Abstract
Background
Biofilm-related periprosthetic infections are catastrophic to patients and clinicians. Data suggest the addition of vitamin E to UHMWPE may have the ability to reduce biofilm formation on the surface of UHMWPE; however, previous studies were performed using stagnant broth solutions that may not have simulated a physiologic environment. In addition, the observed differences in levels of bacterial attachment, though statistically significant, may not be clinically significant.
Questions/purposes
We blended vitamin E with UHMWPE material and tested it for the ability to resist biofilm formation using a clinical isolate of methicillin-resistant Staphylococcus aureus (MRSA). Three additional materials were tested for comparison: highly crosslinked UHMWPE, compression-molded UHMWPE, and polyetheretherketone. We also determined whether the surface roughness of these materials facilitated biofilm formation.
Methods
Using a flow cell system, samples of each material type were placed into separate chambers. A 10% solution of brain-heart infusion broth containing 105 colony-forming units (CFUs)/mL was flowed through the flow cell over 48 hours. The number of bacteria that adhered to the surface was quantified and biofilm formation was observed qualitatively using scanning electron microscopy. Optical profilometry was used to determine the surface roughness of each material type.
Results
Vitamin E-blended UHMWPE did not reduce biofilm formation of a clinically relevant strain of MRSA compared to materials that did not have vitamin E. More specifically, vitamin E-blended materials had similar amounts of biofilm formation (~ 8 log10 CFUs/cm2) compared to materials not containing vitamin E (~ 8.1 log10 CFUs/cm2) (p > 0.4). The roughness of vitamin E-blended material surfaces (mean ± SD: 1.85 ± 0.46 µm) compared to that of materials without vitamin E (2.06 ± 1.24 µm) did not appear to influence biofilm formation.
Conclusions
Under physiologically relevant conditions, vitamin E-blended UHMWPE did not have the ability to reduce the formation of biofilms by MRSA.
Clinical Relevance
These data indicate that the addition of vitamin E to UHMWPE may not reduce clinically relevant rates of biofilm-related periprosthetic infections of total joint arthroplasty devices.
Introduction
Periprosthetic infections that develop as a result of biofilm formation on total joint arthroplasty devices cause catastrophic morbidity. Biofilm implant-related infections are difficult to treat. Multiple factors contribute to this difficulty, including water channels in a biofilm that may remove antibiotics from the community, lower-metabolic-state bacteria (resistant variants) in a biofilm that antibiotics are less effective against, and plasmid gene transfer, which may result in molecules that interfere with antibiotic treatment [8, 13, 18, 23]. As a result, removal of the prosthesis, which results in protracted convalescence periods, pain, and expense, is often required.
In an effort to prevent biofilm-related periprosthetic infections, multiple technologies are currently under development for orthopaedic applications. Examples include passive and active antimicrobial coatings [4, 9, 19], surface modifications [10], and bioabsorbable sleeves that contain antimicrobials [16]. Data suggest that implant materials alone, such as black silicon and silicon nitride, may have the ability to prevent bacterial attachment or eradicate bacteria that come in contact with the material [7, 10]. Additionally, previous reports have suggested that the addition of vitamin E to UHMWPE may prevent the adhesion of bacteria to its surface and thus reduce the risk of biofilm formation and subsequent infection [1, 5, 12]. It has been proposed that bacteria may have increased affinity to adhere to oxidized UHMWPE surfaces and form biofilms. Thus, the addition of vitamin E may reduce oxidation and result in a reduction of biofilm formation on the surface [1].
Notably, at least two limitations have accompanied these previous studies. First, stagnant broth solutions were used, which may not be considered an accurate model for a clinical scenario where liquid flow may be present. It has been shown that the strength of a biofilm is affected by flow conditions [15]. Second, although statistically significant differences were reported in bacterial attachment on UHMWPE surfaces, these differences may not be clinically significant. More specifically, Banche et al. [1] reported a statistically significant difference in bacterial adhesion when comparing the attachment of Staphylococcus epidermidis to oxidized UHMWPE (7.25 × 107 colony-forming units [CFUs]/mL) to vitamin E-blended UHMWPE (1.27 × 107 CFUs/mL). Animal model data and clinical data have indicated that as little as 102 or 105 CFUs/g of tissue may be pathogenic [2, 11]. Thus, if a potential material claim is to be made regarding reduction of biofilm formation in the context of preventing infection, it may be necessary to demonstrate a reduction or kill rate of bacterial biofilms larger than a 0.5 log10 or 1 log10 reduction.
