Abstract
Background
Modular megaprostheses are now the most common method of reconstruction after segmental resection of the long bones in the lower extremities. Previous studies reported variable outcome and failure rates after knee megaprosthetic reconstructions.
Questions/purposes
The objectives of this study were to analyze the results of a modular tumor prosthesis after resection of bone tumor around the knee with respect to (1) survivorship; (2) failure rate; (3) comparative survivorship against different sites of reconstructions and of primary and revision implants; and (4) functional results on the Musculoskeletal Tumor Society (MSTS) scoring system.
Methods
Between 2003 and 2010, 247 rotating-hinge Global Modular Reconstruction System (GMRS) knee prostheses were implanted in our institute for malignant and aggressive benign tumors. During this time, that group represented 23% of the patients who had oncologic megaprosthesis reconstruction about the knee after resection of primary or metastatic bone tumors (247 of 1086 patients). In the other 77% of cases we used other types of oncologic prostheses. Before 2003 we used the older Howmedica Modular Resection System and Kotz Modular Femur/Tibia Replacement from 2003 we used mostly the GMRS but we continued to use the HMRS in some cases such as patients with poor prognoses, elderly patients, or metastatic patients. Sites included 187 distal femurs and 60 proximal tibias. Causes of megaprosthesis failure were classified according to Henderson et al. in five types: Type 1 (soft tissue failure), Type 2 (aseptic loosening), Type 3 (structural failure), Type 4 (infection), and Type 5 (tumor progression). Followup was at a minimum oncologic followup of 2 years (mean, 4 years; range, 2–8 years). Kaplan-Meier actuarial curves of implant survival to major failures were done. Functional results were analyzed according to the MSTS II system; 223 of the 247 were available for functional scoring (81%).
Results
At latest followup, among 175 treated patients for primary reconstruction, 117 are continuously disease-free, 26 have no evidence of disease after treatment of relapse, eight are alive with disease, and 24 died from disease. The overall failure rate of the megaprostheses in our series was 29.1% (72 of 247). Type 1 failure occurred in 8.5% (21 of 247) cases, Type 2 in 5.6% (14 of 247), Type 3 in 0%, Type 4 in 9.3% (23 of 247), and Type 5 in 5.6% (14 of 247). Kaplan-Meier curve showed an overall implant survival rate for all types of failures of 70% at 4 years and 58% at 8 years. Prosthetic survivorship for revisions was 80% at 5 years and for primary reconstructions was 60% at 5 years (p = 0.013). Survivorship to infection was 95% at 5 years for revision patients and 84% at 5 years for primary patients (p = 0.475). The mean MSTS score was 84 (25.2; range, 8–30) with no difference between sites of localization (24.7 in proximal tibia versus 25.4 in distal femur reconstruction; p = 0.306).
Conclusions
Results at a minimum of 2 years with this modular prosthesis are satisfactory in terms of survivorship (both oncologic and reconstructive) and causes and rates of failure. Although these results seem comparable with other like implants, we will continue to follow this cohort, and we believe that comparative trials among the available megaprosthesis designs are called for.
Level of Evidence
Level IV, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
The distal femur and the proximal tibia are common sites for primary and metastatic bone tumors. The introduction of chemotherapy, the advances in surgical and diagnostic techniques, and the multidisciplinary approach allowed limb salvage procedures for sarcoma with no obvious detectable differences in oncologic or functional results compared with amputation [2–5, 8, 11, 12, 26, 28, 31, 38, 39]. Since the early 1980s metallic megaprostheses have been used for the reconstruction of bone defects after tumor resection as a result of their availability, relative ease of use, immediate fixation and ability to allow early weightbearing, relatively rapid restoration of function, excellent cosmetic appearance, and emotional acceptance [1, 31, 39]. Initially the prostheses were custom implants, but currently modular implants are available and these routinely are used for reconstruction.
Despite the advances in materials and implant design that have occurred over the years, all systems have complications and failures, and these remain high compared with conventional total knee systems, making revision surgery relatively common [1, 6, 7, 9, 11, 16–21, 23, 24, 26, 27, 32, 35, 36, 39, 41, 42, 45]. Poorer long-term results may be the result of many factors such as immunosuppression of patients with oncologic diagnoses, extensive resection of the bony and soft tissues about the knee, longer operative time, and general patient condition. Previous studies using different types of megaprostheses have reported on implant survival to failure ranging from 68% (fixed-hinge) to 77% to 100% (rotating-hinge) and a 10-year estimated risk of failure from 22% (rotating-hinge) to 42% (fixed-hinge) [1, 6, 20, 33, 40]. Since 2003, the rotating-hinge Global Modular Reconstruction System (GMRS) has been available, which allows modularity and the availability of a rotating hinge, which provides stability and permits a large amount of rotation that reduces mechanical stresses at the bone-stem interface [40]. We have used this implant since its introduction, but the reported literature to date has with this implant primarily been in small clinical series.
The aims of our study were (1) to analyze survivorship of patients with limb salvage for bone tumors around the knee using this implant system; (2) to evaluate the most common failures after knee arthroplasty using this rotating-hinge modular prosthetic system; (3) to analyze survival of the knee megaprostheses comparing sites of reconstruction and implants done for primary tumor resections or revisions; and (4) to evaluate functional results in patients who had these procedures.
Materials and Methods
Our institutional database was queried for patients receiving a rotating-hinge GMRS (Stryker Inc, Rutherford, NJ, USA) knee megaprosthesis from 2003 until 2010. We chose to initiate this study in 2003 because the first GMRS was implanted in our institution that year. Inclusion criteria were the use of this type of modular knee implant for primary reconstruction of a segmental bone defect or for revision of a failed segmental reconstruction performed with a megaprosthesis, allograft, or other method. Minimum followup was 2 years (mean, 4 years; range, 2–8 years) for inclusion in this study.
From October 2003 to August 2010, 247 knee prostheses were implanted including 175 primary devices and 72 devices implanted for failure of a previous oncologic reconstruction. The mean age at surgery was 32 years (range, 9–81 years). There were 143 male patients and 104 female patients. Anatomic sites of replacement were distal femur in 187 and the proximal tibia in 60. In all cases, intraarticular resection of the knee was performed. Indications for resection and reconstruction included 241 primary bone tumors and six metastatic lesions. Histological diagnoses of primary bone tumors were osteosarcoma in 152 cases, Ewing’s sarcoma in 15, chondrosarcoma in 11, spindle cell sarcoma in 17, other sarcoma in 11, and giant cell tumor in 35 (Table 1).
Table 1.
