Abstract
Agents targeting the insulin-like growth factor receptor type 1 (IGF1R) have shown antitumor activity. Based on the evidence for interaction between the IGF-1 and TRAIL pathways, we hypothesized that the combination of ganitumab (monoclonal antibody to IGF1R) with the pro-apoptotic death receptor 5 agonist, conatumumab, might increase antitumor response. Ganitumab and conatumumab were tested in combination in a Colo-205 xenograft model. Part 1 of the clinical study was a phase Ib program of three doses of conatumumab (1, 3, 15 mg/kg) in combination with 18 mg/kg ganitumab to determine the maximum tolerated dose (MTD) in patients with advanced solid tumors. Part 2 was conducted in six cohorts with advanced non-small cell lung cancer (squamous or nonsquamous histology), colorectal cancer, sarcoma, pancreatic cancer, or ovarian cancer, treated at the recommended doses of the combination. The combination was significantly more active in the Colo-205 xenograft model than either single agent alone (p<0.0015). In part 1 of the clinical study, no dose-limiting toxicities were observed and the MTD of conatumumab was 15 mg/kg in combination with 18 mg/kg ganitumab. In part 2, 78 patients were treated and there were no objective responses but 28 patients (36 %) had stable disease (median 46 days, range 0–261). The combination was well-tolerated with no new toxicities. In conclusion, the combination of ganitumab and conatumumab was well-tolerated but had no objective responses in the population tested. The successful future application of this combination of antitumor mechanisms may rely on the identification of predictive biomarkers.
Keywords: Monoclonal antibodies, Insulin-like growth factor receptor, Death receptor, TRAIL, Apoptosis, Targeted therapy
Introduction
The type I insulin-like growth factor receptor (IGF1R) signaling pathway has been an active area of oncology research for the last decade [1–4]. IGF1R is present on most tumor cells [5], and inhibition of this receptor slowed tumor growth and increased the antitumor activity of chemotherapy, radiation, and biologic agents in xenograft models [6–8]. Moreover, activation of the IGF1R pathway has been postulated as a mechanism of signaling escape from other anticancer treatments including hormones and targeted agents [9–11]. Ganitumab is an investigational, fully human, antagonist IgG1 monoclonal antibody that binds to the extracellular domain of IGF1R, blocking interaction with both of its ligands (IGF-1 and IGF-2) [8]. Ganitumab does not cross react with or inhibit the insulin receptor [8]. In vitro, ganitumab showed anticancer activity against pancreatic cancer [8], endometrial cancer [12], and sarcoma [13] models by pro-apoptotic and antiproliferative mechanisms. In clinical studies, ganitumab monotherapy was well-tolerated and demonstrated anticancer activity [3, 14, 15]. Importantly, the clinical activity of IGF1R antagonists as single agents has been consistently shown to be modest, and therefore combination studies of IGF1R antagonists with other potentially synergistic agents are warranted.
Activation of death receptors (DR) leading to apoptosis is another approach for anticancer-targeted therapy [16–18]. DR5 is a member of the TRAIL family of receptors, and interaction with its endogenous ligand Apo2L/TRAIL induces apoptosis through caspases in transformed cell lines while normal cells are unaffected [16, 19]. Conatumumab is an investigational, fully human, agonist IgG1 monoclonal antibody directed against the extracellular domain of DR5. Conatumumab showed apoptotic activity in tumors [20] and antitumor activity via caspase activation in preclinical xenograft models of multiple types [21]. In the clinical setting, conatumumab was well-tolerated and demonstrated antitumor activity in advanced solid tumors [22, 23]. By inducing apoptosis, DR agonists may enhance the activity of other single agents with diverse mechanisms of action, for example, rituximab, panitumumab, bortezomib, and vorinostat [18, 24].
We hypothesized that the combination of an agent that triggers apoptosis (conatumumab) with an agent that inhibits resistance to apoptosis (ganitumab) could provide a therapeutic advantage. The potential for an antitumor effect when these two mechanisms of action are combined is suggested by work showing that IGF-1 and Akt activation can drive resistance to TRAIL-induced apoptosis. Studies performed in multiple myeloma and thyroid carcinoma cells suggest that IGF-1, via upregulation of the anti-apoptotic (caspase inhibitor) protein FLIP, can decrease TRAIL sensitivity [25, 26]. In addition, αIR3, a monoclonal antibody against IGF1R was shown to potentiate cell death in vitro when combined with TRAIL in SW589 human thyroid carcinoma cells [26]. Other studies have shown additional evidence for the interaction of the IGF-1 and TRAIL pathways [27, 28] including the inhibition of TRAIL-induced apoptosis by IGF-1 [11] and increased sensitivity to DR5-mediated apoptosis through the inhibition of IGF1R [29]. These data provide a direct link involving IGF-1 in TRAIL-induced cell death resistance and suggest that inhibition of IGF1R action with ganitumab could enhance the pro-apoptotic effect of conatumumab in some tumor types. Combination of two monoclonal antibodies targeting these mechanisms has not been previously reported in the literature to our knowledge.
Death receptors have been shown to be expressed and apoptosis signaling pathways appear to be intact in most human tumor types. Pro-apoptotic receptor agonists have been shown to induce apoptosis in vitro and in vivo in a broad spectrum of tumor cell lines, including lung, colorectal, pancreatic, and ovarian cancers, as well as sarcoma [30]. In addition, a growing body of evidence has implicated the IGF pathways in these same tumors types [31–37]. Early phase clinical trials targeting IGF-1R in lung cancer and sarcoma, including Ewing’s sarcoma, have provided evidence of anticancer activity warranting further investigation [38, 39]. Therefore, in the present phase 1b/2 clinical study, we chose to evaluate the safety and efficacy of the ganitumab/conatumumab combination in patients with lung, colorectal, pancreatic, and ovarian cancers and sarcoma. In the phase 1b portion of the study, the primary objective was to identify a dose of conatumumab in combination with ganitumab that is safe and tolerated as determined by the incidence of dose-limiting toxicities (DLTs). In the phase 2 portion of the study, the primary objective was to estimate the efficacy of conatumumab in combination with ganitumab as measured by the objective response rate (ORR).
Methods
Preclinical xenograft model
Four- to 6-week-old, female, athymic nude mice (Harlan Sprague–Dawley Labs) were housed in cages with a 12-h light/dark cycle and met all Association for Assessment and Accreditation of Laboratory Animal Care specifications. All experimental procedures were done in accordance with Institutional Animal Care and Use Committee and the US Department of Agriculture regulations. Mice bearing established (~200 mm3) Colo-205 colorectal cancer (APC, BRAF, SMAD4, and TP53 mutated) xenografts were randomly assigned into four groups (10 mice per group) and treated intraperitoneally twice per week with human(h)IgG1 (300 µg/dose) alone, ganitumab (300 µg/dose) and hIgG1 (3 µg/dose), conatumumab (3 µg/dose) and hIgG1 (300 µg/dose), or ganitumab and conatumumab at the same doses in combination for the duration of the experiment. Tumor volumes and body weights were measured twice per week using calipers and an analytical balance, respectively. Repeated measures ANOVA (RMANOVA) was used to compare tumor growth inhibition throughout the experiment in the combination group versus each single agent group.
Patients
Key inclusion criteria included, in part 1, locally advanced or metastatic, treatment-refractory solid tumors and, in part 2, locally advanced or metastatic non-small cell lung cancer (NSCLC; squamous or non-squamous cell carcinoma; up to two prior treatment regimens), colorectal cancer (up to two prior treatment regimens), pancreatic cancer (up to one prior treatment regimen), ovarian cancer (up to two prior treatment regimens), or sarcoma (up to two prior treatment regimens). In part 2, eligible patients must have had measurable disease (at least one measurable lesion). In both parts, patients had to have been ≥16 years old with a life expectancy ≥3 months, an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1, and adequate organ function (liver, kidneys, bone marrow, coagulation, heart, glycemic function).
Key exclusion criteria included the presence of uncontrolled central nervous system metastasis; prior treatment with DR agonists; prior treatment with IGF1R antagonists; systemic chemotherapy, hormonal therapy, immunotherapy, and experimental or approved anticancer proteins/antibodies therapy within 28 days before enrollment, except in part 1 where patients could continue approved hormonal therapy as medically indicated; any prior or synchronous malignancy (except for non-melanoma skin cancer or in situ cervical cancer) other than the study disease, unless treated with curative intent with no evidence of disease ≥3 years before enrollment (part 2 only); and any clinically significant medical condition other than cancer, including cardiovascular disease or chronic obstructive pulmonary disease, which in the opinion of the investigator could interfere with the safe delivery of study treatment or increase risk of toxicity.
