Abstract
Background
Carpal tunnel syndrome is a common compressive neuropathy of the median nerve. The efficacy and safety of endoscopic versus open carpal tunnel release remain controversial.
Questions/purposes
The purpose of this study was to determine whether endoscopic compared with open carpal tunnel release provides better symptom relief, validated outcome scores, short- and long-term strength, and/or digital sensibility; entails a differential risk of complications such as nerve injury, scar tenderness, pillar pain, and reoperation; allows an earlier return to work; and takes less operative time.
Methods
The English-language literature was searched using MEDLINE, the Cumulative Index to Nursing and Allied Health Literature, and the Cochrane Central Register of Controlled Trials. Randomized controlled trials that compared endoscopic and open carpal tunnel release were included in the meta-analysis. Methodologic quality was assessed with the Consolidated Standards Of Reporting Trials (CONSORT) checklist, and a sensitivity analysis was performed. Symptom relief, Boston Carpal Tunnel Questionnaire (BCTQ) scores, strength, digital sensibility, complications, reoperation, interval to return to work, and operative time were analyzed. Twenty-one randomized controlled trials containing 1859 hands were included.
Results
Endoscopically treated patients showed similar symptom relief and BCTQ scores; better early recovery of grip strength (mean difference [MD], 3.03 kg [0.08–5.98]; p = 0.04) and pinch strength (MD, 0.77 kg [0.33–1.22]; p < 0.001) but no advantage after 6 months; lower risk of scar tenderness (risk ratio [RR], 0.53 [0.35–0.82]; p = 0.005); higher risk of nerve injury (RR, 2.84 [1.08–7.46]; p = 0.03), most of which were transient neurapraxias. Similar risk of pillar pain and reoperation; an earlier return to work (MD, −8.73 days [−12.82 to −4.65]; p < 0.001); and reduced operative time (MD, −4.81 minutes [−9.23 to −0.39]; p = 0.03).
Conclusions
High-level evidence from randomized controlled trials indicates that endoscopic release allows earlier return to work and improved strength during the early postoperative period. Results at 6 months or later are similar according to current data except that patients undergoing endoscopic release are at greater risk of nerve injury and lower risk of scar tenderness compared with open release. While endoscopic release may appeal to patients who require an early return to work and activities, surgeons should be cognizant of its elevated incidence of transient nerve injury amid its similar overall efficacy to open carpal tunnel release. Additional research is required to define the learning curve of endoscopic release and clarify the influence of surgeon volume on its safety.
Introduction
Carpal tunnel syndrome is a compressive neuropathy of the median nerve that can cause hand pain, numbness, and tingling. Initial management of carpal tunnel syndrome involves nonoperative measures such as rest, splinting, NSAIDs, physiotherapy, and corticosteroid injection. Surgical release of the transverse carpal ligament, first described by Learmonth in 1933 [22], is indicated in recalcitrant cases. More than 350,000 surgical operations are performed for carpal tunnel syndrome every year in the United States [31].
Although the standard technique of open carpal tunnel release has proven effective and safe [19, 33], endoscopic carpal tunnel release was introduced in the form of single- [1] and double-portal [6] techniques with the aim of reducing morbidity and expediting recovery from surgery. Endoscopic carpal tunnel release has yet to be as widely adopted as open release [3] but offers the theoretical advantages of reduced postoperative pain, faster recovery of grip strength, earlier return to work and activities of daily living, and fewer wound-related complications associated with open release such as scar tenderness and pillar pain in the thenar and hypothenar eminences [9, 19, 24, 26, 32, 37, 39]. These putative benefits are achieved, in part, by avoiding the traditional midpalmar incision used in the open approach. However, pragmatic concerns relating to endoscopic release include its relative technical difficulty [5, 8, 14], cost-effectiveness [7], time requirement [44], and potential risk of iatrogenic injury to neurovascular structures [13, 18, 30]. A large sample size, as is achievable through a meta-analysis, may be necessary to detect such complications and differences in other outcome measures [4]. Although endoscopic carpal tunnel release has been practiced for more than two decades, controversy persists regarding its safety and overall patient outcomes relative to open release. Previous meta-analyses [40, 43] have been conducted to compare these procedures but have had important methodologic weaknesses in their inclusion criteria, outcome parameters, and validity assessment. Thoma et al. [40] neither evaluated long-term strength after 12 weeks nor validated outcome assessments. Although Vasiliadis et al. [43] analyzed major and minor complications in aggregate, specific complications such as nerve injury, scar tenderness, and pillar pain were not analyzed. Our meta-analysis is the first, to our knowledge, to specifically examine pillar pain and ancillary endpoints like operative time and digital sensibility performance. Several randomized controlled trials (RCTs) also have been recently published [2, 11, 17, 20].
The objective of our meta-analysis was to determine whether clinical outcomes differ among patients with carpal tunnel syndrome treated with an endoscopic versus open approach according to high-level evidence from RCTs, including symptom relief; validated outcome scores; short- and long-term strength; digital sensibility; complications potentially more likely to occur after one of the procedures such as nerve injury, scar tenderness, and pillar pain; interval to return to work; and operative time.
Materials and Methods
Eligibility Criteria
The meta-analysis was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [29]. The inclusion criteria were limited to RCTs that compared open and endoscopic carpal tunnel releases. Studies that evaluated revision surgery, did not report the followup interval, or reported only limited qualitative findings were excluded. Abstracts, laboratory or anatomic studies, review or technique articles, commentaries, and descriptive or nontherapeutic studies also were excluded. No restrictions were imposed on the publication date. Studies that analyzed the same cohort of patients were consolidated to prevent duplication of data, and data from the longer followup were preferentially used.
Literature Search
MEDLINE, the Cumulative Index to Nursing and Allied Health (CINAHL), and the Cochrane Central Register of Controlled Trials (CENTRAL) were queried by two independent reviewers (ETS, RJS) to identify relevant English-language studies, and disagreements were resolved by consensus. The search term was as follows: “carpal tunnel” AND (open OR endoscopic). The search was performed in April 2014. The resulting study titles and abstracts were reviewed according to the eligibility criteria. Full manuscripts were procured and reviewed for eligible studies, and their citations were manually screened to identify additional studies that might have been missed. A PRISMA trial flow shows the study selection algorithm (Fig. 1).
