Abstract
Similarity in oldest parturitions in humans and great apes suggests that we maintain ancestral rates of ovarian aging. Consistent with that hypothesis, previous counts of primordial follicles in postmortem ovarian sections from chimpanzees (Pan troglodytes) showed follicle stock decline at the same rate that human stocks decline across the same ages. Here, we correct that finding with a chimpanzee sample more than three times larger than the previous one, which also allows comparison into older ages. Analyses show depletion rates similar until about age 35, but after 35, the human counts continue to fall with age, while the change is much less steep in chimpanzees. This difference implicates likely effects on ovarian dynamics from other physiological systems that are senescing at different rates, and, potentially, different perimenopausal experience for chimpanzees and humans.
Electronic supplementary material
The online version of this article (doi:10.1007/s11357-015-9746-4) contains supplementary material, which is available to authorized users.
Keywords: Menopause, Reproductive aging, Hominid evolution
Introduction
All mammalian females build finite oocyte stocks during early development that deplete continuously thereafter, mostly through the process of atresia (Baker 1963; McGee and Hsueh 2000; vom Saal 1994). Initial size of the oocyte pool—as well as the rate of its exhaustion with age—varies widely across species (Gosden and Tefler 1987). In humans, this follicle stock reaches maximum size in the fifth fetal month and drops approximately 80 % before birth (Baker 1963). Gosden and Tefler (1987) have shown correlations in stock size with longevity across 19 mammal species, with human stocks of a size expected for longevities of the other great apes.
Striking parallels in reproductive aging between humans and chimpanzees—our nearest living relatives—have been recognized for some time (Gould et al. 1981; Graham 1979, 1981), although such similarities have occasionally been questioned (Finch and Sapolsky 1999; Videan et al. 2006, 2008). Investigating these similarities, Jones et al. (2007) counted primordial follicles in ovarian sections taken at necropsy from chimpanzees ranging in ages at death from 0 to 47 years and compared them with counts in human ovaries across the same ages. Fitting linear models to log-transformed counts, they found rates of decline with age in humans and chimpanzees to be statistically indistinguishable.
Here, we expand the number of chimpanzees represented from 19 to 67. Of those, we use 65 that are within the 0–51 age range of the human sample to model and compare patterns of change with age in the two species. This larger chimpanzee sample allows non-linear modeling, which reveals interspecific differences in rates of follicle stock decline that become especially marked at older ages.
The comparison between chimpanzees and humans is of particular evolutionary interest because oldest parity is in the forties in both species, while women, but not chimpanzee females, usually remain strong and healthy through and beyond their fertile years (Finch 2010; Gurven and Kaplan 2007; Hawkes 2010; Levitis et al. 2013). The follicle counts come—necessarily—from Western women and captive chimpanzees, where mortality is very low compared to other populations of each species. However, the cross-species similarity in terminal fertility and distinctive human postfertile life stage holds across populations with very different overall mortality levels (Hawkes et al. 2009). In all human populations, most females, if they survive childhood, live well beyond their fertile years, but chimpanzees succumb to geriatric morbidities while still cycling (Boesch and Boesch-Achermann 2000; Emery-Thompson et al. 2007; Nishida et al. 2003; Sugiyama 2004; Wich et al. 2004). Even in captivity, chimpanzees rarely survive into their forties (Lacreuse et al. 2008). However, stable diets and extensive veterinary care nearly double average adult life spans (Dyke et al. 1995), and a few achieve sufficiently advanced ages to experience a period of clinical menopause—defined retrospectively after 1 year without menses (Burger 1999; Herndon et al. 2012).
Classic papers in evolutionary life history theory (Hamilton 1966; Williams 1957) saw the notable postmenopausal survival of women as an indication of the importance of this life stage in human evolution. Subsequent lines of evidence are consistent with the grandmother hypothesis that increased longevity evolved in our lineage without a concurrent shift in the ancestral rate of ovarian aging. That hypothesis proposes that when ecological changes restricted the foods young juveniles could manage, the increased dependence of juveniles gave older females, whose fertility was declining, a new way to increase their fitness. By subsidizing those juveniles, ancestral grandmothers allowed mothers to have next babies sooner. As more robust elders left more descendants, longevity increased in subsequent generations ( Hawkes et al. 1998; Hawkes 2003, 2010; Kim et al. 2012, 2014; see Hawkes and Coxworth 2013 for review).
The hypothesis that greater longevity evolved in our lineage without a shift in ovarian aging was bolstered by the similarity in rates of ovarian follicle loss in chimpanzees and humans reported by Jones et al. (2007). Analysis of the larger sample now accumulated corrects that result, showing interspecific differences that become especially marked by the mid-thirties. After reporting these results, we briefly consider the interactions between ovaries and other physiological systems in cycling dynamics, which may help explain this difference.
Materials and methods
Subjects
We requested ovarian tissue samples from routine necropsies performed at deaths unrelated to our project at various primate research centers throughout the USA. All institutions were endorsed by the Association for Accreditation of Laboratory Animal Care International, and all animals represented in this study were provided with exceptional clinical care.
Tissue samples from a total of 67 common chimpanzees (Pan troglodytes) were examined, including the 19 individuals originally analyzed by Jones et al. (2007). In this larger dataset, ages range from 1 day to 56 years (Table S1). We report our full sample, but the human dataset we use for comparison (Hansen et al. 2008, described below) includes ages 0–51, so we use the same age range for chimpanzees—excluding the two oldest—for a total of 65 chimpanzees in our statistical model comparisons. We subsequently include the two oldest to further characterize the change with age in older chimpanzees.