Study design
This was a multicenter, open-label, two-part phase 1b/2 study. All patients provided written informed consent before any study-specific procedure was performed, and the study was approved by the institutional review board or ethics committee for each site.
Both investigational products were administered intravenously (IV) on day 1 every 3 weeks (Q3W) until disease progression, intolerable adverse event, death, withdrawal of consent, or administrative decision, for up to 24 months. Ganitumab was administered first over a 60-min infusion followed by conatumumab over a 60-min infusion.
In part 1, the primary endpoint was the incidence of adverse events and clinical laboratory abnormalities defined as dose-limiting toxicities. In part 2, the primary endpoint was the ORR (confirmed complete response (CR) and partial response (PR)) using modified Response Evaluation Criteria in Solid Tumors (RECIST) version 1. In both parts, predefined secondary endpoints included incidence of adverse events, the presence of antibodies to ganitumab or conatumumab, and pharmacokinetic parameters.
In part 1, conatumumab doses of 1, 3, and 15 mg/kg Q3W were selected for evaluation in combination with ganitumab 18 mg/kg Q3W in sequential dose-escalation cohorts in patients with advanced solid tumors. The schedule of conatumumab for this study was chosen based on pharmacokinetic modeling from the first-in-human study, which supported Q2W and Q3W dosing [22]. The 3 mg/kg Q3W conatumumab dose was predicted to have a Cmin of 10 µg/mL, approximately 4-fold higher than the EC50 value in the xenograft model, and the highest dose of 15 mg/kg Q3W was selected to ensure trough concentrations above the EC90 in most patients. Although a dose up to 20 mg/kg was found safe in a prior monotherapy study, we chose to keep the highest dose at 15 mg/kg in the present combination therapy study. Of note, a partial response had been observed previously in a patient treated with conatumumab at only 0.3 mg/kg [22]; therefore, we also included 1 mg/kg in the present study. The ganitumab dose was also selected based on pharmacokinetic modeling in the first-in-human phase 1 study [3]. The planned sample size was 3 to 6 DLT-evaluable patients per cohort. Up to three patients were enrolled initially in each cohort. If no patient experienced a DLT after all patients completed the first cycle of treatment, then dose escalation to the next cohort occurred. However, if a patient experienced a DLT, then three additional patients were to be treated at the same dose level.
In part 2, the conatumumab dose identified as safe and tolerable in part 1 was evaluated in combination with ganitumab 18 mg/kg IV Q3W in parallel cohorts of the following tumor types: advanced NSCLC (non-squamous histology [cohort 1] or squamous histology [cohort 2]), colorectal cancer (cohort 3), pancreatic cancer (cohort 4), ovarian cancer (cohort 5), and sarcoma (cohort 6). The planned sample size was 90 (15 patients per cohort).
Safety assessments
Adverse events and DLT were recorded at day 1 of every cycle and at every office visit. Physical exam, vital signs, and laboratory assessments, including hematology and blood chemistry, were conducted on day 1 of every cycle. Anticoagulation was also measured in those on anticoagulant therapy and HgbA1c was measured in those with diabetes on day 1 of cycles 1, 3, and every 3 cycles thereafter.
The NCI CTCAE v3.0 event grading scale was used as a guide for grading DLT. DLT was defined as any grade 3 or higher hematologic or non-hematologic toxicity related to conatumumab, or the combination of conatumumab with ganitumab, except for lymphopenia and anemia. To be considered a DLT, fatigue had to be grade 4 or grade 3 for >7 days; anorexia, nausea, vomiting, stomatitis/mucositis, or diarrhea had to be grade 3 or 4 despite maximum supportive care; neutropenia had to be grade 3 or 4 with fever >38.5 °C; thrombocytopenia had to be grade 3 or 4 with grade >1 bleeding; elevated ALT or AST must have been >10 times the upper limit of normal; amylase or lipase elevation had to be grade 4 for >7 days; hyperglycemia must have had lifethreatening consequences (ketoacidosis or hyperosmolar nonketotic coma); and pulmonary embolism must have been symptomatic.
Tumor analysis
In part 2, radiological assessments for disease status were performed according to modified RECIST version 1.0. Radiological imaging by CT or MRI was done every 6 weeks [±7 days] during the first 6 months of the study and every 9 weeks [±7 days] thereafter. PR and CR assessments were confirmed by repeat assessment within 28 days. Stable disease (SD) was defined as neither sufficient shrinkage to qualify for PR nor sufficient increase to qualify for PD, taking as reference the smallest sum longest diameter since the treatment started. To qualify for a response of SD, follow-up measurements must have met the SD criteria at least once by study week 5 (day 35).
Pharmacokinetics
Serum samples for pharmacokinetics were collected before the ganitumab and conatumumab infusions and 5 min before the end of each infusion at day 1 of cycles 1 and 3, and before each of the infusions on day 1 of cycle 5 and every three cycles thereafter. Serum concentrations of conatumumab or ganitumab were measured by validated enzyme-linked immunosorbent assays. The lower limit of quantification for conatumumab and ganitumab was 29.9 and 300 ng/mL, respectively. Serum pharmacokinetic parameters (Cmax and Cmin) were estimated using noncompartmental methods with WinNonlin Enterprise software (version 5.1.1; Pharsight Corp.)
Evaluation of anti-ganitumab and anti-conatumumab antibodies
Serum samples for anti-ganitumab and anti-conatumumab antibodies were collected before the start of day 1 of cycles 1, 3, and 5, and every six cycles thereafter (cycle 11, 17, etc.). Samples were also taken at day 30 and at the day 60 safety follow-up visits. Assay details have been previously published [3, 22].
Statistical analysis
Parts 1 and 2 were analyzed separately. Two interim analyses of part 2 safety data were planned and conducted after 15 and 45 patients had been enrolled and received one cycle of treatment. The proportion of patients with an objective response per local investigator assessment with corresponding 95 % Clopper Pearson method [40] confidence intervals was calculated for each cohort in part 2. All safety data were summarized per cohort and in aggregate over all cohorts using descriptive statistics.
Results
Preclinical studies
Xenograft models
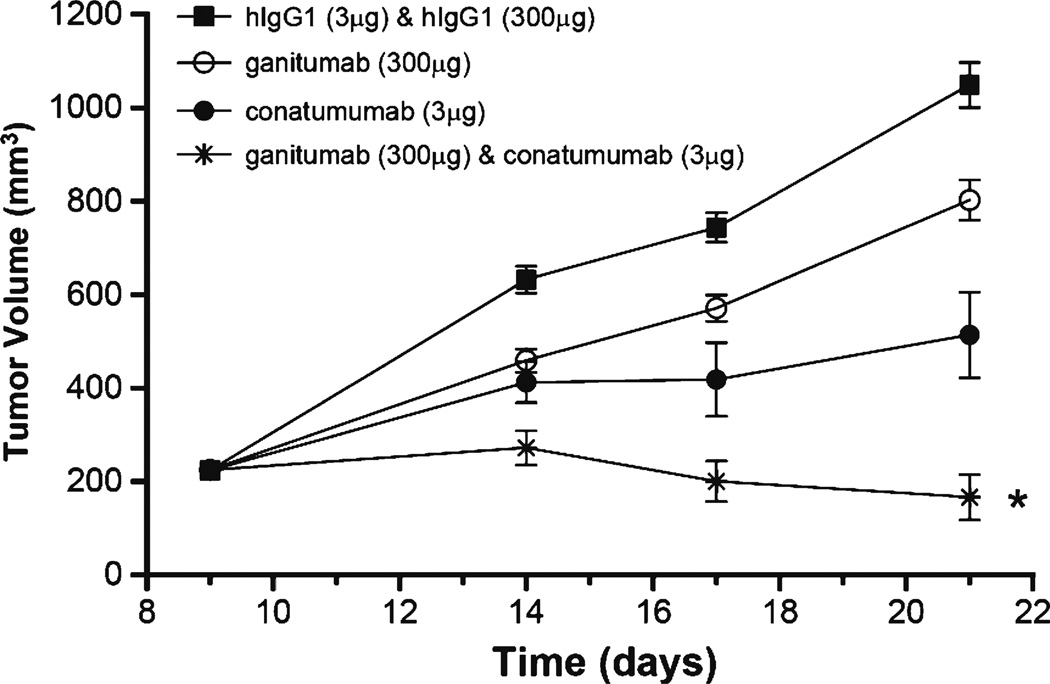
The combination of ganitumab and conatumumab was evaluated in the Colo-205 colorectal carcinoma xenograft model (Fig. 1). In the presence of the control hIgG1 antibodies, tumor volume increased steadily over time. Single-agent treatment with ganitumab or conatumumab resulted in reduction of tumor volume compared to the control. The combination of ganitumab and conatumumab led to strong tumor growth inhibition that was significantly better than either agent alone (p<0.0105 vs either single agent).
