Abstract
Background
Conventional survival analysis for endoprosthetic complications does not consider competing events adequately. Patients who die of their disease are no longer at risk for complications; therefore, death as a competing event may alter survivorship estimates in the orthopaedic-oncological setting.
Questions/purposes
This investigation aimed to compare (1) endoprosthetic survivorship after osteosarcoma by Kaplan-Meier analysis; and (2) by a competing risk model.
Methods
Between 1981 and 2009, we performed 247 modular endoprostheses for patients with extremity osteosarcoma; 73 patients had a followup of less than 2 years but all patients were included in statistical analysis. No patients were lost to followup for reasons other than death. Revision-free endoprosthetic survival until soft tissue failure (Type 1), aseptic loosening (Type 2), structural failure (Type 3), infection (Type 4), and local tumor progression (Type 5) was estimated according to a Kaplan-Meier analysis and a competing risk model. Sixty-four patients died throughout followup; the 5- and 10-year overall survival and metastasis-free survival were 72% and 70% and 70% and 69%, respectively. One hundred twenty-two patients (49%) had complications.
Results
Competing risk analysis consistently resulted in reduced estimates of the frequency of complications and reconstructive failures compared with Kaplan-Meier analysis. Cumulative risks for complication Types 1 to 5 at 10 years without/with death as a competing event revealed a risk of 19%/16% for Type 1, 26%/20% for Type 2, 51%/38% for Type 3, 23%/20% for Type 4, and 4%/3% for Type 5.
Conclusions
A competing risk model reveals considerably reduced risks for every complication compared with Kaplan-Meier analysis when death is included as a competing event. Because it more realistically represents the risks of complications, competing risk models should be used to arrive at risk estimates for purposes of counseling patients about those risks associated with modular endoprosthetic reconstruction.
Level of Evidence
Level III, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
The frequency of limb salvage surgery for patients with primary bone malignancies using modular endoprostheses has steadily increased during the last decades and during this time, endoprosthetic survival has improved because of advances in design, manufacturing, and surgical techniques [15]. However, complication rates are still high as a result of a variety of factors including factors such as the large dead space in the tumor bed surrounding the prosthesis, wide soft tissue exposure, fixation issues of the implants, and immune suppression. Consequently, reconstructive failures remain relatively common and are caused by local tumor recurrence, infection, aseptic loosening, and fracture, among others [1, 4, 5, 12, 14, 16, 19, 20].
Because malignant bone tumors are rare, reports about survivorship and complications of tumor endoprostheses generally are limited to small cohorts with relatively short followup [6, 7, 10, 14, 15] and commonly these investigations apply Kaplan-Meier estimations and Cox regression models to describe endoprosthetic survival. These analyses deal principally with one event as an endpoint, for example revision surgery, independent of its cause [20]. However, in patients undergoing endoprosthetic reconstructions after wide resection of a malignant bone tumor, although interest may focus on a single specific cause of prosthetic failure (eg, revision for infection), other events may take place that can influence the likelihood of this failure occurring or even prevent it from occurring at all (eg, amputation for local recurrence). Over a population, the presence of such “competing risks” may dramatically influence estimates of event frequencies. For example, a patient who has had an amputation for local recurrence cannot subsequently have a revision of an endoprosthesis for infection. More generally, in such competing risk situations, each individual is exposed to two or more causes of failure but the ultimate failure can be attributed to only one of these competing events [12, 19].
The long-term overall survival rates of osteosarcoma remain approximately 60%, so death from disease is a very potent competing factor that must have an influence on endoprosthetic survival [2–4, 20]. However, because Kaplan-Meier estimation does not take into account any kind of competing event, the clinical scenario of a highly malignant bone tumor and a surgical therapy with a considerable revision rate will lead to an overestimation of the respective risks for revision. So for the musculoskeletal oncologic surgeon as well as for the patient, a more reliable estimation of the risk of any revision therefore is called for to guide the decision-making process on the optimal treatment option, especially when evaluating endoprosthetic survival and the risk of certain failures in long-term survivors. A competing risk model offers just such an approach [1–3, 8, 16], but the differences in survival estimates between conventional Kaplan-Meier survivorship and the competing risk approach for one of our more common surgical interventions—osteosarcoma reconstruction—have not been well characterized.