Fig. 1.
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) trial flow shows the inclusion process for the RCTs in the meta-analysis.
Data Abstraction
Data from eligible studies were extracted for study and patient characteristics, symptom relief, outcome scores, strength, digital sensibility performance, complications, reoperation, interval to return to work, and operative time. If outcome data and/or measures of variance were reported only using graphic plots but were omitted from the body of the text, plot-digitizing software (Plot Digitizer Version 2.6.4; Joseph Huwaldt and Scott Steinhorst, http://www.plot-digitizer.com-about.com/) was used to quantify these data.
Data Items
Study and patient characteristics of interest included the sample size, followup interval, age, sex distribution, hand dominance, bilaterality, symptom duration, diagnostic use of electrophysiologic testing, and surgical technique. Symptom relief was assessed according to overall improvement and the presence of postoperative pain, numbness, paresthesia, subjective weakness, and night symptoms. Overall improvement was defined as complete or near complete subjective symptom relief. Pain was analyzed using VAS scores normalized to a 10-point scale. Clinical outcomes were assessed using the symptom severity and functional status components of the validated Boston Carpal Tunnel Questionnaire (BCTQ) [23]. The maximum grip and pinch strength were assessed. Pinch strength was an aggregate of two-point (tip) and key (lateral) pinch strength. Strength was further assessed using only long-term followup data at 6 months or greater. Digital sensibility was quantified using the Semmes-Weinstein monofilament test score, static two-point discrimination, and static two-point sensory threshold. The risk of nerve injury, scar tenderness, pillar pain, and reoperation after each procedure were evaluated. Nerve injury was further analyzed according to single- versus double-portal endoscopic technique and older (1992–2002) versus newer (2003–2013) studies. The interval to return to work was analyzed. Finally, the operative time of the two procedures was compared.
Data Synthesis and Statistical Analysis
Pooled analysis was performed to compare several clinical outcome measures between groups, depending on the availability of data. A random-effects model was selected to account for statistical heterogeneity across the included trials using Review Manager Version 5.2.11 (The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark). If the SD for a given outcome was not reported in a study, it was computed from other provided statistics, including the 95% CI, standard error (SE), interquartile range, or p value. When the SD could not be determined, it was imputed using the mean of the values reported by the other studies [15]; this strategy was used when the SD was available for at least three or ½ of all the studies for a given outcome. Continuous data were analyzed through the inverse-variance statistical method and computation of the standardized mean difference (SMD) or mean difference (MD) and 95% CI. Dichotomous data were analyzed through the Mantel-Haenszel statistical method and computation of the risk ratio (RR) and 95% CI. Pooled analysis was performed for a given outcome when data were reported by at least three studies. Multiple group comparisons were extracted and pooled from studies that compared endoscopic carpal tunnel release with standard and limited open techniques. When multiple studies reported an outcome using the same scale and unit of measurement, use of the MD method allowed aggregation of change from baseline and final data in the same analysis in accordance with the Cochrane Handbook for Systematic Reviews of Interventions [15] under the assumption that between-group differences in both measurements closely approximate each other in RCTs. Otherwise, change from baseline and final data were pooled in separate analyses using the SMD method, which allowed comparison of related data that were reported using disparate scales or units of measurement. Data from the latest followup were preferentially used. Effect sizes were presented in relation to the endoscopic carpal tunnel release group; for instance, a positive RR indicated a higher risk in the endoscopic release group. The z statistic and p value were used to determine the statistical significance of the pooled comparison. Forest plots were generated and presented for the following chief outcomes: overall improvement, BCTQ scores, grip and pinch strength at 6 months or greater, any complications significantly increased after either procedure, and interval to return to work. Computation of weighted means was performed to analyze demographic characteristics for each group. The methodologic quality of each included RCT was assessed using the 22-point Consolidated Standards Of Reporting Trials (CONSORT) checklist [36]. Studies were scored and classified as excellent (18–22), good (13–17), fair (8–12), or poor (≤ 7). A sensitivity analysis [21] was performed using only trials of excellent or good methodologic quality according to the CONSORT score to explore the possibility that consideration of lower-quality RCTs might unduly influence the findings of the main analyses. A funnel plot, which is a visual representation of statistical precision plotted against the treatment effect, was constructed to assess the potential influence of publication bias on the results.
Study Selection
The initial search of MEDLINE, CINAHL, and CENTRAL identified 363 English-language articles, whose titles and abstracts subsequently were screened to determine their eligibility. Citation lists of selected studies were manually cross-referenced to ensure that no additional studies were missed. Twenty-one RCTs [1–5, 10–12, 14, 16, 17, 20, 25, 27, 28, 34, 35, 38, 41, 42, 44] containing a total of 1859 hands met the inclusion criteria, which compared open (n = 913) with endoscopic carpal tunnel release (n = 946).
Study Characteristics
All included studies were RCTs published from 1992 to 2013 (Table 1). Each trial was designed to compare open and endoscopic carpal tunnel releases. The size of the open and endoscopic release treatment groups in the trials ranged from 10 to 95 hands and 10 to 97 hands, respectively. The frequency-weighted followup for the included studies was 11 ± 14 months. The methodologic quality was excellent in two studies, good in five, and fair in 14, yielding a mean CONSORT score of 12.0 (range, 8–21). A funnel plot (Fig. 2) of the analysis of nerve injury appeared essentially symmetric in relation to the pooled estimate from the meta-analysis, indicating minimal publication bias.
Table 1.