In this study, vitamin E was blended with UHMWPE and crosslinked using two separate levels of gamma radiation (150 kGy and 75 kGy). A flow cell system was used to expose the materials to conditions that may model a physiologically relevant environment for biofilm formation on biomaterials [19]. The primary hypothesis was that the vitamin E-blended and radiation-crosslinked UHMWPE would resist the adhesion and formation of clinically relevant methicillin-resistant Staphylococcus aureus (MRSA) biofilms when compared to other polymeric materials that are currently used in clinical applications. The roughness of each material that resulted from the machining process was analyzed as a secondary outcome measure to determine the potential influence that roughness had on biofilm formation.
Materials and Methods
Materials and Machining
Five sample types were manufactured, machined, and sterilized for this study: highly crosslinked UHMWPE (HXL), vitamin E-loaded HXL crosslinked with 150 kGy and 75 kGy gamma radiation (HXL VE 150 kGy and HXL VE 75 kGy), compression-molded UHMWPE (CM), and polyetheretherketone (PEEK) (ASTM F2026 compliant; PEEK-OPTIMA®; Invibio Biomaterial Solutions, West Conshohocken, PA, USA). All of the UHMWPE materials were processed from resin type GUR 1020 and were compression molded. Other processes used to manufacture, machine, and sterilize each material were proprietary to the company (DJO Surgical, Austin, TX, USA). Each material was machined using the same conditions. However, surface roughness, Ra, was not specifically controlled. Rather, the resulting roughness from the machining process was determined for each material and analyzed for its influence on biofilm formation.
The rationale for selecting PEEK as a test material was twofold. First, our previously published studies have indicated that the MRSA isolate used in this study produces biofilms on PEEK material [19–22]. Second, PEEK is commonly used in spinal implant surgeries and these data may provide an indication of MRSA biofilm formation on PEEK materials that may be relevant to other clinical applications.
Each sample had dimensions of 2 cm × 2 cm × 1 cm (Fig. 1A). To determine whether the growth of MRSA biofilms would be reduced or prevented on the surface of the two vitamin E-loaded samples (HXL VE 150 kGy and HXL VE 75 kGy) in comparison to the other three clinically relevant material types (HXL, CM, and PEEK), each material type was tested for biofilm formation using a flow cell system developed by the Bone and Joint Research Laboratory at the George E. Wahlen Department of Veterans Affairs (Salt Lake City, UT, USA) [19]. The flow cell consisted of six chambers, each having a dimension of 4 cm × 4 cm × 2 cm (Fig. 1B).
Fig. 1A–B.
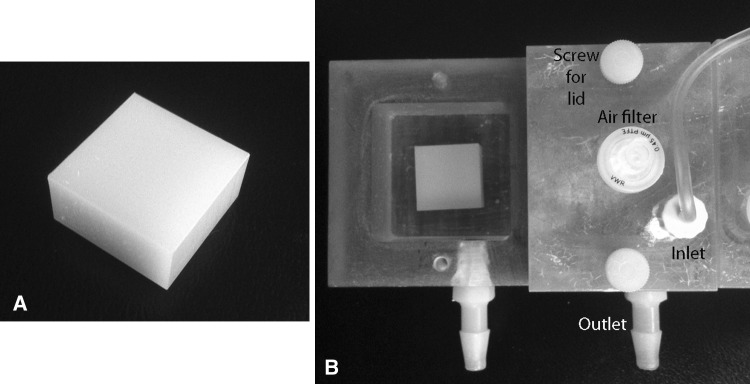
(A) A representative image of a vitamin E-blended material manufactured to 2 cm × 2 cm × 1 cm is shown. (B) The vitamin E-blended material is shown inside a chamber of the flow cell unit. A lid is shown covering the adjoining chamber. The lid is secured in place by two screws. The inlet allowed broth to be flowed into the chamber. The broth then flowed into an effluent container through the outlet.