We therefore sought to (1) analyze the failure mechanisms and revision rates of modular endoprosthetic reconstruction after wide resection of osteosarcoma by conventional survival analysis; and (2) to compare the results with a competing risk model to investigate altered outcomes. We theorized that endoprosthetic survival rates would be considerably reduced by including death as a competing event.
Patients and Methods
Patients
From 1981 to 2009, we surgically treated 570 cases of osteosarcoma by wide resection. Two hundred forty-seven patients (43%) also have received modular endoprosthetic reconstruction including 143 male (58%) and 104 female (42%) patients with a mean age of 22 years (range, 7–73 years; median, 18 years). The minimum followup was 1 month (average, 86 months; range, 1–143 months); 73 patients (30%) had a followup of less than 2 years because of death resulting from tumor or the result of any complication of Types 1 to 5. No patients were lost to followup. All patients were included in statistical analysis.
Tumors were located in the femur (146 [59%]), tibia (76 [31%]), and humerus (25 [10%]), including 222 (90%) conventional osteosarcomas and 25 (10%) other subtypes with metastases at diagnosis detected in 34 (14%). There were 110 (45%) reconstructions of the distal femur, 45 (18%) of the proximal tibia, 31 (13%) combined, and 61 (25%) others (Table 1).
Table 1.
Demographic factors of the patients in the present study
| Characteristics | Number |
|---|---|
| Number of patients (male/female) | 247 (143/104) |
| Age (years; mean; range) | 22; 7–73 |
| Followup (months; mean; range) | 86; 1–143 |
| Site | |
| Proximal humerus | 18 |
| Distal humerus | 3 |
| Total humerus | 4 |
| Proximal femur | 21 |
| Distal femur | 110 |
| Total femur | 15 |
| Distal femur and proximal tibia | 31 |
| Proximal tibia | 45 |
| Implants | |
| Kotz Modular Femur Tibia Reconstruction System (KMFTR®; Stryker-Howmedica Inc, Mahwah, NJ, USA) | 26 |
| Howmedica Modular Resection System (HMRS; Howmedica GmBH, Kiel, Germany) | 188 |
| Global Modular Replacement System (GMRS; Stryker GmbH & Co KG, Duisburg, Germany) | 33 |
| Method of fixation | |
| Uncemented | 236 |
| Cemented | 11 |
Two hundred thirty-nine patients received chemotherapy, 206 according to the Cooperative OsteoSarcoma Study Group (COSS) regimen and 23 according to the European and American Osteosarcoma Study Group (EURAMOS 1) regimen [13, 21].
Implants
Modular endoprosthetic devices were used in all patients. These included 26 Kotz Modular Femur Tibia Reconstruction System (KMFTR®; Stryker-Howmedica Inc, Mahwah, NJ, USA), 188 Howmedica Modular Resection System (HMRS; Howmedica GmBH, Kiel, Germany), and 33 Global Modular Replacement System (GMRS, Stryker GmbH & Co KG, Duisburg, Germany). The major differences are represented by continuously adapted stem designs and the modular rotating hinge mechanism of the more recent generations that replaced the fixed hinge mechanism of the older devices for replacement of the knee [6, 11, 14, 15, 18]. In all but 11 implants, cementless fixation was used. For reconstruction of the knee, 161 fixed hinge and 39 rotating hinge implants were used. In 48 patients, the ligament advanced reconstruction system (LARS® JK Orthomedic Ltd, Quebec, Canada) was used for soft tissue reconstruction (Table 1) [5].
Failure Mode
The failure modes of the modular endoprostheses were classified according to the ISOLS classification system distinguishing five different types of failure. Type 1 represents soft tissue failures (eg, rupture of the patella ligament), Type 2 is aseptic loosening, Type 3 includes structural failures (eg, implant breakage, periprosthetic fracture), Type 4 is infection, and Type 5 local tumor progression. Types 1 to 3 can be summarized as mechanical failure, whereas Types 4 and 5 are nonmechanical failure modes [9].
Oncologic Outcomes
Sixty-four patients (25.9%) died throughout followup. The 5-year and 10-year overall survival rates were 72% and 70%, respectively. Metastasis-free survival at 5 and 10 years in patients with no metastasis detected at diagnosis was 70% and 69%, respectively. Six patients (2%) developed local recurrence.
Complications
Complications occurred in a total of 122 patients (49%). Sixty-seven patients (27%) required multiple revision surgeries for repetitive complications ranging from two to 12. Seven patients (3%) had to undergo amputation as a consequence of complications. Consequently, the overall limb salvage rate was 97%.