Study design and patient characteristics of included studies
| Study | Year | Followup interval | Groups (number) | Technique | CONSORT score | Outcomes |
|---|---|---|---|---|---|---|
| Kang et al. [17] | 2013 | 3 months | ECTR OCTR |
One-portal limited | 16 | Treatment preference, BCTQ-S, BCTQ-F, DASH, complications, reoperation |
| Larsen et al. [20] | 2013 | 1, 2, 3, 6, 12, 24 weeks | ECTR OCTR OCTR |
One-portal standard limited | 11 | VAS (pain), VAS (paresthesia), time to RTW, probability of RTW, grip strength, ROM, complications |
| Aslani et al. [2] | 2012 | 4 weeks, 4 months | ECTR OCTR OCTR |
NR standard limited | 9 | Operative time, time to RTW, pain, night pain, numbness, weakness, stiffness, Phalen and Tinel signs, complications |
| Ejiri et al. [11] | 2012 | 12, 52 weeks | ECTR OCTR |
One-portal standard | 10 | VAS (pain), numbness and nighttime pain, grip strength, pinch strength, key pinch strength, SW monofilament test, static 2PD, distal latency, complications |
| Atroshi et al. [3] | 2009 | 5 years | ECTR OCTR |
Two-portal standard | 17 | Operative time, satisfaction, symptom relief, BCTQ-S, BCTQ-F, complications, reoperation |
| Atroshi et al. [4] | 2006 | 52 weeks | ECTR OCTR |
Two-portal standard | 21 | Time to RTW, BCTQ-S, BCTQ-F, SF-12, grip strength, pinch strength, SW monofilament test, static 2PD, distal latency, complications |
| Tian et al. [41] | 2007 | ECTR OCTR |
One-portal standard | 8 | Operative time, time to RTW, length of hospital stay, symptom relief, static 2PD, complications, reoperation | |
| Malhotra et al. [28] | 2007 | 26 weeks | ECTR OCTR |
One-portal limited | 9 | Symptom relief, time to symptom relief, satisfaction, time to RTW, pain, grip strength, distal latency, NCV, CMAP, SNAP, complications |
| Rab et al. [34] | 2006 | 12, 52 weeks | ECTR OCTR |
Two-portal limited | 11 | VAS (pain), BCTQ-S, BCTQ-F, grip strength, pinch strength, key pinch strength, static 2PD, static 2PST, distal latency, NCV, complications |
| MacDermid et al. [25] | 2003 | 1, 6, 12 weeks | ECTR OCTR |
Two-portal standard | 10 | Satisfaction rating, BCTQ-S, MPQ, SF-36, grip strength, key pinch strength, tripod pinch strength, static 2PST, complications, reoperation |
| Saw et al. [35] | 2003 | 1, 3, 6, 12 weeks | ECTR OCTR |
One-portal standard | 18 | Operative time, anterior carpal tenderness, time to RTW, BCTQ-S, BCTQ-F, grip strength, complications, reoperation |
| Wong et al. [44] | 2003 | 2, 4, 8, 12, 16, 26, 52 weeks | ECTR OCTR |
Two-portal limited | 11 | Operative time, VAS (pain), ADL score, symptom relief, grip strength, pinch strength, distal latency, NCV, complications |
| Ferdinand & MacLean [14] | 2002 | 6, 12, 26, 52 weeks | ECTR OCTR |
One-portal standard | 13 | Operative time, satisfaction rating, grip strength, Jebson score, static 2PD, complications |
| Trumble et al. [42] | 2002 | 2, 4, 8, 12, 26, 52 weeks | ECTR OCTR |
One-portal standard | 14 | Operative time, satisfaction rating, time to RTW, BCTQ-S, BCTQ-F, grip strength, key pinch strength, tripod pinch strength, Jebson score, Purdue pegboard score, SW monofilament test, distal latency, complications, reoperation |
| Mackenzie et al. [27] | 2000 | 1, 2, 4 weeks | ECTR OCTR |
One-portal Limited | 8 | Grip strength, pinch strength, complications |
| Jacobsen & Rahme [16] | 1996 | 2, 26, 52 weeks | ECTR OCTR |
Two-portal standard | 11 | Satisfaction, time to RTW, symptom relief, analgesic requirement, static 2PD, NCV, complications |
| Dumontier et al. [10] | 1995 | 2 weeks; 1, 3, 6 months | ECTR OCTR |
Two-portal standard | 10 | Rate of RTW, paresthesia, pain, grip strength, ROM, complications |
| Sennwald & Benedetti [38] | 1995 | 4, 8, 12 weeks | ECTR OCTR |
One-portal standard | 9 | Operative time, rate of RTW, time to normal hand use, symptom relief, grip strength, key pinch strength, complications, reoperation |
| Erdmann [12] | 1994 | 1, 2 weeks; 1, 3, 6, 12 months | ECTR OCTR |
Two-portal standard | 10 | VAS (pain), time to RTW, time to symptom relief, grip strength, time to normal grip strength, pinch strength, time to normal pinch strength, distal latency, complications |
| Brown et al. [5] | 1993 | 3, 6, 12 weeks | ECTR OCTR |
Two-portal standard | 16 | Satisfaction rating, time to RTW, rate of ADL impairment, ADL score, paresthesia, pain, symptom relief, grip strength, key pinch strength, APB strength, thenar atrophy, SW score, static 2PD, ICTP, complications |
| Agee et al. [1] | 1992 | 1, 2, 3, 6, 9, 13, 26 weeks | ECTR OCTR |
One-portal standard | 9 | Time to RTW, time to normal ADLs, numbness, paresthesia, pain, weakness, night symptoms, grip strength, pinch strength, key pinch strength, monofilament motor and sensory testing, Phalen and Tinel signs, complications, reoperation |
CONSORT = Consolidated Standards Of Reporting Trials; ECTR = endoscopic carpal tunnel release; OCTR = open carpal tunnel release; NR = not reported; BCTQ-S = Boston Carpal Tunnel Questionnaire (BCTQ) symptom severity score; BCTQ-F = BCTQ functional status score; RTW = return to work; SW = Semmes-Weinstein; 2PD = two-point discrimination; NCV = nerve conduction velocity; CMAP = compound muscle action potential; SNAP = sensory nerve action potential; 2PST = two-point sensory threshold; MPQ = McGill Pain Questionnaire; ADL = activity of daily living; APB = abductor pollicis brevis; ICTP = interstitial carpal tunnel pressure.
Fig. 2.
A funnel plot shows relative symmetry in relation to the pooled estimate from the meta-analysis, indicating minimal publication bias. SE = standard error; RR = risk ratio.
