Summary
Background
Treatment options for metastatic colorectal cancer (CRC) are limited after a fluoropyrimi-dine, oxaliplatin and irinotecan; novel agents need to be explored in this setting. Dasatinib, an oral inhibitor of Src family kinases, inhibits proliferation in CRC cell lines and has antitumor activity in CRC xenograft models.
Patients and methods
We conducted a multi-center phase II trial of dasatinib in unresectable, previously-treated metastatic CRC patients. No more than 2 prior chemotherapy regimens were permitted, which must have contained a fluoropyrimidine, oxaliplatin and irinotecan. The primary endpoint was progression-free survival (PFS) at 4 months. The Simon two-stage design required that at least 5 of the first 19 patients be progression-free at 4 months to expand to a second stage.
Results
Nineteen patients enrolled at 9 centers. The study was terminated after the first stage due to lack of efficacy. There were no objective responses; 1 patient (5%) had stable disease for 7.3 months. The PFS rate at 4 months was 5.3% (90% CI: 0.3, 22.6). Median PFS was 1.6 months (90% CI: 1.4, 1.8). Median overall survival was 5.1 months (90% CI: 2.4, 6.3). Grade 3/4 toxicities included fatigue in 16% of patients, and anemia, anorexia, nausea/vomiting and dyspnea in 11%.
Conclusion
Dasatinib is inactive as a single agent in previously treated metastatic CRC patients.
Keywords: Colorectal cancer, Phase II trial, Dasatinib
Introduction
Despite major advances in the treatment of metastatic colorectal cancer (CRC) over the past two decades, novel agents are still needed for patients with treatment-refractory disease. The median progression-free survival of patients who have progressed on a fluoropyrimidine, oxaliplatin and irinotecan is only 1.7 months; their median overall survival is only 6 months [1]. Anti-EGFR monoclonal antibodies (cetuximab or panitumumab) are an additional treatment option for patients with KRAS wild-type tumors, but their impact on PFS and OS is quite modest [2, 3].
Dasatinib (Sprycel®, Bristol-Myers Squibb), is an oral protein tyrosine kinase inhibitor that targets the Src family kinases including Fyn, Yes, Src, and Lyk, as well as BCR ABL, c-kit, EphA2, and platelet derived growth factor receptor [4]. Src regulates a number of signal transduction pathways that are important for tumor cell survival, including resistance to detachment-induced apoptosis (anoikis) [5]. Src activity is upregulated in greater than 80% of primary CRC, is even higher in liver metastases [6], and correlates with a poor prognosis [7]. An antisense expression vector specific for c-Src decreases the tumorigenicity of CRC cell lines [8], and specifically downregulates the expression of vascular endothelial growth factor (VEGF) [9]. Dasatinib, and the Src inhibitor SKI-606 (bosutinib), have antiproliferative activity in CRC cell lines and antitumor activity in CRC xenograft models [10, 11].
The preclinical data regarding dasatinib in CRC prompted us to evaluate it as a single agent in a phase II trial in patients with treatment-refractory metastatic CRC.
Patients and methods
Eligible patients had histologically or cytologically confirmed metastatic CRC that was not amenable to potentially curative surgery, measurable disease as defined by RECIST version 1.0 [12], ECOG performance status of 0 to 2, adequate bone marrow (granulocytes ≥1,500/μL, platelets ≥100,000/μL), hepatic (total bilirubin ≤1.5 times institutional upper limit of normal; transaminases ≤2.5 times institutional upper limit of normal if no hepatic metastases, or ≤5 times institutional upper limit of normal if hepatic metastases), and renal function (serum creatinine within normal institutional limits or creatinine clearance ≥60 mL/min/1.73 m2). Patients must have received no more than two prior chemotherapy regimens, given in the adjuvant or metastatic settings. The chemotherapy regimens must have included a fluoropyrimidine (5-fluorouracil or capecitabine), oxaliplatin, and irinotecan, and disease progression must have been documented (per RECIST version 1.0) either during or after chemotherapy treatment. Prior treatment with VEGF and/or epidermal growth factor receptor (EGFR) inhibitors was permitted but not required. Prior radiation therapy was allowed, if measurable disease was located outside of the radiation port and more than 4 weeks had elapsed since completion. Exclusion criteria included brain metastases, pregnancy or lactation, concurrent use of potent inducers or inhibitors of CYP3A4, baseline QTc prolongation, clinically significant cardiovascular disease, or uncontrolled inter-current illnesses. All subjects provided written informed consent according to federal and institutional guidelines.
