Abstract
Background
The majority of published functional outcome data for tumor megaprostheses comes in the form of subjective functional outcome scores. Sparse objective data exist demonstrating functional results, activity levels, and efficiency of gait after endoprosthetic reconstruction in patients treated for orthopaedic tumors. Patients embarking on massive surgical operations, often in the setting of debilitating medical therapies, face mortality and a myriad of unknowns. Objective functional outcomes provide patients with reasonable expectations and a means to envision life after treatment. Objective outcomes also provide a means for surgeons to compare techniques, rehabilitation protocols, and implants.
Questions/purposes
We asked the following questions: (1) What is the efficiency of gait (ie, oxygen consumption) at final recovery from endoprosthetic reconstruction for oncologic resections? (2) What is the knee strength after lower extremity endoprosthetic reconstruction as compared with the contralateral limb? (3) How active are patients with tumor megaprostheses at home and in the community?
Methods
Sixty-nine patients with endoprosthetic reconstructions for primary lower extremity bone sarcoma met inclusion criteria and were invited by mailing to undergo oxygen cost study and strength testing. Twenty-four patients (seven proximal femoral replacements, nine distal femoral replacements, and eight proximal tibia replacements) underwent evaluation in the gait laboratory at a mean of 13.2 years after their reconstruction. All patients were then asked to wear step activity monitors at home and in the community for 7 consecutive days.
Results
Median O2 consumption (in mL/kg/m) among the endoprothesis groups was not different from the control patients with the numbers available (proximal femoral replacement 0.17, distal femoral replacement 0.16, proximal tibia replacement 0.18, control 0.15, p = 0.21). With the numbers available, there was no difference in walking speed as compared with the control group (proximal femoral replacement 1.20 m/s, distal femoral replacement 1.27 m/s, proximal tibia replacement 1.12 m/s, control 1.27 m/s, p = 0.08). Patients with proximal tibia replacements had reduced knee extension and flexion strength compared with patients in other reconstruction groups (84% reduction in extension versus those with proximal femoral replacements, 35%, and distal femoral replacement, 53%, p = 0.001, and 43% reduction in flexion versus proximal femoral replacement, 11%, distal femoral replacement, 2%, p = 0.006). With the numbers available, mean strides per day were not different among the reconstruction groups (proximal femoral replacement = 4709 strides/day [3094–6696], distal femoral replacement = 2854 [2461–6015], and proximal tibia replacement = 4411 [3093–6215], p = 0.53).
Conclusions
Although knee strength was reduced in patients with proximal tibia replacements compared with femoral reconstructions, all groups had an efficient gait and were active at home and in the community at a mean of 13.2 years after surgery. Despite the magnitude of these surgeries, these patients are similarly active as patients after standard total hip arthroplasty. These findings provide objective data from which patients undergoing tumor megaprosthesis reconstructions of the lower extremity can reasonably base expectations of efficient gait and active lifestyles outside of the hospital setting. These data may provide hope and long-term goals for patients facing the uncertainty of chemotherapy and surgical treatment.
Level of Evidence
Level III, therapeutic study. See Instructions for Authors for a complete description of levels of evidence.
Introduction
Endoprosthetic reconstruction after resection of bone tumors is an established and widely used technique for obtaining limb salvage. Functional outcomes were of secondary importance in the early experience with limb salvage because patients with malignant disease had extremely poor survival rates [8, 11, 12]. With improvements in medical therapies, long-term patient survival is often the expectation and, thus, the longevity and functional outcomes of reconstructions are becoming a more important consideration. Many studies have assessed endoprosthetic functional outcomes after reconstructions [7, 16, 17] and most used subjective assessments such as the Musculoskeletal Tumor Society (MSTS) score [6].
Several studies have gone further by using more objective measurements such as gait analysis to evaluate reconstruction outcomes. Kawai et al. [9, 10] reported gait analysis data for patients with distal femoral replacements and those with proximal femoral replacements in two separate studies, attempting to correlate functional outcome with specific anatomic units resected. Cammisa et al. [4] described a cohort of distal femoral replacement endoprosthetic reconstructions and compared them with patients undergoing rotationplasty. Benedetti et al. [2] reported gait analysis data for patients who underwent distal femoral replacement to attempt to correlate amount of quadriceps removed to functional outcomes. These studies showed average increased oxygen consumption of 30% to 45% and 10% to 20% decrease in gait velocity in patients with a respective endoprosthesis as compared with control subjects [2, 4, 9, 10]. However, with no minimum followup exclusion criteria, no patients with proximal tibia replacements, nor the implementation of pedometers or other wireless activity tracking devices to assess “real-world” function, a question is raised of to whom and at what time point these findings could be applied.