The majority of complications that required revision surgery were structural failures (Type 3) (n = 64 [26%]) followed by infection (Type 4) (n = 49 [20%]), soft tissue complications (Type 1) (n = 38 [15%]), aseptic loosening (Type 2) (n = 64 [26%]), and local recurrence (Type 5) (n = 6 [2%]). Infection (Type 4) occurred in 13% as a primary complication and in an additional 7%, it occurred after revision surgery.
Statistical Considerations
Statistical analysis of the data focused on survival of the implant and oncological survival, respectively. Investigation endpoints were progression of disease, dead of disease, and revision resulting from any cause. Descriptive summary statistics included means and frequencies. The number of complications was assessed as well as the aforementioned types of complications. Survivorship analysis included Kaplan-Meier estimations. Additionally, competing risk analysis was carried out with death as a competing event. We compared survivorship of both analytic methods with respect to the different types of complications. The difference between the analyses was expressed as absolute reduction of risk for the respective complication expressed as percentage. The level of statistical significance level was 0.05; however, it is important to note that there is no mechanism of which we are aware to statistically compare the two statistical approaches in question here (Kaplan-Meier survivorship and competing risk analysis). Accordingly, we present raw survivorship data for both approaches. All calculations were made with SPSS (Version 13.0, 2004; SPSS Inc, Chicago, IL, USA) and R, an open-source statistical software project under the GNU (www.gnu.org) General Public License.
Results
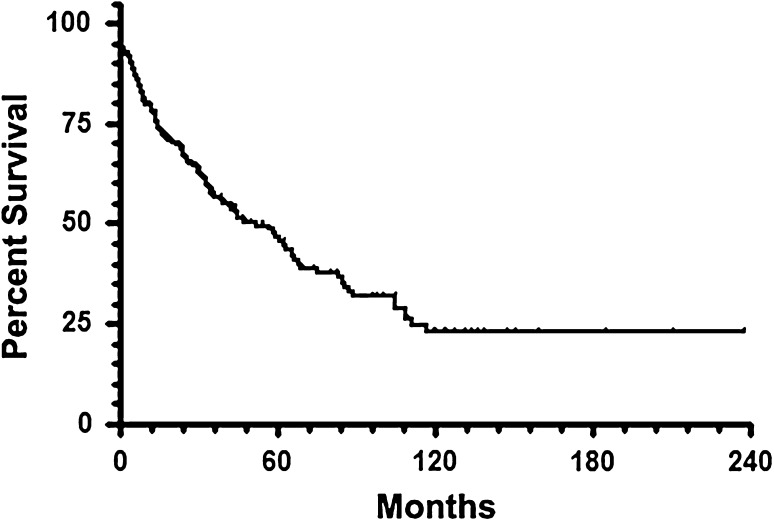
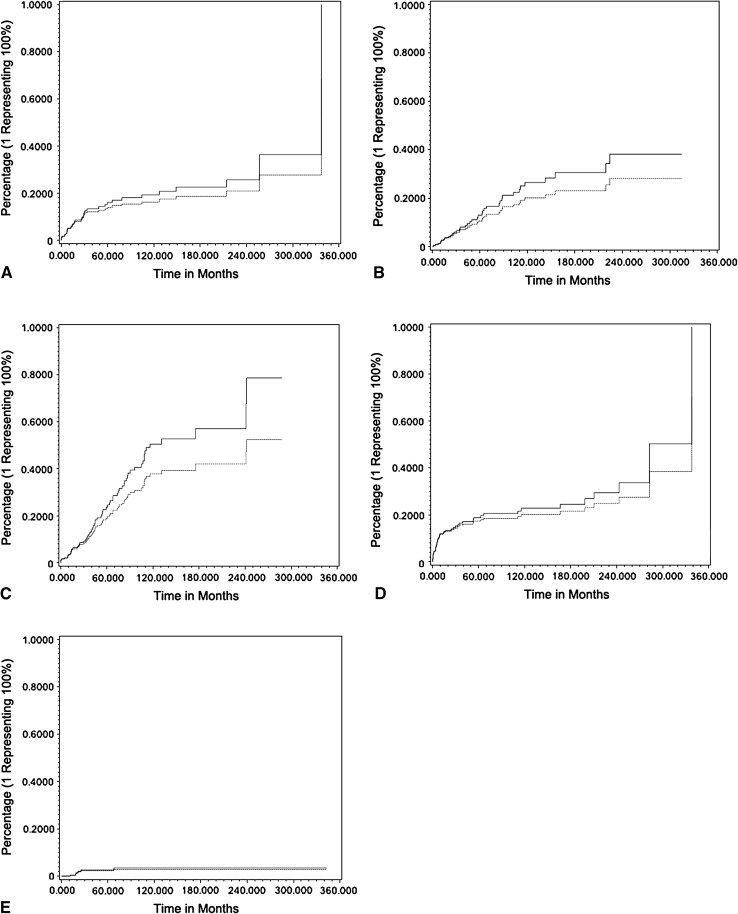
Competing risk analysis consistently resulted in reduced estimates of the frequency of complications and reconstructive failures. Kaplan-Meier estimation revealed a median revision-free survival (RFS) of 52 months. At 5 years, RFS was 47% and decreased to 23% at 10 years and stayed the same at 15 years (Fig. 1). With respect to type of complication, 10-year RFS was 96% for local recurrence (Type 5), 81% for soft tissue failure (Type 1), 77% for infection (Type 3), 74% for aseptic loosening (Type 2), and 49% for structural failure (Type 3) (Fig. 2).