Study evaluations
Pretreatment evaluation included a medical history and physical exam, complete blood count with differential, chemistry panel, pregnancy test, and a computed tomography scan of the chest, abdomen and pelvis. One cycle equaled 28 days. A history and physical exam was repeated every 14 days for the first 2 cycles, then on day 1 of each subsequent cycle. A complete blood count with differential and chemistry panel was repeated every 7 days. Patients received a minimum of 2 cycles unless unacceptable toxicity or rapid progression of disease occurred. Response was evaluated by CT scan according to RECIST criteria [12] every 2 cycles.
Drug administration
Dasatinib was initially dosed at 100 mg orally twice daily (200 mg total daily dose) continuously. Following an amendment in October of 2007 mandated by the National Cancer Institute (NCI) due to drug toxicity observed in other trials, all subsequent patients received either 70 mg twice daily (140 mg total daily dose) or 100 mg in the morning and 50 mg in the evening (150 mg total daily dose).
Adverse effects were graded according to NCI Common Toxicity Criteria version 3.0. For grade 3 or greater hematologic or non-hematologic toxicity attributable to dasatinib at the post-amendment dose, the drug was held for a maximum of 14 days until recovery to grade 1 or 2, and the dose was reduced to 50 mg twice daily. For a recurrent grade 3 or greater toxicity, dasatinib was again held until the toxicity decreased to grade 1 or 2, and the dose was further reduced to 100 mg once daily. No further dose reductions were permitted. If additional dose reductions were required, the patient was removed from the study.
Statistical design
The primary endpoint of this study was the progression-free survival (PFS) rate at 4 months. Secondary endpoints included objective response rate (complete response + partial response), toxicity, and overall survival. PFS and overall survival were calculated from the start of study treatment. The trial was conducted using a Simon optimal 2-stage design to test the null hypothesis that the 4-month PFS rate was less than or equal to 20% versus the alternative that it was at least 40% [13]. Nineteen patients were to be enrolled in the first stage, and all patients were to be evaluable for the primary endpoint. Those with early discontinuation of treatment or early death were considered treatment failures. If four or fewer patients were alive and progression-free at 4 months, the trial would be terminated for lack of efficacy. Otherwise, an additional 35 patients would be accrued, and if 16 or more patients (out of the 54) were alive and progression-free at 4 months, the drug would be considered worthy of further study. This design yields a 0.90 probability of a positive result if the true 4- month PFS rate is at least 40%. Progression-free and overall survival curves were constructed using the method of Kaplan and Meier [14]. Confidence intervals for the median PFS and overall survival times were derived as described by Brookmeyer and Crowley [15].
Results
Patient characteristics
Nineteen patients were enrolled at 9 centers between October 2007 and November 2008. Patient characteristics are listed in Table 1. Fifty-eight percent of patients were male. The median age was 64. The majority of patients (58%) had an ECOG performance status of 1. Most had liver metastases (84%), and 42% had metastases to both liver and lung. All patients had received two prior lines of chemotherapy, and the majority had been previously treated with bevacizumab (89%) and either cetuximab or panitumumab (68%). Only 16% had received adjuvant chemotherapy.
Table 1.