Fig. 1.
Ganitumab and conatumumab in the Colo 205 human colorectal cancer xenograft model. RMANOVA: *p<0.015 vs conatumumab, *p<0.001 vs ganitumab
The combination of ganitumab and conatumumab was also tested in two other xenograft models (HCT-116 colorectal cancer and H460 non-small cell lung cancer) but did not provide any benefit over each single agent alone (data not shown).
Clinical study
Patients and disposition
In part 1, nine patients (three per dose cohort) were enrolled from five centers between January and July 2009. All patients received both study medications and discontinued treatment either due to death (n=1) or disease progression (n=8). Baseline demographics and disease characteristics are shown in Table 1.
Table 1.
Baseline demographics and disease characteristics—part 1
| All patients (n=9) | |
|---|---|
| Gender, n (%) | |
| Male | 4 (44) |
| Female | 5 (56) |
| Median age (range), years | 58 (24, 66) |
| ECOG performance status at screening, n (%) | |
| 0 | 2(22) |
| 1 | 7(78) |
| Primary tumor type, n (%) | |
| Colorectal | 3 (33) |
| Soft tissue sarcoma | 4 (44) |
| Pancreatic | 1 (11) |
| Breast | 1 (11) |
| Stage at enrollment, n (%) | |
| III | 1 (11) |
| IV | 8 (89) |
| Prior chemotherapy, n (%) | 9 (100) |
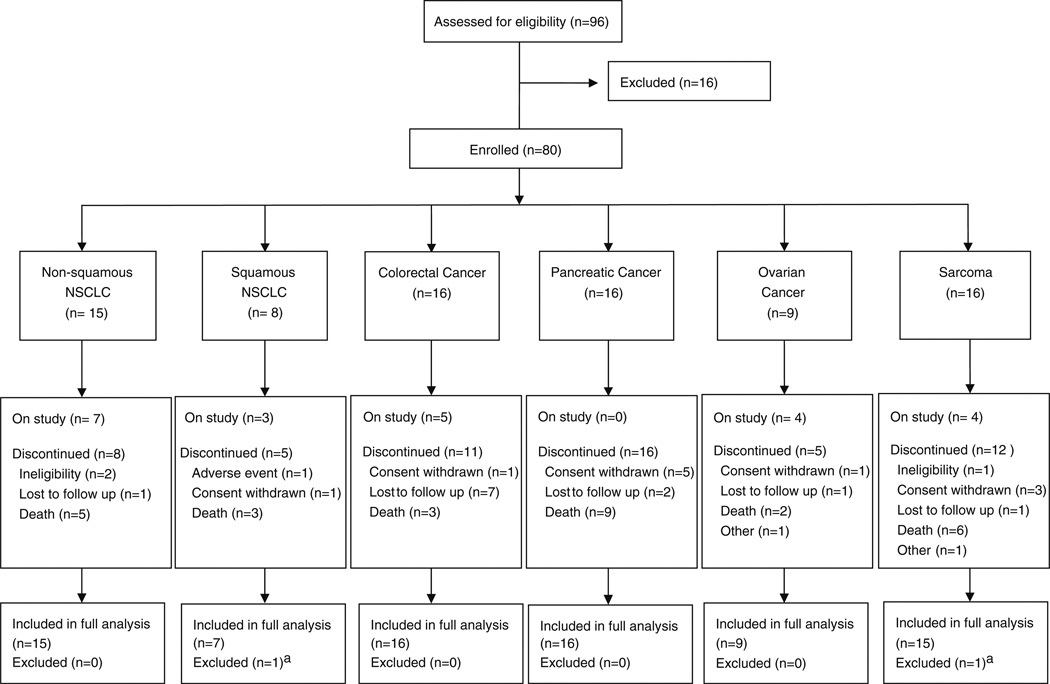
In part 2, 80 patients were enrolled from 16 centers (including all 5 centers from part 1) in the USA and Spain beginning in September 2009 and ending May 2010. Of these, 78 patients received both study medications (full analysis set) (Fig. 2). A total of 16 patients were ineligible due to the following: lab values outside acceptable range (n=8), unwilling or unable to comply with study procedures (n=3), had to remain on anticoagulation therapy (n=2), had received more than one prior anticancer treatment (n=1), general deterioration (n=1), and ECOG score >1 (n=1).
Fig. 2.
CONSORT diagram; aReason for exclusion: did not receive treatment
Fewer patients than planned were enrolled into the squamous cell NSCLC and ovarian cancer cohorts because further enrollment to the study was closed following a protocol-planned tumor response analysis (after the second safety interim analysis) that showed lack of activity in the colorectal cancer and sarcoma cohorts. As of the primary analysis data cutoff date (May 2010), 23 patients remained on study and 57 discontinued due to ineligibility (n=3), adverse event (n=1), withdrawal of consent (n=11), lost to follow-up (n=12), death (n=28), or other (n=2). Disposition for each cohort is shown in Fig. 2. Baseline demographics and disease characteristics are shown in Table 2.
Table 2.
Baseline demographics and disease characteristics—part 2
| All patients (N=78) | |
|---|---|
| Gender, n (%) | |
| Male | 39 (50) |
| Female | 39 (50) |
| Median age (range), years | 59 (29, 83) |
| Disease stage at time of enrollment, n (%) | |
| Stage III | 13 (17) |
| Stage IIIa | 1 (1) |
| Stage IIIb | 1 (1) |
| Stage IV | 62 (79) |
| Unknown | 1 (1) |
| ECOG performance status at screening, n (%) | |
| 0 | 34 (44) |
| 1 | 42 (54) |
| Unknown | 2 (3) |
| Prior radiotherapy, n (%) | 35 (45) |
| Prior chemotherapy, n (%) | 77 (99) |
Safety
No DLTs were experienced in any of the dose cohorts in part 1; therefore, the dose identified for part 2 was 15 mg/kg conatumumab IV every 3 weeks, the highest dose tested.
In part 1, the most common treatment-emergent events (occurring in two or more patients) were fatigue (n=4; 44 %), dyspnea (n=3; 33%), anemia (n=3; 33%), pericardial effusion (n=2; 22 %), nausea (n=2; 22 %), pain (n=2; 22%), drug hypersensitivity (n=2; 22 %), decreased appetite (n=2; 22 %), hyperglycemia (n=2; 22 %); hyperalbuminemia (n=2; 22 %), and hyperkalemia (n=2; 22 %). Eight patients (89 %) experienced at least one treatment-related event including fatigue (33 %), drug hypersensitivity (22 %), adverse drug reaction (11 %), arthralgia (11 %), dyspepsia (11 %), dyspnea (11 %), infusion-related reaction (11 %), myalgia (11 %), nausea (11 %), night sweats (11 %), anemia (11 %), decreased appetite (11 %), hyperglycemia (11 %), rash (11 %), drug eruption (11 %), neutropenia (11 %), and pain (11 %). In part 2, the most common treatment-emergent adverse events overall were fatigue (36 %; 4 %≥grade 3), chills (29 %; 0 %≥grade 3), decreased appetite (29 %; 3 %≥grade 3), nausea (23 %; 3 %≥grade 3), asthenia (14 %; 3 %≥grade 3), dyspnea (14 %; 5 %≥grade 3), pyrexia (14 %;1 %≥grade 3), back pain (12 %; 3 %≥grade 3), and vomiting (10 %; 1 %≥grade 3) (Table 3). The most common treatment-related events ≥5 % in frequency were chills (27 %; 0 %≥grade 3), fatigue (15 %; 1 %≥grade 3), nausea (12 %; 0 %≥grade 3), pyrexia (6 %; 0 %≥grade 3), asthenia (5 %; 0 %≥grade 3), and rash (5 %; 0 %≥grade 3). There was one treatment-related serious adverse event: grade 3 hemoptysis in a patient with squamous NSCLC. Adverse events previously associated with IGF1R inhibitors, including ganitumab, were hyperglycemia (5 %; 3 %≥grade 3), thrombocytopenia (1 %; 1 % grade 3); and neutropenia (3 %; 1 % grade 3). Adverse events known to be associated with conatumumab were amylase increase (3 %; 1 % grade 3) and lipase increase (3 %; 0 %≥grade 3). There were three fatal adverse events, none of which were deemed related to treatment, including acute respiratory failure in the setting of pulmonary embolism and disease progression in a patient with ovarian cancer, disease progression in a patient with squamous NSCLC, and respiratory distress in a patient with non-squamous NSCLC.