Bacterial Isolate
A clinically relevant isolate of MRSA was used in this study. It was collected from the knee of an infected patient who underwent arthroscopic surgery and was kindly provided by ARUP Laboratories (Salt Lake City, UT, USA). This isolate has been confirmed to contain the icaADBC gene operon and has been used in previous studies to confirm its pathogenic and biofilm-forming properties [19, 21, 22]. The isolate was stored at −80°C and passaged fewer than two times on Columbia blood agar (Hardy Diagnostics, Midvale, UT, USA) before each use.
To perform each experiment, a 0.5 McFarland standard (concentration of ~ 1 × 108 CFUs/mL) of the isolate was made. Three milliliters of the McFarland standard was added to 2997 mL 10% modified brain-heart infusion broth for a final bacterial concentration of approximately 1 × 105 CFUs/mL.
Biofilm Formation and Quantification
Samples (n = 7) of each material type were placed individually into a chamber of the flow cell. The brain-heart infusion broth containing 105 MRSA CFUs/mL was pumped through each chamber at a rate of 4.5 mL/hour using a peristaltic pump (Masterflex® L/S; Cole-Parmer, Vernon Hills, IL, USA). To prevent the premature growth of bacteria/biofilm formation, the broth with bacteria was kept on ice outside the incubator and pumped into the chambers of the flow cell within the incubator.
Using previously established protocols [19–22], after 48 hours, each sample was removed from a chamber of the flow cell, rinsed three times in phosphate-buffered saline, placed into 20 mL phosphate-buffered saline, vortexed for 1 minute, and sonicated at 42 kHz for 10 minutes. A 10-fold dilution series was used to quantify the number of CFUs that were attached to and/or formed into biofilms on the surface. The rationale for using a 48-hour time point was based on preliminary data indicating, that by 24 hours, a monolayer of cells had formed on each material type, but mature, three-dimensional biofilms had not yet formed (data not shown). The objective of this study was to assess the formation of biofilms. Thus, 48 hours was the time point selected.
Scanning Electron Microscopy
Using the same protocol as above, after the 48-hour incubation period, samples (n = 7) of each material type were fixed in 0.25% glutaraldehyde, dehydrated in ascending concentrations of ethanol (70%, 95%, 100%), coated with carbon, and imaged using scanning electron microscopy (SEM).
Surface Analysis
Samples (n = 7) of each of the five material types were characterized using a noncontact Zygo® Optical Profilometer 10x objective (Zygo Corp, Middlefield, CT, USA) at the University of Utah NanoFab Laboratory (Salt Lake City, UT, USA). Five images were collected from each sample, one in each of the four corners and middle section. Surface roughness, Ra, data were analyzed using MetroPro® 8.3.5 software (Zygo Corp).
Statistical Analysis
Bacterial quantification data were compared using one-way ANOVA with post hoc Tukey analysis. Effect size (η2) and 95% CI were determined. A p value of less than 0.05 was considered significant. We used SPSS® 17.0 software (SPSS Inc, Chicago, IL, USA) for all statistical analyses.