Patient characteristics (N=19)
| Characteristics | No. of Patients (%) |
|---|---|
| Age | |
| Median | 64 |
| Range | 38-83 |
| Sex | |
| Male | 11 (58) |
| Female | 8 (42) |
| ECOG Performance Status | |
| 0 | 8 (42) |
| 1 | 11 (58) |
| 2 | 0 (0) |
| Sites of visceral metastatic disease | |
| Liver only | 8 (42) |
| Lung only | 3 (16) |
| Liver and lung | 8 (42) |
| Prior therapies | |
| 2 lines of chemotherapy | 19 (100) |
| Adjuvant FOLFOX | 3 (16) |
| Bevacizumab | 17 (89) |
| Cetuximab or panitumumab | 13 (68) |
The first 2 patients, treated prior to the amendment, received 100 mg twice daily. All subsequent patients received either 70 mg twice daily (140 mg total daily dose; 6 patients) or 100 mg in the morning and 50 mg in the evening (150 mg total daily dose; 11 patients). Tablets were available in 20 mg or 50 mg strengths, and the unavailability of 20 mg tablets at several centers resulted in the use of the 100 mg and 50 mg regimen.
A total of 34 cycles were delivered (median 1.9, range <1– 8). Dose reductions and/or interruptions were required in 32% of patients. Six patients withdrew from the study before completing 2 cycles of treatment. One patient developed grade 5 ventricular fibrillation 5 days after starting dasatinib. One patient died of disease progression. Three patients withdrew from the trial for toxicity before the first CT scan: one patient developed grade 3 anorexia, nausea, and fatigue, one experienced grade 3 pleural effusion, and one had grade 3 abdominal pain. An additional patient withdrew for personal reasons and died 4 weeks later.
Response and survival
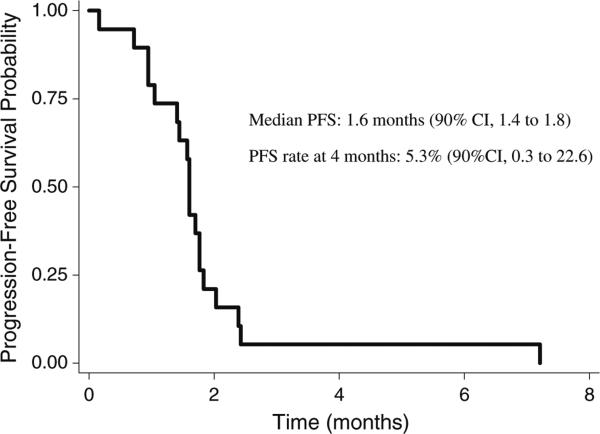
There were no objective responses. One patient (5%) had stable disease, maintained for 7.3 months. Twelve patients (63%) had progressive disease, while the remaining 6 patients (32%) discontinued treatment before the first CT scan. The 4-month PFS rate was 5.3% (90% CI, 0.3 to 22.6%). The Kaplan-Meier curve for PFS is presented in Fig. 1. The estimated median PFS was 1.6 months (90% CI, 1.4 to 1.8). The Kaplan-Meier curve for overall survival is shown in Fig. 2. The estimated median overall survival was 5.1 months (90% CI, 2.4 to 6.3). The six-month survival probability was 0.37 (90% CI, 0.19 to 0.54).
Fig. 1.
Kaplan-Meier estimate of progression-free survival (PFS)
Fig. 2.
Kaplan-Meier estimate of overall survival
Accrual to the study was terminated early due to lack of efficacy, according to the Simon 2-stage trial design. Even if the 2 patients with early discontinuation of treatment who survived more than 4 months were counted as being progression-free at 4 months, criteria for continuing to the second stage would not have been met.
Toxicity
All patients were evaluable for toxicity. The most common toxicities are summarized in Table 2. Anemia was the most frequent hematologic toxicity, developing in 48% of patients, though only 11% of patients experienced grade 3 anemia. Dyspnea, which developed in 26% of patients, was grade 3 or greater in 11% of patients. Other grade 3/4 toxicities potentially attributable to dasatinib included: fatigue (16%), nausea/vomiting (11%), anorexia (11%), diarrhea (5%), abdominal pain (5%), and pleural effusion (5%). There was a single grade 5 toxicity, cardiac arrhythmia (5%), which was potentially attributable to dasatinib. Both patients treated at 100 mg twice daily developed at least one grade 3 or greater toxicity. Five of the 6 (83%) patients treated at 70 mg twice daily and 6 of 11 (55%) patients treated at 100 mg in the morning and 50 mg in the evening experienced grade 3 or greater toxicities.