Table 3.
Incidence of treatment-emergent adverse events in ≥5 % of all patients—part 2
| Non-squamous NSCLC (N=15) |
Squamous NSCLC (N=7) |
Colorectal cancer (N=16) |
Pancreatic cancer (N=16) |
Ovarian cancer (N=9) |
Sarcoma (N=15) |
All patients (N=78) |
|
|---|---|---|---|---|---|---|---|
| Patients reporting at least one adverse event, n (%) | 15 (100) | 7 (100) | 15 (94) | 14 (88) | 9 (100) | 11 (73) | 71 (91) |
| Fatigue | 7 (47) | 2 (29) | 1 (6) | 9 (56) | 6 (67) | 3 (20) | 28 (36) |
| ≥Grade 3 | 0 (0) | 0 (0) | 0 (0) | 3 (19) | 0 (0) | 0 (0) | 3 (4) |
| Chills | 6 (40) | 1 (14) | 7 (44) | 4 (25) | 2 (22) | 3 (20) | 23 (29) |
| ≥Grade 3 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Decreased appetite | 7 (47) | 2 (29) | 2 (13) | 7 (44) | 3 (33) | 2 (13) | 23 (29) |
| ≥Grade 3 | 0 (0) | 0 (0) | 0 (0) | 1 (6) | 1 (11) | 0 (0) | 2 (3) |
| Nausea | 4 (27) | 1 (14) | 0 (0) | 5 (31) | 5 (56) | 3 (20) | 18 (23) |
| ≥Grade 3 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (22) | 0 (0) | 2 (3) |
| Asthenia | 0 (0) | 1 (14) | 5 (31) | 3 (19) | 1 (11) | 1 (7) | 11 (14) |
| ≥Grade 3 | 0 (0) | 0 (0) | 1 (6) | 0 (0) | 1 (11) | 0 (0) | 2 (3) |
| Dyspnea | 3 (20) | 1 (14) | 1 (6) | 2 (13) | 3 (33) | 1 (7) | 11 (14) |
| ≥Grade 3 | 0 (0) | 1 (14) | 0 (0) | 1 (6) | 2 (22) | 0 (0) | 4 (5) |
| Pyrexia | 1 (7) | 0 (0) | 2 (13) | 3 (19) | 1 (11) | 4 (27) | 11 (14) |
| ≥Grade 3 | 0 (0) | 0 (0) | 1 (6) | 0 (0) | 0 (0) | 0 (0) | 1 (1) |
| Back pain | 2 (13) | 0 (0) | 0 (0) | 1 (6) | 2 (22) | 4 (27) | 9 (12) |
| ≥Grade 3 | 1 (7) | 0 (0) | 0 (0) | 0 (0) | 1 (11) | 0 (0) | 2 (3) |
| Vomiting | 1 (7) | 0 (0) | 1 (6) | 2 (13) | 2 (22) | 2 (13) | 8 (10) |
| ≥Grade 3 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (11) | 0 (0) | 1 (1) |
| Abdominal pain | 2 (13) | 0 (0) | 1 (6) | 2 (13) | 2 (22) | 0 (0) | 7 (9) |
| ≥Grade 3 | 0 (0) | 0 (0) | 1 (6) | 1 (6) | 0 (0) | 0 (0) | 2 (3) |
| Upper abdominal pain | 1 (7) | 0 (0) | 2 (13) | 1 (6) | 1 (11) | 2 (13) | 7 (9) |
| ≥Grade 3 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (7) | 1 (1) |
| Chest pain | 1 (7) | 0 (0) | 1 (6) | 1 (6) | 3 (33) | 1 (7) | 7 (9) |
| ≥Grade 3 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Dehydration | 1 (7) | 0 (0) | 0 (0) | 2 (13) | 4 (44) | 0 (0) | 7 (9) |
| ≥Grade 3 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Diarrhea | 1 (7) | 0 (0) | 2 (13) | 1 (6) | 3 (33) | 0 (0) | 7 (9) |
| ≥Grade 3 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Constipation | 2 (13) | 0 (0) | 0 (0) | 2 (13) | 1 (11) | 1 (7) | 6 (8) |
| ≥Grade 3 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (7) | 1 (1) |
| Cough | 1 (7) | 1 (14) | 1 (6) | 1 (6) | 2 (22) | 0 (0) | 6 (8) |
| ≥Grade 3 | 0 (0) | 1 (14) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (1) |
| Insomnia | 2 (13) | 0 (0) | 0 (0) | 2 (13) | 1 (11) | 0 (0) | 5 (6) |
| ≥Grade 3 | 0 (0) | 0 (0) | 0 (0) | 1 (6) | 0 (0) | 0 (0) | 1 (1) |
| Anemia | 1 (7) | 0 (0) | 2 (13) | 1 (6) | 0 (0) | 0 (0) | 4 (5) |
| ≥Grade 3 | 1 (7) | 0 (0) | 0 (0) | 1 (6) | 0 (0) | 0 (0) | 2 (3) |
| Hyperglycemia | 0 (0) | 2 (29) | 0 (0) | 1 (6) | 1 (11) | 0 (0) | 4 (5) |
| ≥Grade 3 | 0 (0) | 2 (29) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (3) |
| Pain | 1 (7) | 0 (0) | 0 (0) | 2 (13) | 1 (11) | 0 (0) | 4 (5) |
| ≥Grade 3 | 0 (0) | 0 (0) | 0 (0) | 2 (13) | 0 (0) | 0 (0) | 2 (3) |
| Pleural effusion | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 4 (44) | 0 (0) | 4 (5) |
| ≥Grade 3 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (22) | 0 (0) | 2 (3) |
| Rash | 0 (0) | 0 (0) | 1 (6) | 1 (6) | 0 (0) | 2 (13) | 4 (5) |
| ≥Grade 3 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Weight decreased | 3 (20) | 0 (0) | 0 (0) | 0 (0) | 1 (11) | 0 (0) | 4 (5) |
| ≥Grade 3 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
Efficacy
In part 1, one confirmed PR was observed and two patients had SD. The PR (duration, 186 days) occurred in a 62-year-old woman on 1 mg/kg conatumumab plus ganitumab with stage IV well-differentiated myxofibrosarcoma who had received 13 prior anticancer therapies. One of the patients with SD (female; age 66; stage IV colorectal cancer) had prolonged SD (177 days).
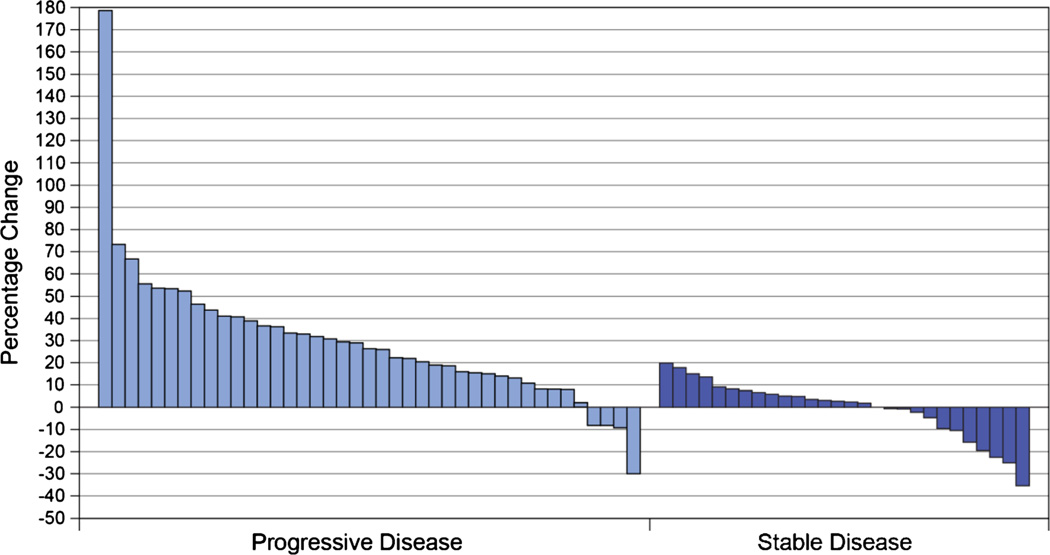
In part 2, no objective responses were observed (Table 4). Twenty-eight patients (36 %) had SD including one patient (female, age 77) with an unconfirmed PR (duration unknown; had progressed by next visit) who had stage III colorectal cancer at enrollment and had received nine prior anticancer therapies. The median duration of SD was 46 days (range 0, 261). Several patients experienced prolonged SD including a 38-year-old woman with stage IV synovial sarcoma (duration 261 days), a 58-year-old man with stage IV leiomyosarcoma (duration 227 days), a 47-year-old man with stage IV non-squamous NSCLC (duration 210 days), and a 45-year-old man with stage IV colorectal cancer (duration 190 days). Individual patients’ best tumor response showed that several patients with PD and several with SD experienced tumor regression (Fig. 3). Median PFS times ranged from 1.3months in sarcoma up to 3.3 months in squamous NSCLC (Table 4).
