Abstract
Background
Lumbar total disc replacement (L-TDR) is a procedure used to relieve back pain and maintain mobility. Contemporary metal-on-polyethylene (MoP) L-TDRs were developed to address wear performance concerns about historical designs, but wear debris generation and periprosthetic tissue reactions for these newer implants have not been determined.
Questions/purposes
The purpose of this study was to determine (1) whether periprosthetic ultrahigh-molecular-weight polyethylene (UHMWPE) wear debris and biological responses were present in tissues from revised contemporary MoP L-TDRs that contain conventional cores fabricated from γ-inert-sterilized UHMWPE; (2) how fixed- versus mobile-bearing design affected UHMWPE wear particle number, shape, and size; and (3) how these wear particle characteristics compare with historical MoP L-TDRs that contain cores fabricated from γ-air-sterilized UHMWPE.
Methods
We evaluated periprosthetic tissues from 11 patients who received eight fixed-bearing ProDisc-L and four mobile-bearing CHARITÉ contemporary L-TDRs with a mean implantation time of 4.1 and 2.7 years, respectively. Histologic analysis of tissues was performed to assess biological responses and polarized light microscopy was used to quantify number and size/shape characteristics of UHMWPE wear particles from the fixed- and mobile-bearing devices. Comparisons were made to previously reported particle data for historical L-TDRs.
Results
Five of seven (71%) fixed-bearing and one of four mobile-bearing L-TDR patient tissues contained at least 4 particles/mm2 wear with associated macrophage infiltration. Tissues with wear debris were highly vascularized, whereas those without debris were more necrotic. Given the samples available, the tissue around mobile-bearing L-TDR was observed to contain 87% more, 11% rounder, and 11% less-elongated wear debris compared with tissues around fixed-bearing devices; however, there were no significant differences. Compared with historical L-TDRs, UHMWPE particle number and circularity for contemporary L-TDRs were 99% less (p = 0.003) and 50% rounder (p = 0.003).
Conclusions
In this preliminary study, short-term results suggest there was no significant influence of fixed- or mobile-bearing designs on wear particle characteristics of contemporary L-TDRs, but conventional UHMWPE has notably improved the wear resistance of these devices compared with historical UHMWPE.
Introduction
Lumbar total disc replacement (L-TDR) is an established alternative to spinal fusion for degenerative disc disease and its associated back and leg pain. With the goal to preserve natural segmental motion in the spine, commonly used implant designs incorporate cobalt-chromium (CoCr) metallic endplates, which are fixed to the adjacent vertebral bodies, articulating against a polymer core made of ultrahigh-molecular-weight polyethylene (UHMWPE). As a result of the decreased sliding distance in metal-on-polyethylene (MoP) L-TDRs compared with THA and TKA, wear and osteolysis of L-TDRs were originally thought to be negligible in the anterior column of the lumbar spine [21, 22]. However, studies of historical L-TDRs with γ-air-sterilized UHMWPE cores have demonstrated wear of the UHMWPE core along with several cases of osteolysis in the lumbar spine [18, 35]. Additionally, both submicron (0.05–2 µm) and large UHMWPE wear particles (≥ 2 µm) were present in periprosthetic tissues from historical TDRs [29, 30]. The presence of wear debris was associated with an innate inflammatory response and in one case contributed to osteolysis. The particle shapes were similar to those observed in revision tissues from THA and were generally round to oval in morphology, whereas the TKA particles were more needle-shaped [29]. The mean particle numbers were similar and ranged from 0 to 1002 particles/mm2 [29]. Additionally, the extent of impingement of the implant positively correlated with increased submicron wear debris and biological activity [4]. Collectively, these studies and others have established the clinically relevant complication of UHMWPE core wear for historical L-TDRs [17, 19, 28, 33, 34] and served as an impetus for improving bearing surface materials and designs.
Contemporary L-TDR designs incorporate γ-inert-sterilized UHMWPE cores and air-impermeable packaging to improve oxidation resistance and thus enhance the wear performance of the cores [16, 36]. The ProDisc-L (DePuy Synthes Spine, West Chester, PA, USA) and CHARITÉ (originally Waldemar Link, Hamburg, Germany, later fabricated by DePuy Spine, Raynham, MA, USA, and currently discontinued) are two established contemporary designs. The biomaterials used in the fabrication of the ProDisc-L prosthesis are quite similar to the mobile-bearing CHARITÉ in that they consist of two CoCr alloy endplates articulating against a conventional UHMWPE core component. Although the materials used in these L-TDRs are similar, there are differences in the designs of these implants. Unlike the mobile-bearing design of the CHARITÉ, the core of ProDisc-L is fixed through a locking mechanism into the inferior endplate, thus allowing relative motion only between the core and the superior endplate [15]. Other contemporary L-TDRs designs currently in clinical use are the Mobidisc (LDR Spine, Troyes, France) and Activ-L (Aesculap AG, Tuttlingen, Germany), which also use similar biomaterials in fabrication but differ particularly from the CHARITÉ in the amount of constraint presented by the bearing. To our knowledge, only one case report has evaluated retrievals of Mobidisc and Activ-L from two patients [1], and there is still limited understanding of implant wear or periprosthetic tissue reactions for contemporary MoP L-TDRs. Additionally, it remains unclear whether the bearing design of L-TDRs will influence the generation of UHMWPE particles and their associated biologic response.
In this study, we analyzed retrievals of two contemporary designs of fixed-bearing and mobile-bearing L-TDRs to evaluate γ-inert-sterilized UHMWPE performance in vivo and to compare design differences. We asked: (1) are periprosthetic UHMWPE wear debris and associated biological responses present in tissues from revised contemporary MoP L-TDRs that contain cores fabricated from γ-inert sterilized UHMWPE; (2) what is the influence of bearing design (ie, fully mobile versus fixed designs) on wear particle number, size, and shape; and (3) how do the wear UHMWPE particles from contemporary MoP L-TDRs compare with historical MoP L-TDRs that contain cores fabricated from γ-air-sterilized UHMWPE?