Table 2.
Most frequent toxicities, according to the National Cancer Institute common toxicity criteria version 3.0 (N= 19 patients)
| Grade 1 % | Grade 2 % | Grade 3 % | Grade 4 % | |
|---|---|---|---|---|
| Hematologic | ||||
| Anemia | 21 | 16 | 11 | 0 |
| Neutropenia | 21 | 5 | 0 | 0 |
| Thrombocytopenia | 32 | 0 | 0 | 0 |
| Non-hematologic | ||||
| Fatigue | 21 | 42 | 16 | 0 |
| Nausea/vomiting | 21 | 21 | 11 | 0 |
| Anorexia | 16 | 16 | 11 | 0 |
| Dyspnea | 11 | 5 | 5 | 5 |
| Diarrhea | 32 | 5 | 5 | 0 |
| Abdominal pain | 5 | 26 | 5 | 0 |
| Pleural effusion | 5 | 5 | 5 | 0 |
| Cardiac arrhythmia | 0 | 0 | 0 | 5a |
Grade 5 toxicity: ventricular fibrillation with cardiac arrest
Discussion
We evaluated dasatinib as a single agent in patients with previously-treated metastatic CRC, and observed a PFS rate at 4 months that was consistent with the null hypothesis that the PFS rate at 4 months is less than or equal to 20%. Furthermore, we observed no objective responses, and a median PFS and overall survival that are comparable to those seen with best supportive care in previous studies [1]. We therefore conclude that dasatinib is inactive in patients with previously treated metastatic CRC.
It is disappointing that the preclinical data suggesting activity for dasatinib in CRC was not substantiated in this phase II study. Unfortunately, our results are similar to those observed with dasatinib monotherapy in other solid tumors, such as relapsed chemo-sensitive small cell lung cancer [16] and advanced head and neck cancer [17]. It may be that Src inhibition alone is not enough to disrupt the tumorigenicity of metastatic lesions.
This was a heavily pretreated population, as all patients had progressed on standard chemotherapy and the majority had also failed available biologic therapies (the VEGF inhibitor bevacizumab and the EGFR inhibitors cetuximab or panitumumab). The lack of efficacy of dasatinib monotherapy for metastatic CRC in this heavily pretreated setting does not necessarily preclude its activity in combination with other agents in this disease, however. Preclinical data suggests that dasatinib may work synergistically with oxaliplatin [18] and curcumin [19]. Early results of a phase IB study of dasatinib in combination with FOLFOX and cetuximab in metastatic CRC were promising, with a median PFS of 4.6 months in all patients and a partial response rate of 17% in patients previously refractory to FOLFOX and cetuximab [20]. A follow-up phase II study at the recommended phase II dose of dasatinib 150 mg daily is ongoing [21].
The ability of patients to tolerate dasatinib was limited by fatigue, gastrointestinal side effects (anorexia, nausea/vomiting, diarrhea) and pulmonary toxicities (dyspnea and pleural effusion). Dose reductions and/or interruptions were not uncommon (occurring in 32% of patients), and 3 patients withdrew from the trial prior to the first CTscan due to grade 3 treatment-related toxicities. We observed more grade 3–4 toxicity than was reported in a phase I dose-escalation study of dasatinib in patients with advanced solid tumors, in which none of the 5 patients treated at 70 mg twice daily continuously (the recommended phase II dose) experienced grade 3–4 toxicity [22]. Since the baseline patient characteristics were similar in the two studies, one reason for the discrepancy might be the longer median duration of treatment (2 cycles, or 2 months) in our study compared to the phase I study (1 month) at this dose level. Additionally, the first two patients in our study (prior to the amendment) were treated at an initial dose of 100 mg twice daily, and both experienced grade 3 toxicities. Once daily dosing may be better tolerated than twice daily dosing. The maximum tolerated dose of dasatinib was 180 mg daily in a phase I study, with pleural effusion as the dose-limiting toxicity [23]. A randomized study of once daily versus twice daily dosing of dasatinib in patients with chronic-phase chronic myeloid leukemia demonstrated that 100 mg once daily yielded less hematologic and non-hematologic toxicity than 70 mg twice daily, with comparable efficacy [24].