Table 4.
Objective response rate and progression-free survival—part 2
| Non-squamous NSCLC (N=15) |
Squamous NSCLC (N=7) |
Colorectal cancer (N=16) |
Pancreatic cancer (N=16) |
Ovarian cancer (N=9) |
Sarcoma (N=15) |
All patients (N=78) |
|
|---|---|---|---|---|---|---|---|
| Best overall response assessment, n (%) | |||||||
| Complete or partial response (CR or PR) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Stable disease (SD)a | 6 (40) | 5 (71) | 6 (38) | 1 (7) | 5 (56) | 5 (33) | 28 (36) |
| Unconfirmed PR | 0 (0) | 0 (0) | 1 (6) | 0 (0) | 0 (0) | 0 (0) | 1 (1) |
| Progressive disease (PD) | 9 (60) | 2 (29) | 9 (56) | 11 (73) | 3 (33) | 10 (67) | 44 (57) |
| Not evaluable | 0 (0) | 0 (0) | 1 (6) | 3 (20) | 1 (11) | 0 (0) | 5 (6) |
| PFS (months), median (95 % CI) | 1.6 (1.3, 3.7) | 3.3 (1.9, 5.4) | 1.5 (1.3, 2.6) | 1.4 (1.2, 2.3) | 2.8 (1.6, 3.9) | 1.3 (1.2,1.7) | – |
A best overall response of SD requires a radiologically determined response of SD or better no earlier than study day 35
Fig. 3.
Individual patients’ best overall tumor response (maximum percentage decrease or minimum percentage increase from baseline in the sum of the longest diameter)
Pharmacokinetics
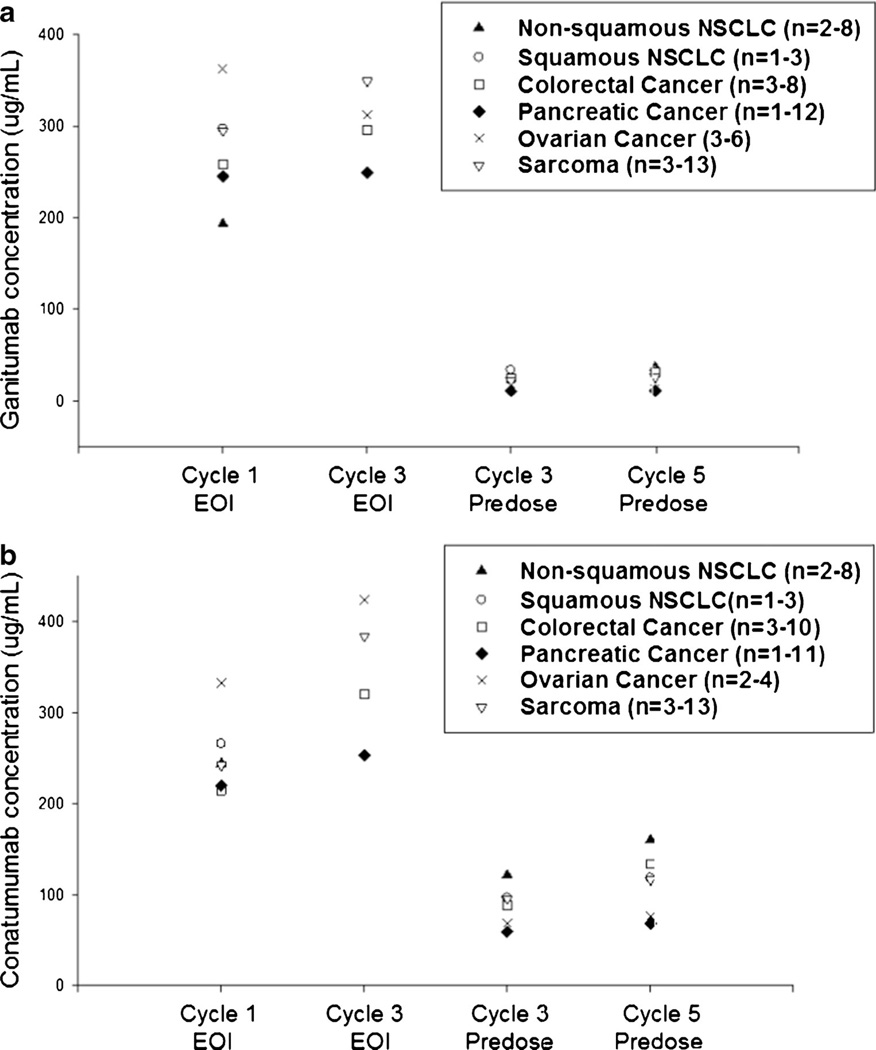
Mean predose and end-of-infusion concentrations of conatumumab and ganitumab in part 2 are shown in Fig. 4. Mean ganitumab concentrations at the end of infusion ranged from 193 to 362 µg/mL after cycle 1 and from 249 to 334 µg/mL following cycle 3 dosing. The predose concentrations of ganitumab in cycles 3 and 5 ranged from 11 to 37 µg/mL. Mean conatumumab concentrations at the end of infusion ranged from 215 to 332 µg/mL after cycle 1 and from 253 to 423 µg/mL following cycle 3 dosing. The predose concentrations of conatumumab in cycles 3 and 5 ranged from 59 to 160 µg/mL.
Fig. 4.
Mean predose and end-of-infusion concentrations in part 2. a Mean ganitumab concentration; b Mean conatumumab concentration. n=the range of patient numbers at four time points
Immunogenicity
No neutralizing antibodies were detected.
Discussion
This is the first study to report on the combination of an IGF1R inhibitor and a DR5 agonist in patients with cancer. Despite a strong scientific rationale and evidence for an additive effect for the ganitumab/conatumumab combination in a xenograft model, this clinical trial did not show evidence for activity of the combination in any of the tumor types tested, though one patient with sarcoma had a PR, and several patients with colorectal cancer (n=2), sarcoma (n=2), and NSCLC (n=1) experienced prolonged stable disease. Of note, recent review articles summarizing the trial data from multiple candidates have concluded that both IGF1R-directed [41, 42, 35] and DR5 agonist agents [43] have shown modest activity as single agents in the clinical setting. Results from a prior single-agent study with ganitumab were encouraging, with responses observed in 2 of 12 patients with Ewing’s sarcoma (1 durable CR and 1 PR), and 2 of 5 patients with neuroendocrine tumors (1 PR and 1 durable minor response) [3]. Another ganitumab study in sarcoma reported 2 PRs and SD in 17 of 35 evaluable patients [15]. Prior phase 1 studies of conatumumab yielded a best response of SD in 14 of 37 patients with 1 sustained PR over 4 years in a patient with NSCLC [22], and in a Japanese study, a best response of SD occurred in 9 of 18 patients with solid tumors [23].
Given the support for interaction between the TRAIL and IGF-1 pathways [29, 28, 11, 26] and the evidence that inhibition of IGF-1 sensitizes cells for death ligand-induced apoptosis [27, 29], we postulated that the combination of ganitumab and conatumumab might increase antitumor response in the clinical setting. The clinical study was conducted in five tumor types, which were selected based on the body of evidence that overexpression of IGF-1, IGF-2, and/or their receptor IGF1R plays an important role in tumor growth and survival in NSCLC, colorectal cancer [33], sarcoma [34, 35], pancreatic cancer [36], and ovarian cancer [37]. Preclinical data supported an effect of the ganitumab/conatumumab combination based on the Colo 205 xenograft model, and although the NSCLC xenograft model did not give supportive evidence, there were other reports of NSCLC sensitivity to the IGF-1/DR antitumor mechanisms in the literature. As part 1 was designed to evaluate the MTD and not efficacy, the tumor types for part 2 were preselected based on preclinical and clinical evidence and were not chosen based on part 1 results. Although there was some prior evidence of ganitumab activity in Ewing’s sarcoma as mentioned above, Ewing’s sarcoma was not specifically evaluated in the present study, although sarcoma was selected as one of the cohorts.