Materials and Methods
Spine tissues from regions adjacent to the implanted device were obtained at the time of revision surgery. Tissues, along with their respective devices, were collected as part of a public, multicenter retrieval research program initiated in 2004 [14, 19]. Contemporary TDRs were classified as modern device designs incorporating components made of γ-inert-sterilized UHMWPE GUR 1020 resin; this study investigated tissue retrievals from around two contemporary lumbar designs: the fixed-bearing ProDisc-L and mobile-bearing CHARITÉ. The fixed-bearing L-TDR cohort included seven patients and eight implants (implantation time, 1–6 years; mean, 4.1 years), whereas the mobile-bearing cohort included four patients and four implants (implantation time, 2–3 years; mean, 2.7 years). All were revised primarily for persistent back and/or leg pain and, for two of the patients, osteolysis was observed (Table 1). The osteolytic conditions were rare occurrences described in a recently published case report [35]. Implant subsidence or migration was a complication noted in three patients with fixed-bearing and one patient with mobile-bearing L-TDRs. Primary surgical tissues were obtained from other L-TDR patients and served as controls. We previously reported on 16 patients’ periprosthetic tissue responses around revised historical TDRs called SB CHARITÉ III, in which components were made of either γ-air-sterilized UHMWPE GUR 412 resin or γ-inert-sterilized UHMWPE GUR 1020 resin with polymer barrier packing that allowed exposure to air [28]. Quantitative findings on particle number and shape characteristics from this previous study were quantitatively compared with the current contemporary L-TDRs under investigation in this study.
Table 1.
Clinical information of MoP L-TDR retrievals
| Patient ID | Device | Bearing design | Patient sex | Age at implantation (years) | Primary diagnosis | Level | Year of index surgery | Year of removal surgery | Implantation time (years) | Revision reason(s) | Complications | Osteolysis |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BHSP 022 | ProDisc-L (DePuy Synthes Spine, West Chester, PA, USA) | Fixed | Male | 41 | Herniated disc; lumbar pain/radiculopathy | L4-L5 | 2007 | 2012 | 5.0 | Right L4-L5 arthropathy; facet pain; progressive degeneration; radiculopathy | Yes | |
| BHSP 023 | ProDisc-L | Fixed | Male | 56 | Herniated disc; lumbar pain/radiculopathy | L4-L5 | 2009 | 2012 | 3.0 | Increasing pain in the back, lower back and left quad | Subsidence | Yes |
| BHSP 025a BHSP 025b |
ProDisc-L ProDisc-L |
Fixed Fixed |
Male | 49 | Disc degeneration; discogenic back pain | L4-L5; L5-S1 |
2009 | 2013 | 4.0 | Severe pain | Posterior migration; compressing nerve roots | No |
| BHSP 026 | ProDisc-L | Fixed | Female | N/A | N/A | L5-S1 | N/A | 2012 | N/A | Persistent pain | No | |
| BHSP 027 | ProDisc-L | Fixed | Female | 33 | Herniated disc w/degeneration; back pain | L4-L5 | 2008 | 2013 | 5.0 | Lumbar pain; radiculopathy | No | |
| BHSP 0032 | ProDisc-L | Fixed | Male | 46 | N/A | L5-S1 | 2008 | 2014 | 6.0 | Severe lower back pain; L4-L5 disc injury; radiculopathy | Subsidence; upper level degeneration | No |
| PDL 004 | ProDisc-L | Fixed | Female | 27 | Unremitting lower back pain | L5-S1 | 2008 | 2009 | 1.3 | Pain; ventral encroachment into spinal canal | Partial dissociation | No |
| BRSP 003 | CHARITÉ (DePuy Spine, Raynham, MA, USA) | Mobile | Male | 28 | Disc degeneration | L4-L5 | 2006 | 2008 | 1.5 | Discogenic pain | No | |
| BRSP 004 | CHARITÉ | Mobile | Female | 22 | Disc degeneration | L5-S1 | 2004 | 2008 | 3.3 | Discogenic pain | No | |
| BRSP 006 | CHARITÉ | Mobile | N/A | N/A | Disc degeneration | L4-L5 | 2005 | 2008 | 2.7 | Painful instrumentation | Subsidence; scarring in disc space | No |
| BRSP 007 | CHARITÉ | Mobile | N/A | N/A | Painful retained hardware | L5-S1 | 2005 | 2008 | 3.3 | Pain | Periprosthetic scarring | No |
MoP = metal-on-polyethylene; L-TDR = lumbar total disc replacement; N/A = not available.
Retrieval Analyses
All implants were cleaned with 10% bleach and examined under a stereomicroscope equipped with a digital camera (Leica DFC490, Wetzlar, Germany) to assess for surface damage and impingement mechanisms such as plastic deformation, scratching, burnishing, pitting, and embedded debris. Retrieved tissues were fixed in Universal Molecular Fixative (UMFIX; Sakura Finetek USA, Inc, Torrance, CA, USA) and representative regions were selected from each tissue, embedded in paraffin, and 6-µm serial sections were mounted onto Superfrost/Plus (Fischer Scientific Co, Pittsburgh, PA, USA) slides. Slides were dewaxed, rehydrated, and stained with hematoxylin, eosin, and Alcian blue. Subsequently, Wright-Giemsa and Prussian blue stains were used for further in-depth histological evaluation when inflammation was present. Transmitted and polarized light images were captured using an Olympus BX50 microscope (Olympus, Melville, NY, USA) equipped with a stepper motor-controlled stage, an elliptically polarized light imaging system, and a Jenoptik ProgRes Speed XT Core5 camera (Jenoptik, Jena, Germany). A 36-image (× 20 objective) composite, that spanned the entire tissue section, was created for each section under transmitted light to grade tissue reactions by at least two individuals (SYV, MJS) using a scoring system scaled from 0 to 3 (Table 2). The scoring criteria were based on the Oxford method that is presently used for grading total joint arthroplasty tissues for macrophage and lymphocyte inflammation, aseptic lymphocyte-dominated vasculitis associated lesion (ALVAL), and necrosis [7, 27] but modified to exclude ALVAL responses to metal wear debris and include hemosiderin deposition and vascularization, which are more predominant in spine tissue.
Table 2.
Criteria for scoring biological responses in periprosthetic tissues of L-TDRs
| Score | Necrosis | Hemosiderin | Innate inflammation (macrophages) | Vascularization | ||
|---|---|---|---|---|---|---|
| Tissue percent area | Tissue percent area | Number of cells | Tissue percent area | Number of blood vessels | Tissue percent area | |
| 0+ | 0 | 0 | 0 | 0 | 0 | 0 |
| 1+ | Scattered or Isolated | < 10 | 1–9 | < 10 | < 10 | < 10 |
| 2+ | < 25 | 10–50 | 10–50 | 10–50 | 10–50 | 10–25 |
| 3+ | > 25 | > 50 | > 50 | > 50 | > 50 | > 25 |
L-TDRs = lumbar total disc replacements.