The median PFS and overall survival observed in our study emphasize the poor prognosis of this heavily pre-treated patient population, despite reasonably good performance status at the time of enrollment. We conclude that dasatinib given as a single agent does not appear to be active in advanced CRC. Other novel agents for these patients need to be developed.
Acknowledgments
Funding source NCI Grant N01-CM-62201.
Footnotes
Presented at the 2011 Gastrointestinal Cancers Symposium, January 20–22, 2011, San Francisco, CA, USA.
Contributor Information
Manish R. Sharma, Section of Hematology/Oncology, University of Chicago, 5841 S. Maryland Avenue, MC 2115, Chicago, IL 60637-1470, USA
Kristen Wroblewski, Department of Health Studies, University of Chicago, Chicago, IL, USA.
Blase N. Polite, Section of Hematology/Oncology, University of Chicago, 5841 S. Maryland Avenue, MC 2115, Chicago, IL 60637-1470, USA
James A. Knost, Illinois Cancer Care, Peoria, IL, USA
James A. Wallace, Section of Hematology/Oncology, University of Chicago, 5841 S. Maryland Avenue, MC 2115, Chicago, IL 60637-1470, USA
Sanjiv Modi, Joliet Hematology/Oncology, Joliet, IL, USA.
Bethany G. Sleckman, St Johns Mercy Medical Center, St Louis, MO, USA
David Taber, Michiana Hematology/Oncology, South Bend, IN, USA.
Everett E. Vokes, Section of Hematology/Oncology, University of Chicago, 5841 S. Maryland Avenue, MC 2115, Chicago, IL 60637-1470, USA
Walter M. Stadler, Section of Hematology/Oncology, University of Chicago, 5841 S. Maryland Avenue, MC 2115, Chicago, IL 60637-1470, USA
Hedy L. Kindler, Section of Hematology/Oncology, University of Chicago, 5841 S. Maryland Avenue, MC 2115, Chicago, IL 60637-1470, USA
References
- 1.Van Cutsem E, Peeters M, Siena S, et al. Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol. 2007;25(13):1658–1664. doi: 10.1200/JCO.2006.08.1620. [DOI] [PubMed] [Google Scholar]
- 2.Amado RG, Wolf M, Peeters M, et al. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26(10):1626–1634. doi: 10.1200/JCO.2007.14.7116. [DOI] [PubMed] [Google Scholar]
- 3.Dahabreh IJ, Terasawa T, Castaldi PJ, Trikalinos TA. Systematic review: anti-epidermal growth factor receptor treatment effect modification by KRAS mutations in advanced colorectal cancer. Ann Intern Med. 2011;154(1):37–49. doi: 10.7326/0003-4819-154-1-201101040-00006. [DOI] [PubMed] [Google Scholar]
- 4.Mayer EL, Krop IE. Advances in targeting SRC in the treatment of breast cancer and other solid malignancies. Clin Cancer Res. 2010;16(14):3526–3532. doi: 10.1158/1078-0432.CCR-09-1834. [DOI] [PubMed] [Google Scholar]
- 5.Windham TC, Parikh NU, Siwak DR, et al. Src activation regulates anoikis in human colon tumor cell lines. Oncogene. 2002;21(51):7797–7807. doi: 10.1038/sj.onc.1205989. [DOI] [PubMed] [Google Scholar]
- 6.Talamonti MS, Roh MS, Curley SA, Gallick GE. Increase in activity and level of pp 60c-src in progressive stages of human colorectal cancer. J Clin Invest. 1993;91(1):53–60. doi: 10.1172/JCI116200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aligayer H, Boyd DD, Heiss MM, et al. Activation of Src kinase in primary colorectal carcinoma: an indicator of poor clinical prognosis. Cancer. 2002;94(2):344–351. doi: 10.1002/cncr.10221. [DOI] [PubMed] [Google Scholar]
- 8.Staley CA, Parikh NU, Gallick GE. Decreased tumorigenicity of a human colon adenocarcinoma cell line by an antisense expression vector specific for c-Src. Cell Growth Differ. 1997;8(3):269–274. [PubMed] [Google Scholar]
- 9.Ellis LM, Staley CA, Liu W, et al. Down-regulation of vascular endothelial growth factor in a human colon carcinoma cell line transfected with an antisense expression vector specific for c-src. J Biol Chem. 1998;273(2):1052–1057. doi: 10.1074/jbc.273.2.1052. [DOI] [PubMed] [Google Scholar]
- 10.Golas JM, Lucas J, Etienne C, et al. SKI-606, a Src/Abl inhibitor with in vivo activity in colon tumor xenograft models. Cancer Res. 2005;65(12):5358–5364. doi: 10.1158/0008-5472.CAN-04-2484. [DOI] [PubMed] [Google Scholar]
- 11.Dasatinib Investigator's Brochure 2006.