Sex, type of implant, site of reconstruction, and histology
| Sex | Type of implant | Sites | Diagnoses | ||||
|---|---|---|---|---|---|---|---|
| Male | 144 | Primary | 175 | Distal femur | 187 | Osteosarcoma | 152 |
| Female | 103 | Revision | 72 | Proximal tibia | 60 | Spindle cell sarcoma | 17 |
| Ewing sarcoma | 15 | ||||||
| Chondrosarcoma | 11 | ||||||
| Metastases | 6 | ||||||
| Leiomyosarcoma | 6 | ||||||
| Lymphoma | 2 | ||||||
| Liposarcoma | 1 | ||||||
| Radiation-induced sarcoma | 1 | ||||||
| Myeloma | 1 | ||||||
| Giant cell tumor | 35 |
Complete followup, including radiographic analysis and Musculoskeletal Tumor Society (MSTS) scores, was available on 223 patients (81%) at a minimum of 2 years. Oncologic outcome, clinical evaluation without radiographic followup, and MSTS scores were available in all patients.
During this time, we performed 1086 other knee megaprostheses for oncologic indications, making the group with this implant 23% of that part of our practice. In the other 77% of cases we used other types of oncologic prostheses. Before 2003 we used the older Howmedica Modular Resection System (HMRS, Howmedica, Kiel, Germany) and the Kotz Modular Femur/Tibia Replacement (KMFR, Howmedica) and from 2003 we used mostly the GMRS but we continued to use the HMRS in some cases such as patients with poor prognoses, elderly patients, or metastatic patients. During this period, our general indications for using this type of modular prosthesis system were reconstruction of the limb after resection of bone malignant tumors, aggressive (Stage 3) benign tumors, and solitary bone metastases with bone destruction and impending or actual pathological fracture.
Metastatic lesions resulting in megaprosthetic replacement were lung cancer in one case, breast cancer in one case, renal cancer in one case, Ewing’s sarcoma in one case, and metastasis from dedifferentiated tumor in two cases.
This prosthetic system uses metallic housings constructed of cobalt-chromium-molybdenum and titanium. The knee articulation is a rotating-hinge design with two polyethylene bushings in all cases. Stems were cemented in 22 patients and uncemented in 225. Stem designs were straight with flutes in 241 cases and curved in six cases according to the femoral anatomy and with hydroxyapatite coating in all cases. The tibial component was metal in all cases. Adaptors are available to allow linkage to previous generation implants. Candidates for cemented fixation were patients with bone metastases and extensive osteolytic defects such as hemoproliferative lesions. Candidates for cementless fixation were younger patients and patients with primary bone tumors.
Primary implants were used in 175 patients for reconstruction of the distal femur in 126 cases and reconstruction of the proximal tibia in 49 cases.
Revision implants were used in 72 patients, previously treated in our center or in another center, after failure of a previous distal femur replacement in 61cases and after failure of a previous proximal tibia replacement in 11 cases. Indications for revision procedures included 56 failed megaprostheses, 11 failed allograft reconstructions, and five failed allograft-prosthesis composites. In all cases we implanted standard modular implants; custom implants were not included in the series. All patients had preoperative staging and planning. Patients with tumor were staged according to Enneking’s system [14]. Twelve patients with sarcoma were Stage IA, 106 were Stage IIA, 80 Stage IIB, and 14 were Stage III. Thirty-five patients had giant cell tumors and all were considered to have Stage 3 benign-aggressive lesions; one patient with a giant cell tumor had lung metastases.
In all proximal tibial reconstructions, the extensor mechanism and wound coverage were performed with rotation of the gastrocnemius muscle flap in all cases. In five cases, the gastrocnemius muscle flap and augmentation with an artificial ligament and/or equine pericardium were used.
Patients were administered preoperative intravenous antibiotics and continued to receive antibiotics for 5 days. Postoperative management included bed rest, analgesia, and mobilization with a walker or crutches after the second postoperative day. Patients with proximal tibial replacement were kept in a straight-leg brace for 6 weeks before initiation of ROM under the guidance of a physical therapist. Chemical antithrombosis prophylaxis was given until complete weightbearing and included low-molecular-weight heparin or warfarin. Perioperative adjuvant treatments were administered to patients with malignant tumors as indicated by tumor histology and dictated by medical oncology consultants.
All patients received supervised physical therapy for a minimum of 6 weeks after discharge from the hospital. Routine followup examinations occurred at 3-month intervals for 3 years, then every 6 months for 2 years, and then annually. Followup evaluations included physical examination, radiographs, and disease-specific chest imaging. Physical functioning was assessed using the MSTS functional rating system.
Causes of megaprosthesis failure were classified according to Henderson et al. [21] in soft tissues failure (Type 1), aseptic loosening (Type 2), structural fracture (Type 3), infection (Type 4), and local tumor recurrence (Type 5). Major failures were considered unplanned revision of any portion of an implant, removal of the prosthesis, or amputation of the limb. Periprosthetic infection was diagnosed through clinical examination, radiographic studies, and laboratory values including erythrocyte sedimentation rate, C-reactive protein, white blood cell count in joint fluid analysis, and bacterial culture.
When revision surgery was required, we used either a one- or two-stage procedure. One-stage revision involves removal of all modular components and polyethylene parts and accurate débridement of all infected surrounding soft tissues and the periprosthetic scar tissues. One-stage revision of infected tumor prostheses has been recommended for patients with early or low-grade infection and antibiotic-sensitive pathogens, poor general condition of the patient, and long delay of chemotherapy. Two-stage revision involves complete exchange of all prosthetic components and use of systemic antibiotics and antibiotic-loaded bone cement. Two-stage revision is recommended for patients with persistent and higher-grade infections, antibiotic-resistant pathogens, or a failed one-stage procedure. Antibiotic-loaded bone cement was substituted every 30 to 45 days until the C-reactive protein or white blood cell count was normal and labeled leukocytes–technetium 99m sulphur colloid marrow imaging became negative; then a revision prostheses was implanted. Systemic antibiotics were administered for at least 6 weeks according to the cultures. The prostheses used for revisions may be cemented (if no adequate bone stock remained) or cementless (after infection healed and there remained a satisfactory quality of bone).
All patients were analyzed with regard to local recurrence, implant survival, and functional outcome. Megaprostheses that were performed for revision of a failed reconstruction were analyzed in terms of the preexisting construct in addition to the outcome of the revision implant.
Survival was evaluated with Kaplan-Meier actuarial curves and comparative statistical analysis with the log rank test [25]. Statistical significance was defined as a p value of ≤ 0.05. Patients who died with their original implant in place were censored. Survival time zero was considered the date of implantation and endpoints were considered implant failure requiring revision or amputation. Statistical analysis was performed using MedCalc Software Version 11.1 (MedCalc Software Broekstraat 52, Mariakerke, Belgium). Statistical analysis of variance for MSTS score was performed using one-way analysis of variance.