Results
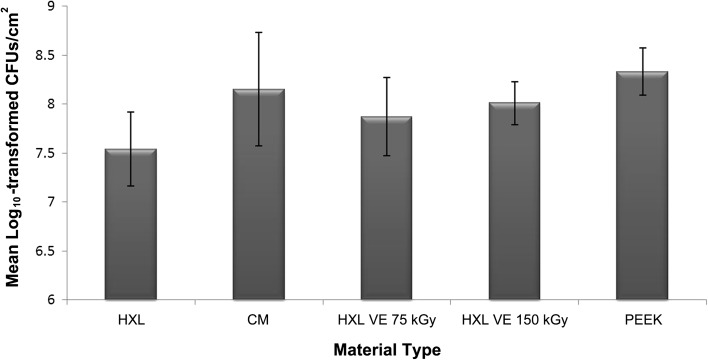
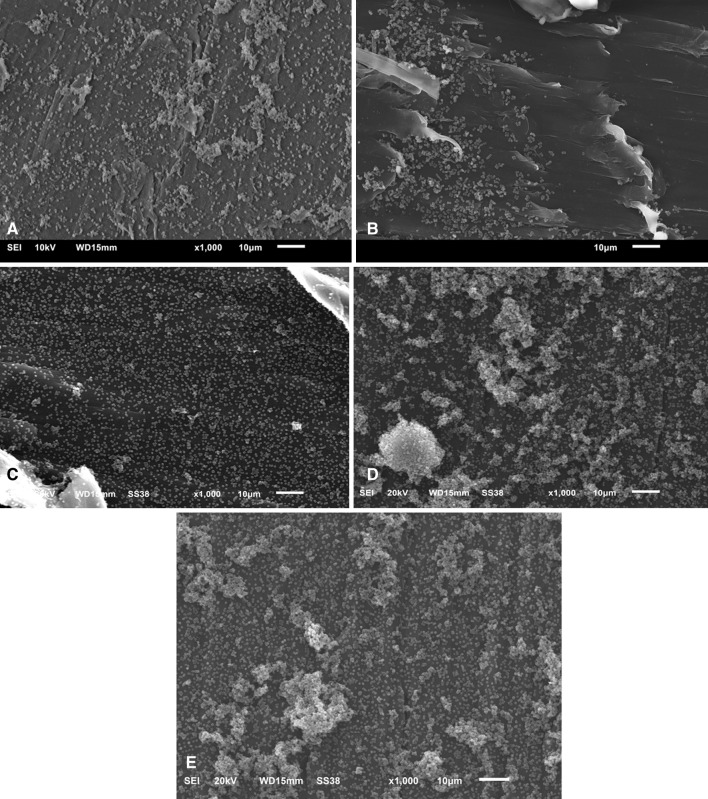
The two vitamin E-blended materials did not resist the attachment/formation of MRSA biofilms to any greater degree than the other three material types used clinically in total joint arthroplasties. All materials had greater than 107 CFUs/cm2 (Fig. 2). There was a significant difference in the number of bacteria between the HXL and PEEK materials (η2 = 0.479; 95% CI: 7.43 × 108, 3.23 × 109; p = 0.014), but this reduction was not considered clinically relevant as per the description given in the Introduction. No significant differences in the number of adhered bacteria were observed among the other material types, ie, CM, HXL VE 75 kGy, and HXL VE 100 kGy (η2 = 0.09; 95% CI: 3.33 × 108, 9.93 × 108; p = 0.43). SEM corroborated the quantification data, which indicated that each material type had greater than 107 CFUs/cm2, by showing areas of substantial biofilm formation on each of the five material types (Fig. 3). The SEM and quantification data together did not support our primary hypothesis, ie, that vitamin E-blended materials would have the ability to reduce/prevent the formation of MRSA biofilms on the surface.
Fig. 2.
A bar graph shows the number of log10 transformed CFUs of bacteria per cm2 of each material type. The HXL material has the lowest amount of biofilm formation. There was a significant difference between the HXL and PEEK materials (p = 0.014) but not among the other three material types (p = 0.43). Data are presented as mean ± SD.
Fig. 3A–E.
Representative SEM images collected from each of the five material types after 48 hours of incubation are shown: (A) HXL, (B) CM, (C) HXL VE 75 kGy, (D) HXL VE 150 kGy, and (E) PEEK. Each material type had areas wherein bacteria attached to the surface and biofilms formed.