Wear Particle Characterization
For UHMWPE particle analysis, a 36-image (× 20 objective) composite was created from each tissue section under polarized light that corresponded to the transmitted light tissue composites. UHMWPE wear particle number, size, and shape were determined by first using a customized image threshold operation programmed in MATLAB® (MathWorks Inc, Natick, MA, USA) followed by counting/measuring particles through NIH ImageJ (National Institutes of Health, Bethesda, MD, USA). In brief, polarized light images were split into three eight-bit channels (red, green, and blue). Signal from blue channels were converted into masks based on a threshold value relative to the average signal intensity of each image. All images were visually reviewed to ensure that false-positive signals from birefringent collagen or dust did not contribute to particle counts. The resulting particle number was then converted to number/mm2 area of tissue using a measured conversion factor of 3.887 µm/pixel. Initial validation of this technique was performed using an environmental scanning electron microscope (ESEM) to study histomorphologic changes and wear debris in periprosthetic tissues of THAs [2]. Particle size and shape were characterized by measuring the equivalent circular diameter (ECD), perimeter-based-circularity, and aspect ratio as described in an earlier study by Baxter et al. [3].
Statistical Analysis
Descriptive statistics were used for evaluating wear debris-induced tissue responses, which were semiquantitatively compared based on histology and tissue scores. When quantifying UHMWPE particle numbers, the total number of particles in all tissue sections for each patient was normalized to total tissue sectional area, minimizing the effect of region-specific heterogeneity of particle distribution. Size and shape measurements of particles were averaged for each patient when wear was present. To statistically compare UHMWPE particle number, ECD, perimeter, circularity, and aspect ratio between the two different bearing designs, the Mann-Whitney U test was employed using IBM SPSS Statistics V22 software package (IBM Corporation, Armonk, NY, USA). Significance was based on p < 0.05. Because we were unable to detect statistical differences in particle measurements between the two bearing designs, fixed- and mobile-bearing patients were combined into a single contemporary L-TDR cohort before comparing it with the historical L-TDR cohort. To compare these two groups, the Mann-Whitney U test was used to test differences in UHMWPE particle number, circularity, and aspect ratio. Particle size measurements of ECD and perimeter were not statistically analyzed because particles larger than 2 µm were not evaluated in the historical L-TDR cohort.
Results
UHMWPE Wear Debris and Biological Tissue Responses
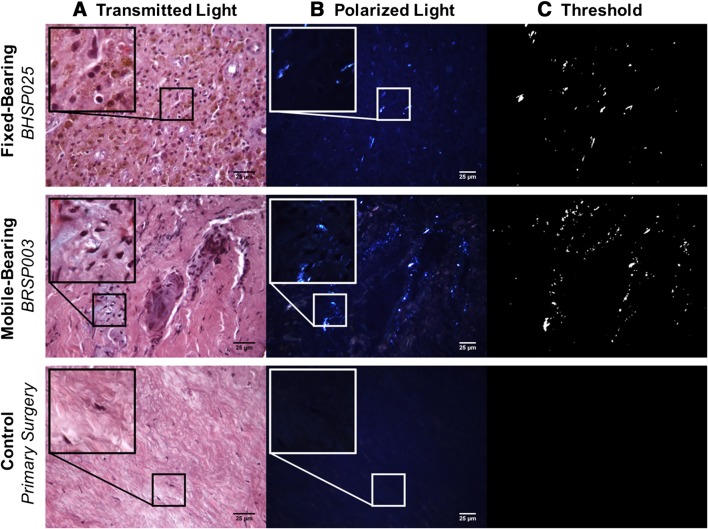
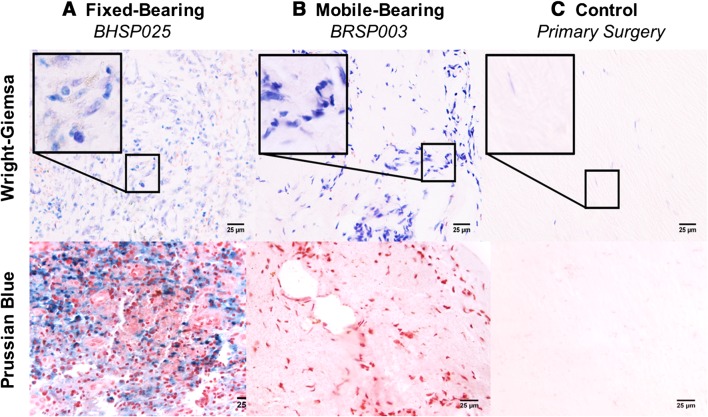
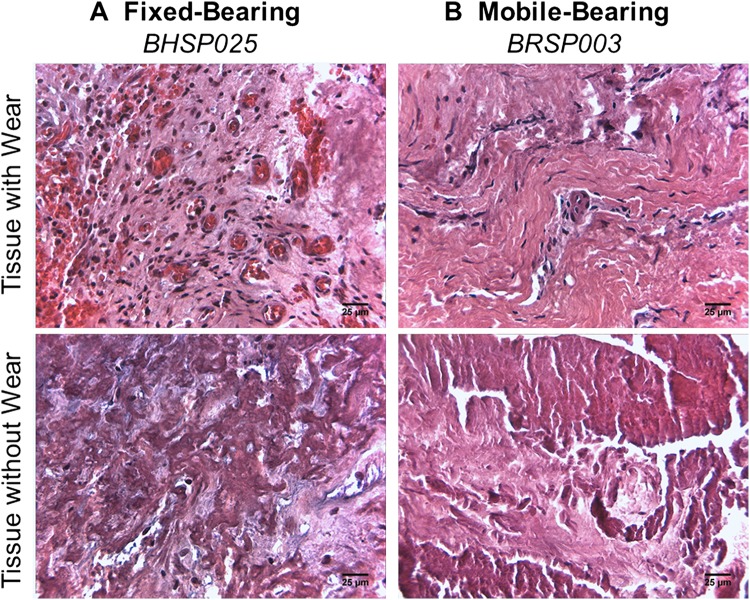
Periprosthetic UHMWPE wear debris with corresponding macrophage infiltration was observed in five of seven patients with a fixed-bearing L-TDR and one of four patients with a mobile-bearing L-TDR. Generally, the wear debris was associated with low to moderate biological tissue responses as compared with tissues (controls) from L-TDR patients undergoing primary surgery (Fig. 1). For the fixed-bearing L-TDR revisions, tissues from three of seven (43%) patients contained at least 15 UHMWPE particles/mm2 with an associated macrophage infiltration score > 1.5 (Table 3). Tissues from two other patients (29%) contained < 5 UHMWPE particles/mm2 and a variable macrophage infiltration (0.5 and 2). Tissues from patients BHSP 023 and BHSP 025, who had their implants revised for malpositioning and device impingement, contained metal wear debris, lymphocytes, plasma cells, and hemosiderin that occupied > 10% of the total tissue area (Fig. 2). Tissue from patient BHSP 0032 also contained metal particles and lymphocytes but no hemosiderin deposits. Patient PDL 004 had one tissue sample with an isolated area containing 21 UHMWPE particles/mm2 but no detectable inflammation. For the mobile-bearing L-TDR cohort, tissue that contained UHMWPE wear debris was found in only one of four patients. None of the tissues associated with the mobile-bearing devices contained metallic debris, and none of the implants exhibited rim impingement. A tissue sample from patient BRSP 003 contained 107 UHMWPE wear particles/mm2 and it had a high macrophage infiltration score (3.0). For the fixed-bearing cohort, tissues with wear debris were consistently more vascularized with mean scores as high as 3 (range, 0–3), whereas necrotic/acellular regions were scarce (Fig. 3). In contrast, the majority of tissue samples around these devices that did not contain wear debris had low vascularity and more prominent regions of necrosis (mean necrosis score, 2) (Table 4). Tissues around mobile-bearing devices, with or without wear, were moderately vascularized with isolated necrotic regions.