- 12.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92(3):205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 13.Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials. 1989;10(1):1–10. doi: 10.1016/0197-2456(89)90015-9. [DOI] [PubMed] [Google Scholar]
- 14.Kaplan E, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 15.Brookmeyer R, Crowley J. A confidence interval for the median survival time. Biometrics. 1982;38:29–41. [Google Scholar]
- 16.Miller AA, Pang H, Hodgson L, et al. A phase II study of dasatinib in patients with chemosensitive relapsed small cell lung cancer (Cancer and Leukemia Group B 30602). J Thorac Oncol. 2010;5(3):380–384. doi: 10.1097/JTO.0b013e3181cee36e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brooks HD, Glisson BS, Bekele BN, et al. Phase 2 study of dasatinib in the treatment of head and neck squamous cell carcinoma. Cancer. 2010 doi: 10.1002/cncr.25769. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kopetz S, Lesslie DP, Dallas NA, et al. Synergistic activity of the SRC family kinase inhibitor dasatinib and oxaliplatin in colon carcinoma cells is mediated by oxidative stress. Cancer Res. 2009;69(9):3842–3849. doi: 10.1158/0008-5472.CAN-08-2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nautiyal J, Banerjee S, Kanwar SS, et al. Curcumin enhances dasatinib-induced inhibition of growth and transformation of colon cancer cells. Int J Cancer. 2011;128(4):951–961. doi: 10.1002/ijc.25410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lieu CH, Wolff RA, Eng C, et al. Phase IB study of the Src inhibitor dasatinib with FOLFOX and cetuximab in metastatic colorectal cancer. J Clin Oncol. 2010;28:3536. [Google Scholar]
- 21. [January 17, 2011]; http://www.clinicaltrials.gov/ct2/show/NCT00501410?term=dasatinib+FOLFOX+cetuximab&rank=1.
- 22.Demetri GD, Lo Russo P, MacPherson IR, et al. Phase I dose-escalation and pharmacokinetic study of dasatinib in patients with advanced solid tumors. Clin Cancer Res. 2009;15(19):6232–6240. doi: 10.1158/1078-0432.CCR-09-0224. [DOI] [PubMed] [Google Scholar]
- 23.Johnson FM, Agrawal S, Burris H, et al. Phase 1 pharmacokinetic and drug-interaction study of dasatinib in patients with advanced solid tumors. Cancer. 2010;116(6):1582–1591. doi: 10.1002/cncr.24927. [DOI] [PubMed] [Google Scholar]
- 24.Shah NP, Kantarjian HM, Kim DW, et al. Intermittent target inhibition with dasatinib 100 mg once daily preserves efficacy and improves tolerability in imatinib-resistant and intolerant chronic-phase chronic myeloid leukemia. J Clin Oncol. 2008;26(19):3204–3212. doi: 10.1200/JCO.2007.14.9260. [DOI] [PubMed] [Google Scholar]