Because we could not always reliably determine exact ages, we rounded each to the nearest year for statistical analyses. In most cases (n = 65), mounted ovarian tissue samples had been archived by the research institution for pathological examination. In others (n = 2), the research center granted us immediate access to the entire ovary upon the death of an animal. In these cases, tissues were sent to AML Laboratories to be grossed, stained, and mounted. In archived tissues, one to three slides were examined for primordial follicle counts. In cases where we had access to the entire organ, we fixed and analyzed a minimum of two slides per ovary.
Histology
Following the methods of Jones et al. (2007), we focused on primordial follicles, defined as oocytes surrounded by one layer of flattened granulosa cells (Gougeon 1996; Miller et al. 1997) because they provide the best assay of remaining follicular stocks (Miller et al. 1999). Primordial follicles tend to cluster in the superficial cortex of the ovary, just beneath the dense tunica albuginea; as a result, archived sections were only included if they showed part of an edge. For the two cases in which we had whole ovaries, the 5-μm sections were cut perpendicular to the longest dimension and we chose sections for counting from near the center of each ovary.
Comparative analyses
A single counter (CTC) examined 137 slides that met these criteria from 67 individuals, counting each slide twice to ensure accuracy. In order to determine count reliability, we estimated the coefficient of variation (CV) for counts of the same slides. Averaged across the entire sample, we found insignificant differences in follicle counts (CV 7.3 %).
To characterize follicle loss in chimpanzees, we first calculated mean follicle counts for each individual. This involved averaging across multiple counts of each slide and, in some cases, multiple slides for the same individual. We report the means, standard deviations, raw counts, and confidence intervals for each individual in Table S1.
Our human comparison is from Hansen et al. (2008), who report the estimated number of non-growing follicles by age in whole ovaries in American women aged 0–51 years. In this case, ovaries were collected from individuals who had elected to undergo oophorectomies or through collaboration with organ donation agencies. Chimpanzee counts come from cross sections, each of which represents approximately 1/2000 of an ovary. This difference between whole organs and the fraction sampled in sections makes age-specific counts differ between the species by more than three orders of magnitude and precludes a comparison of absolute follicle stock size between the two species. As in Jones et al. (2007), we focus our attention not on comparing the totals themselves but on the change in follicle counts with age.
During data exploration, we plotted the individual averages across ages. Then, following Jones et al. (2007), we transformed the data. Because two individuals had zero follicles in all counts, we added one to the average of all individuals (a convention for transforming counts that include zeros) and log10 transformed them; we then modeled the relationship between transformed follicle count averages and subject age excluding the two oldest chimpanzees, so the age ranges modeled for both species are the same, 0–51. Models estimated with these data agree closely with estimates generated through a more rigorous resampling protocol, which we do not report here.
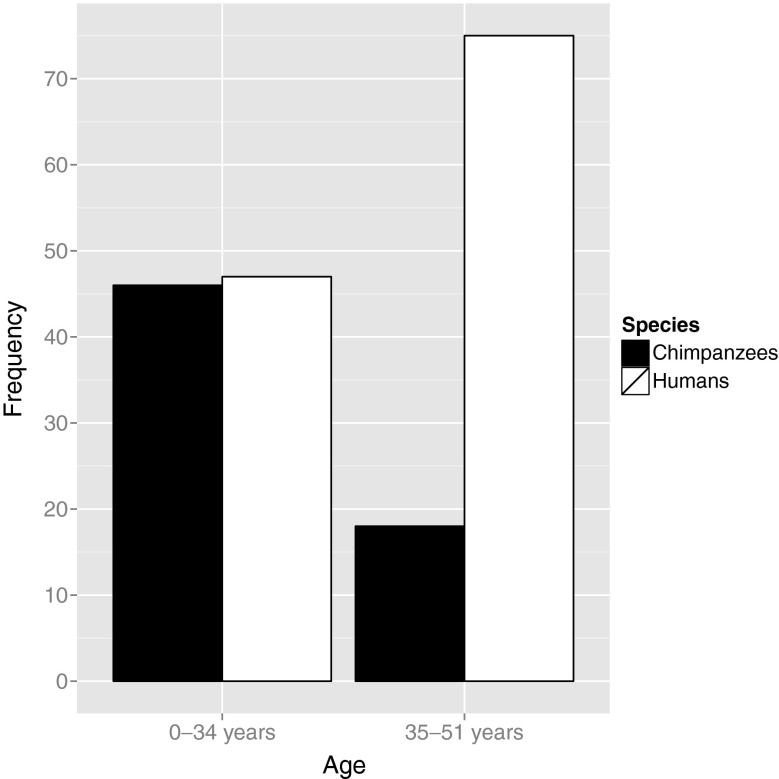
Sample sizes are similar for the two species under the age of 35, but the human sample is three times as large over the age of 35 (Fig. 1). After fitting and comparing models across the entire age range, we divided the datasets and analyzed the under- and over-35 samples separately. This is not a revival of the broken stick model (Faddy et al. 1992) that has been recognized by many (Hawkes and Smith 2010; Leidy et al. 1998), including the authors themselves (Faddy and Gosden 1996), as biologically implausible. Instead, we divided the age classes to allow more reliable and transparent comparisons of age-related patterns of follicle loss in our sample.
Fig. 1.