Fig. 1A–C.
Transmitted light images (A) and polarized microscopy (B) of tissue sections revealed the presence of UHMWPE wear and corresponding macrophage infiltration. Insets for the L-TDR patient tissues show higher magnifications of these regions. There was no inflammation present in control tissue obtained from primary surgery. Particles were characterized using a MATLAB threshold (C). Tissue sections were stained with hematoxylin and eosin (Original magnification × 400; inset, × 1000).
Table 3.
Histologic evaluation and mean scores of retrieved tissues with wear debris*
| Patient ID | Implant bearing design | Tissue samples with wear debris | UHMWPE wear debris (particles/mm2) | Metal wear debris | Inflammation (macrophages) |
Type of inflammatory cells | Necrosis | Hemosiderin | Vascularization |
|---|---|---|---|---|---|---|---|---|---|
| BHSP 022 | Fixed | 2/4 | 4.3 | No | 0.5 | Macrophages | 1.0 | 0.5 | 0 |
| BHSP 023 | Fixed | 6/14 | 4.8 | Yes | 1.7 | Predominantly macrophages with lymphocytes and plasma cells | 0.9 | 1.2 | 1.3 |
| BHSP 025a | Fixed | 2/6 | 21.8 | Yes | 2.0 | Predominantly macrophages with lymphocytes and plasma cells | 1.5 | 2.0 | 2 |
| BHSP 025b | Fixed | 6/12 | 1.74 | Yes | 1.8 | Predominantly macrophages with lymphocytes and plasma cells | 0.0 | 1.7 | 2.5 |
| BHSP 027 | Fixed | 3/6 | 29.1 | No | 1.7 | Macrophages | 0.5 | 0.0 | 1.5 |
| BHSP 0032 | Fixed | 1/1 | 15.0 | Yes | 1.5 | Predominantly macrophages with lymphocytes | 1.0 | 0.0 | 2.5 |
| PDL 004 | Fixed | 1/3 | 20.9 | No | 0.0 | None | 1.0 | 0.0 | 0 |
| BRSP 003 | Mobile | 1/4 | 107.3 | No | 3.0 | Macrophages | 1.0 | 0.0 | 1 |
* Inflammation, necrosis, hemosiderin, and vascularization scores on a 0–3 scale.
Fig. 2A–C.
Wright Giemsa and Prussian blue stains were used to identify inflammatory cells and hemosiderin deposits, respectively. There were macrophages, plasma cells, and lymphocytes with hemosiderin deposits in tissues around fixed-bearing L-TDRs (A), but only macrophages in mobile-bearing L-TDR tissues (B). Insets for the L-TDR patient tissues show higher magnifications of the inflammatory cells. There was no inflammation or hemosiderin present in control tissue (C) obtained from primary surgery (Original magnification × 400; inset, × 1000).
Fig. 3A–B.
In alcian blue, hematoxylin and eosin-stained tissue sections, representative fixed-bearing L-TDR tissue images (A) showed increased vascularization in tissues with wear debris and necrosis in tissues without wear debris. Mobile-bearing L-TDR tissues (B) had lower vascularization in tissues with wear debris and a mix of moderate vascularization and isolated necrosis in tissues without wear debris (Original magnification, × 400).
Table 4.
Histologic evaluation and mean scores of retrieved tissues without wear*
| Patient ID | Implant bearing design | Tissue samples without wear debris | Necrosis | Hemosiderin | Vascularization |
|---|---|---|---|---|---|
| BHSP 022 | Fixed | 2/4 | 0.5 | 0.5 | 0.0 |
| BHSP 023 | Fixed | 8/14 | 1.2 | 0.8 | 0.8 |
| BHSP 025a | Fixed | 4/6 | 2.0 | 0.0 | 0.0 |
| BHSP 025b | Fixed | 6/12 | 1.0 | 1.0 | 1.2 |
| BHSP 026 | Fixed | 4/4 | 0.8 | 0.8 | 1.0 |
| BHSP 027 | Fixed | 3/6 | 1.5 | 0.0 | 0.0 |
| PDL 004 | Fixed | 2/3 | 1.0 | 0.0 | 0.0 |
| BRSP 003 | Mobile | 3/4 | 0.7 | 0.0 | 1.0 |
| BRSP 004 | Mobile | 4/4 | 0.5 | 0.0 | 1.5 |
| BRSP 006 | Mobile | 6/6 | 0.7 | 0.0 | 1.3 |
| BRSP 007 | Mobile | 2/2 | 0.0 | 0.0 | 0.0 |
* Necrosis, hemosiderin, and vascularization scores on a 0–3 scale.
Wear Particle Number, Size, and Shape for Contemporary Total Disc Replacement Designs
For the mobile-bearing L-TDR patient tissue with UHMWPE wear debris, particle number was observed to be increased by 87% compared with fixed-bearing patient tissues, and the particles were 11% rounder and 11% less elongated (Table 5), but, with the number of samples available, these differences were not significant. Qualitative observations revealed the area percentage or amount of tissue occupied by particles from the mobile-bearing device appeared more extensive than the particles from fixed-bearing devices with the exception of tissues from patient BHSP 022, which contained large (> 10 µm) particles (highest ECD and perimeter values). Nonetheless, the overall distribution of particle sizes was similar for both cohorts. The majority of the particles were between 1 and 10 µm (75% and 83% for the fixed-bearing and mobile-bearing L-TDR cohorts, respectively). Submicron particles (< 1 μm) represented 20% of the particles from fixed-bearing devices and 16% from the mobile-bearing device. Large particles (> 10 μm) were rarely observed and represented less than 2% of the particles in both cohorts.
Table 5.