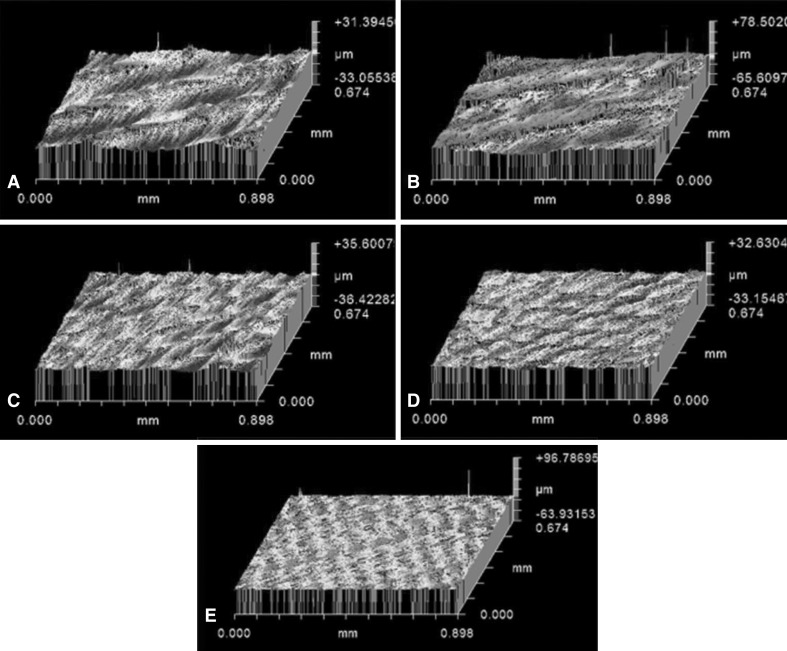
As regards our secondary outcome measure, the influence of surface roughness on biofilm formation, optical profilometry measurements (Fig. 4) indicated that the CM material had the greatest surface roughness whereas the PEEK material had the least surface roughness (Table 1). Notably, these two materials had similar amounts of biofilm formation per cm2 (Fig. 2). The HXL material, which had a surface roughness in the middle range of the five materials, had the lowest amount of biofilm formation (Fig. 2). However, the reduction of approximately 0.5 to 1 log10 from greater than 107 CFUs that were seen in the HXL material compared to the other four materials may not be considered clinically significant, as noted in the Introduction.
Fig. 4A–E.
Representative images of surface roughness (Ra) output for each of the five material types imaged by optical profilometry are shown: (A) HXL, (B) CM, (C) HXL VE 75 kGy, (D) HXL VE 150 kGy, and (E) PEEK. The CM material had the greatest surface roughness whereas the PEEK material had the least surface roughness.
Table 1.
Surface roughness of each material type as indicated by optical profilometry
| Material type | Surface roughness (µm) |
|---|---|
| HXL | 2.38 ± 0.68 |
| CM | 3.11 ± 1.23 |
| HXL VE 75 kGy | 2.18 ± 0.83 |
| HXL VE 150 kGy | 1.53 ± 0.45 |
| PEEK | 0.69 ± 0.14 |
Values are expressed as mean ± SD; HXL = highly crosslinked UHMWPE; CM = compression-molded UHMWPE; HXL VE 150 kGy = vitamin E-loaded HXL crosslinked with 150 kGy gamma radiation; HXL VE 75 kGy = vitamin E-loaded HXL crosslinked with 75 kGy gamma radiation; PEEK = polyetheretherketone.
Discussion
Biofilm-related periprosthetic infections are difficult to treat and result in serious patient morbidity and sometimes even mortality. Vitamin E has been investigated as an additive to UHMWPE materials with a potential secondary benefit to resist the formation of biofilms on the surface; however, previous studies have been limited in their use of stagnant broth solutions and also made an assumption that a statistically significant reduction from 7.25 × 107 CFUs to 1.27 × 107 CFUs adhering to a material surface might translate to a clinically significant difference. In this study, using a flow cell system to model a physiologically relevant scenario, we examined the ability of vitamin E-blended UHMWPE to resist the formation of MRSA biofilms, compared to several materials that are in common use in orthopaedic implants, HXL, CM, and PEEK. We also evaluated the influence of the surface roughness of these materials on biofilm formation.