Fig. 1.
Endoprosthetic revision-free survival of 247 patients with resection of extremity osteosarcoma and modular endoprosthetic reconstruction is shown.
Fig. 2A–E.
Cumulative risk of Types 1 to 5 (A–E) complications over time was assessed by Kaplan-Meier estimation (solid line) and competing risk analysis (dotted line).
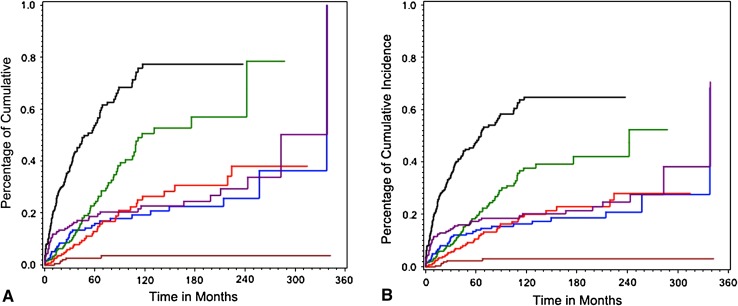
The overall risk of any complication after 10 years was 77% according to Kaplan-Meier estimation and 65% by cumulative risk analysis. This represents an absolute risk reduction of 12% when considering death of disease as a competing risk factor. The probability of occurrence of soft tissue failure (Type 1) was 19% at 10 years and 26% at 20 years according to Kaplan-Meier estimation. Cumulative risk analysis revealed a probability of 16% and 21%, respectively, for this type of complication. The difference between the assessment methods was 3% and 5%, respectively; as noted earlier, differences between these statistical approaches could not themselves be statistically compared, and so small differences like this need to be considered carefully. For aseptic loosening (Type 2), there is a risk of 26% and 38%, respectively, according to Kaplan-Meier estimation. Cumulative risk analysis represented 20% and 28%, respectively, leading to a difference of 6% and 10%. Structural failure (Type 3) probability was estimated with 51% at 10 years and 57% at 20 years according to Kaplan-Meier estimation. There was a probability of 38% and 42%, respectively, when cumulative risk analysis was performed. For Type 3 complications, the difference between both assessment methods reached 13% and 15%, respectively. The probability of occurrence of infection (Type 4) was 23% at 10 years and 29% at 20 years according to Kaplan-Meier estimation. Cumulative risk analysis revealed 20% and 24%, respectively, resulting in a difference of 3% and 4%. Survival analysis showed 4% probability for occurrence of local recurrence (Type 5) for Kaplan-Meier estimation and 3% for cumulative risk analysis irrespective of postoperative interval (10 or 20 years) leading to a difference of 1% (Figs. 2, 3).
Fig. 3A–B.
Cumulative risk of any complication was assessed by Kaplan-Meier estimation (A) and competing risk analysis (B). The black line indicates the first complication and the colored lines represent the following types of complication: Type 1 (blue), Type 2 (red), Type 3 (green), Type 4 (purple), and Type 5 (brown).