UHMWPE particle number and characteristics from tissues with wear debris
| Patient ID | Implant bearing design | Tissue samples with wear debris | < 0.1–1 µm (particles/mm2) | 1–10 µm (particles/mm2) | > 10 µm (particles/mm2) | All sizes (particles/mm2) | Percent area of particles* | Equivalent circular diameter (µm)† | Perimeter (µm)† | Circularity† | Aspect ratio† |
|---|---|---|---|---|---|---|---|---|---|---|---|
| BHSP 022 | Fixed | 2/4 | 0.5 | 3.1 | 0.6 | 4.3 | 0.12% | 6.6 ± 10.8 | 28.1 ± 47.0 | 0.7 ± 0.2 | 1.8 ± 0.5 |
| BHSP 023 | Fixed | 6/14 | 1.2 | 3.1 | 0.5 | 4.8 | 0.05% | 2.7 ± 5.6 | 13.4 ± 39.4 | 0.7 ± 0.3 | 2.0 ± 1.0 |
| BHSP 025a | Fixed | 2/6 | 4.7 | 16.7 | 0.4 | 21.8 | 0.03% | 2.9 ± 3.1 | 12.0 ± 19.1 | 0.8 ± 0.2 | 1.9 ± 0.7 |
| BHSP 025b | Fixed | 6/12 | 0.2 | 1.5 | 0.1 | 1.7 | 0.02% | 2.6 ± 2.7 | 10.3 ± 15.7 | 0.8 ± 0.2 | 1.9 ± 0.7 |
| BHSP 027 | Fixed | 3/6 | 6.8 | 21.8 | 0.6 | 29.1 | 0.07% | 2.9 ± 5.2 | 11.8 ± 38.1 | 0.8 ± 0.2 | 1.9 ± 0.8 |
| BHSP 0032 | Fixed | 1/1 | 2.6 | 10.6 | 1.3 | 15.0 | 0.06% | 3.8 ± 4.7 | 8.4 ± 11.4 | 0.8 ± 0.2 | 1.9 ± 0.8 |
| PDL 004 | Fixed | 1/3 | 4.6 | 15.9 | 0.5 | 20.9 | 0.02% | 2.3 ± 2.4 | 8.4 ± 11.4 | 0.9 ± 0.2 | 1.7 ± 0.4 |
| BRSP 003 | Mobile | 1/4 | 17.7 | 89.1 | 0.5 | 107.3 | 0.11% | 2.2 ± 2.9 | 8.2 ± 14.1 | 0.9 ± 0.1 | 1.7 ± 0.5 |
* Percent area of particles is the ratio of the total area of all particles to the total area of tissue; †mean ± SD.
Comparison of Wear Particle Characteristics to Metal-on-polyethylene Historical Lumbar Total Disc Replacements
Compared with five historical L-TDR patients identified in a previous study [28], UHMWPE particle number and circularity in the seven contemporary L-TDR patient tissues were statistically significantly different, but aspect ratio was not (Table 6). Specifically, the number of particles per gram of tissue was 99% less (p = 0.003) and their shape was 50% rounder (p = 0.003) in contemporary L-TDR cohorts, which included both fixed- and mobile-bearing designs. Qualitative observations of particle size revealed that tissues from contemporary L-TDR patients contained more submicron and small (< 10 µm) wear debris and less associated inflammation in comparison to the historical L-TDR cohort (not shown).
Table 6.
Comparing UHMWPE particle number and characteristics in patients with wear debris
| Comparison | Fixed-bearing L-TDR | Mobile-bearing L-TDR | Contemporary L-TDR (fixed and mobile) | Historical mobile-bearing L-TDR* (Punt et al., 2011) [28] |
|---|---|---|---|---|
| UHMWPE core | γ-inert-sterilized | γ-inert-sterilized | γ-inert-sterilized | γ-air-sterilized |
| Patients with wear debris (number) | 6 of 7 | 1 of 4 | 7 of 11 | 5 of 5 |
| Particle number† (particles/g × 107; mean ± SD) | 1.2 ± 3.1 | 9.0 | 2.1 ± 2.9‡ | 162.1 ± 27.1 |
| Circularity (mean ± SD) | 0.8 ± 0.2 | 0.9 ± 0.2 | 0.8 ± 0.2‡ | 0.4± 0.2 |
| Aspect ratio (mean ± SD) | 1.9 ± 0.8 | 1.7 ± 0.5 | 1.8 ± 0.7 | 2.0 ± 1.0 |
* Particle measurements were generated using ESEM and a cutoff of 2 µm so size characteristics were not comparable; †measurements from contemporary L-TDR cohorts were converted from particles/mm2 for comparison purposes; ‡significant differences between combined contemporary L-TDRs and historical L-TDRs (p < 0.01); L-TDR = lumbar total disc replacement; N/A = not available; ESEM = environmental scanning electron microscope.
Discussion
L-TDR was developed as an alternative to spinal fusion for the treatment of degenerative disc disease in the lumbar spine and a device that would preserve or restore segmental function and motion. However, historical generations of L-TDR devices with γ-air-sterilized UHMWPE cores raised concerns regarding wear debris generation and subsequent immunological responses that may adversely affect clinical outcomes. Today, modern L-TDR designs incorporate γ-inert-sterilized UHMWPE cores to improve wear resistance and minimize wear debris generation in an effort to reduce the risk of revision surgery. The aims of this study were to evaluate wear debris and tissue reactions in tissues from revised contemporary MoP L-TDRs and determine the influence of bearing design on wear particle number, size, and shape. Furthermore, we wanted to know how UHMWPE wear particle densities and characteristics compared with previous historical MoP L-TDRs. After analyzing retrieved tissues around eight fixed- and four mobile-bearing L-TDRs from 11 patients revised primarily for pain, we found measurable UHMWPE wear debris with corresponding macrophage infiltration in five patients who had a fixed- and one patient who had a mobile-bearing L-TDR. The frequency, amount, and shape of wear debris suggested the bearing design of contemporary devices did not influence wear particle characteristics. Furthermore, particle comparisons with a retrieval study of historical devices suggested that γ-inert-sterilized UHMWPE has improved wear resistance and reduced wear-induced periprosthetic tissue reactions.
