–254. doi: 10.1016/j.maturitas.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 22.Nuzzo V, Zuccoli A, de Terlizzi F, Colao A, Tauchmanova L. Low 25-hydroxyvitamin D levels and low bone density assessed by quantitative ultrasonometry in a cohort of postmenopausal Italian nuns. Journal of clinical densitometry : the official journal of the International Society for Clinical Densitometry. 2013;16:308–312. doi: 10.1016/j.jocd.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 23.Lips P, van Schoor NM, de Jongh RT. Diet, sun, and lifestyle as determinants of vitamin D status. Annals of the New York Academy of Sciences. 2014;1317:92–98. doi: 10.1111/nyas.12443. [DOI] [PubMed] [Google Scholar]
- 24.Looker AC, Pfeiffer CM, Lacher DA, Schleicher RL, Picciano MF, Yetley EA. Serum 25-hydroxyvitamin D status of the US population: 1988–1994 compared with 2000–2004. The American journal of clinical nutrition. 2008;88:1519–1527. doi: 10.3945/ajcn.2008.26182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brett KM, Cooper GS. Associations with menopause and menopausal transition in a nationally representative US sample. Maturitas. 2003;45:89–97. doi: 10.1016/s0378-5122(03)00139-7. [DOI] [PubMed] [Google Scholar]
- 26.Gold EB, Bromberger J, Crawford S, et al. Factors associated with age at natural menopause in a multiethnic sample of midlife women. Am J Epidemiol. 2001;153:865–874. doi: 10.1093/aje/153.9.865. [DOI] [PubMed] [Google Scholar]
- 27.Gold EB, Crawford SL, Avis NE, et al. Factors related to age at natural menopause: longitudinal analyses from SWAN. Am J Epidemiol. 2013;178:70–83. doi: 10.1093/aje/kws421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Henderson KD, Bernstein L, Henderson B, Kolonel L, Pike MC. Predictors of the timing of natural menopause in the Multiethnic Cohort Study. Am J Epidemiol. 2008;167:1287–1294. doi: 10.1093/aje/kwn046. [DOI] [PubMed] [Google Scholar]
- 29.Kato I, Toniolo P, Akhmedkhanov A, Koenig KL, Shore R, Zeleniuch-Jacquotte A. Prospective study of factors influencing the onset of natural menopause. Journal of clinical epidemiology. 1998;51:1271–1276. doi: 10.1016/s0895-4356(98)00119-x. [DOI] [PubMed] [Google Scholar]
- 30.Stanford JL, Hartge P, Brinton LA, Hoover RN, Brookmeyer R. Factors influencing the age at natural menopause. Journal of chronic diseases. 1987;40:995–1002. doi: 10.1016/0021-9681(87)90113-5. [DOI] [PubMed] [Google Scholar]
- 31.Plante BJ, Cooper GS, Baird DD, Steiner AZ. The impact of smoking on antimullerian hormone levels in women aged 38 to 50 years. Menopause (New York, NY. 2010;17:571–576. doi: 10.1097/gme.0b013e3181c7deba. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Willett W, Stampfer MJ, Bain C, et al. Cigarette smoking, relative weight, and menopause. Am J Epidemiol. 1983;117:651–658. doi: 10.1093/oxfordjournals.aje.a113598. [DOI] [PubMed] [Google Scholar]
- 33.Cooper GS, Baird DD, Hulka BS, Weinberg CR, Savitz DA, Hughes CL., Jr Follicle-stimulating hormone concentrations in relation to active and passive smoking. Obstet Gynecol. 1995;85:407–411. doi: 10.1016/0029-7844(94)00381-M. [DOI] [PubMed] [Google Scholar]