Sample size comparisons between the chimpanzee (dark bars) and human (open bars) data used in statistical model comparisons
Results
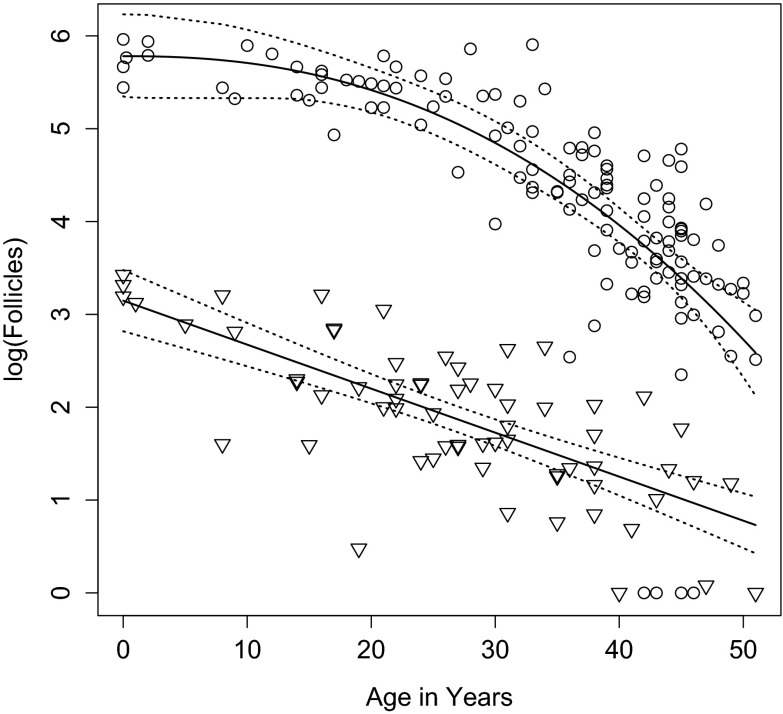
Jones et al. (2007) log-transformed chimpanzee data and regressed them on age. For that sample, measured that way, the rates of decline with age in the two species were statistically indistinguishable. Here, we log-transformed the Hansen counts and the counts in the larger chimpanzee sample (after adding one to each count), fitted linear models to those counts by age, and replicated the previous result. As in Jones et al. (2007), the confidence intervals of the slopes (b) of the best-fit linear models overlapped. Then, we fitted power and exponential models to the transformed counts and compared them with linear models using Akaike information criteria (AICc). In this three-way model comparison, a non-linear model proved to be a better fit for the human, but not the chimpanzee sample, highlighting differences between the two species (Fig. 2).
Fig. 2.
A non-linear model best fits the human (circles)—but not the chimpanzee (triangles)—data, highlighting differences between the species. The figure shows log-transformed counts ages 0–51, best-fit models, and their confidence intervals
Up to age 35, but not at older ages, sample sizes for the two species are similar (Fig. 1), so we divided samples from each species into two age classes, fit linear, power, and exponential models to the data in each age class and compared them. Table 1 provides the parameter values, AICc, and relative likelihoods for the best-fit models.
Table 1.
Model parameters and comparisons
| Species | Age | Modela | No. of parameters | AICc | dAICc | rLIK | Weight | a (CI) | b (CI) | c (CI) |
|---|---|---|---|---|---|---|---|---|---|---|
| Pan troglodytes | 0–51 | Linear | 3 | 113.5 | 0.00 | 1.00 | 0.635 | 3.148 (2.845, 3.471) | −0.047 (−0.058, −0.036) | – |
| Power | 4 | 115.8 | 2.30 | 0.32 | 0.201 | −0.052 (−0.315, −) | 0.979 (0.538, 1.821) | 3.165 (2.641, 3.765) | ||
| Exponential | 3 | 116.2 | 2.70 | 0.26 | 0.165 | 1.202 (1.071, 1.321) | −0.023 (−0.028, −0.017) | – | ||
| 0–34 | Linear | 3 | 79.40 | 1.10 | 0.58 | 0.275 | 3.025 (2.644, 3.407) | −.040 (−0.056, −0.023) | – | |
| Power | 4 | 79.6 | 1.30 | 0.52 | 0.249 | −0.268 (−0.950, −0.020) | 0.493 (0.159, 1.289) | 3.329 (2.776, 3.917) | ||
| Exponential | 3 | 78.3 | 0.00 | 1.00 | 0.476 | 1.140 (0.995, 1.269) | −0.018 (−0.024, −0.011) | – | ||
| 35–51 | Linear | 3 | 40.1 | 0.00 | 1.00 | 0.421 | 2.680 (0.356, 5.004) | −0.038 (−0.095, 0.018) | – | |
| Power | 4 | 41.6 | 1.50 | 0.47 | 0.199 | 4.926e − 16 (−, −) | 1.030 (10.300, 10.300) | 1.307 (1.307, 1.307) | ||