We acknowledge the limitations of our study. First, although the primary revision reason for all patients was pain, implant malpositioning and impingement were reported in three of six fixed-bearing and none of the mobile-bearing L-TDR patients. This complication may serve as a confounding variable when comparing the two designs. However, the issue of impingement is not an uncommon finding for L-TDRs because it was previously reported for mobile-bearing L-TDR retrievals and contributed to wear debris generation and the immune responses [4]. Second, the implantation times varied between the ProDisc-L and CHARITÉ cohorts. The times ranged from 1 to 6 years (mean, 4.1 years) for the ProDisc-L cohort and 2 to 3 years (mean, 2.7 years) for the CHARITÉ cohort, although both cohorts were short-term revisions. Third, the cohort sizes were small and wear particle characteristics of mobile-bearing devices were extrapolated from particles that were observed in only one of four patients who had wear debris. Nevertheless, to our knowledge, there are no published retrieval studies for contemporary MoP L-TDRs. Lastly, comparisons of particle number and characteristics are provided for historical MoP L-TDRs; however, the previous study used ESEM and excluded wear particles larger than 2 µm, whereas in this study, we used polarized light microscopy and were able to detect particles as small as 0.461 µm. Although polarized light microscopy has been used in other related studies to investigate UHWMPE particles of particular sizes [29, 30], the different approaches make it difficult to make direct comparisons.
Not all periprosthetic tissue from the two contemporary MoP L-TDRs examined in this study contained UHMWPE wear debris; this was true for tissues from all patients and different tissues from the same patient. However, those tissues that contained UHMWPE wear debris generally had an associated macrophage inflammation. This type of colocalization of wear debris and macrophages is an established phenomenon in total joint arthroplasty and more recently noted in historical L-TDRs [30]. One noteworthy difference observed for the contemporary L-TDRs was a decrease in particles > 10 µm, so unlike historical L-TDRs, giant cells were not observed in these tissues. Periprosthetic UHMWPE particles from both contemporary L-TDR cohorts resulted primarily in a macrophage response, except for three patients with metallic wear debris from fixed-bearing devices and an associated lymphocytic response. These patient tissues also had higher macrophage infiltration scores. The presence of metallic debris may be attributed to the unintended wear mechanism of impingement between the metallic endplates arising from malpositioning and/or subsidence, which was noted in more than 50% of contemporary fixed-bearing device retrievals in a separate retrieval study [20]. Interestingly, considerable amounts of hemosiderin were present in many of these tissues, indicative of phagocytosis of erythrocytes and degradation of hemoglobin by macrophages [13, 32]. The exact contribution of hemosiderin to revision remains unclear; however, a previous study has associated the deposition with the accumulation of activated macrophages that are positive for osteoclastic cell markers [25]. Although the amount of UHMWPE wear debris in the spine may not be severe enough to directly contribute to osteolysis [18], vertebral osteolysis was noted as a clinical complication in two patients with fixed-bearing L-TDRs, both of whom had tissues containing hemosiderin. The effect of hemosiderin in these tissues on UHMWPE wear-induced inflammation requires further investigation. Other biological tissue responses noted around the fixed-bearing devices included increased vascularization in tissues with wear and necrosis in tissues without wear. In contrast, tissues around mobile-bearing L-TDRs, with and without wear debris, had low to moderate vascularization and necrosis. The presence of these reactions in both cohorts is noteworthy because these reactions have been implicated in the development of pain. Specifically, increased vascularization (angiogenesis) and sensory nerve growth are closely linked processes [5, 24]; and tissue necrosis or cell death results in the release of proinflammatory cytokines and other factors that initiate persistent pain by directly activating nociceptive sensory neurons [6, 38]. Both reactions can lead to maladaptive plasticity and neural disease states, which raises the question whether these tissue responses contributed to neuropathic pain in both fixed- and mobile-bearing L-TDR patients.
UHMWPE wear debris in tissues around fixed-bearing devices qualitatively appeared smaller, less concentrated, and less round than debris in tissues around the one mobile-bearing retrieval; however, no statistical difference was observed. Austen et al. recently reported a case study of two patients revised for a different set of fixed- and mobile-bearing contemporary L-TDRs, and observed larger UHMWPE particles in tissues from the patient with the mobile-bearing design [1]. Interestingly, retrieval studies of TKA also found comparable findings in which larger UHMWPE particles were found in tissues surrounding failed mobile-bearing TKRs than in tissues around failed fixed-bearing TKRs [9, 26]. Design-dependent differences in loading and wear mechanisms may explain observed qualitative differences of wear particles between designs. All fixed-bearing retrievals in our study showed signs of scratching on dome regions, and the tissues with metal wear corresponded with implant components that had metallic and endplate burnishing as a result of impingement attributable to malposition and/or subsidence. The mobile-bearing patient tissue retrieval with wear corresponded to an implant core that had burnishing, pitting, and multidirectional scratching. The overall density of UHMWPE particles was relatively low in tissues from both cohorts, but the majority was 1 to 10 µm, which falls within a size range that activates macrophages [8]. Further research and larger sample sizes are necessary to determine whether design-dependent differences significantly influence particle size and shape differences.
UHMWPE particles from the contemporary L-TDR cohorts were less numerous and rounder in comparison to the historical L-TDR group, suggesting that modern L-TDR designs have improved wear properties. A separate study investigating particles > 2 µm in historical L-TDR cohorts reported a mean of 231 particles/mm2 [29], which was roughly 10-fold higher than the amount of similar-sized particles from contemporary TDRs (mean, 22 particles/mm2). This comparison paralleled findings from a case report, which noted that the mean number of UHMWPE particles was two orders of magnitude lower in a different set of revised contemporary L-TDRs [1]. Our study also showed that mean particle circularity (roundness) was noticeably higher in the contemporary L-TDR cohort (mean, 0.8 versus mean, 0.4), but aspect ratios were within the same range as those of historical L-TDR particles [28]. Interestingly, UHMWPE particles from conventional THAs fabricated with γ-inert-sterilized UHMWPE acetabular liners have been reported to have shapes similar to the contemporary L-TDR group [3, 10–12, 23]. Multiple studies have reported that particles with more rounded morphologies trigger less robust macrophage activation compared with fibrillar-shaped particles [11, 31, 37]. Thus, with the samples available in this study, both the decreased number and increased roundness of the particles suggest that contemporary L-TDRs will have a reduced inflammatory response.
In summary, the concentration of wear debris and subsequent tissue responses were greatly reduced particle numbers in tissues from contemporary L-TDRs when compared with historical L-TDRs. Although we investigated short-term revisions within 5 years of implantation, we showed that periprosthetic tissues from both fixed- and mobile-bearing L-TDR patients contained UHMWPE particles within size ranges known to elicit a macrophage response. Because artificial discs are intended to last the lifetime of the patient, further retrieval studies are still necessary to elucidate the long-term role of UHMWPE wear and its association, if any, to the clinical performance of lumbar disc arthroplasty.