Patient Characteristics
Patient demographic and clinical characteristics were similar between the treatment groups (Table 2). The diagnosis of carpal tunnel syndrome was electrophysiologically confirmed with routine electromyography and/or nerve conduction studies in 19 of 21 studies [1–5, 11, 12, 14, 16, 17, 20, 25, 27, 28, 34, 38, 41, 42, 44]. Four studies included only patients with bilateral carpal tunnel syndrome [14, 17, 34, 44]. There were 16 and seven trial arms managed with standard [1–5, 10–12, 14, 16, 20, 25, 35, 38, 41, 42] and limited open carpal tunnel release [2, 17, 20, 27, 28, 34, 44], respectively. There were 11 and nine trial arms managed with single- [1, 11, 14, 17, 20, 27, 28, 35, 38, 41, 42] and double-portal [3–5, 10, 12, 16, 25, 34, 44] endoscopic carpal tunnel release, respectively, whereas one trial did not clearly specify the endoscopic technique [2].
Table 2.
Pooled patient characteristics of treatment groups
| Patient characteristics | Number of studies | ECTR | OCTR |
|---|---|---|---|
| Followup (months) | 21 | 11 ± 14 | 11 ± 14 |
| Age (years) | 18 | 52 ± 5 | 52 ± 4 |
| Male (%) | 18 | 29 | 24 |
| Dominant or right hand (%) | 13 | 71 | 69 |
| Symptom duration (months) | 8 | 32 ± 15 | 30 ± 16 |
ECTR = endoscopic carpal tunnel release; OCTR = open carpal tunnel release.
Results
Symptom Relief
Overall improvement of carpal tunnel syndrome symptoms was equally likely after endoscopic and open releases (RR, 1.00 [0.95–1]; n = 656; p = 0.98) [3, 5, 11, 16, 28, 38, 41, 44] (Fig. 3A) (Table 3). There was no difference between groups in the rate of persistent symptoms, including pain (RR, 0.76 [0.53–1]; n = 483; p = 0.14) [1, 2, 5, 10, 28], numbness (RR, 0.78 [0.29–2]; n = 402; p = 0.63) [1, 2, 11, 28], paresthesia (RR, 0.78 [0.51–1]; n = 294; p = 0.24) [1, 5, 10], subjective weakness (RR, 0.93 [0.34–3]; n = 302; p = 0.89) [1, 2, 28], or night symptoms (RR, 1.15 [0.45–3]; n = 341; p = 0.77) [1, 2, 11]. Both procedures similarly improved the VAS pain score (MD, −0.13 [−0.55 to 0.28]; n = 455; p = 0.53) [12, 20, 34, 35, 44] (Fig. 3B).
Fig. 3A–B.
The forest plots show the (A) risk ratio of overall improvement and (B) mean difference for the VAS pain score. ECTR = endoscopic carpal tunnel release; OCTR = open carpal tunnel release; IV = inverse variance; M-H = Mantel-Haenszel; df = degrees of freedom.
Table 3.
Summary of results of pooled analyses
| Analysis | Sample size | Treatment versus no treatment | Statistical significance |
|---|---|---|---|
| Interval to return to work (days) | 1234 | MD = −9 [−13 to −5] (favors ECTR) | SS (Z = 4.19; p < 0.001) |
| Overall improvement | 656 | RR = 1.00 [0.95–1.05] | NS (Z = 0.03; p = 0.98) |
| Persistent pain | 483 | RR = 0.76 [0.53–1.09] | NS (Z = 1.48; p = 0.14) |
| Persistent numbness | 402 | RR = 0.78 [0.29–2.11] | NS (Z = 0.48; p = 0.63) |
| Persistent paresthesia | 294 | RR = 0.78 [0.51–1.19] | NS (Z = 1.17; p = 0.24) |
| Persistent weakness | 302 | RR = 0.93 [0.34–2.56] | NS (Z = 0.14; p = 0.89) |
| Persistent night symptoms | 341 | RR = 1.15 [0.45–2.90] | NS (Z = 0.29; p = 0.77) |
| Pain (VAS) | 455 | MD = 0 [−1 to 0] | NS (Z = 0.63; p = 0.53) |
| Satisfaction rating | 534 | MD = 1 [−4 to 7] | NS (Z = 0.49; p = 0.62) |
| BCTQ symptom severity score | 715 | SMD = 0 [0 to 0] | NS (Z = 0.46; p = 0.64) |
| BCTQ functional status score | 592 | SMD = 0 [0 to 0] | NS (Z = 0.45; p = 0.65) |
| Grip strength (kg) | 1196 | MD = 3 [0 to 6] (favors ECTR) | SS (Z = 2.02; p = 0.04) |
| Pinch strength (kg) | 920 | MD = 1 [0 to 1] (favors ECTR) | SS (Z = 3.42; p < 0.001) |
| Grip strength (kg) (≥ 6 months) | 571 | MD = 1 [−1 to 3] | NS (Z = 0.74; p = 0.46) |
| Pinch strength (kg) (≥ 6 months) | 614 | MD = 0 [0 to 1] | NS (Z = 1.57; p = 0.12) |
| Semmes-Weinstein monofilament test score | 589 | MD = 0 [0 to 0] | NS (Z = 0.30; p = 0.76) |
| Static two-point discrimination (mm) | 579 | MD = 0 [0 to 0] | NS (Z = 0.20; p = 0.84) |
| Static two-point sensory threshold (mm) | 143 | MD = 0 [−1 to 1] | NS (Z = 0.32; p = 0.75) |
| Nerve injury | 1573 | RR = 2.84 [1.08–7.46] (favors OCTR) | SS (Z = 2.12; p = 0.03) |
| Scar tenderness | 581 | RR = 0.53 [0.35–0.82] (favors ECTR) | SS (Z = 2.84; p = 0.005) |
| Pillar pain | 449 | RR = 0.93 [0.45–1.95] | NS (Z = 0.18; p = 0.85) |
| Reoperation | 957 | RR = 1.77 [0.64–4.93] | NS (Z = 1.10; p = 0.27) |
| Operative time (minutes) | 695 | MD = −5 [−9 to 0] (favors ECTR) | SS (Z = 2.13; p = 0.03) |
SMD and MD refer to (treatment)—(no treatment); RR refers to (treatment)/(no treatment); 95% CI is provided in brackets; BCTQ = Boston Carpal Tunnel Questionnaire; MD = mean difference; ECTR = endoscopic carpal tunnel release; RR = risk ratio; OCTR = open carpal tunnel release; SMD = standardized mean difference; SS = statistically significant; NS = nonsignificant.