| Exponential | 3 | 40.3 | 0.20 | 0.90 | 0.381 | 1.422 (−0.649, 1.792) | −0.032 (−0.090, −0.007) | – | ||
| Homo sapiens | 0–51 | Linear | 3 | 314.3 | 11.60 | 0.00 | 0.003 | 6.512 (6.086, 6.947) | −0.066 (−0.078, −0.054) | – |
| Power | 4 | 302.7 | 0.00 | 1.00 | 0.997 | −0.0004 (−5.350, −0.008) | 2.316 (1.531, 2.386) | 5.871 (5.390, 6.252) | ||
| Exponential | 3 | 322.4 | 19.70 | 0.00 | 0.000 | 1.887 (1.805, 1.959) | −0.013 (−0.015, −0.011) | – | ||
| 0–34 | Linear | 3 | 46.8 | 0.00 | 1.00 | 0.373 | 5.858 (5.618, 6.099) | −0.026 (−0.037, −0.016) | – | |
| Power | 4 | 47.1 | 0.30 | 0.86 | 0.321 | −0.001 (−0.002, 0.0001) | 0.702 (−0.309, 1.830) | 5.712 (5.471, 6.026) | ||
| Exponential | 3 | 47.2 | 0.40 | 0.82 | 0.306 | 1.769 (1.726, 1.810) | −0.005 (−0.007, −0.003) | – | ||
| 35–51 | Linear | 3 | 215.7 | 0.10 | 0.95 | 0.432 | 7.753 (5.403, 10.103) | −0.097 (−0.152, −0.042) | – | |
| Power | 4 | 217.6 | 2.03 | 0.36 | 0.411 | −4.610 (−4.648, −4.539) | 0.036 (0.010, 0.069) | 4.775 (4.738, 4.864) | ||
| Exponential | 3 | 215.6 | 0.00 | 1.00 | 0.157 | 2.532 (1.785, 3.000) | −0.030 (−0.043, −0.012) | – |
Best-fit models are indicated in boldface
dAICc delta AICc, rLIK relative likelihood
aModels have the following form: linear is y = a + bx, power is y = ax b + c, exponential is y = e a + bx, with y = log10 follicle count and x = age in all three
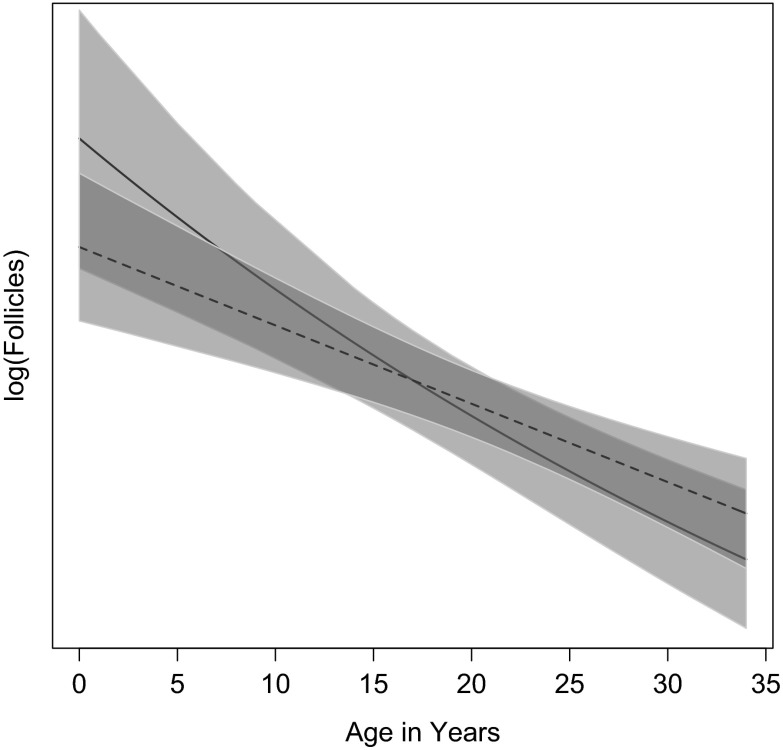
Since it is not intercepts but slopes that are of interest, we focus attention on the slopes by centering the best-fit models for the 0–34 samples at the median age in Fig. 3. The chimpanzee slope is slightly steeper (in linear models, for these ages, the slope (b) for chimpanzees = −0.04 (CI −0.06, −0.02), for humans −0.03 (CI − 0.04, −0.02)
Fig. 3.
Best-fit model comparisons between the chimpanzee and human 0–34-year data, centered by age class means. The chimpanzee model is the solid line, the human model is the dashed line
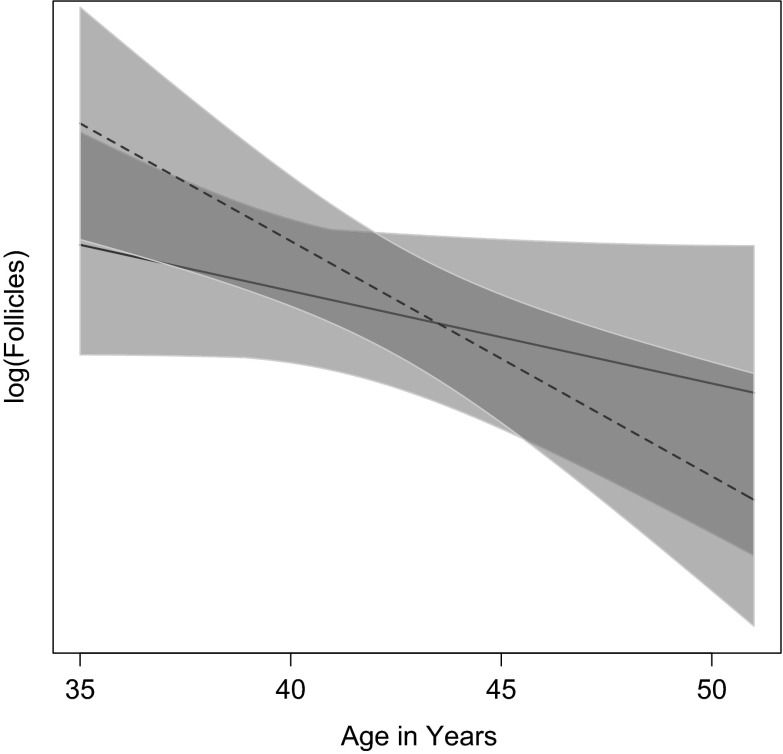
For the 35–51 samples, Fig. 4 shows the best-fit models centered in the same way. In this age range, women’s counts decline with age while chimpanzee counts show little age-related decline. The linear model for humans in this range is almost as good as the exponential model, with a slope of −0.097 (CI −0.152, −0.042)—twofold to threefold steeper than the −0.038 slope of the linear model for chimpanzees in that age range where the confidence interval overlaps zero. Adding the counts from the two oldest chimpanzees, ages 54 and 56, and fitting a linear model to ages 35–56 drops the slope more than threefold to −0.012 (95 % CI = −0.06, 0.03), essentially flat.