Discussion
Many musculoskeletal malignancies behave aggressively and often result in the death of patients who experience them. Because of this, death from disease is a potent competing risk factor to consider when evaluating endoprosthetic durability and other clinical endpoints apart from death in patients with malignant bone tumors. Because Kaplan-Meier estimation does not take into account competing events, the clinical scenario of a highly malignant bone tumor and a surgical therapy with a considerable revision rate will lead to an overestimation of revision risk when Kaplan-Meier estimation is performed. However, the degree to which this is the case for a common, important indication—osteosarcoma reconstruction—has not been well characterized. Therefore, in the present study, we compared conventional Kaplan-Meier estimation and a competing risk analysis of endoprosthetic survival in 247 patients with osteosarcoma at a single center. Competing risk analysis consistently resulted in reduced estimates of the frequency of complications and reconstructive failures.
There are several limitations to this investigation. First, the retrospective nature and the single-center design are considerable limitations associated with the present study. A followup of less than 2 years in some patients still will capture all deaths but will tend to deemphasize oncologic failures relative to deaths. Furthermore, we do are not able to provide deeper insight into other factors or covariates that might be related to endoprosthetic failure such as implant design, cementation technique, joint anatomy, body weight, activity level, and many others, therefore representing a potential bias of failure modes. Still, we feel that our population forms a homogenous and strongly representative group of patients with endoprosthetic reconstruction after osteosarcoma. In our model, we did not include covariates influencing survival by use of a Cox regression model. This model would remain valid even in the presence of competition; however, the influence of covariates in a competing risk model is no longer regarded as easily interpretable [2]. Despite the application of a competing risk analysis, the outcomes still represent an estimation based on a relatively large study cohort, which might be valuable to take into account when counselling individual patients. Finally, because we have applied two different ways of calculating survival estimations, we can indicate an absolute difference between the survival functions; however, we cannot provide a mathematical comparison of the functions (like, for example, in the case of comparing two Kaplan-Meier estimations by use of a log-rank test). Therefore, it is not possible to identify statistically differences but to identify potential differences.
In the assessment of survival of endoprosthetic devices, one is interested in the time to the occurrence of a particular event such as the death of a patient, the recurrence of a cancer, or the revision of an implant. Until recently, such data have been generically referred to as survival (or survivorship) data, and survival analysis refers to the analysis of these data in the form of delays from a time origin to the occurrence of an event of interest [17]. However, for many patients, the event is not observed during the course of the study and their observations, deprived of a survival time, are said to be censored. These censored observations are particular to survival data and require specific methods for estimation [2]. One of the assumptions underlying the Kaplan-Meier estimator is that patients whose observations are censored such as those of patients who have not been revised and die during followup have the same risk of occurrence of the event (such as reconstructive failure) as patients remaining alive and in the study. Plainly, in orthopaedic oncology research, this assumption is violated with considerable frequency (patients who have died are no longer at risk of reconstructive failure), and it is this issue that drives the concern we raise in the present report.
Although death from oncological disease will withdraw patients from the risk pool of living patients subject to certain complications (such as endoprosthetic failure), and so make the Kaplan-Meier estimator inappropriate for use in this setting, we still need to be able to counsel our patients with realistic estimates of likelihoods of particular outcomes for those who do survive. The competing risk model provides this approach, and our results provide further or even more realistic information about endoprosthetic failures in musculoskeletal oncology. Although in this context competing risk analysis is a well-established tool and may not require a further proof of principle, we feel that these considerations do provide new information that could be helpful to doctors and patients alike in different clinical scenarios. For example, in primary malignant bone tumors, we find high endoprosthetic revision rates in patients who survive their disease. The RFS of modular endoprosthetic reconstruction in our patients with osteosarcoma has continuously dropped over time to a 15-year RFS of only 23% in Kaplan-Meier analysis, suggesting that the vast majority of osteosarcoma long-term survivors will live long enough to experience a revision of their implant. These rates, however, drop considerably when analyzed by a competing risk model, from 77% to 65% in the present series. This seems essential when it comes to informing patients about the expected outcomes of their complex treatment or the evaluation of performance of different endoprosthetic designs or implantation techniques. A competing risks approach is important also to compare data from the sarcoma reconstruction literature with that of the general arthroplasty literature. Since their widespread introduction in the late 1980s, modular endoprosthetic reconstructions have not provided comparable long-term results to endoprostheses used for the treatment of degenerative joint disease. Because tumor reconstructions are exposed to competing risks (such as death or tumor recurrence resulting from the underlying oncologic disease), the RFS of these endoprostheses must be described by a competing risk model to be compared with survival of standard endoprostheses. To our knowledge, the present study offers the first data set for osteosarcoma that addresses this question in this way. Finally, patients with advanced metastatic bone disease often have a poor prognosis, sometimes measured in months, and so any treatment approach should seek to help these patients avoid further revisions. Insofar as many of these patients may die of disease shortly after surgery, it is especially important to analyze their reconstructive results using a competing risks model.