The reason for the failure to replicate the preclinical results in the Colo 205 xenograft model might be related to the previous treatment of most of the patients, thus perhaps resulting in tumors that had already developed mechanisms to circumvent the DR5 pro-apoptotic pathway and IGF-1-mediated inhibition of apoptosis. Another potential explanation could be the fact that the population tested, despite the selection for specific tumor type, was molecularly unselected, as biomarkers associated with vulnerability or resistance to these mechanisms of action have not yet been identified. We did not select for tumors expressing IGF1R; however, in a study of dalotuzumab, another IGF1R monoclonal antibody, IGF1R selection did not markedly increase antitumor efficacy, as only 1 of 80 patients showed a response [4]. It is possible that conducting the trial in a single tumor type, such as colon cancer, might have increased the likelihood of finding a responsive population.
Sensitivity to ganitumab and conatumumabmay depend on molecular characteristics of the tumor that have yet to be identified. The Colo 205 model has mutations in APC, BRAF, SMAD4, and TP53; therefore, evaluation of the mutation status of the patients in this trial could provide additional information but has not been undertaken at this time. Some progress in identification of a potential biomarker for conatumumab activity was recently reported in an analysis of three phase 2 studies which showed that carriers of the Fc gamma receptor IIIa V allele had significantly longer overall survival versus control in patients with metastatic colorectal cancer, a trend toward longer survival in NSCLC, and no effect in patients with pancreatic cancer [44]. Determination and appropriate application of biomarkers is complex andmay emerge only after a new drug has been in use in patients for some time, as exemplified by the case of epidermal growth factor receptor inhibitor resistance in tumors harboring KRAS mutations [45]. Progress in the area of identification of biomarkers in earlier stage clinical trials is clearly desirable, and earlier application of biomarkers may reduce the occurrence of negative clinical trials [46] such as the present one.
In addition, valid preclinical models are important. In this case, a more thorough evaluation of the mechanism of the effect of ganitumab and conatumumab in the Colo 205 xenograft model might have led to a better understanding of the characteristics required for response. Additional testing of the combination in other xenograft models from the other tumor types included in the clinical trial might also have given a better indication of the likelihood of success in the clinical setting. We note that in vitro study of the combination was not possible due to the requirement of specific culture conditions for each agent. The presence of these culture conditions affects the activity of the other agent; thus, we were unable to produce culture conditions without confounding the results.
The combination of ganitumab and conatumumab was well-tolerated with no dose-limiting toxicities, suggesting that concomitant IGF1R inhibition and DR5 stimulation does not lead to novel toxicities. Consistent with prior studies [3, 4, 17, 22, 47], the most common treatment-emergent adverse events were fatigue, chills, decreased appetite, nausea, asthenia, dyspnea, pyrexia, back pain, and vomiting. Thrombocytopenia has also been observed previously with ganitumab [3] and dalotuzumab [48]; in the present study, one grade 3 event (1 %) was observed. Amylase increases or hepatic toxicity have been observed with other investigational products targeting TRAIL receptors [49, 50], and lipase increase was identified as an adverse event associated with conatumumab [22, 23]. As seen in prior studies, the incidence of amylase/lipase increase was low in the present study and was not dose-limiting.
Pharmacokinetic analyses showed no large differences for ganitumab or conatumumab concentrations across different tumor types and only slight accumulation of both agents occurred over time. Exposures of ganitumab and conatumumab in this study were similar to those observed in first-in-human studies [3, 22]. In relation to the effective doses studied in preclinical models, the clinical trough concentrations of ganitumab and conatumumab reported here were around or above the concentrations observed at the doses required to achieve 90 % maximal tumor growth inhibition (ED90) in the xenograft models.
In conclusion, this study showed that the combination of the IGF1R inhibitor ganitumab and the DR5 agonist conatumumab was tolerable but not active in the population tested, suggesting that future research of these mechanisms in combination may not yield evidence of anticancer activity unless predictive biomarkers are identified.
Acknowledgments
This work was sponsored by Amgen Inc., Thousand Oaks CA, USA. Wanda J. Krall, PhD, on behalf of Amgen Inc., and Kathryn Boorer, PhD, of Amgen Inc. assisted the authors in the writing of the manuscript.
Footnotes
Conflict of interest Josep Tabernero, Hedy Kindler, Karen Reckamp, E. Gabriela Chiorean, and A. Craig Lockhart received research funding for their respective institutions for the conduct of this study. Josep Tabernero was a paid consultant for Amgen, Bristol-Myers Squibb, Genentech, Merck KGaA, Millennium, Novartis, Onyx, Pfizer, Roche, Sanofi, Imclone, and Bayer. Hedy Kindler was a paid consultant and received travel support from Amgen, and received consultancy fees from Bristol-Myers Squibb, OSI/Astellas, Genentech/Roche, Infinity, and Clovis. Sant P. Chawla and Karen Reckamp received consultancy fees from Amgen. Nilofer Azad had no relationships to disclose. José Baselga received consultancy fees from Astra Zeneca, Bayer Healthcare, Exelixis, Glaxo SmithKline, Intellikine, Janssen Oncology, Novartis, Merck, Roche/Genentech, and Sanofi Aventis. Cheng-Pang Hsu, Nigel F. Baker, and Pedro Beltran are employees of Amgen and hold Amgen stock. Francesco Galimi was an employee of Amgen while the study was conducted.
Francesco Galimi’s address at the time the work was conducted.
Contributor Information
Josep Tabernero, Email: jtabernero@vhio.net, Vall d’Hebron University Hospital and Institute of Oncology (VHIO), Universitat Autònoma de Barcelona, Passeig Vall d’Hebron 119-129, Barcelona 08035, Spain.
Sant P. Chawla, Sarcoma Oncology Center, Santa Monica, CA, USA
Hedy Kindler, University of Chicago Cancer Research Center, Chicago, IL, USA.
Karen Reckamp, City of Hope Comprehensive Cancer Center Medical Oncology, Duarte, CA, USA.
E. Gabriela Chiorean, Indiana University Simon Cancer Center, Indianapolis, IN, USA.
Nilofer S. Azad, John Hopkins Medical Center, Baltimore, MD, USA
A. Craig Lockhart, Washington University School of Medicine, St. Louis, MO, USA.
Cheng-Pang Hsu, Amgen Inc., Thousand Oaks, CA, USA.
Nigel F. Baker, Amgen Ltd., Cambridge, UK
Francesco Galimi, Amgen Inc., Thousand Oaks, CA, USA.
Pedro Beltran, Amgen Inc., Thousand Oaks, CA, USA.
José Baselga, Massachusetts General Hospital Cancer Center, Boston, MA, USA.