Results
Oncologic Outcome
At latest followup, among 175 patients treated at initial diagnosis for a tumor, 117 are continuously disease-free, 26 have no evidence of disease after treatment of relapse, eight are alive with disease, and 24 dead with disease. Estimated 4-year and 8-year survival rates for primary treated patients were 88% and 85%, respectively. Of 206 patients with a low- or high-grade sarcoma, 14 (6.8%) developed a recurrence, and of those, 42 (20.4%) had concomitant systemic metastases. All patients with benign tumors were alive.
Failures and Implant Survival
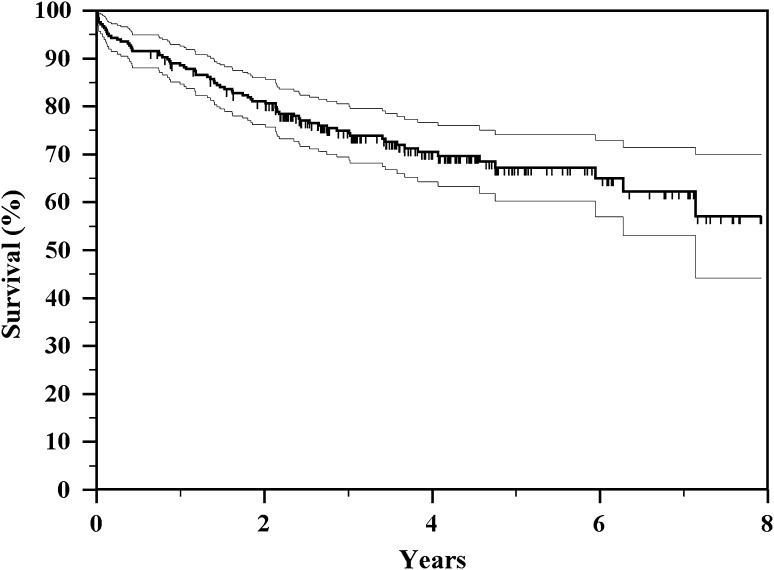
The overall incidence of failure in our series was 29.1% (72 of 247); failure occurred at a mean of 3 years (range, 1 month to 7 years) (Table 2). Estimated 4-year and 8-year survival rates for all types of failure were 70% and 58%, respectively (Fig. 1; Table 3). The failure rate of primary implants in our series was 33% (58 of 175) and in revision implants was 19.4% (14 of 72) (Table 2); in primary implants, the mean prostheses followup was 3.1 years and in revision implants was 3.9 years with a significantly better time to failure for revision implants (one-way analysis of variance, p = 0.003). Megaprosthesis failures by anatomic location included 50 distal femoral replacements and 22 proximal tibial replacements. Megaprosthesis failures by type of reconstruction included 58 primary implants and 14 revision implants (Table 2).
Table 2.
Causes of endoprosthesis failure
| Type or site of implant (number of cases) | Type 1 (soft tissue failure) | Type 2 (aseptic loosening) | Type 3 (structural failure) | Type 4 (infection) | Type 5 (tumor recurrence) | All types (risk) |
|---|---|---|---|---|---|---|
| Primary implants (175) | 18 (10.3%) | 9 (5.1%) | – | 20 (11.4%) | 11 (6.3%) | 58 (33%) |
| Revisions (72) | 3 (4.2%) | 5 (6.9%) | – | 3 (4.1%) | 3 (4.1%) | 14 (19.4%) |
| Distal femur (187) | 13 (7%) | 10 (5.3%) | – | 16 (8.5%) | 11 (5.9%) | 50 (26.7%) |
| Proximal tibia (60) | 8 (13.3%) | 4 (6.6%) | – | 7 (11.6%) | 3 (5%) | 22 (36.7%) |
| Overall (247) | 21 (8.5%) | 14 (5.7%) | – | 23 (9.3%) | 14 (5.7%) | 72 (29.1%) |
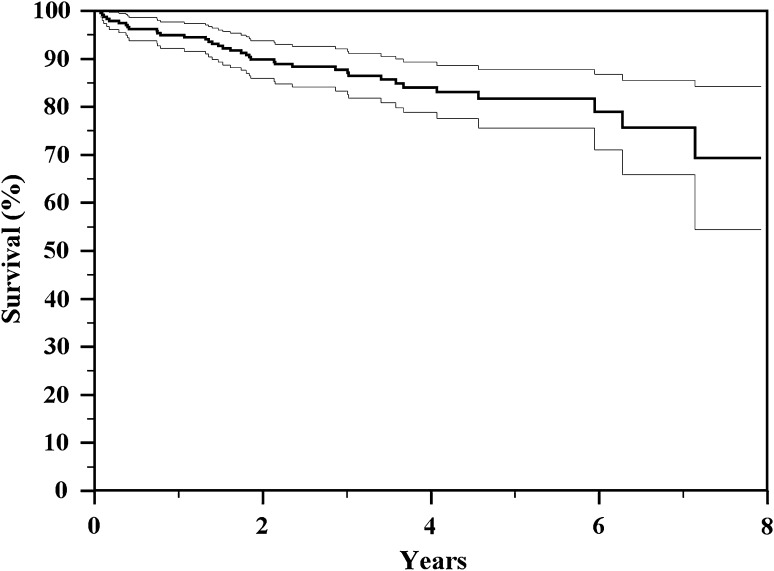
Fig. 1.
The Kaplan-Meier actuarial curve shows overall implant survival to all types of failure of 70% and 58%, respectively, at 4 and 8 years.
Table 3.