Boston Carpal Tunnel Questionnaire (BCTQ)
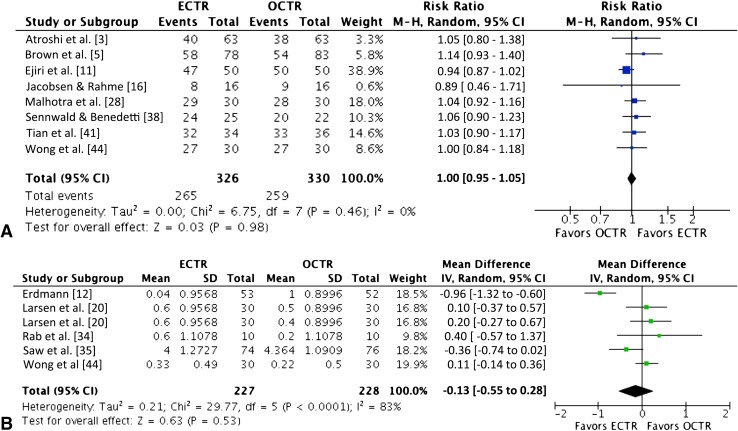
Both groups showed similar improvement in the components of the BCTQ, including symptom severity (SMD, 0.04 [−0.11 to 0.18]; n = 715; p = 0.64) [3, 17, 25, 34, 35, 42] and functional status scores (SMD, −0.06 [−0.30 to 0.19]; n = 592; p = 0.65) [3, 17, 34, 35, 42] (Fig. 4).
Fig. 4A–B.
The forest plots show the standardized mean difference for the (A) symptom severity and (B) functional status scores of the Boston Carpal Tunnel Questionnaire. ECTR = endoscopic carpal tunnel release; OCTR = open carpal tunnel release; IV = inverse variance; df = degrees of freedom.
Strength
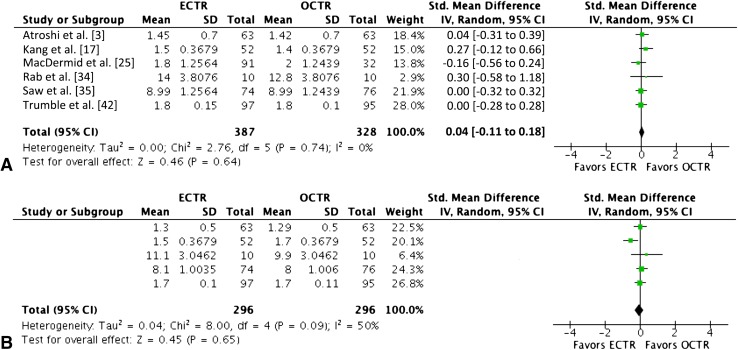
Patients treated with endoscopic carpal tunnel release showed greater grip strength (MD, 3.03 kg [0.08–6]; n = 1196; p = 0.04) [4, 5, 10–12, 14, 25, 27, 28, 34, 35, 38, 42] and pinch strength (MD, 0.77 kg [0.33–1]; n = 920; p = 0.001) [4, 5, 11, 12, 25, 27, 34, 38, 42]. However, when only long-term followup data at 6 months or greater were used, no differences remained between the techniques in grip strength (MD, 0.90 kg [−1 to 3]; n = 571; p = 0.46) [4, 10, 12, 14, 28, 34, 42] or pinch strength (MD, 0.37 kg [−0.09 to 0.84]; n = 614; p = 0.12) [4, 5, 12, 34, 42] (Fig. 5).
Fig. 5A–B.
The forest plots show the mean difference in (A) grip strength (kg) and (B) pinch strength (kg) using only long-term followup data at 6 months or greater. ECTR = endoscopic carpal tunnel release; OCTR = open carpal tunnel release; IV = inverse variance; df = degrees of freedom.
Digital Sensibility
Digital sensibility testing showed no differences between groups according to the Semmes-Weinstein monofilament test score (MD, −0.02 [−0.16 to 0.12]; n = 589; p = 0.76) [4, 5, 11, 42], static two-point discrimination (MD, 0.04 mm [−0.35 to 0.43]; n = 579; p = 0.84) [4, 5, 11, 14, 16, 34, 41], or static two-point sensory threshold (MD, 0.13 mm [−0.67 to 0.93]; n = 143; p = 0.75) [25, 34] at followup.
Complications
The risk of nerve injury was increased in endoscopically treated patients (RR, 2.84 [1–7]; n = 1573; p = 0.03) [1, 4, 11, 12, 14, 16, 20, 25, 27, 28, 34, 35, 38, 41, 42, 44] (Fig. 6A), though the majority were temporary neurapraxias. The risk of nerve injury did not vary for one- versus two-portal endoscopic techniques (p = 0.79) or studies published before or after 2003 (p = 0.30). Scar tenderness was less common after endoscopic release (RR, 0.53 [0.35–0.82]; n = 581; p = 0.005) [3, 5, 12, 14, 28, 41] (Fig. 6B). Patients in both groups were similarly susceptible to pillar pain (RR, 0.93 [0.45–2]; n = 449; p = 0.85) [2, 12, 20, 27, 44]. There was no difference in the reoperation risk between the groups (RR, 1.77 [0.64–5]; n = 957; p = 0.27) [1, 3, 17, 25, 35, 38, 41, 42].
Fig. 6A–B.
The forest plots show the risk ratios for (A) nerve injury and (B) scar tenderness. . ECTR = endoscopic carpal tunnel release; OCTR = open carpal tunnel release; M-H = Mantel-Haenszel; df = degrees of freedom.
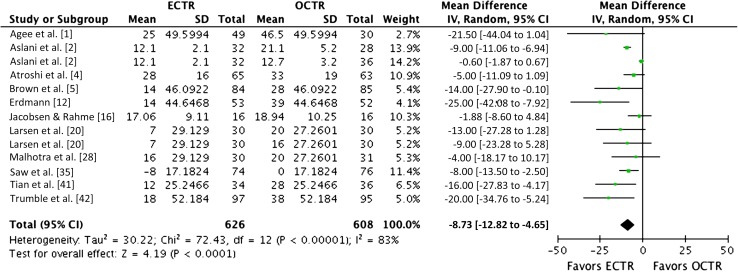
Return to Work
Patients treated with endoscopic carpal tunnel release returned to work earlier than patients who had open release (MD, −9 days [−13 to −5]; n = 1234; p < 0.001) [1, 2, 4, 5, 12, 16, 20, 28, 35, 41, 42] (Fig. 7).