Footnotes
Funding provided by the National Institutes of Health (NIAMS) R01 AR56264. One of the authors (SMK) is an employee and shareholder of Exponent, Inc (Philadelphia, PA, USA), and institutional support for SMK is received as a Principal Investigator from Smith & Nephew (Memphis, TN, USA); Stryker Orthopaedics (Mahwah, NJ, USA); Zimmer Inc (Warsaw, IN, USA); Biomet (Warsaw, IN, USA); DePuy Synthes (West Chester, PA, USA); Medtronic (Minneapolis, MN, USA); Invibio (Lancashire, UK); Stelkast (McMurray, PA, USA); Formae (Paoli, PA, USA); Kyocera Medical (Osaka, Japan); Wright Medical Technology (Memphis, TN, USA); CeramTec (Plochingen, Germany); DJO (Austin, TX, USA); Celanese (Bedminster Township, NJ, USA); Aesculap (Tuttlingen, Germany); SpinalMotion, Inc (Mountain View, CA, USA); and Active Implants (Memphis, TN, USA) outside of the submitted work. One of the authors (THL) certifies that he has received royalties, during the study period of USD 100,001 to USD 1,000,000 from Medtronic (Memphis, TN, USA).
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research ® editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research ® neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA-approval status, of any drug or device prior to clinical use.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This work was performed at the Implant Research Center, Philadelphia, PA, USA.
References
- 1.Austen S, Punt IM, Cleutjens JP, Willems PC, Kurtz SM, MacDonald DW, van Rhijn LW, van Ooij A. Clinical, radiological, histological and retrieval findings of Activ-L and Mobidisc total disc replacements: a study of two patients. Eur Spine J. 2012;21(Suppl 4):S513–S520. doi: 10.1007/s00586-011-2141-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baxter RM, Ianuzzi A, Freeman TA, Kurtz SM, Steinbeck MJ. Distinct immunohistomorphologic changes in periprosthetic hip tissues from historical and highly crosslinked UHMWPE implant retrievals. J Biomed Mater Res A. 2010;95:68–78. doi: 10.1002/jbm.a.32813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baxter RM, MacDonald DW, Kurtz SM, Steinbeck MJ. Characteristics of highly cross-linked polyethylene wear debris in vivo. J Biomed Mater Res B Appl Biomater. 2013;101:467–475. doi: 10.1002/jbm.b.32902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baxter RM, MacDonald DW, Kurtz SM, Steinbeck MJ. Severe impingement of lumbar disc replacements increases the functional biological activity of polyethylene wear debris. J Bone Joint Surg Am. 2013;95:e751–e759. doi: 10.2106/JBJS.K.00522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown MF, Hukkanen MV, McCarthy ID, Redfern DR, Batten JJ, Crock HV, Hughes SP, Polak JM. Sensory and sympathetic innervation of the vertebral endplate in patients with degenerative disc disease. J Bone Joint Surg Br. 1997;79:147–153. doi: 10.1302/0301-620X.79B1.6814. [DOI] [PubMed] [Google Scholar]
- 6.Cuellar JM, Scuderi GJ, Cuellar VG, Golish SR, Yeomans DC. Diagnostic utility of cytokine biomarkers in the evaluation of acute knee pain. J Bone Joint Surg Am. 2009;91:2313–2320. doi: 10.2106/JBJS.H.00835. [DOI] [PubMed] [Google Scholar]
- 7.Grammatopoulos G, Pandit H, Kamali A, Maggiani F, Glyn-Jones S, Gill HS, Murray DW, Athanasou N. The correlation of wear with histological features after failed hip resurfacing arthroplasty. J Bone Joint Surg Am. 2013;95:e81. doi: 10.2106/JBJS.L.00775. [DOI] [PubMed] [Google Scholar]
- 8.Green TR, Fisher J, Stone M, Wroblewski BM, Ingham E. Polyethylene particles of a ‘critical size’ are necessary for the induction of cytokines by macrophages in vitro. Biomaterials. 1998;19:2297–2302. doi: 10.1016/S0142-9612(98)00140-9. [DOI] [PubMed] [Google Scholar]
- 9.Hirakawa K, Bauer TW, Stulberg BN, Wilde AH, Borden LS. Characterization of debris adjacent to failed knee implants of 3 different designs. Clin Orthop Relat Res. 1996;331:151–158. doi: 10.1097/00003086-199610000-00021. [DOI] [PubMed] [Google Scholar]
- 10.Howling GI, Barnett PI, Tipper JL, Stone MH, Fisher J, Ingham E. Quantitative characterization of polyethylene debris isolated from periprosthetic tissue in early failure knee implants and early and late failure Charnley hip implants. J Biomed Mater Res. 2001;58:415–420. doi: 10.1002/jbm.1036. [DOI] [PubMed] [Google Scholar]
- 11.Ingham E, Fisher J. The role of macrophages in osteolysis of total joint replacement. Biomaterials. 2005;26:1271–1286. doi: 10.1016/j.biomaterials.2004.04.035. [DOI] [PubMed] [Google Scholar]
- 12.Kobayashi A, Bonfield W, Kadoya Y, Yamac T, Freeman MA, Scott G, Revell PA. The size and shape of particulate polyethylene wear debris in total joint replacements. Proc Inst Mech Eng H. 1997;211:11–15. doi: 10.1243/0954411971534638. [DOI] [PubMed] [Google Scholar]
- 13.Kockx MM, Cromheeke KM, Knaapen MW, Bosmans JM, De Meyer GR, Herman AG, Bult H. Phagocytosis and macrophage activation associated with hemorrhagic microvessels in human atherosclerosis. Arterioscler Thromb Vasc Biol. 2003;23:440–446. doi: 10.1161/01.ATV.0000057807.28754.7F. [DOI] [PubMed] [Google Scholar]
- 14.Kurtz S, Steinbeck M, Ianuzzi A, Van Ooij A, Punt I, Isaza J, Ross ER. Retrieval analysis of motion preserving spinal devices and periprosthetic tissues. SAS. 2009;3:161–177. doi: 10.1016/j.esas.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kurtz SM. The UHMWPE Biomaterials Handbook. Burlington, MA, USA: Academic Press; 2009. [Google Scholar]
- 16.Kurtz SM, MacDonald D, Ianuzzi A, van Ooij A, Isaza J, Ross ER, Regan J. The natural history of polyethylene oxidation in total disc replacement. Spine (Phila Pa 1976). 2009;34:2369–2377. [DOI] [PubMed]