Fig. 4.
Best-fit model comparisons between the chimpanzee and human 35–51-year data, centered by age class means. The chimpanzee model is the solid line, the human model is the dashed line
The conclusion from the samples and analysis of Jones et al. (2007) was that the rates of follicular decline in humans and chimpanzees are statistically indistinguishable. In contrast, the analysis here indicates important differences. Before age 35, the rates of loss are similar. After age 35, the rate of decline is much less steep in chimpanzees than in humans. While the small size of our over-35 chimpanzee sample contributes to the wide confidence interval for those ages, the between-species difference is substantial.
Discussion
Not only are chimpanzees our closest living relatives, their similarity in body and brain size to australopithecines—the fossil genus ancestral to our own—makes them the favored model for estimating ancestral maturation and aging rates (Plavcan 2012; Robson and Wood 2008; Smith and Tompkins 1995). Improved characterizations of the similarities and differences between humans and chimpanzees are indispensible for justifying hypotheses about the evolution of hominin life histories. Here, we have added data from an additional 48 chimpanzees to the counts from 19 individuals reported by Jones et al. (2007) and compared the decline in primordial follicle counts with age in chimpanzees to the decline in humans. Results show similarity in the rate of decline before the age of 35 but a notably slower chimpanzee decline after it.
Other recent findings provide further context for these results. Herndon et al. (2012) compared age-specific percentages of menopause in chimpanzees and humans and found that the chimpanzee “percentages are numerically lower than the percentages of women that are menopausal at equivalent ages” (p. 1151). Their sample is small, however, and chimpanzees surviving into their forties may represent a subset that was potentially more robust and fertile to older ages all along (Hawkes and Smith 2010). This mortality selection likely affects our older age sample of ovarian sections as well. It is only unusually robust females that survive to these ages. Still, the slower decline in follicle stocks we report here parallels the hint in Herndon and colleagues’ analysis that progression to menopause may be slower in chimpanzees than in humans.
Differences between the two species in the aging of physiological systems that directly interact with the ovaries would be grounds to expect a difference. Although menopause is typically ascribed to depletion of ovarian follicular stocks, ovarian cycles depend on feedback relationships between ovarian steroid hormones and gonadotropins regulated by the brain (O’Connor et al. 2001; Park et al. 2002). Menopausal changes in women involve hypothalamic and pituitary alterations (Rehman and Masson 2005; Santoro et al. 1998; Wise 1999; Wise et al. 2002; Yin and Gore 2006). Interaction along the hypothalamic-pituitary-ovarian (HPO) axis has been the focus of attention for some time (Wise 1999; Wise et al. 2002; Yin and Gore 2006) with the multifactorial determinants of these interactions an increasingly rich area of study (Kenealy et al. 2013), especially in rodents (Bonavera et al. 1998; Downs and Wise 2009; Maffucci and Gore 2006; Reame 2000; Wise 2001).
Yet, the aging phenotypes that lead researchers to classify chimpanzees as geriatric in their mid-thirties (Goodall 1986; Herndon et al. 2012) suggest that declines in performance along the HPO axis would be earlier in chimpanzees than in humans. Gosden (1996), describing women, said that the “reproductive system ages faster than the body as a whole and by age 45 can be said to be in the state that a woman’s other organs have reached by eighty” (p. 353–354). The converse expectation for female chimpanzees would be that as they approach the end of fertility their other organs would have reached a state similar to the organs of women decades older.
This points to the importance of species comparisons between chimpanzees and humans that have been growing areas of interest. In particular, gene expression and glial cells play a key role in the control of gonadotropin-releasing hormone (GnRH) and in hormonal feedback on GnRH neurons (Garcia-Segura et al. 2008; Kenealy et al. 2013; Zhang et al. 2013). Sherwood and colleagues (2006) report species differences between humans and chimpanzees in glial cell densities in area 32 of the prefrontal cortex (PFC), although these data are largely derived from considerably different human/chimpanzee age comparisons. Area 32 has been shown to be the site of significant projections between the hypothalamus and the PFC (Ongur et al. 1998; Rempel-Clower and Barbas 1998). Therefore, potential differences in glial cell densities may affect the functionality of the HPO axis and differences in the rate of recruitment of primary oocytes (Wise et al. 2002).
Gene expression in the hypothalamus is also of paramount importance for aging in general because GnRH from the hypothalamus promotes neurogenesis, and aging is characterized by diminished neurogenesis (Zhang et al. 2013). Thus, in postmenopausal women, where GnRH levels are especially high, we should expect to see slower rates of overall somatic decline. If the function of the HPO axis changes in the same way with age in chimpanzees and humans, then gonadotropin levels should rise with age as ovarian steroid hormones fall (O’Connor et al. 2001; Park et al. 2002). Our data suggest this may not be the case.