In this series, the observed failure rates as estimated by competing risks modeling were consistently lower for all five types of endoprosthetic failure than those estimated using Kaplan-Meier survivorship. In fact, the cumulative risk of requiring revision surgery after 10 years was 77% when assessment was performed with Kaplan-Meier estimation and 65% when competing risk analysis was performed. This represents a difference of 12%. With respect to the different types of complications, the difference between the two aforementioned methods ranged from 3% to 15%. This is consistent with the work of Biau et al. [1, 3], who demonstrated a 67% risk of revision at 15 years in 91 patients who underwent TKA after tumor resection when Kaplan-Meier analysis had been performed. In contrast, with the cumulative incidence function, they found a 47% risk of revision. In their study, the Kaplan-Meier method overestimated the risk of revision by 43% at 15 years.
In conclusion, the results of the present study indicate that competing risk analysis considerably reduces the estimated risk for all types of endoprosthetic complications in patients with osteosarcoma compared with Kaplan-Meier analysis, and we believe that these lower failure rates better reflect reality resulting from the high competing risk of death of disease. Therefore, competing risk may work as a more realistic description of endoprosthetic survival in oncologic patients. Despite these observations, this statistical model will do nothing to reduce actual complication rates, but competing risk analysis may provide a more appropriate tool than Kaplan-Meier survivorship for estimating survival in patient populations at high risk for mortality in the months or years after surgical treatments.
Footnotes
Each author certifies that he or she, or a member of his or her immediate family, has no funding or commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research ® editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research ® neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA-approval status, of any drug or device prior to clinical use.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
References
- 1.Biau D, Faure F, Katsahian S, Jeanrot C, Tomeno B, Anract P. Survival of total knee replacement with a megaprosthesis after bone tumor resection. J Bone Joint Surg Am. 2006;88:1285–1293. doi: 10.2106/JBJS.E.00553. [DOI] [PubMed] [Google Scholar]
- 2.Biau DJ, Hamadouche M. Estimating implant survival in the presence of competing risks. Int Orthop. 2011;35:151–155. doi: 10.1007/s00264-010-1097-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biau DJ, Latouche A, Porcher R. Competing events influence estimated survival probability: when is Kaplan-Meier analysis appropriate? Clin Orthop Relat Res. 2007;462:229–233. doi: 10.1097/BLO.0b013e3180986753. [DOI] [PubMed] [Google Scholar]
- 4.Bielack SS, Kempf-Bielack B, Delling G, Exner GU, Flege S, Helmke K, Kotz R, Salzer-Kuntschik M, Werner M, Winkelmann W, Zoubek A, Jurgens H, Winkler K. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: an analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J Clin Oncol. 2002;20:776–790. doi: 10.1200/JCO.20.3.776. [DOI] [PubMed] [Google Scholar]
- 5.Dominkus M, Sabeti M, Toma C, Abdolvahab F, Trieb K, Kotz RI. Reconstructing the extensor apparatus with a new polyester ligament. Clin Orthop Relat Res. 2006;453:328–334. doi: 10.1097/01.blo.0000229368.42738.b6. [DOI] [PubMed] [Google Scholar]
- 6.Funovics PT, Schuh R, Adams SB, Jr, Sabeti-Aschraf M, Dominkus M, Kotz RI. Modular prosthetic reconstruction of major bone defects of the distal end of the humerus. J Bone Joint Surg Am. 2011;93:1064–1074. doi: 10.2106/JBJS.J.00239. [DOI] [PubMed] [Google Scholar]