Implant survival by type of failure, comparison between types, and sites of reconstruction
| Type of failure | Implant survival at 4 years | Implant survival at 8 years | p value (primary versus revision) | p value (distal femur versus proximal tibia) |
|---|---|---|---|---|
| All modes of failure, all series | 70% | 58% | 0.013 | 0.143 |
| All modes of failure, primary implants | 65% | 52% | ||
| All modes of failure, revision implants | 80% | 72% | ||
| All modes of failure, distal femur | 73% | 60% | ||
| All modes of failure, proximal tibia | 64% | 47% | ||
| Type 1, all series | 90% | 90% | 0.084 | 0.112 |
| Type 2 | 92% | 85% | 0.985 | 0.557 |
| Type 3 | – | – | – | – |
| Type 4 | 90% | 80% | 0.047 | 0.399 |
| Type 5 | 93% | 91% | 0.332 | 0.828 |
| Type 2–3–4 | 84% | 69% | 0.124 | 0.304 |
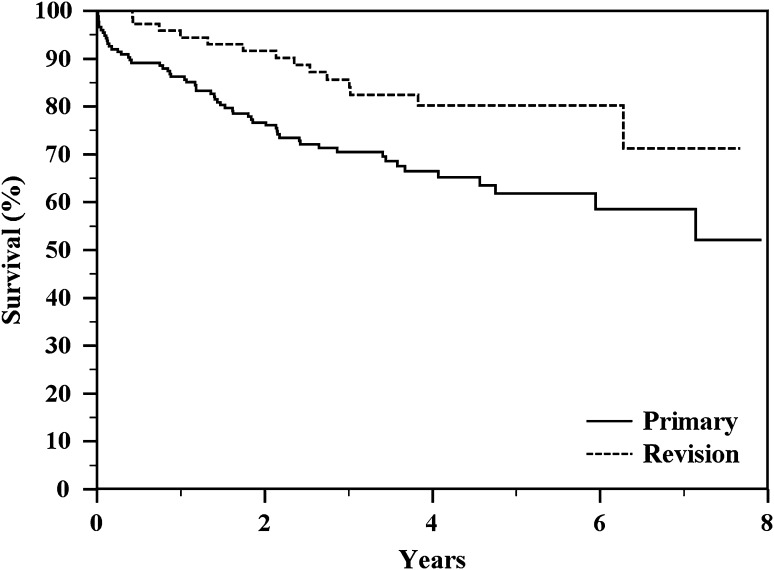
Survivorship was better in revision reconstructions than primary reconstructions (p = 0.013; Fig. 2; Table 3). The failure rate of revision implants in our series was 19.4% (14 of 72); failure occurred at a mean of 3.7 years (range, 1 month to 7 years).
Fig. 2.
The Kaplan-Meier actuarial curves show a significant difference in implant survival to all complications between the types of reconstruction (p = 0.013) with better results for revision implants.
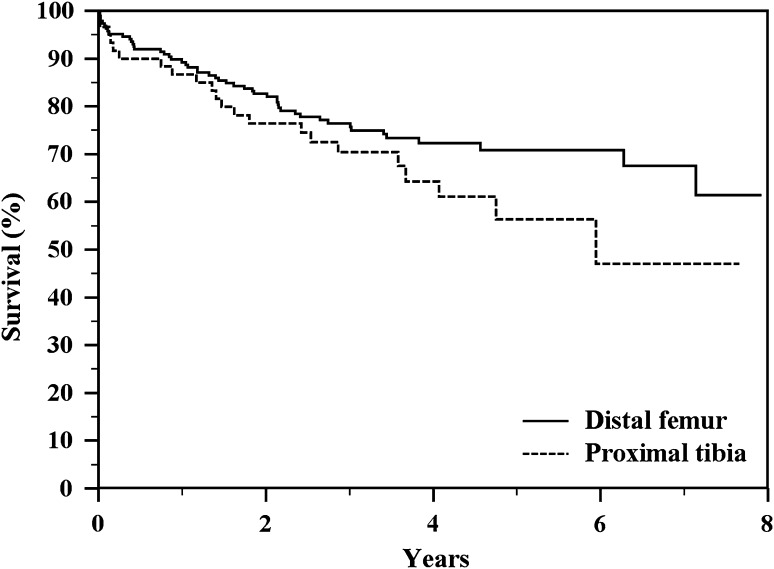
The failure rate of distal femoral replacements was 26.7% (50 of 187); failure occurred at a mean of 3 years (range, 1 month to 8 years). The failure rate of proximal tibia replacements was 36.7% (22 of 60); failure occurred at a mean of 2.7 years (range, 1 month to 8 years). There was not a difference in implant survival rates between the sites of reconstruction (p = 0.1432) (Fig. 3; Table 3).
Fig. 3.
The Kaplan-Meier actuarial curves show a nonsignificant difference in implant survival to all complications between distal femur and proximal tibia reconstructions (p = 0.1432).
Failed primary megaprostheses requiring revision included 18 Type 1 failures (10.3%), nine Type 2 failures (5.1%), 20 Type 4 failures (11.4%), and 11 Type 5 failures (6.3%); there were no cases of Type 3 failures (Table 2).
Type 1 failure (soft tissue failure) occurred at a mean of 1 year (range, 1 month to 3.8 years). Estimated 4-year and 8-year survival rates to Type 1 failure were 90% (Table 3). Soft tissue failure occurred in 18 primary implants (10.3%) and in three revision implants (4.2%), in 13 distal femur (7%), and in eight proximal tibia (13.3%). In distal femur reconstruction, the most frequent Type 1 complication was superficial infection, hematoma, and wound dehiscence (eight of 13 cases) that was treated with débridement. In proximal tibia reconstructions, the most frequent Type 1 failure was superficial infection and hematoma (five of 10 cases) and detachment of the patellar tendon (four of 10 cases); in all cases of patellar tendon detachment, revision surgery was done with reattachment of the tendon; in three cases, the extension lag was less than 20°, whereas in one case, the extension lag was 40°.
Type 2 failure (aseptic loosening) occurred at mean of 4.5 years (range, 1 month to 8 years). Estimated 4-year and 8-year survival rates to Type 2 failure were 92% and 85%, respectively (Table 3). Aseptic loosening occurred in nine primary implants (5.1%) and in five revision implants (6.9%), in 10 distal femur (5.3%), and in four proximal tibia (6.6%) with no difference in survival to Type 2 failure between the types and the sites of reconstruction (Table 3). In all cases, revision surgery was performed with excellent functional results.