Given the earlier onset of geriatric morbidities in chimpanzees, it is unlikely that senescent dysregulation of the hypothalamus occurs later in that species than in humans. But, to better characterize the apparent puzzle of slower decline in follicle stocks, with menopause “a late-life event in the chimpanzee” (Herndon et al. 2012), we need additional comparisons of age-specific HPO hormones between humans and our sister species. The limited data available are consistent with differences between chimpanzees and humans in HPO axis aging. One geriatric chimpanzee’s endocrine profile (Cloutier 2010) indicated suppression of estrone conjugates (E1C) and pregnanediol (PdG), as would be expected for a menopausal woman. However, gonadotropins, as expressed through luteinizing hormone (LH) levels were also low. This combination was unexpected. In postfertile women, levels of gonadotropins are high because ovarian steroid hormones no longer rise to curtail their production by the hypothalamus. Such a mismatch with typical human profiles is consistent with the possibility that HPO aging differs between the species; chimpanzees do not experience menopause in the same way humans do.
Future age-matched endocrine comparisons between the species are necessary to improve our understanding of the physiology of perimenopause. Such data will clarify functional life history comparisons and contribute to understanding distinctive features of menopausal experience that evolved in our lineage along with our derived postfertile life stage (Alberts et al. 2013; Levitis et al. 2013).
Electronic supplementary material
(PDF 119 kb)
Acknowledgments
We thank Dr. Kirtly Jones for technical advice and training, and the Yerkes National Primate Research Center of Emory University; the Southwest National Primate Research Center; the New Iberia Research Center at the University of Louisiana; the Keeling Center for Research at MD Anderson, University of Texas; and Chimp Haven for their assistance in the acquisition of tissue samples for this study. Research reported here was supported by the National Science Foundation (award number BCS0717886) and the National Institutes of Health (award numbers P51RR000165, P51RR013986, ODP51OO011133, and 9U42OD014838-11).
References
- Alberts S, et al. Reproductive aging patterns in primates reveal that humans are distinct. Proc Natl Acad Sci. 2013;110:13440–13445. doi: 10.1073/pnas.1311857110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker T. A quantitative and cytological study of germ cells in human ovaries. Proc Biol Sci. 1963;158:417–433. doi: 10.1098/rspb.1963.0055. [DOI] [PubMed] [Google Scholar]
- Boesch C, Boesch-Achermann H. The chimpanzees of Tai Forest. Cambridge: Cambridge University Press; 2000. [Google Scholar]
- Bonavera J, Swerdloff R, Sinhahikim A, Lue Y, Wang C. Aging results in attenuated gonadotropin releasing hormone-luteinizing hormone axis responsiveness to glutamate receptor agonist N-methyl-d-aspartate. J Neuroendocrinol. 1998;10:93–99. doi: 10.1046/j.1365-2826.1998.00177.x. [DOI] [PubMed] [Google Scholar]
- Burger H. The endocrinology of the menopause. J Steroid Biochem Mol Biol. 1999;69:31–35. doi: 10.1016/S0960-0760(98)00145-9. [DOI] [PubMed] [Google Scholar]
- Cloutier C (2010) Exploring the endocrine profile of a geriatric female chimpanzee (Pan troglodytes). Florida Atlantic University
- Downs J, Wise P. The role of the brain in female reproductive aging. Mol Cell Endocrinol. 2009;299:32–38. doi: 10.1016/j.mce.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyke B, Gage T, Alford P, Swenson B, Williams-Blangero S. Model life table for captive chimpanzees. Am J Primatol. 1995;37:25–37. doi: 10.1002/ajp.1350370104. [DOI] [PubMed] [Google Scholar]
- Emery-Thompson M, et al. Aging and fertility patterns in wild chimpanzees provide insights into the evolution of menopause. Curr Biol. 2007;17:2150–2156. doi: 10.1016/j.cub.2007.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faddy M, Gosden R. A model conforming the decline in follicle numbers to the age of menopause in women. Hum Reprod. 1996;11:1484–1486. doi: 10.1093/oxfordjournals.humrep.a019422. [DOI] [PubMed] [Google Scholar]
- Faddy M, Gosden R, Gougeon A, Richardson S, Nelson J. Accelerated disappearance of ovarian follicles in mid-life: implications for forecasting menopause. Hum Reprod. 1992;7:1342–1346. doi: 10.1093/oxfordjournals.humrep.a137570. [DOI] [PubMed] [Google Scholar]
- Finch C. Evolution of the human lifespan and diseases of aging: roles of infection, inflammation, and nutrition. Proc Natl Acad Sci U S A. 2010;107:1718–1724. doi: 10.1073/pnas.0909606106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch C, Sapolsky R. The evolution of Alzheimer disease, the reproductive schedule, and apoE isoforms. Neurobiol Aging. 1999;20:407–428. doi: 10.1016/S0197-4580(99)00053-6. [DOI] [PubMed] [Google Scholar]
- Garcia-Segura L, Lorenz B, DonCarlos L. The role of glia in the hypothalamus: implications for gonadal steroid feedback and reproductive neuroendocrine output. Reproduction. 2008;135:419–429. doi: 10.1530/REP-07-0540. [DOI] [PubMed] [Google Scholar]