Fig. 7.
The forest plot shows the mean difference in the interval to return to work (days). ECTR = endoscopic carpal tunnel release; OCTR = open carpal tunnel release; IV = inverse variance; df = degrees of freedom.
Operative Time
The operative time was shorter for endoscopic compared with open release (MD, −5 minutes [−9 to −0.39]; n = 695; p = 0.03) [3, 14, 35, 38, 41, 42, 44].
Sensitivity Analysis
The sensitivity analysis confirmed the statistical significance of the analyses of scar tenderness (p = 0.003) and interval to return to work (p < 0.001), where both parameters favor endoscopic release. However, statistical significance was no longer present for nerve injury (p = 0.43) or operative time (p = 0.49). The findings of the remaining comparisons were confirmed.
Discussion
Surgical release of the transverse carpal ligament, which is indicated when conservative treatment fails, is a commonly performed orthopaedic procedure [31]. Although the standard technique of open carpal tunnel release is known to be effective and safe, endoscopic release was introduced more than two decades ago with the potential advantages of lower morbidity and quicker recovery from surgery. However, concerns regarding endoscopic release have included its relative technical difficulty, cost-effectiveness, time requirement, and potential risk of iatrogenic injury to neurovascular structures. Furthermore, several RCTs have been conducted to date without showing clear superiority of any procedure, and neither of these techniques is clearly favored at present. Thus, the purpose of our meta-analysis was to determine whether endoscopic compared with open release provides better symptom relief, validated outcome scores, short- and long-term strength, and/or digital sensibility; entails a differential risk of complications such as nerve injury, scar tenderness, pillar pain, and reoperation; allows an earlier return to work; and takes less operative time.
There are limitations to this meta-analysis. Some pooled analyses were based on data from a small number of studies, increasing the likelihood of bias. Studies varied in their outcome parameters, such as BCTQ scores, which were not used in the included RCTs until 2002. As there are virtually no validated instruments to assess patient satisfaction, this endpoint was omitted from the meta-analysis even though it was reported in some of the studies. The use of data imputation to address unreported SDs or other measures of variance required that assumptions be made regarding missing data but allowed a larger number of primary studies to be pooled in the analyses, increasing their statistical power and lessening the influence of any single study on the findings. Type II error, or failure to reject a false null hypothesis, was possible for some analyses where there may have been a trend toward statistically significant differences between groups. Additionally, heterogeneity with respect to the choice of endoscopic (single- versus double-portal) and open (standard versus limited) techniques may underlie some of the variability witnessed in the outcomes.
Strengths of this meta-analysis are the exclusive use of high-level evidence from RCTs, the large sample sizes of the pooled study groups, and the large number of analyses performed. Methodologic bias was minimized in this study owing to the exclusive use of data from RCTs whose methodologic quality was excellent or good in seven of 21 cases according to the CONSORT checklist. Furthermore, a sensitivity analysis was performed using only studies of excellent or good methodologic quality according to the CONSORT score to test whether the findings of the analyses might have been influenced by inclusion of low-quality trials. Publication bias also was minimal in this meta-analysis, as shown through funnel plot analysis.
The findings of this meta-analysis suggest that symptom relief and clinical outcomes, according to the validated BCTQ symptom severity and functional status indices, were no different for patients treated with endoscopic versus open release. Endoscopic release provides improved grip and pinch strength during the early postoperative period, an earlier return to work, and a lower risk of scar tenderness. Return-to-work data are difficult to interpret because patients often return to work when indicated by their surgeon, regardless of procedure type. However, superior improvement of the grip and pinch strength with endoscopic release during the early postoperative period compared with open release may explain why patients undergoing endoscopic release can return to work sooner. However, when only long-term followup data at 6 months or greater were considered, no differences in strength remained between the groups. Compared with previously published meta-analyses [40, 43], ours is the first to establish that long-term grip and pinch strength are equal after both procedures. Despite the analyses that favored endoscopic release, patients undergoing this method have a statistically significant, threefold increased risk of nerve injury after the procedure. The higher risk of temporary nerve injury associated with endoscopic release was reported in a previous meta-analysis on this topic [40]. Despite the greater risk of nerve injury after endoscopic release, the reoperation rate was not increased in our meta-analysis, and the sensitivity analysis did not confirm a higher risk of nerve injury with the endoscopic technique. The reduction in scar tenderness after endoscopic release is plausible owing to the generally small incisions with the endoscopic technique. Interestingly, pillar pain was no different between the open and endoscopic groups, although reduction of pillar pain initially was posited as one of the advantages of the endoscopic technique. Finally, the 5-minute reduction in the overall operative time of the endoscopic procedure is likely clinically unimportant.
The findings of our meta-analysis of RCTs suggest that endoscopic carpal tunnel release may have clinically important advantages with respect to earlier return to work, improved strength during the early postoperative period, and reduction of scar tenderness, although pillar pain is not reduced with the endoscopic technique. However, long-term followup data suggest similar results for final strength regardless of the technique. Endoscopic release also increases the risk of transient nerve injury. Although the methodologic quality of these RCTs proved variable, they represent the highest level of evidence available for making treatment recommendations. Questions remain regarding the influence of surgeon volume on the safety of the endoscopic procedure. The learning curve for this technique should be more clearly defined. While endoscopic release may appeal to patients who require an early return to work and activities, surgeons should be cognizant of its elevated incidence of reversible nerve injury despite its similar overall efficacy to open release.
Footnotes
Each author certifies that he or she, or a member of his or her immediate family, has no funding or commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research ® editors and board members are on file with the publication and can be viewed on request.