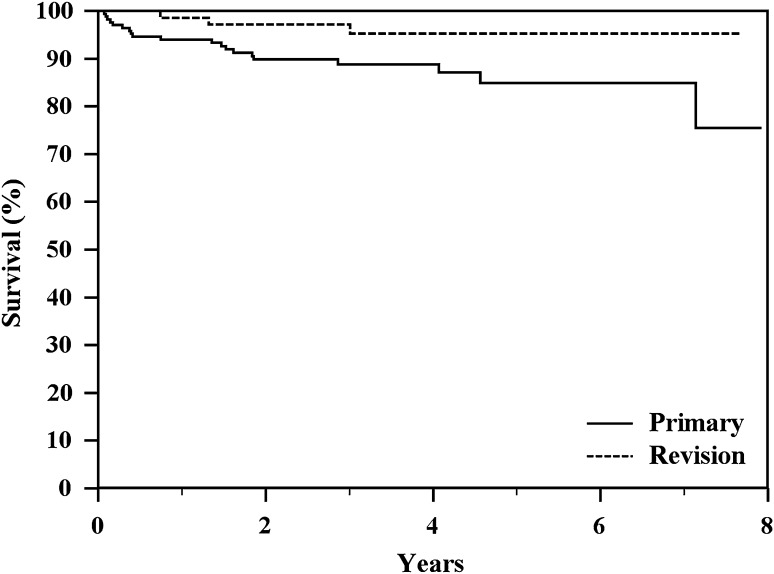
Type 4 failure (infection) occurred at a mean of 3.2 years (range, 1 month to 7.3 years). Estimated 4-year and 8-year survival rates to Type 4 failure were 90% and 80%, respectively (Table 3). Deep infection occurred in 20 primary implants (11.4%) and in three revision implants (4.1%), in 16 distal femur (8.5%), and in seven proximal tibia (11.6%). Survivorship to infection was better in revision reconstructions than primary reconstructions (p = 0.0475; Fig. 4; Table 3). Infection was the most common failure mode (Table 2); however, 20 patients with a deep infection were able to have successful revision of their prosthesis. Six patients were managed with a one-stage revision and 18 patients had a two-stage procedure. Four patients had salvage with conversion of the prosthesis to an arthrodesis.
Fig. 4.
The Kaplan-Meier actuarial curves show a significant different of survival to Type 4 failure between the types of reconstruction (p = 0.0475) with better results for revision implants.
Type 5 failure (local tumor recurrence) occurred at mean of 3.4 years (range, 1 month to 8 years). Estimated 4-year and 8-year survival rates to Type 5 failure were 93% and 91%, respectively (Table 3). Local recurrence occurred in 11 primary implants (6.3%) and in three revision implants (4.1%), in 11 distal femur (5.9%), and in three proximal tibia (5%) with no difference in survival to Type 5 failure between the types and the sites of reconstruction (Table 3). Excision of local recurrence was done in nine patients, whereas five patients underwent amputation.
When only the “classically” defined major causes of failures were considered (infection, aseptic loosening, and breakage), that is in the absence of Type 1 (soft tissue failure) and Type 5 (local tumor recurrence) failures, the overall implant survival was 84% and 69%, respectively, at 4 and 8 years with no difference in survival between the types and the sites of reconstructions (p = 0.124 and p = 0.304, respectively) (Fig. 5; Table 3).
Fig. 5.
The Kaplan-Meier actuarial curve shows overall implant survival to all major complications (infection, aseptic loosening, and breakage) of 84% and 69%, respectively, at 4 and 8 years.
Functional Results
Functional MSTS II scores were obtained in 223 of 247 (81%) patients; the average overall score was 25.2 (range, 8–30). Results were excellent (from 76% to 100%) in 179 (80.2%), good (from 51% to 75%) in 37 patients (16.6%), fair (from 26% to 50%) in six patients (2.7%), and poor in one patient (0.5%); results were good or excellent in 97% of cases. There was no difference related to reconstructive sites; the mean MSTS score was 24.7 in proximal tibia reconstruction and 25.4 in distal femur reconstruction (p = 0.306). There was no difference related to primary implants (mean MSTS II score of 25.3) and in revision implants (mean MSTS score of 24.8) (p = 0.435).
Discussion
The introduction of adjuvant and neoadjuvant chemotherapy for bone sarcomas allowed resection and reconstruction instead of amputation as surgical treatment in most cases of bone sarcomas around the knee [2–5, 8, 12, 28]. Currently, limb salvage procedures are performed in 85% to 95% of patients without impairment of oncologic result compared with amputation in uncontrolled studies [2, 5, 12, 13, 16, 45]. Megaprostheses have been used more frequently in the last three decades and are now the most common method of reconstruction after segmental resection of the long bones in the lower extremity [7, 11, 12, 41, 42]. Several types of modular prostheses are available; however, failures such as infection, aseptic loosening, and mechanical failure can occur with metallic megaprostheses. In the literature, failures of megaprosthetic reconstructions range from 40% to 73% at 5 to 15 years [16, 30, 36, 45]. We therefore sought to evaluate the results of a modular tumor prosthesis after resection of bone tumor around the knee with respect to (1) survivorship; (2) failures; (3) comparative survivorship between different sites of reconstructions and of primary and revision implants; and (4) functional results on the MSTS scoring system. We hypothesized that infection would be the most common mode of failure and higher in proximal tibia and primary implants and that rotating-hinge knees reduce the incidence of aseptic loosening and breakage.
This investigation has several limitations that require discussion. First, it is a retrospective, nonrandomized case series subjecting it to potential recall and selection biases. Second, although comparisons were drawn between patients with primary and revision implants and distal femur and proximal tibia reconstructions, this study lacked a true control group. Third, the tumors of the patients included in this study are heterogeneous in terms of biological behavior and stage; this also precluded us from including adjuvants as variables in our survival analysis. The present study includes patients with metastatic bone disease and aggressive benign bone tumors at both ends of the biologic spectrum and patients with all three stages of sarcoma. Some patients were administered chemotherapy that may affect the results. Some had predictably long life expectancy and others quite short. Unavoidably, the interpretation of the reconstructive outcomes in this study is affected by the oncologic interventions and results. Fourth we did not have radiographs at followup on all patients, and we had MSTS scores only on 84% of patients.
As a result of uncontrolled variables such as adjuvant treatments and cemented/cementless fixation, the study is underpowered to detect some differences and the risk of a statistical error is substantial. However, we opted to include all our patients with distal femur and proximal tibial resections treated with this megaprosthetic reconstructions aiming to address important questions regarding the outcome of the reconstructions in this location. Furthermore, we believe because randomized controlled trials to determine the optimum reconstruction methods after distal femur and proximal tibial resections are not available, even with these limitations, our results may be useful.
Previous studies reported that the 5-year survival rate for bone sarcomas was approximately 60% to 70% after limb salvage procedures [2, 5, 13, 36, 45]. In line with the literature, in our series, overall survival for primary treated patients was 88% and 85%, respectively, at 4 and 8 years without impairment of local recurrence results compared with amputation [8, 26, 31, 38]. Admittedly, because our series is a heterogeneous group of neoplasms, we cannot directly compare our results with those in the literature.
Megaprostheses have a higher failure risk than conventional total knee systems [20] as a result of the high incidence of infection, mechanical failure, and loosening. The failure rates in the literature range from 25% to 92% [6, 7, 18–20, 23, 28, 31, 32, 36, 41, 42], but a comparison is not always possible as a result of the different definitions and classification systems used. We classified the causes of megaprosthesis failure as described previously by Henderson et al. [21] in a multicentric study.