- Goodall J (1986) The chimpanzees of Gombe. Harvard University Press, Cambridge
- Gosden R. Cheating time: science, sex & aging. 1. New York: Freeman; 1996. [Google Scholar]
- Gosden R, Tefler E. Numbers of follicles and oocytes in mammalian ovaries and their allometric relationships. J Zool. 1987;211:169–175. doi: 10.1111/j.1469-7998.1987.tb07460.x. [DOI] [Google Scholar]
- Gougeon A. Regulation of ovarian follicular development in primates: facts and hypotheses. Endocr Rev. 1996;17:121–155. doi: 10.1210/edrv-17-2-121. [DOI] [PubMed] [Google Scholar]
- Gould K, Flint M, Graham C. Chimpanzee reproductive senescence: a possible model for evolution of the menopause. Maturitas. 1981;3:157–166. doi: 10.1016/0378-5122(81)90007-4. [DOI] [PubMed] [Google Scholar]
- Graham C. Reprodutive function in aged female chimpanzees. Am. J. Phys. Anthropol. 1979;50:291–300. doi: 10.1002/ajpa.1330500302. [DOI] [PubMed] [Google Scholar]
- Graham C. Menstural cycle variability in the great apes. In: Graham C, editor. Reproductive biology of the great apes. NY: Academic Press; 1981. pp. 1–43. [Google Scholar]
- Gurven M, Kaplan H. Hunter-gatherer longevity: cross-cultural perspectives. Popul Dev Rev. 2007;33:321–365. doi: 10.1111/j.1728-4457.2007.00171.x. [DOI] [Google Scholar]
- Hamilton W. The moulding of senescence by natural selection. J Theor Biol. 1966;12:12–45. doi: 10.1016/0022-5193(66)90184-6. [DOI] [PubMed] [Google Scholar]
- Hansen K, Knowlton N, Thyer A, Charleston J, Soules M, Klein N. A new model of reproductive aging: the decline in ovarian non-growing follicle number from birth to menopause. Hum Reprod. 2008;23:699–708. doi: 10.1093/humrep/dem408. [DOI] [PubMed] [Google Scholar]
- Hawkes K. Grandmothers and the evolution of human longevity. Am J Hum Biol. 2003;15:380–400. doi: 10.1002/ajhb.10156. [DOI] [PubMed] [Google Scholar]
- Hawkes K. Grandmother effects, heterogeneity, and the evolution of human aging: guidance from human-chimpanzee comparisons. Proc Natl Acad Sci. 2010;107:8977–8984. doi: 10.1073/pnas.0914627107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkes K, Coxworth J. Grandmothers and the evolution of human longevity: a review of findings and future directions. Evol Anthropol. 2013;22:294–302. doi: 10.1002/evan.21382. [DOI] [PubMed] [Google Scholar]
- Hawkes K, Smith K. Do women stop early? Similarities in fertility decline between humans and chimpanzees. Ann. N. Y. Acad. Sci. 2010;1204:43–53. doi: 10.1111/j.1749-6632.2010.05527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkes K, O’Connell J, Jones NB, Alvarez H, Charnov E. Grandmothering, menopause and the evolution of human life histories. Proc Natl Acad Sci. 1998;95:1336–1339. doi: 10.1073/pnas.95.3.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkes K, Smith K, Robson S. Mortality and fertility rates in humans and chimpanzees: how within-species variation complicates cross-species comparisons. Am J Hum Biol. 2009;21:578–586. doi: 10.1002/ajhb.20890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herndon J, Paredes J, Wilson M, Bloomsmith M, Chennareddi L, Walker M (2012) Menopause occurs late in life in the captive chimpanzee (Pan troglodytes). Age 34:1145–1156 [DOI] [PMC free article] [PubMed]
- Jones K, Walker L, Anderson D, Lacreuse A, Robson S, Hawkes K. Depletion of ovarian follicles with age in chimpanzees: similarities to humans. Biol Reprod. 2007;77:247–251. doi: 10.1095/biolreprod.106.059634. [DOI] [PubMed] [Google Scholar]
- Kenealy B, et al. Neuroestradiol in the hypothalamus contributes to the regulation of gonadotropin releasing hormone release. J Neurosci. 2013;33:19051–19059. doi: 10.1523/JNEUROSCI.3878-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim P, Coxworth J, Hawkes K. Increased longevity evolves from grandmothering. Proc R Soc B. 2012;279:4880–4884. doi: 10.1098/rspb.2012.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim P, McQueen J, Coxworth J, Hawkes K. Grandmothering drives the evolution of longevity in a probabilistic model. J Theor Biol. 2014;353:84–94. doi: 10.1016/j.jtbi.2014.03.011. [DOI] [PubMed] [Google Scholar]
- Lacreuse A et al (2008) Menstrual cycles continue into advanced old age in the common chimpanzee (Pan troglodytes). Biol Reprod 79:407–412 [DOI] [PMC free article] [PubMed]
- Leidy L, Godfrey L, Sutherland M. Is follicular atresia biphasic? Fertil Steril. 1998;70:851–859. doi: 10.1016/S0015-0282(98)00316-1. [DOI] [PubMed] [Google Scholar]
- Levitis D, Burger O, Lackey L. The human post-fertile lifespan in comparative evolutionary context. Evol Anthropol. 2013;22:66–79. doi: 10.1002/evan.21332. [DOI] [PubMed] [Google Scholar]
- Maffucci J, Gore A. Age-related changes in hormones and their receptors in animal models of female reproductive senescence. In: Conn M, editor. Handbook of models for human aging. Amsterdam: Elsevier; 2006. pp. 533–552. [Google Scholar]