References
- 1.Rodon J, DeSantos V, Ferry RJ, Jr, Kurzrock R. Early drug development of inhibitors of the insulin-like growth factor-I receptor pathway: lessons from the first clinical trials. Mol Cancer Ther. 2008;7(9):2575–2588. doi: 10.1158/1535-7163.MCT-08-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Molife LR, Fong PC, Paccagnella L, Reid AH, Shaw HM, Vidal L, Arkenau HT, Karavasilis V, Yap TA, Olmos D, Spicer J, Postel-Vinay S, Yin D, Lipton A, Demers L, Leitzel K, Gualberto A, de Bono JS. The insulin-like growth factor-I receptor inhibitor figitumumab (CP-751,871) in combination with docetaxel in patients with advanced solid tumours: results of a phase Ib dose-escalation, open-label study. Br J Cancer. 2010;103(3):332–339. doi: 10.1038/sj.bjc.6605767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tolcher AW, Sarantopoulos J, Patnaik A, Papadopoulos K, Lin CC, Rodon J, Murphy B, Roth B, McCaffery I, Gorski KS, Kaiser B, Zhu M, Deng H, Friberg G, Puzanov I. Phase I, pharmacokinetic, and pharmacodynamic study of AMG 479, a fully human monoclonal antibody to insulin-like growth factor receptor 1. J Clin Oncol. 2009;27(34):5800–5807. doi: 10.1200/JCO.2009.23.6745. [DOI] [PubMed] [Google Scholar]
- 4.Atzori F, Tabernero J, Cervantes A, Prudkin L, Andreu J, Rodriguez-Braun E, Domingo A, Guijarro J, Gamez C, Rodon J, Di Cosimo S, Brown H, Clark J, Hardwick JS, Beckman RA, Hanley WD, Hsu K, Calvo E, Rosello S, Langdon RB, Baselga J. A phase I pharmacokinetic and pharmacodynamic study of dalotuzumab (MK-0646), an anti-insulin-like growth factor-1 receptor monoclonal antibody, in patients with advanced solid tumors. Clin Cancer Res. 2011;17(19):6304–6312. doi: 10.1158/1078-0432.CCR-10-3336. [DOI] [PubMed] [Google Scholar]
- 5.Sachdev D, Yee D. Disrupting insulin-like growth factor signaling as a potential cancer therapy. Mol Cancer Ther. 2007;6(1):1–12. doi: 10.1158/1535-7163.MCT-06-0080. [DOI] [PubMed] [Google Scholar]
- 6.Flanigan SA, Pitts TM, Eckhardt SG, Tentler JJ, Tan AC, Thorburn A, Leong S. The insulin-like growth factor I receptor/insulin receptor tyrosine kinase inhibitor PQIP exhibits enhanced antitumor effects in combination with chemotherapy against colorectal cancer models. Clin Cancer Res. 2010;16(22):5436–5446. doi: 10.1158/1078-0432.CCR-10-2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sabbatini P, Rowand JL, Groy A, Korenchuk S, Liu Q, Atkins C, Dumble M, Yang J, Anderson K, Wilson BJ, Emmitte KA, Rabindran SK, Kumar R. Antitumor activity of GSK1904529A, a small-molecule inhibitor of the insulin-like growth factor-I receptor tyrosine kinase. Clin Cancer Res. 2009;15(9):3058–3067. doi: 10.1158/1078-0432.CCR-08-2530. [DOI] [PubMed] [Google Scholar]
- 8.Beltran PJ, Mitchell P, Chung YA, Cajulis E, Lu J, Belmontes B, Ho J, Tsai MM, Zhu M, Vonderfecht S, Baserga R, Kendall R, Radinsky R, Calzone FJ. AMG 479, a fully human anti-insulin-like growth factor receptor type I monoclonal antibody, inhibits the growth and survival of pancreatic carcinoma cells. Mol Cancer Ther. 2009;8(5):1095–1105. doi: 10.1158/1535-7163.MCT-08-1171. [DOI] [PubMed] [Google Scholar]
- 9.Guix M, Faber AC, Wang SE, Olivares MG, Song Y, Qu S, Rinehart C, Seidel B, Yee D, Arteaga CL, Engelman JA. Acquired resistance to EGFR tyrosine kinase inhibitors in cancer cells is mediated by loss of IGF-binding proteins. J Clin Invest. 2008;118(7):2609–2619. doi: 10.1172/JCI34588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jones HE, Gee JM, Barrow D, Tonge D, Holloway B, Nicholson RI. Inhibition of insulin receptor isoform-A signalling restores sensitivity to gefitinib in previously de novo resistant colon cancer cells. Br J Cancer. 2006;95(2):172–180. doi: 10.1038/sj.bjc.6603237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hilmi C, Larribere L, Giuliano S, Bille K, Ortonne JP, Ballotti R, Bertolotto C. IGF1 promotes resistance to apoptosis in melanoma cells through an increased expression of BCL2, BCL-X(L), and survivin. J Investig Dermatol. 2008;128(6):1499–1505. doi: 10.1038/sj.jid.5701185. [DOI] [PubMed] [Google Scholar]
- 12.Mendivil A, Zhou C, Cantrell LA, Gehrig PA, Malloy KM, Blok LJ, Burger CW, Bae-Jump VL. AMG 479, a novel IGF-1-R antibody, inhibits endometrial cancer cell proliferation through disruption of the PI3K/Akt and MAPK pathways. Reprod Sci. 2011;18(9):832–841. doi: 10.1177/1933719111398501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beltran PJ, Chung YA, Moody G, Mitchell P, Cajulis E, Vonderfecht S, Kendall R, Radinsky R, Calzone FJ. Efficacy of ganitumab (AMG 479), alone and in combination with rapamycin, in Ewing's and osteogenic sarcoma models. J Pharmacol Exp Ther. 2011 doi: 10.1124/jpet.110.178400. [DOI] [PubMed] [Google Scholar]
- 14.Chung CH, Pohlmann PR, Rothenberg ML, Burkey BB, Parker J, Palka K, Aulino J, Puzanov I, Murphy B. Insulin-like growth factor-1 receptor inhibitor, AMG-479, in cetuximab-refractory head and neck squamous cell carcinoma. Head Neck. 2011;33(12):1804–1808. doi: 10.1002/hed.21478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tap WD, Demetri G, Barnette P, Desai J, Kavan P, Tozer R, Benedetto PW, Friberg G, Deng H, McCaffery I, Leitch I, Badola S, Chang S, Zhu M, Tolcher A. Phase II study of ganitumab, a fully human anti-type-1 insulin-like growth factor receptor antibody, in patients with metastatic Ewing family tumors or desmoplastic small round cell tumors. J Clin Oncol. 2012 doi: 10.1200/JCO.2011.37.2359. [DOI] [PubMed] [Google Scholar]
- 16.Kelley SK, Ashkenazi A. Targeting death receptors in cancer with Apo2L/TRAIL. Curr Opin Pharmacol. 2004;4(4):333–339. doi: 10.1016/j.coph.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 17.Rosevear HM, Lightfoot AJ, Griffith TS. Conatumumab, a fully human mAb against death receptor 5 for the treatment of cancer. Curr Opin Investig Drugs. 2010;11(6):688–698. [PubMed] [Google Scholar]
- 18.Wiezorek J, Holland P, Graves J. Death receptor agonists as a targeted therapy for cancer. Clin Cancer Res. 2010;16(6):1701–1708. doi: 10.1158/1078-0432.CCR-09-1692. [DOI] [PubMed] [Google Scholar]
- 19.Ashkenazi A, Pai RC, Fong S, Leung S, Lawrence DA, Marsters SA, Blackie C, Chang L, McMurtrey AE, Hebert A, DeForge L, Koumenis IL, Lewis D, Harris L, Bussiere J, Koeppen H, Shahrokh Z, Schwall RH. Safety and antitumor activity of recombinant soluble Apo2 ligand. J Clin Invest. 1999;104(2):155–162. doi: 10.1172/JCI6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zoog SJ, Ma CY, Kaplan-Lefko PJ, Hawkins JM, Moriguchi J, Zhou L, Pan Y, Hsu CP, Friberg G, Herbst R, Hill J, Juan G. Measurement of conatumumab-induced apoptotic activity in tumors by fine needle aspirate sampling. Cytometry A. 2010;77(9):849–860. doi: 10.1002/cyto.a.20940. [DOI] [PubMed] [Google Scholar]
- 21.Kaplan-Lefko PJ, Graves JD, Zoog SJ, Pan Y, Wall J, Branstetter DG, Moriguchi J, Coxon A, Huard JN, Xu R, Peach ML, Juan G, Kaufman S, Chen Q, Bianchi A, Kordich JJ, Ma M, Foltz IN, Gliniak BC. Conatumumab, a fully human agonist antibody to death receptor 5, induces apoptosis via caspase activation in multiple tumor types. Cancer Biol Ther. 2010;9(8):618–631. doi: 10.4161/cbt.9.8.11264. [DOI] [PubMed] [Google Scholar]
- 22.Herbst RS, Kurzrock R, Hong DS, Valdivieso M, Hsu CP, Goyal L, Juan G, Hwang YC, Wong S, Hill JS, Friberg G, LoRusso PM. A first-in-human study of conatumumab in adult patients with advanced solid tumors. Clin Cancer Res. 2010;16(23):5883–5891. doi: 10.1158/1078-0432.CCR-10-0631. [DOI] [PubMed] [Google Scholar]
- 23.Doi T, Murakami H, Ohtsu A, Fuse N, Yoshino T, Yamamoto N, Boku N, Onozawa Y, Hsu CP, Gorski KS, Friberg G, Kawaguchi T, Sasaki T. Phase 1 study of conatumumab, a pro-apoptotic death receptor 5 agonist antibody, in Japanese patients with advanced solid tumors. Cancer Chemother Pharmacol. 2011;68(3):733–741. doi: 10.1007/s00280-010-1544-1. [DOI] [PubMed] [Google Scholar]