Four important limitations accompanied this study. First, only one bacterial isolate was examined. Additional clinical isolates, including S epidermidis, Propionibacterium acnes, and gram negative organisms such as Pseudomonas aeruginosa, Klebsiella pneumonia, and Escherichia coli, will need to be tested to analyze the ability of vitamin E-blended UHMWPE to resist biofilm formation of other species. Second, as is common with the majority of in vitro investigations, no biologic components were present such as antibodies or membrane attack complex components of the complement system that may help to fight infection in patients. To more accurately model a clinical scenario, an animal model wherein vitamin E-blended UHMWPE is implanted and bacteria are inoculated will be needed to determine whether the material would prevent or reduce biofilm-related periprosthetic infection. Third, biofilm growth was examined at a single time point in this study, 48 hours. However, it is recognized that bacterial levels and time of exposure in a clinical setting may vary. For example, low levels of bacteria may not survive for 48 hours in an in vivo setting. To more fully determine the effect of initial inocula, time of exposure, biofilm formation, and resulting infection, additional in vitro and in vivo data would be required. Finally, this study did not control for surface roughness and its influence on biofilm formation. To more accurately determine the influence of surface roughness on biofilm formation, a controlled study with varying roughnesses of each material type would need to be performed. Herein, the roughness of each material type that resulted from the machining process alone was analyzed for its effect on biofilm formation.
In this study, vitamin E-blended UHMWPE did not prevent or reduce the attachment or formation of bacterial biofilms on their surfaces under the specified conditions, nor did any of the other materials tested. One of the most commonly used clinical material types, HXL, had the fewest number of bacteria adhere to its surface in a 48-hour period. An average of approximately 5 × 107 CFUs/cm2 adhered to this material whereas the other four material types had between approximately 1 × 108 and 5 × 108 CFUs/cm2. Notably, a clinically accepted standard for the number of planktonic bacteria that may cause infection is 105 CFUs/g of tissue [3, 11]. Bernthal et al. [2] have even shown that an inoculum of 102 CFUs can cause low-grade, chronic infection in a mouse model of joint arthroplasty infection. Thus, a biofilm consisting of 107 or 108 CFUs/cm2 would be very likely to cause biofilm-related periprosthetic infection, and a reduction from 108 to 107 CFUs/cm2 would likely have no clinical relevance.
Previous studies have examined bacterial adherence to vitamin E-blended materials. Molina-Manso et al. [12] and Gomez-Barrena et al. [5] performed a rapid adherence test wherein materials were exposed to bacterial suspensions containing 108 bacteria for 90 minutes. Their results indicated that clinical isolates of S aureus showed no difference in the amount that adhered to control or vitamin E-blended samples, which is consistent with our data. An important limitation of that study was that 90 minutes of bacterial exposure may not have provided an accurate measure for biofilm formation on the surface of a material since bacteria require roughly 24 hours to form a mature biofilm [23]. In a study by Banche et al. [1], three material types were exposed to an initial inoculum of 107 CFUs/mL for various times including 24 hours and 48 hours. These tests were not performed under flow but rather under static conditions. In that study, fewer S epidermidis adhered to vitamin E-loaded materials than to UHMWPE-only samples. However, the observed difference may not be considered clinically relevant, as a biofilm with a difference of 107 to 108 (one log10 unit) likely would have similar potential to cause infection. The authors suggested that their data indicated that vitamin E-loaded samples may reduce bacterial adhesion but also recognized that additional data would be needed to confirm the results.
Surface roughness was not controlled for in this study but was analyzed as a secondary outcome measure to determine the influence that roughness, created by machining, may have had on biofilm formation. The roughnesses of HXL, CM, and the two vitamin E-blended materials were similar and did not appear to affect biofilm formation. The PEEK material had the smoothest surface after being exposed to the same machining process as the other four materials yet had the most biofilm formation. This was particularly interesting given that multiple studies have indicated that an increase in surface roughness may promote or encourage biofilm formation [6, 14, 17]. Future experiments will need to be performed to determine whether rougher surfaces of PEEK enhance or promote biofilm formation compared to smoother surfaces of PEEK. Such experiments would help to determine whether the surface chemistry of PEEK influences biofilm formation more so than roughness. A review of the literature indicated that such studies have not yet been performed. It is also difficult to draw conclusions related to PEEK biofilm formation and how this might affect total joint arthroplasty applications as PEEK is primarily used in spinal applications and not total joint devices. The use of PEEK, however, served as a material type on which MRSA had been shown previously to form biofilms [18–20, 22]. Overall, based on the optical profilometry data obtained along with quantified biofilm data, surface roughness did not appear to influence bacterial adhesion and biofilm formation for materials currently used in total joint arthroplasty devices or the vitamin E-blended materials.