- McGee E, Hsueh A. Initial and cyclical recruitment of ovarian follicles. Endocr Rev. 2000;21:200–214. doi: 10.1210/edrv.21.2.0394. [DOI] [PubMed] [Google Scholar]
- Miller P, Charleston J, Battaglia D, Klein N, Soules M. An accurate, simple method for unbiased determination of primordial follicle number in the primate ovary. Biol Reprod. 1997;56:909–915. doi: 10.1095/biolreprod56.4.909. [DOI] [PubMed] [Google Scholar]
- Miller P, Charleston J, Battaglia D, Klein N, Soules M. Morphometric analysis of primordial follicle number in pigtailed monkey ovaries: symmetry and relationship with age. Biol Reprod. 1999;61:553–556. doi: 10.1095/biolreprod61.2.553. [DOI] [PubMed] [Google Scholar]
- Nishida T, et al. Demography, female life history, and reproductive profiles among chimpanzees of Mahale. Am J Primatol. 2003;59:99–121. doi: 10.1002/ajp.10068. [DOI] [PubMed] [Google Scholar]
- O’Connor K, Holman D, Woods J. Menstrual cycle variability and the perimenopause. Am J Hum Biol. 2001;13:465–478. doi: 10.1002/ajhb.1078. [DOI] [PubMed] [Google Scholar]
- Ongur D, An X, Price J. Prefrontal projections to the hypothalamus in Macaque monkeys. J Comp Neurol. 1998;401:480–505. doi: 10.1002/(SICI)1096-9861(19981130)401:4<480::AID-CNE4>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Park S, Goldsmith L, Weiss G. Age-related changes in the regulation of luteinizing hormone secretion by estrogen in women. Exp Biol Med. 2002;227:455–464. doi: 10.1177/153537020222700709. [DOI] [PubMed] [Google Scholar]
- Plavcan J (2012) Body size, size Variation, and sexual size dimorphism in early Homo. Curr Anthropol 53:S409–S423
- Reame N. Neuroendocrine regulation of the perimenopausal transition. In: Lobo R, Kelsey J, Marcus R, editors. Menopause: biology & pathology. San Diego: Academic Press; 2000. pp. 95–110. [Google Scholar]
- Rehman H, Masson E. Neuroendocrinology of female aging. Gend Med. 2005;2:41–56. doi: 10.1016/S1550-8579(05)80008-7. [DOI] [PubMed] [Google Scholar]
- Rempel-Clower N, Barbas H. Topographic organization of connections between the hypothalamus and prefrontal cortex in the rhesus monkey. J Comp Neurol. 1998;398:393–419. doi: 10.1002/(SICI)1096-9861(19980831)398:3<393::AID-CNE7>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Robson S, Wood B. Hominin life history: reconstruction and evolution. J Anat. 2008;212:394–425. doi: 10.1111/j.1469-7580.2008.00867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro N, Banwell T, Tortoriello S, Lieman H, Adel T, Skurnick J. Effects of aging and gonadal failure on the hypothalamic-pituitary axis in women. Am J Obstet Gynecol. 1998;178:732–741. doi: 10.1016/S0002-9378(98)70483-1. [DOI] [PubMed] [Google Scholar]
- Sherwood C, et al. Evolution of increased glia-neuron ratios in the human frontal cortex. Proc Natl Acad Sci. 2006;103:13606–13611. doi: 10.1073/pnas.0605843103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith B, Tompkins R. Toward a life history of the Hominidae. Annu. Rev. Anthropol. 1995;24:257–279. doi: 10.1146/annurev.an.24.100195.001353. [DOI] [Google Scholar]
- Sugiyama Y. Demographic parameters and life history of chimpanzees at Bossou, Guinea. Am. J. Phys. Anthropol. 2004;124:154–165. doi: 10.1002/ajpa.10345. [DOI] [PubMed] [Google Scholar]
- Videan E, Fritz J, Heward C, Murphy J. The effects of aging on hormone and reproductive cycles in female chimpanzees (Pan troglodytes) Comp Med. 2006;56:291–299. [PubMed] [Google Scholar]
- Videan E, Fritz J, Heward C, Murphy J. Reproductive aging in female chimpanzees (Pan troglodytes) In: Atsalis S, Margulis S, Hof P, editors. Primate reproductive aging, vol 36. Interdisciplin Top Gerontol. Basel: Karger; 2008. pp. 103–118. [DOI] [PubMed] [Google Scholar]
- vom Saal F (1994) The physiology of reproduction. In: Knobil E, Neill J (eds) The physiology of reproduction, 2nd edn. Raven, NY, pp 1213–1314
- Wich S, Utami-Atmoko S, Setia TM, Rijksen H, Schurmann C, van Hooff JARAM, van Schaik CP (2004) Life history of wild Sumatran orangutans (Pongo abelii). J Hum Evol 47:385–398 [DOI] [PubMed]
- Williams G. Pleitropy, natural selection, and the evolution of senescence. Evolution. 1957;11:398–411. doi: 10.2307/2406060. [DOI] [Google Scholar]
- Wise P. Neuroendocrine modulation of the “menopause”: insights into the aging brain. Am J Physiol. 1999;277:E965–E970. doi: 10.1152/ajpendo.1999.277.6.E965. [DOI] [PubMed] [Google Scholar]
- Wise P. The ‘meopause’ and the aging brain: causes and repurcussions of hypoestrogenicity. Biogerontol. 2001;2:113–115. doi: 10.1023/A:1011537201380. [DOI] [PubMed] [Google Scholar]
- Wise P, et al. Neuroendocrine modulation and repercussions of female reproductive aging. Endocr Rev. 2002;57:235–256. doi: 10.1210/rp.57.1.235. [DOI] [PubMed] [Google Scholar]
- Yin W, Gore A. Neuroendocrine control of reproductive aging: roles of GnRH neurons. Reprod. 2006;131:403–414. doi: 10.1530/rep.1.00617. [DOI] [PubMed] [Google Scholar]
- Zhang G, et al. Hypothalamic programming of systemic ageing involving IKK-b, NF-kB and GnRH. Nature. 2013;497:211–218. doi: 10.1038/nature12143. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 119 kb)