References
- 1.Agee JM, McCarroll HR, Jr, Tortosa RD, Berry DA, Szabo RM, Peimer CA. Endoscopic release of the carpal tunnel: a randomized prospective multicenter study. J Hand Surg Am. 1992;17:987–995. doi: 10.1016/S0363-5023(09)91044-9. [DOI] [PubMed] [Google Scholar]
- 2.Aslani HR, Alizadeh K, Eajazi A, Karimi A, Karimi MH, Zaferani Z, Hosseini Khameneh SM. Comparison of carpal tunnel release with three different techniques. Clin Neurol Neurosurg. 2012;114:965–968. doi: 10.1016/j.clineuro.2012.02.017. [DOI] [PubMed] [Google Scholar]
- 3.Atroshi I, Hofer M, Larsson GU, Ornstein E, Johnsson R, Ranstam J. Open compared with 2-portal endoscopic carpal tunnel release: a 5-year follow-up of a randomized controlled trial. J Hand Surg Am. 2009;34:266–272. doi: 10.1016/j.jhsa.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 4.Atroshi I, Larsson GU, Ornstein E, Hofer M, Johnsson R, Ranstam J. Outcomes of endoscopic surgery compared with open surgery for carpal tunnel syndrome among employed patients: randomised controlled trial. BMJ. 2006;332:1473. doi: 10.1136/bmj.38863.632789.1F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown RA, Gelberman RH, Seiler JG, 3rd, Abrahamsson SO, Weiland AJ, Urbaniak JR, Schoenfeld DA, Furcolo D. Carpal tunnel release: a prospective, randomized assessment of open and endoscopic methods. J Bone Joint Surg Am. 1993;75:1265–1275. doi: 10.2106/00004623-199309000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Chow JC. Endoscopic release of the carpal ligament: a new technique for carpal tunnel syndrome. Arthroscopy. 1989;5:19–24. doi: 10.1016/0749-8063(89)90085-6. [DOI] [PubMed] [Google Scholar]
- 7.Chung KC, Walters MR, Greenfield ML, Chernew ME. Endoscopic versus open carpal tunnel release: a cost-effectiveness analysis. Plast Reconstr Surg. 1998;102:1089–1099. doi: 10.1097/00006534-199809020-00026. [DOI] [PubMed] [Google Scholar]
- 8.Cobb TK, Knudson GA, Cooney WP. The use of topographical landmarks to improve the outcome of Agee endoscopic carpal tunnel release. Arthroscopy. 1995;11:165–172. doi: 10.1016/0749-8063(95)90062-4. [DOI] [PubMed] [Google Scholar]
- 9.Cseuz KA, Thomas JE, Lambert EH, Love JG, Lipscomb PR. Long-term results of operation for carpal tunnel syndrome. Mayo Clin Proc. 1966;41:232–241. [PubMed] [Google Scholar]
- 10.Dumontier C, Sokolow C, Leclercq C, Chauvin P. Early results of conventional versus two-portal endoscopic carpal tunnel release: a prospective study. J Hand Surg Br. 1995;20:658–662. doi: 10.1016/S0266-7681(05)80130-5. [DOI] [PubMed] [Google Scholar]
- 11.Ejiri S, Kikuchi S, Maruya M, Sekiguchi Y, Kawakami R, Konno S. Short-term results of endoscopic (Okutsu method) versus palmar incision open carpal tunnel release: a prospective randomized controlled trial. Fukushima J Med Sci. 2012;58:49–59. doi: 10.5387/fms.58.49. [DOI] [PubMed] [Google Scholar]
- 12.Erdmann MW. Endoscopic carpal tunnel decompression. J Hand Surg Br. 1994;19:5–13. doi: 10.1016/0266-7681(94)90038-8. [DOI] [PubMed] [Google Scholar]
- 13.Erhard L, Ozalp T, Citron N, Foucher G. Carpal tunnel release by the Agee endoscopic technique: results at 4 year follow-up. J Hand Surg Br. 1999;24:583–585. doi: 10.1054/jhsb.1999.0226. [DOI] [PubMed] [Google Scholar]
- 14.Ferdinand RD, MacLean JG. Endoscopic versus open carpal tunnel release in bilateral carpal tunnel syndrome: a prospective, randomised, blinded assessment. J Bone Joint Surg Br. 2002;84:375–379. doi: 10.1302/0301-620X.84B3.12224. [DOI] [PubMed] [Google Scholar]
- 15.Higgins JP, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration, 2009. Version 5.0.2 [updated September 2009]. Available at: www.cochrane-handbook.org. Accessed April 1, 2014.
- 16.Jacobsen MB, Rahme H. A prospective, randomized study with an independent observer comparing open carpal tunnel release with endoscopic carpal tunnel release. J Hand Surg Br. 1996;21:202–204. doi: 10.1016/S0266-7681(96)80097-0. [DOI] [PubMed] [Google Scholar]
- 17.Kang HJ, Koh IH, Lee TJ, Choi YR. Endoscopic carpal tunnel release is preferred over mini-open despite similar outcome: a randomized trial. Clin Orthop Relat Res. 2013;471:1548–1554. doi: 10.1007/s11999-012-2666-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kelly CP, Pulisetti D, Jamieson AM. Early experience with endoscopic carpal tunnel release. J Hand Surg Br. 1994;19:18–21. doi: 10.1016/0266-7681(94)90040-X. [DOI] [PubMed] [Google Scholar]
- 19.Kulick MI, Gordillo G, Javidi T, Kilgore ES, Jr, Newmeyer WL., 3rd Long-term analysis of patients having surgical treatment for carpal tunnel syndrome. J Hand Surg Am. 1986;11:59–66. doi: 10.1016/S0363-5023(86)80104-6. [DOI] [PubMed] [Google Scholar]
- 20.Larsen MB, Sorensen AI, Crone KL, Weis T, Boeckstyns ME. Carpal tunnel release: a randomized comparison of three surgical methods. J Hand Surg Eur Vol. 2013;38:646–650. doi: 10.1177/1753193412475247. [DOI] [PubMed] [Google Scholar]
- 21.Lau J, Ioannidis JP, Schmid CH. Quantitative synthesis in systematic reviews. Ann Intern Med. 1997;127:820–826. doi: 10.7326/0003-4819-127-9-199711010-00008. [DOI] [PubMed] [Google Scholar]