Taken together, our data indicated that vitamin E-blended UHMWPE was not able to prevent the growth/formation of biofilms of a clinically relevant strain of MRSA. Surface roughness did not appear to influence biofilm formation for those polymeric materials that are relevant to total joint arthroplasty devices. However, additional data are needed using multiple types of gram-positive and gram-negative bacteria to gain a deeper understanding of the role that vitamin E or surface roughness plays in biofilm formation. In conclusion, any rationale for adding vitamin E to UHMWPE may need to derive from properties other than the ability to prevent infection.
Acknowledgments
The authors thank DJO Surgical for funding and supporting this project. We also thank Bryan Haymond BS, Ralph Gochnour, Gina Allyn, Leslie Florian, and Logan Horne for their technical support and Dennis Romney for machining the flow cell system.
Footnotes
The institution of one or more of the authors (DLW, JML, RDB) has received, during the study period, funding from DJO Surgical (Austin, TX, USA). One of the authors (JV) is an employee of DJO Surgical.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research ® editors and board members are on file with the publication and can be viewed on request.
This work was performed at George E. Wahlen Department of Veterans Affairs, Salt Lake City, UT, USA.
References
- 1.Banche G, Bracco P, Bistolfi A, Allizond V, Boffano M, Costa L, Cimino A, Cuffini AM, Del Prever EM. Vitamin E blended UHMWPE may have the potential to reduce bacterial adhesive ability. J Orthop Res. 2011;29:1662–1667. doi: 10.1002/jor.21432. [DOI] [PubMed] [Google Scholar]
- 2.Bernthal NM, Stavrakis AI, Billi F, Cho JS, Kremen TJ, Simon SI, Cheung AL, Finerman GA, Lieberman JR, Adams JS, Miller LS. A mouse model of post-arthroplasty Staphylococcus aureus joint infection to evaluate in vivo the efficacy of antimicrobial implant coatings. PLoS One. 2010;5:e12580. doi: 10.1371/journal.pone.0012580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clinical and Laboratory Standards Institute . Methods for Determining Bactericidal Activity of Antimicrobial Agents. Wayne, PA: Clinical and Laboratory Standards Institute; 1999. pp. 1–28. [Google Scholar]
- 4.Fuchs T, Stange R, Schmidmaier G, Raschke MJ. The use of gentamicin-coated nails in the tibia: preliminary results of a prospective study. Arch Orthop Trauma Surg. 2011;131:1419–1425. doi: 10.1007/s00402-011-1321-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gómez-Barrena E, Esteban J, Molina-Manso D, Adames H, Martínez-Morlanes M, Terriza A, Yubero F, Puértolas J. Bacterial adherence on UHMWPE doped with vitamin E: an in vitro study. J Mater Sci Mater Med. 2011;22:1701–1706. doi: 10.1007/s10856-011-4340-5. [DOI] [PubMed] [Google Scholar]
- 6.Gorman SP, Mawhinney WM, Adair CG, Issoukis M. Confocal laser scanning microscopy of peritoneal catheter surfaces. J Med Microbiol. 1993;38:411–417. doi: 10.1099/00222615-38-6-411. [DOI] [PubMed] [Google Scholar]
- 7.Gorth DJ, Puckett S, Ercan B, Webster TJ, Rahaman M, Bal BS. Decreased bacteria activity on Si3N4 surfaces compared with PEEK or titanium. Int J Nanomedicine. 2012;7:4829–4840. doi: 10.2147/IJN.S35190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gristina AG, Costerton JW. Bacteria-laden biofilms: a hazard to orthopedic prostheses. Infect Surg. 1984;3:655–662. [Google Scholar]
- 9.Hetrick EM, Schoenfisch MH. Reducing implant-related infections: active release strategies. Chem Soc Rev. 2006;35:780–790. doi: 10.1039/b515219b. [DOI] [PubMed] [Google Scholar]