In our series the infection rate in the primary implant was 11.4% and in revisions 4.1%; the survivorship to infection for revision implants was longer than primary reconstructions (p = 0.0475). In the literature, infection has been reported to be the most common mode of failure, ranging between 5% and 40%, for megaprostheses and has a substantial effect on ultimate patient outcome [1, 16–18, 21, 22, 24, 27, 43, 45]. The infection rate of primary megaprostheses in the literature was reported from 2% to 20% and increases to 43% after revision surgery [1, 6, 7, 9, 16–19, 23, 24, 27, 32, 35, 36, 41, 42, 45]. This supports the contention that the immunosuppression caused by the chemotherapy in those patients who receive drugs, extensive resection of bone and soft tissues, longer operative time for the resection of the tumor, and the patient’s general conditions increase the risk of infection in primary treated patients [1, 15–18, 22, 24, 27, 29, 33, 34, 44, 45].
The infection rate of distal femur megaprostheses in the literature was reported as approximately 5.5% and in proximal tibia ranged from 3.6% to 40% [17, 21, 22, 43]. In our series the infection rate in distal femurs was 8.5% and in proximal tibias 11.6% with no difference between the sites of reconstruction (p = 0.399). In the proximal tibia, the risk of infection has been related to the relative lack of wound coverage and unreliable options for extensor mechanism reconstruction [30]. The medial gastrocnemius flap is considered valuable for both reconstruction of the extensor mechanism and adequate coverage of the prostheses [30], which likely reduces the infection rate.
A two-stage revision was performed in 18 cases of deep infection and a new prosthesis was implanted in 20 of 25 cases of deep infection (80%). Two-stage revision is recommended for deep infection of megaprostheses when infection is diagnosed late (ie, greater than 1 month after the initial placement of the prosthesis), whereas one-stage revision may have success in acutely diagnosed infection (ie, those with infections diagnoses less than 1 month from the initial procedure) [17, 18, 40].
In this study, the incidence of Type 2 failure (aseptic loosening) was 5.7% overall at a mean of 4.5 years postoperatively with no difference between sites (distal femur versus proximal tibia) and types (primary versus revision implant). Aseptic loosening remains a common cause of implant failure in the current generation of implants. In the literature, an incidence of aseptic loosening from 4.9% to 9.6% has been reported [1, 15, 32, 37, 40, 41, 43]. In distal femur replacement, aseptic loosening is approximately 6% [21] and in the proximal tibia, the range in the literature is between 5% and 6% [17, 18, 29, 30].
Breakage of the prosthetic component (Type 3 failure) did not occur in our series. In the meta-analysis of the literature published by Henderson et al. [21], the rate of Type 3 failure in distal femur reconstruction was 6.3%, whereas in the proximal tibia, the range in the literature was between 2% and 12% [1, 15–17, 22].
Most of the studies concerning prosthetic reconstructions previously published considered only the major failures such as infection, aseptic loosening, and breakage [1, 6, 7, 10, 16, 19, 23, 26, 30, 32–35, 37, 40, 42, 45]. The survival to major complications range in the literature was from 45% to 93% at 5 years; in particular, from 45% to 70% in the proximal tibia [17, 30, 43] and from 77 to 93% in distal femur replacements [1, 16, 21, 41].
For the reader to compare our results with those of the prior literature, we excluded soft tissue failures and local recurrence to see more directly how our results compare with the literature. In this study, the overall implant survival to all major causes of failure (infection, aseptic loosening, and breakage) was 84% and 69%, respectively, at 4 and 8 years with no difference between the sites (Table 3). These survival rates are higher than survival to aseptic loosening and breakage in fixed-hinge knee megaprostheses [40], likely as a result of reduced mechanical torsional stresses at the bone-stem interface provided by the rotating hinge of the knee [6, 7].
In rotating-hinge knee prostheses, the hinge component provides stability and the rotating component allows a fair amount of rotation (when the knee is flexed, it allows 15° of internal rotation and 15° of external rotation), providing functional advantages compared with a fixed hinge [30, 40].
In our series, functional results were good or excellent in 97% of the cases with no difference between sites (distal femur, proximal tibia) (p = 0.306) or types (primary, revision) (p = 0.435) of reconstruction. The mean MSTS function for the patients with distal femur megaprosthetic reconstructions ranges in the literature from 78% to 86% [10, 16, 35] and in our series, it is 85% (25.4 of 30). The mean MSTS function for the patients with proximal tibial megaprosthetic reconstructions ranges in the literature from 65% to 95% [13, 17, 22, 30] and in our series, it is 82% (24.7 of 30).
In conclusion, the results at a minimum of 2 years with the GMRS modular prosthesis for the distal femur and proximal tibia are promising with a relatively low incidence of failures. Infection was the most frequent cause of failure. We noted better results for revision implants and in our series, there were no differences in survival time to infection between distal femur and proximal tibia reconstruction. The incidence of aseptic loosening with this prosthesis appears to be low without a difference between distal femur and proximal tibia reconstruction and there were no mechanical failures of a prosthetic component in our series. We believe these results are likely the result of the use of a rotating-hinge knee system for the knee and cementless stems that reduce mechanical torsional stresses at the bone-stem interface that cause loosening and breakage. Functional results were good or excellent in 97% and satisfactory in both sites of reconstruction.