- 22.Learmonth J. The principle of decompression in the treatment of certain diseases of peripheral nerves. Surg Clin North Am. 1933;13:905–913. [Google Scholar]
- 23.Levine DW, Simmons BP, Koris MJ, Daltroy LH, Hohl GG, Fossel AH, Katz JN. A self-administered questionnaire for the assessment of severity of symptoms and functional status in carpal tunnel syndrome. J Bone Joint Surg Am. 1993;75:1585–1592. doi: 10.2106/00004623-199311000-00002. [DOI] [PubMed] [Google Scholar]
- 24.Louis DS, Greene TL, Noellert RC. Complications of carpal tunnel surgery. J Neurosurg. 1985;62:352–356. doi: 10.3171/jns.1985.62.3.0352. [DOI] [PubMed] [Google Scholar]
- 25.MacDermid JC, Richards RS, Roth JH, Ross DC, King GJ. Endoscopic versus open carpal tunnel release: a randomized trial. J Hand Surg Am. 2003;28:475–480. doi: 10.1053/jhsu.2003.50080. [DOI] [PubMed] [Google Scholar]
- 26.MacDonald RI, Lichtman DM, Hanlon JJ, Wilson JN. Complications of surgical release for carpal tunnel syndrome. J Hand Surg Am. 1978;3:70–76. doi: 10.1016/S0363-5023(78)80118-X. [DOI] [PubMed] [Google Scholar]
- 27.Mackenzie DJ, Hainer R, Wheatley MJ. Early recovery after endoscopic vs. short-incision open carpal tunnel release. Ann Plast Surg. 2000;44:601–604. doi: 10.1097/00000637-200044060-00004. [DOI] [PubMed] [Google Scholar]
- 28.Malhotra R, Kiran EK, Dua A, Mallinath SG, Bhan S. Endoscopic versus open carpal tunnel release: a short-term comparative study. Indian J Orthop. 2007;41:57–61. doi: 10.4103/0019-5413.30527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 30.Murphy RX, Jr, Jennings JF, Wukich DK. Major neurovascular complications of endoscopic carpal tunnel release. J Hand Surg Am. 1994;19:114–118. doi: 10.1016/0363-5023(94)90233-X. [DOI] [PubMed] [Google Scholar]
- 31.Owings MF, Kozak LJ. Ambulatory and inpatient procedures in the United States, 1996. Vital and Health Statistics. Series 13, Data from the National Health Care Survey. No 139. Available at: http://www.cdc.gov/nchs/data/series/sr_13/sr13_139.pdf. Accessed April 1, 2014. [PubMed]
- 32.Palmer DH, Paulson JC, Lane-Larsen CL, Peulen VK, Olson JD. Endoscopic carpal tunnel release: a comparison of two techniques with open release. Arthroscopy. 1993;9:498–508. doi: 10.1016/S0749-8063(05)80396-2. [DOI] [PubMed] [Google Scholar]
- 33.Phalen GS. The carpal-tunnel syndrome: seventeen years’ experience in diagnosis and treatment of six hundred fifty-four hands. J Bone Joint Surg Am. 1966;48:211–228. [PubMed] [Google Scholar]
- 34.Rab M, Grunbeck M, Beck H, Haslik W, Schrogendorfer KF, Schiefer HP, Mittlbock M, Frey M. Intra-individual comparison between open and 2-portal endoscopic release in clinically matched bilateral carpal syndrome. J Plast Reconstr Aesthet Surg. 2006;59:730–736. doi: 10.1016/j.bjps.2005.11.018. [DOI] [PubMed] [Google Scholar]
- 35.Saw NL, Jones S, Shepstone L, Meyer M, Chapman PG, Logan AM. Early outcome and cost-effectiveness of endoscopic versus open carpal tunnel release: a randomized prospective trial. J Hand Surg Br. 2003;28:444–449. doi: 10.1016/S0266-7681(03)00097-4. [DOI] [PubMed] [Google Scholar]
- 36.Schulz KF, Altman DG, Moher D, CONSORT Group CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMC Med. 2010;2010(8):18. doi: 10.1186/1741-7015-8-18. [DOI] [PubMed] [Google Scholar]
- 37.Semple JC, Cargill AO. Carpal-tunnel syndrome: results of surgical decompression. Lancet. 1969;1:918–919. doi: 10.1016/S0140-6736(69)92548-3. [DOI] [PubMed] [Google Scholar]
- 38.Sennwald GR, Benedetti R. The value of one-portal endoscopic carpal tunnel release: a prospective randomized study. Knee Surg Sports Traumatol Arthrosc. 1995;3:113–116. doi: 10.1007/BF01552386. [DOI] [PubMed] [Google Scholar]
- 39.Seradge H, Seradge E. Piso-triquetral pain syndrome after carpal tunnel release. J Hand Surg Am. 1989;14:858–862. doi: 10.1016/S0363-5023(89)80090-5. [DOI] [PubMed] [Google Scholar]
- 40.Thoma A, Veltri K, Haines T, Duku E. A meta-analysis of randomized controlled trials comparing endoscopic and open carpal tunnel decompression. Plast Reconstr Surg. 2004;114:1137–1146. doi: 10.1097/01.PRS.0000135850.37523.D0. [DOI] [PubMed] [Google Scholar]
- 41.Tian Y, Zhao H, Wang T. Prospective comparison of endoscopic and open surgical methods for carpal tunnel syndrome. Chin Med Sci J. 2007;22:104–107. [PubMed] [Google Scholar]
- 42.Trumble TE, Diao E, Abrams RA, Gilbert-Anderson MM. Single-portal endoscopic carpal tunnel release compared with open release: a prospective, randomized trial. J Bone Joint Surg Am. 2002;84:1107–1115. doi: 10.2106/00004623-200207000-00003. [DOI] [PubMed] [Google Scholar]
- 43.Vasiliadis HS, Georgoulas P, Shrier I, Salanti G, Scholten RJ. Endoscopic release for carpal tunnel syndrome. Cochrane Database Syst Rev. 2014;1:CD008265. [DOI] [PMC free article] [PubMed]
- 44.Wong KC, Hung LK, Ho PC, Wong JM. Carpal tunnel release: a prospective, randomised study of endoscopic versus limited-open methods. J Bone Joint Surg Br. 2003;85:863–868. [PubMed] [Google Scholar]