- 10.Ivanova EP, Hasan J, Webb HK, Gervinskas G, Truong VK, Wu AH, Lamb RN, Baulin VA, Watson GS, Watson JA, Mainwaring DE, Crawford RJ. Bactericidal activity of black silicon. Nat Commun. 2013;4:2838. doi: 10.1038/ncomms3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krizek TJ, Robson MC, Kho E. Bacterial growth and skin graft survival. Surg Forum. 1967;18:518–520. [Google Scholar]
- 12.Molina-Manso D, Gómez-Barrena E, Esteban J, Adames H, Martínez MJ, Cordero J, Fernandéz-Roblas R, Puértolas JA. Bacterial adherence on UHMWPE doped with vitamin E: an in vitro study. J Phys Conf Ser. 2010;252:1–7. [Google Scholar]
- 13.Nickel JC, Ruseska I, Wright JB, Costerton JW. Tobramycin resistance of Pseudomonas aeruginosa cells growing as a biofilm on urinary catheter material. Antimicrob Agents Chemother. 1985;27:619–624. doi: 10.1128/AAC.27.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quirynen M, Bollen CM. The influence of surface roughness and surface free energy on supragingival and subgingival plaque formation in man: a review of the literature. J Clin Periodont. 1995;22:1–14. doi: 10.1111/j.1600-051X.1995.tb01765.x. [DOI] [PubMed] [Google Scholar]
- 15.Stoodley P, Jacobsen A, Dunsmore BC, Purevdorj B, Wilson S, Lappin-Scott HM, Costerton JW. The influence of fluid shear and AlCl3 on the material properties of Pseudomonas aeruginosa PAO1 and Desulfovibrio sp. EX265 biofilms. Water Sci Technol. 2001;43:113–120. [PubMed] [Google Scholar]
- 16.von Plocki SC, Armbruster D, Klein K, Kampf K, Zlinszky K, Hilbe M, Kronen P, Gruskin E, Von Rechenberg B. Biodegradable sleeves for metal implants to prevent implant-associated infection: an experimental in vivo study in sheep. Vet Surg. 2012;41:410–421. doi: 10.1111/j.1532-950X.2011.00943.x. [DOI] [PubMed] [Google Scholar]
- 17.Wilkins KM, Martin GP, Hanlon GW, Marriot C. The influence of critical surface tension and microrugosity on the adhesion of bacteria to polymer monofilaments. Int J Pharmaceut. 1989;57:1–7. doi: 10.1016/0378-5173(89)90256-1. [DOI] [Google Scholar]
- 18.Williams DL, Costerton JW. Using biofilms as initial inocula in animal models of biofilm-related infections. J Biomed Mater Res B Appl Biomater. 2012;100:1163–1169. doi: 10.1002/jbm.b.31979. [DOI] [PubMed] [Google Scholar]
- 19.Williams DL, Haymond BS, Beck JP, Savage PB, Chaudhary V, Epperson RT, Kawaguchi B, Bloebaum RD. In vivo efficacy of a silicone-cationic steroid antimicrobial coating to prevent implant-related infection. Biomaterials. 2012;33:8641–8656. doi: 10.1016/j.biomaterials.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Williams DL, Haymond BS, Bloebaum RD. Use of delrin plastic in a modified CDC biofilm reactor. Res J Microbiol. 2011;6:425–429. doi: 10.3923/jm.2011.425.429. [DOI] [PubMed] [Google Scholar]
- 21.Williams DL, Haymond BS, Woodbury KL, Beck JP, Moore DE, Epperson RT, Bloebaum RD. Experimental model of biofilm implant-related osteomyelitis to test combination biomaterials using biofilms as initial inocula. J Biomed Mat Res A. 2012;100:1888–1900. doi: 10.1002/jbm.a.34123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Williams DL, Woodbury KL, Haymond BS, Parker AE, Bloebaum RD. A modified CDC biofilm reactor to produce mature biofilms on the surface of PEEK membranes for an in vivo animal model application. Curr Microbiol. 2011;62:1657–1663. doi: 10.1007/s00284-011-9908-2. [DOI] [PubMed] [Google Scholar]
- 23.Wolcott RD, Rumbaugh KP, James G, Schultz G, Phillips P, Yang Q, Watters C, Stewart PS, Dowd SE. Biofilm maturity studies indicate sharp debridement opens a time-dependent therapeutic window. J Wound Care. 2010;19:320–328. doi: 10.12968/jowc.2010.19.8.77709. [DOI] [PubMed] [Google Scholar]