Footnotes
Each author certifies that he or she, or a member of his or her immediate family, has no funding or commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research ® editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research ® neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA-approval status, of any drug or device prior to clinical use.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
References
- 1.Ahlmann ER, Menendez LR, Kermani C, Gotha H. Survivorship and clinical outcome of modular endoprosthetic reconstruction for neoplastic disease of the lower limb. J Bone Joint Surg Br. 2006;88:790–795. doi: 10.1302/0301-620X.88B6.17519. [DOI] [PubMed] [Google Scholar]
- 2.Bacci G, Ferrari S, Bertoni F, Ruggieri P, Picci P, Longhi A, Casadei R, Fabbri N, Forni C, Versari M, Campanacci M. Long-term outcome for patients with nonmetastatic osteosarcoma of the extremity treated at the Istituto Ortopedico Rizzoli according to the Istituto Ortopedico Rizzoli/osteosarcoma-2 protocol: an updated report. J Clin Oncol. 2000;18:4016–4027. doi: 10.1200/JCO.2000.18.24.4016. [DOI] [PubMed] [Google Scholar]
- 3.Bacci G, Ferrari S, Comandone A, Zanone A, Ruggieri P, Longhi A, Bertoni F, Forni C, Versari M, Rimondini S. Neoadjuvant chemotherapy for Ewing’s sarcoma of bone in patients older than thirty-nine years. Acta Oncol. 2000;39:111–116. doi: 10.1080/028418600431076. [DOI] [PubMed] [Google Scholar]
- 4.Bacci G, Picci P, Ferrari S, Avella M, Prever BA, Ruggieri P, Casadei R, Lari S, Monti C, Cazzola A. Neoadjuvant chemotherapy for nonmetastatic osteosarcoma for the extremities: the recent experience at the Rizzoli Institute. Cancer Treat Res. 1993;62:299–308. doi: 10.1007/978-1-4615-3518-8_36. [DOI] [PubMed] [Google Scholar]
- 5.Bacci G, Picci P, Ferrari S, Ruggieri P, Casadei R, Tienghi A, Brach del Prever A, Gherlinzoni F, Mercuri M, Monti C. Primary chemotherapy and delayed surgery for nonmetastatic osteosarcoma of the extremities. Results in 164 patients preoperatively treated with high doses of methotrexate followed by cisplatin and doxorubicin. Cancer. 1993;72:3227–3238. doi: 10.1002/1097-0142(19931201)72:11<3227::AID-CNCR2820721116>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 6.Bhangu AA, Kramer MJ, Grimer RJ, O’Donnell RJ. Early distal femoral endoprosthetic survival: cemented stems versus the compress implant. Int Orthop. 2006;30:465–472. doi: 10.1007/s00264-006-0186-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Biau D, Faure F, Katsahian S, Jeanrot C, Tomeno B, Anract P. Survival of total knee replacement with a megaprosthesis after bone tumor resection. J Bone Joint Surg Am. 2006;88:1285–1293. doi: 10.2106/JBJS.E.00553. [DOI] [PubMed] [Google Scholar]
- 8.Campanacci M. Bone and Soft Tissue Tumors. New York, NY, USA: Springer-Verlag; 1999. pp. 1–70. [Google Scholar]
- 9.Cannon SR. Massive prosthesis for malignant bone tumours of the limbs. J Bone Joint Surg Br. 1997;79:497–506. doi: 10.1302/0301-620X.79B3.14191. [DOI] [PubMed] [Google Scholar]
- 10.Capanna R, Morris HG, Campanacci D, Del Ben M, Campanacci M. Modular uncemented prosthetic reconstruction after resection of tumor of the distal femur. J Bone Joint Surg Br. 1994;76:178–186. [PubMed] [Google Scholar]
- 11.Capanna R, Ruggieri P, Biagini R, Ferraro A, DeCristofaro R, McDonald D, Campanacci M. The effect of quadriceps excision on functional results after distal femoral resection and prosthetic replacement of bone tumors. Clin Orthop Relat Res. 1991;267:186–196. [PubMed] [Google Scholar]
- 12.Capanna R, van Horn JR, Biagini R, Ruggieri P, Bettelli G, Campanacci M. Reconstruction after resection of the distal fibula for bone tumor. Acta Orthop Scand. 1986;57:290–294. doi: 10.3109/17453678608994394. [DOI] [PubMed] [Google Scholar]
- 13.Eckardt JJ, Eilber FR, Dorey FJ, Mirra JM. The UCLA experience in limb salvage surgery for malignant tumours. Orthopedics. 1985;8:612–621. doi: 10.3928/0147-7447-19850501-15. [DOI] [PubMed] [Google Scholar]
- 14.Enneking WF, Spanier SS, Goodman MA. A system for the surgical staging of musculoskeletal sarcoma. Clin Orthop Relat Res. 1980;153:106–120. [PubMed] [Google Scholar]
- 15.Flint MN, Griffin AM, Bell RS, Ferguson PC, Wander JC. Aseptic loosening is uncommon with uncemented proximal tibia tumor prostheses. Clin Orthop Relat Res. 2006;450:52–59. doi: 10.1097/01.blo.0000229300.67394.77. [DOI] [PubMed] [Google Scholar]
- 16.Gosheger G, Gebert C, Ahrens H, Streitbuerger A, Winkelmann W, Hardes J. Endoprosthetic reconstruction in 250 patients with sarcoma. Clin Orthop Relat Res. 2006;450:164–171. doi: 10.1097/01.blo.0000223978.36831.39. [DOI] [PubMed] [Google Scholar]
- 17.Grimer RJ, Belthur M, Chandrasekar C, Carter SR, Tillman RM. Two-stage revision for infected endoprostheses used in tumor surgery. Clin Orthop Relat Res. 2002;395:193–203. doi: 10.1097/00003086-200202000-00022. [DOI] [PubMed] [Google Scholar]
- 18.Hardes J, Gebert C, Schwappach A, Ahrens H, Streitburger A, Winkelmann W, Gosheger G. Characteristics and outcome of infections associated with tumor endoprostheses. Arch Orthop Trauma Surg. 2006;126:289–296. doi: 10.1007/s00402-005-0009-1. [DOI] [PubMed] [Google Scholar]
- 19.Heisel C, Breusch SJ, Schmid G, Bernd L. Lower limb salvage surgery with MUTARS endoprostheses: 2 to 7 year results. Acta Orthop Belg. 2004;70:142–147. [PubMed] [Google Scholar]
- 20.Heisel C, Kinkel S, Bernd L, Ewerbeck V. Megaprostheses for the treatment of malignant bone tumours of the lower limbs. Int Orthop. 2006;30:452–457. doi: 10.1007/s00264-006-0207-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Henderson ER, Groundland JS, Pala E, Dennis JA, Wooten R, Cheong D, Windhager R, Kotz RI, Mercuri M, Funovics PT, Hornicek FJ, Temple HT, Ruggieri P, Letson GD. Failure mode classification for tumor endoprostheses: retrospective review of five institutions and a literature review. J Bone Joint Surg Am. 2011;93:418–429. doi: 10.2106/JBJS.J.00834. [DOI] [PubMed] [Google Scholar]