- 17.Kurtz SM, Patwardhan A, MacDonald D, Ciccarelli L, van Ooij A, Lorenz M, Zindrick M, O’Leary P, Isaza J, Ross R. What is the correlation of in vivo wear and damage patterns with in vitro TDR motion response? Spine. 2008;33:481–489. doi: 10.1097/BRS.0b013e318165e3be. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kurtz SM, Toth JM, Siskey R, Ciccarelli L, Macdonald D, Isaza J, Lanman T, Punt I, Steinbeck M, Goffin J, van Ooij A. The latest lessons learned from retrieval analyses of ultra-high molecular weight polyethylene, metal-on-metal, and alternative bearing total disc replacements. Semin Spine Surg. 2012;24:57–70. doi: 10.1053/j.semss.2011.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kurtz SM, van Ooij A, Ross R, de Waal Malefijt J, Peloza J, Ciccarelli L, Villarraga ML. Polyethylene wear and rim fracture in total disc arthroplasty. Spine J. 2007;7:12–21. [DOI] [PubMed]
- 20.Lebl DR, Cammisa FP, Girardi FP, Wright T, Abjornson C. In vivo functional performance of failed Prodisc-L devices: retrieval analysis of lumbar total disc replacements. Spine (Phila Pa 1976). 2012;37:E1209–1217. [DOI] [PubMed]
- 21.Link HD, Keller A. Biomechanics of total disc replacement. In: Buttner-Janz K, Hochschuler SH, McAfee PC, editors. The Artificial Disc. Berlin, Germany: Springer; 2003. pp. 33–52. [Google Scholar]
- 22.Link HD, McAfee PC, Pimenta L. Choosing a cervical disc replacement. Spine J. 2004;4:294S–302S. doi: 10.1016/j.spinee.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 23.Mabrey JD, Afsar-Keshmiri A, McClung GA, 2nd, Pember MA, 2nd, Wooldridge TM, Mauli Agrawal C. Comparison of UHMWPE particles in synovial fluid and tissues from failed THA. J Biomed Mater Res. 2001;58:196–202. doi: 10.1002/1097-4636(2001)58:2<196::AID-JBM1007>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 24.Mapp PI, Walsh DA. Mechanisms and targets of angiogenesis and nerve growth in osteoarthritis. Nat Rev Rheumatol. 2012;8:390–398. doi: 10.1038/nrrheum.2012.80. [DOI] [PubMed] [Google Scholar]
- 25.Margevicius KJ, Bauer TW, McMahon JT, Brown SA, Merritt K. Isolation and characterization of debris in membranes around total joint prostheses. J Bone Joint Surg Am. 1994;76:1664–1675. doi: 10.2106/00004623-199411000-00010. [DOI] [PubMed] [Google Scholar]
- 26.Minoda Y, Kobayashi A, Iwaki H, Miyaguchi M, Kadoya Y, Ohashi H, Takaoka K. Characteristics of polyethylene wear particles isolated from synovial fluid after mobile-bearing and posterior-stabilized total knee arthroplasties. J Biomed Mater Res B Appl Biomater. 2004;71:1–6. doi: 10.1002/jbm.b.30005. [DOI] [PubMed] [Google Scholar]
- 27.Phillips E, Klein GR, Cates HE, Kurtz SM, Steinbeck MJ. Histological characterization of periprosthetic tissue responses for metal-on-metal hip replacement. J Long Term Eff Med Implants. 2014;24:13–23. doi: 10.1615/JLongTermEffMedImplants.2014010275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Punt I, Baxter R, van Ooij A, Willems P, van Rhijn L, Kurtz S, Steinbeck M. Submicron sized ultra-high molecular weight polyethylene wear particle analysis from revised SB Charite III total disc replacements. Acta Biomater. 2011;7:3404–3411. doi: 10.1016/j.actbio.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Punt IM, Austen S, Cleutjens JP, Kurtz SM, ten Broeke RH, van Rhijn LW, Willems PC, van Ooij A. Are periprosthetic tissue reactions observed after revision of total disc replacement comparable to the reactions observed after total hip or knee revision surgery? Spine (Phila Pa 1976). 2012;37:150–159. [DOI] [PMC free article] [PubMed]
- 30.Punt IM, Cleutjens JP, de Bruin T, Willems PC, Kurtz SM, van Rhijn LW, Schurink GW, van Ooij A. Periprosthetic tissue reactions observed at revision of total intervertebral disc arthroplasty. Biomaterials. 2009;30:2079–2084. doi: 10.1016/j.biomaterials.2008.12.071. [DOI] [PubMed] [Google Scholar]
- 31.Ren W, Yang SY, Fang HW, Hsu S, Wooley PH. Distinct gene expression of receptor activator of nuclear factor-kappaB and rank ligand in the inflammatory response to variant morphologies of UHMWPE particles. Biomaterials. 2003;24:4819–4826. doi: 10.1016/S0142-9612(03)00384-3. [DOI] [PubMed] [Google Scholar]
- 32.Schrijvers DM, De Meyer GR, Herman AG, Martinet W. Phagocytosis in atherosclerosis: Molecular mechanisms and implications for plaque progression and stability. Cardiovasc Res. 2007;73:470–480. doi: 10.1016/j.cardiores.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 33.van Ooij A, Kurtz SM, Stessels F, Noten H, van Rhijn L. Polyethylene wear debris and long-term clinical failure of the Charite disc prosthesis: a study of 4 patients. Spine (Phila Pa 1976). 2007;32:223–229. [DOI] [PubMed]
- 34.van Ooij A, Oner FC, Verbout AJ. Complications of artificial disc replacement: a report of 27 patients with the SB Charite disc. J Spinal Disord Tech. 2003;16:369–383. doi: 10.1097/00024720-200308000-00009. [DOI] [PubMed] [Google Scholar]
- 35.Veruva SY, Lanman TH, Hanzlik JA, Kurtz SM, Steinbeck MJ. Rare complications of osteolysis and periprosthetic tissue reactions after hybrid and non-hybrid total disc replacement. Eur Spine J. 2014 Aug 28 [Epub ahead of print]. [DOI] [PubMed]
- 36.Veruva SY, Steinbeck MJ, Toth J, Alexander DD, Kurtz SM. Which design and biomaterial factors affect clinical wear performance of total disc replacements? A systematic review. Clin Orthop Relat Res. 2014 Jul 8 [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 37.Yang SY, Ren W, Park Y, Sieving A, Hsu S, Nasser S, Wooley PH. Diverse cellular and apoptotic responses to variant shapes of UHMWPE particles in a murine model of inflammation. Biomaterials. 2002;23:3535–3543. doi: 10.1016/S0142-9612(02)00032-7. [DOI] [PubMed] [Google Scholar]
- 38.Zhang JM, An J. Cytokines, inflammation, and pain. Int Anesthesiol Clin. 2007;45:27–37. doi: 10.1097/AIA.0b013e318034194e. [DOI] [PMC free article] [PubMed] [Google Scholar]