- 24.Goetsch L, Gonzalez A, Leger O, Beck A, Pauwels PJ, Haeuw JF, Corvaia N. A recombinant humanized anti-insulin-like growth factor receptor type I antibody (h7C10) enhances the antitumor activity of vinorelbine and anti-epidermal growth factor receptor therapy against human cancer xenografts. Int J Cancer. 2005;113(2):316–328. doi: 10.1002/ijc.20543. [DOI] [PubMed] [Google Scholar]
- 25.Mitsiades CS, Treon SP, Mitsiades N, Shima Y, Richardson P, Schlossman R, Hideshima T, Anderson KC. TRAIL/Apo2L ligand selectively induces apoptosis and overcomes drug resistance in multiple myeloma: therapeutic applications. Blood. 2001;98(3):795–804. doi: 10.1182/blood.v98.3.795. [DOI] [PubMed] [Google Scholar]
- 26.Poulaki V, Mitsiades CS, Kotoula V, Tseleni-Balafouta S, Ashkenazi A, Koutras DA, Mitsiades N. Regulation of Apo2L/tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis in thyroid carcinoma cells. Am J Pathol. 2002;161(2):643–654. doi: 10.1016/S0002-9440(10)64220-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fischer-Posovszky P, Tornqvist H, Debatin KM, Wabitsch M. Inhibition of death-receptor mediated apoptosis in human adipocytes by the insulin-like growth factor I (IGF-I)/IGF-I receptor autocrine circuit. Endocrinology. 2004;145(4):1849–1859. doi: 10.1210/en.2003-0985. [DOI] [PubMed] [Google Scholar]
- 28.Wang Q, Wang X, Hernandez A, Hellmich MR, Gatalica Z, Evers BM. Regulation of TRAIL expression by the phosphatidylinositol 3-kinase/Akt/GSK-3 pathway in human colon cancer cells. J Biol Chem. 2002;277(39):36602–36610. doi: 10.1074/jbc.M206306200. [DOI] [PubMed] [Google Scholar]
- 29.Pennarun B, Kleibeuker JH, Oenema T, Stegehuis JH, de Vries EG, de Jong S. Inhibition of IGF-1R-dependent PI3K activation sensitizes colon cancer cells specifically to DR5-mediated apoptosis but not to rhTRAIL. CellOncol (Dordr) 2011;34(3):245–259. doi: 10.1007/s13402-011-0033-9. [DOI] [PubMed] [Google Scholar]
- 30.Ashkenazi A, Herbst RS. To kill a tumor cell: the potential of proapoptotic receptor agonists. J Clin Invest. 2008;118(6):1979–1990. doi: 10.1172/JCI34359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Larsson O, Girnita A, Girnita L. Role of insulin-like growth factor 1 receptor signalling in cancer. Br J Cancer. 2005;92(12):2097–2101. doi: 10.1038/sj.bjc.6602627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Samani AA, Yakar S, LeRoith D, Brodt P. The role of the IGF system in cancer growth and metastasis: overview and recent insights. Endocr Rev. 2007;28(1):20–47. doi: 10.1210/er.2006-0001. [DOI] [PubMed] [Google Scholar]
- 33.Ewing GP, Goff LW. The insulin-like growth factor signaling pathway as a target for treatment of colorectal carcinoma. Clin Colorectal Cancer. 2010;9(4):219–223. doi: 10.3816/CCC.2010.n.032. [DOI] [PubMed] [Google Scholar]
- 34.Olmos D, Martins AS, Jones RL, Alam S, Scurr M, Judson IR. Targeting the insulin-like growth factor 1 receptor in Ewing's sarcoma: reality and expectations. Sarcoma. 2011:402508. doi: 10.1155/2011/402508. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ho AL, Schwartz GK. Targeting of insulin-like growth factor type 1 receptor in Ewing sarcoma: unfulfilled promise or a promising beginning? J Clin Oncol. 2011;29(34):4581–4583. doi: 10.1200/JCO.2011.38.2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rieder S, Michalski CW, Friess H, Kleeff J. Insulin-like growth factor signaling as a therapeutic target in pancreatic cancer. Anticancer Agents Med Chem. 2011;11(5):427–433. doi: 10.2174/187152011795677454. [DOI] [PubMed] [Google Scholar]
- 37.Beauchamp MC, Yasmeen A, Knafo A, Gotlieb WH. Targeting insulin and insulin-like growth factor pathways in epithelial ovarian cancer. J Oncol. 2010 doi: 10.1155/2010/257058. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Karp DD, Paz-Ares LG, Novello S, Haluska P, Garland L, Cardenal F, Blakely LJ, Eisenberg PD, Langer CJ, Blumenschein G, Jr, Johnson FM, Green S, Gualberto A. Phase II study of the anti-insulin-like growth factor type 1 receptor antibody CP-751,871 in combination with paclitaxel and carboplatin in previously untreated, locally advanced, or metastatic non-small-cell lung cancer. J Clin Oncol. 2009;27(15):2516–2522. doi: 10.1200/JCO.2008.19.9331. [DOI] [PubMed] [Google Scholar]
- 39.Olmos D, Postel-Vinay S, Molife LR, Okuno SH, Schuetze SM, Paccagnella ML, Batzel GN, Yin D, Pritchard-Jones K, Judson I, Worden FP, Gualberto A, Scurr M, de Bono JS, Haluska P. Safety, pharmacokinetics, and preliminary activity of the anti-IGF-1R antibody figitumumab (CP-751,871) in patients with sarcoma and Ewing's sarcoma: a phase 1 expansion cohort study. Lancet Oncol. 2010;11(2):129–135. doi: 10.1016/S1470-2045(09)70354-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Clopper CJ, Pearson ES. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometricka. 1934;26(4):404–413. [Google Scholar]
- 41.Gombos A, Metzger-Filho O, Dal Lago L, Awada-Hussein A. Clinical development of insulin-like growth factor receptor-1 (IGF-1R) inhibitors: at the crossroad? Investig New Drugs. 2012 doi: 10.1007/s10637-012-9811-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boone DN, Lee AV. Targeting the insulin-like growth factor receptor: developing biomarkers from gene expression profiling. Crit Rev Oncog. 2012;17(2):161–173. doi: 10.1615/critrevoncog.v17.i2.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Camidge DR, Herbst RS, Gordon MS, Eckhardt SG, Kurzrock R, Durbin B, Ing J, Tohnya TM, Sager J, Ashkenazi A, Bray G, Mendelson D. A phase I safety and pharmacokinetic study of the death receptor 5 agonistic antibody PRO95780 in patients with advanced malignancies. Clin Cancer Res. 2010;16(4):1256–1263. doi: 10.1158/1078-0432.CCR-09-1267. [DOI] [PubMed] [Google Scholar]
- 44.Pan Y, Haddad V, Sabin T, Baker N, Hei YJ, Galimi F, Graves J, Huang C, Cottrell S. Predictive value of Fc gamma receptor IIIa genotype in response to conatumumab in three phase II studies. J Clin Oncol. 2011;29(suppl) Abstract 3103. [Google Scholar]
- 45.De Roock W, Biesmans B, De Schutter J, Tejpar S. Clinical biomarkers in oncology: focus on colorectal cancer. Mol Diagn Ther. 2009;13(2):103–114. doi: 10.1007/BF03256319. [DOI] [PubMed] [Google Scholar]
- 46.Tan DS, Thomas GV, Garrett MD, Banerji U, de Bono JS, Kaye SB, Workman P. Biomarker-driven early clinical trials in oncology: a paradigm shift in drug development. Cancer J. 2009;15(5):406–420. doi: 10.1097/PPO.0b013e3181bd0445. [DOI] [PubMed] [Google Scholar]
- 47.Haluska P, Shaw HM, Batzel GN, Yin D, Molina JR, Molife LR, Yap TA, Roberts ML, Sharma A, Gualberto A, Adjei AA, de Bono JS. Phase I dose escalation study of the anti insulin-like growth factor-I receptor monoclonal antibody CP-751,871 in patients with refractory solid tumors. Clin Cancer Res. 2007;13(19):5834–5840. doi: 10.1158/1078-0432.CCR-07-1118. [DOI] [PubMed] [Google Scholar]
- 48.Hidalgo M, Tirado Gomez M, Lewis N, Vuky JL, Taylor G. A phase I study of MK-0646, a humanized monoclonal antibody against the insulin-like growth factor receptor type 1 (IGF1R) in advanced solid tumor patients in a q2 wk schedule. J Clin Oncol. 2008;26(Suppl):3520. [Google Scholar]
- 49.Tolcher AW, Mita M, Meropol NJ, von Mehren M, Patnaik A, Padavic K, Hill M, Mays T, McCoy T, Fox NL, Halpern W, Corey A, Cohen RB. Phase I pharmacokinetic and biologic correlative study of mapatumumab, a fully human monoclonal antibody with agonist activity to tumor necrosis factor-related apoptosis-inducing ligand receptor-1. J Clin Oncol. 2007;25(11):1390–1395. doi: 10.1200/JCO.2006.08.8898. [DOI] [PubMed] [Google Scholar]
- 50.Plummer R, Attard G, Pacey S, Li L, Razak A, Perrett R, Barrett M, Judson I, Kaye S, Fox NL, Halpern W, Corey A, Calvert H, de Bono J. Phase 1 and pharmacokinetic study of lexatumumab in patients with advanced cancers. Clin Cancer Res. 2007;13(20):6187–6194. doi: 10.1158/1078-0432.CCR-07-0950. [DOI] [PubMed] [Google Scholar]