- 22.Horowitz SM, Lane JM, Otis JC, Healy JH. Prosthetic arthroplasty of the knee after resection of a sarcoma in the proximal end of the tibia: a report of sixteen cases. J Bone Joint Surg Am. 1991;73:286–293. [PubMed] [Google Scholar]
- 23.Ilyas I, Kurar A, Moreau PG, Younge DA. Modular megaprosthesis for distal femoral tumors. Int Orthop. 2001;25:375–377. doi: 10.1007/s002640100290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jeys LM, Grimer RJ, Carter SR, Tillman RM. Periprosthetic infection in patients treated for an oncological orthopaedic condition. J Bone Joint Surg Am. 2005;87:842–849. doi: 10.2106/JBJS.C.01222. [DOI] [PubMed] [Google Scholar]
- 25.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1985;53:457–481. doi: 10.1080/01621459.1958.10501452. [DOI] [Google Scholar]
- 26.Kotz R, Dominkus M, Zettl T, Ritschl P, Windhager R, Gadner H, Zielinski C, Salzer-Kuntschik M. Advances in bone tumour treatment in 30 years with respect to survival and limb salvage. A single institution experience. Int Orthop. 2002;26:197–202. doi: 10.1007/s00264-002-0365-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee SH, Oh JH, Lee KS, Yoo KH, Kim HS. Infection after prosthetic reconstruction in limb salvage surgery. Int Orthop. 2002;26:179–184. doi: 10.1007/s00264-001-0328-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luksch R, Tienghi A, Hall KS, Fagioli F, Picci P, Barbieri E, Gandola L, Eriksson M, Ruggieri P, Daolio P, Lindholm P, Prete A, Bisogno G, Tamburini A, Grignani G, Abate ME, Podda M, Smeland S, Ferrari S. Primary metastatic Ewing’s family tumors: results of the Italian Sarcoma Group and Scandinavian Sarcoma Group ISG/SSG IV Study including myeloablative chemotherapy and total-lung irradiation. Ann Oncol. 2012;23:2970–2976. doi: 10.1093/annonc/mds117. [DOI] [PubMed] [Google Scholar]
- 29.Malawer MM, Chou LB. Prosthetic survival and clinical results with use of large-segment replacements in the treatment of high-grade bone sarcomas. J Bone Joint Surg Am. 1995;77:1154–1165. doi: 10.2106/00004623-199508000-00003. [DOI] [PubMed] [Google Scholar]
- 30.Mavrogenis AF, Pala E, Angelini A, Ferraro A, Ruggieri P. Proximal tibial resections and reconstructions: clinical outcome of 225 patients. J Surg Oncol. 2013;107:335–342. doi: 10.1002/jso.23216. [DOI] [PubMed] [Google Scholar]
- 31.Mirra JM, Picci P, Gold RH, eds. Bone Tumors: Clinical, Radiologic and Pathologic Correlations. Vol. 1. Philadelphia, PA, USA: Lea & Febiger; 1989:248–262.
- 32.Mittermayer F, Krepler P, Dominkus M, Schwameis E, Sluga M, Heinzl H, Kotz R. Long-term followup of uncemented tumor endoprostheses for the lower extremity. Clin Orthop Relat Res. 2001;388:167–177. doi: 10.1097/00003086-200107000-00024. [DOI] [PubMed] [Google Scholar]
- 33.Myers GJC, Abudu AT, Carter SR, Tillman RM, Grimer RJ. Endoprosthetic replacement of the distal femur for bone tumors. Long term results. J Bone Joint Surg Br. 2007;89:521–526. doi: 10.1302/0301-620X.89B4.18631. [DOI] [PubMed] [Google Scholar]
- 34.Myers GJC, Abudu AT, Carter SR, Tillman RM, Grimer RJ. The long-term results of endoprosthetic replacement of the proximal tibia for bone tumors. J Bone Joint Surg Br. 2007;89:1632–1637. doi: 10.1302/0301-620X.89B12.19481. [DOI] [PubMed] [Google Scholar]
- 35.Natarajan MV, Sivaseelam A, Ayyappan S, Bose JC, Sampath Kumar M. Distal femoral tumours treated by resection and custom mega-prosthetic replacement. Int Orthop. 2005;29:309–313. doi: 10.1007/s00264-005-0677-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Orlic D, Smerdelj M, Kolundzic R, Bergovec M. Lower limb salvage surgery: modular endoprosthesis in bone tumour treatment. Int Orthop. 2006;30:458–464. doi: 10.1007/s00264-006-0193-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Plotz W, Rechl H, Burgkart R, Messmer C, Schelter R, Hipp E, Gradinger R. Limb salvage with tumor endoprostheses for malignant tumors of the knee. Clin Orthop Relat Res. 2002;405:207–215. doi: 10.1097/00003086-200212000-00027. [DOI] [PubMed] [Google Scholar]
- 38.Rougraff BT, Simon MA, Kneisl JS, Greenberg DB, Mankin HJ. Limb salvage compared with amputation for osteosarcoma of the distal end of the femur: a long-term oncological, functional and quality of life study. J Bone Joint Surg Am. 1994;76:649–656. doi: 10.2106/00004623-199405000-00004. [DOI] [PubMed] [Google Scholar]
- 39.Ruggieri P, Bosco G, Pala E, Errani C, Mercuri M. Local recurrence, survival and function after total femur resection and megaprosthetic reconstruction for bone sarcomas. Clin Orthop Relat Res. 2010;468:2860–2866. doi: 10.1007/s11999-010-1476-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ruggieri P, Mavrogenis AF, Pala E, Abdel-Mota’al M, Mercuri M. Long term results of fixed-hinge megaprostheses in limb salvage for malignancy. Knee. 2012;19:543–549. doi: 10.1016/j.knee.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 41.Sharma S, Turcotte RE, Isler MH, Wong C. Experience with cemented large segment endoprostheses for tumors. Clin Orthop Relat Res. 2007;459:54–59. doi: 10.1097/BLO.0b013e3180514c8e. [DOI] [PubMed] [Google Scholar]
- 42.Turcotte RE. Endoprosthetic replacements for bone tumors: review of the most recent literature. Curr Opin Orthop. 2007;18:572–578. doi: 10.1097/BCO.0b013e3282ef6eaf. [DOI] [Google Scholar]
- 43.Unwin PS, Cannon SR, Grimer RJ, Kemp HB, Sneath RS, Walker PS. Aseptic loosening in cemented custom-made prosthetic replacements for bone tumours of the lower limb. J Bone Joint Surg Br. 1996;78:5–13. [PubMed] [Google Scholar]
- 44.Wunder JS, Leitch K, Griffin AM, Davis AM, Bell RS. Comparison of two methods of reconstruction for primary malignant tumors at the knee: a sequential cohort study. J Surg Oncol. 2001;77:89–99. doi: 10.1002/jso.1076. [DOI] [PubMed] [Google Scholar]
- 45.Zeegen EN, Aponte-Tinao LA, Hornicek FJ, Gebhardt MC, Mankin HJ. Survivor analysis of 141 modular metallic endoprostheses at early followup. Clin Orthop Relat Res. 2004;420:239–250. doi: 10.1097/00003086-200403000-00034. [DOI] [PubMed] [Google Scholar]