- 7.Gosheger G, Gebert C, Ahrens H, Streitbuerger A, Winkelmann W, Hardes J. Endoprosthetic reconstruction in 250 patients with sarcoma. Clin Orthop Relat Res. 2006;450:164–171. doi: 10.1097/01.blo.0000223978.36831.39. [DOI] [PubMed] [Google Scholar]
- 8.Hamadouche M, Boutin P, Daussange J, Bolander ME, Sedel L. Alumina-on-alumina total hip arthroplasty: a minimum 18.5-year follow-up study. J Bone Joint Surg Am. 2002;84:69–77. [PubMed] [Google Scholar]
- 9.Henderson ER, Groundland JS, Pala E, Dennis JA, Wooten R, Cheong D, Windhager R, Kotz RI, Mercuri M, Funovics PT, Hornicek FJ, Temple HT, Ruggieri P, Letson GD. Failure mode classification for tumor endoprostheses: retrospective review of five institutions and a literature review. J Bone Joint Surg Am. 2011;93:418–429. doi: 10.2106/JBJS.J.00834. [DOI] [PubMed] [Google Scholar]
- 10.Ilyas I, Pant R, Kurar A, Moreau PG, Younge DA. Modular megaprosthesis for proximal femoral tumors. Int Orthop. 2002;26:170–173. doi: 10.1007/s00264-002-0335-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kotz R. [Tumor endoprosthesis in malignant bone tumors] [in German] Der Orthopade. 1993;22:160–166. [PubMed] [Google Scholar]
- 12.Lazo-Langner A, Rodger MA, Barrowman NJ, Ramsay T, Wells PS, Coyle DA. Comparing multiple competing interventions in the absence of randomized trials using clinical risk-benefit analysis. BMC Med Res Methodol. 2012;12:3. doi: 10.1186/1471-2288-12-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marina N, Bielack S, Whelan J, Smeland S, Krailo M, Sydes MR, Butterfass-Bahloul T, Calaminus G, Bernstein M. International collaboration is feasible in trials for rare conditions: the EURAMOS experience. Cancer Treat Res. 2009;152:339–353. doi: 10.1007/978-1-4419-0284-9_18. [DOI] [PubMed] [Google Scholar]
- 14.Mittermayer F, Krepler P, Dominkus M, Schwameis E, Sluga M, Heinzl H, Kotz R. Long-term followup of uncemented tumor endoprostheses for the lower extremity. Clin Orthop Relat Res. 2001;388:167–177. doi: 10.1097/00003086-200107000-00024. [DOI] [PubMed] [Google Scholar]
- 15.Mittermayer F, Windhager R, Dominkus M, Krepler P, Schwameis E, Sluga M, Kotz R, Strasser G. Revision of the Kotz type of tumour endoprosthesis for the lower limb. J Bone Joint Surg Br. 2002;84:401–406. doi: 10.1302/0301-620X.84B3.12204. [DOI] [PubMed] [Google Scholar]
- 16.Putter H, Fiocco M, Geskus RB. Tutorial in biostatistics: competing risks and multi-state models. Stat Med. 2007;26:2389–2430. doi: 10.1002/sim.2712. [DOI] [PubMed] [Google Scholar]
- 17.Ranstam J, Robertsson O. Statistical analysis of arthroplasty register data. Acta Orthop. 2010;81:10–14. doi: 10.3109/17453671003587168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ritschl P, Capanna R, Helwig U, Campanacci M, Kotz R. [KMFTR (Kotz Modular Femur Tibia Reconstruction System) modular tumor endoprosthesis system for the lower extremity] [in German] Z Orthop Ihre Grenzgeb. 1992;130:290–293. doi: 10.1055/s-2008-1039620. [DOI] [PubMed] [Google Scholar]
- 19.Schwartz AJ, Kabo JM, Eilber FC, Eilber FR, Eckardt JJ. Cemented distal femoral endoprostheses for musculoskeletal tumor: improved survival of modular versus custom implants. Clin Orthop Relat Res. 2010;468:2198–2210. doi: 10.1007/s11999-009-1197-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scrucca L, Santucci A, Aversa F. Competing risk analysis using R: an easy guide for clinicians. Bone Marrow Transplant. 2007;40:381–387. doi: 10.1038/sj.bmt.1705727. [DOI] [PubMed] [Google Scholar]
- 21.Winkler K, Bielack SS, Delling G, Jurgens H, Kotz R, Salzer-Kuntschik M. Treatment of osteosarcoma: experience of the Cooperative Osteosarcoma Study Group (COSS) Cancer Treat Res. 1993;62:269–277. doi: 10.1007/978-1-4615-3518-8_32. [DOI] [PubMed] [Google Scholar]