Objective assessments of long-term functional outcomes for endoprosthetic reconstructions therefore remain lacking. Although several small reports exist focusing on a specific endoprosthetic reconstruction, no study has reviewed objective functional outcomes of lower extremity megaprosthesis reconstructions from multiple anatomic locations, showing relative efficiency of gait and muscle strength among reconstruction groups at a mean followup of more than 13 years. Step activity monitors can provide additional insight into activity level among patients in the community and home environment: “real-world” data of how functional our patients truly are. These objective functional data will be of value to provide a framework from which patients can generate reasonable expectations for function once recovery is complete. This serves both to provide the patient with meaningful expectations for function as well as a concrete, tangible window into “life beyond cancer.” Additionally, objective functional data points are comparable across reconstruction techniques and among different orthopaedic oncology centers. As more centers adopt objective outcome metrics, surgeons will have an ability to more critically assess their own outcomes in an attempt to improve technique.
Therefore, we sought to determine: (1) What is the efficiency of gait at final recovery from endoprosthetic reconstruction for oncologic resections? (2) What is the knee strength after lower extremity endoprosthetic reconstruction as compared with the contralateral limb? (3) How active are patients with tumor megaprostheses at home and in the community?
Patients and Methods
Study Design and Setting
After institutional review board approval, patients who had undergone endoprosthetic reconstruction of the lower extremity for primary bone sarcoma were retrospectively identified and invited to return to UCLA for an O2 cost study and strength evaluation. In addition, they were asked to wear a commercially available pedometer for 1 week (a minimum of 3 weekdays and 2 weekend days) to assess home and community walking activity. All invited patients had undergone endoprosthetic reconstructions for primary bone sarcomas, received chemotherapy, and underwent wide resection. All patients were treated by a single surgeon (JJE) with a consistent surgical technique: proximal femoral replacements were performed with a lateral approach and no attempt to secure the greater trochanter to the implant was made, distal femoral replacements were performed with a medial approach, and proximal tibia replacements were performed with an extended medial approach and medial gastrocnemius flap coverage. All reconstructions included resurfacing of the patella. The extent of muscle resection was contingent on tumor size. All implants were Howmedica, Techmedica, or Stryker (Kalamazoo, MI, USA) implants with rotating hinge knee components and cemented stems.
Participants/Study Subjects
Patients with lower extremity endoprosthetic reconstructions were identified from our institutional sarcoma database (n = 487). Exclusion criteria were (1) revision surgery in which the original implant was removed; (2) endoprosthetic reconstruction for a nononcologic diagnosis; (3) original placement of an expandable prosthesis; (4) age younger than 18 years; (5) and followup less than 2 years after limb salvage. Recruitment letters were not sent to those confirmed as dead, lost to followup, or residing outside of the state of California, because expenses and travel time for analysis were expected to be onerous. Sixty-nine patients were thus identified to be eligible for inclusion and were invited to participate by mailing. Additional recruitment was by flyers placed in outpatient clinics. Twenty-four patients (seven with proximal femoral replacements, nine with distal femoral replacements, and eight with proximal tibia replacements) underwent analysis. Normative data from healthy adults, collected in the same testing environment and under the same conditions served as a control population (n = 8). No attempt was made to match this cohort to the endoprosthesis groups.
Description of Experiment, Treatment, or Surgery
On arrival to the gait laboratory, each patient underwent the MSTS score survey administered by a physical therapist (MG, EGF) [6]. Energy consumption testing was performed using a portable breath-by-breath pulmonary gas exchange unit (K4 b2; Cosmed, Rome, Italy). Baseline resting data were collected for 10 minutes. Overground walking data were collected at a self-selected speed for a minimum of 6 minutes and continued until steady-state had been maintained for at least 4 minutes. Walking took place on a 58-m oval pathway to minimize accelerations and decelerations. Steady-state oxygen consumption data were normalized to body weight and divided by walking speed to obtain O2 cost (oxygen consumed per kilogram body weight per meter walked).
Isokinetic strength testing of the knee extensors and flexors was performed using an isokinetic dynamometer (System 4 Pro; Biodex, Shirley, NY, USA) at 60°/s. The nonsurgical limb was tested first for all participants. Data were normalized to body weight and percent deficits were expressed relative to the nonsurgical limb.
Patients were asked to wear a Stepwatch Activity Monitor (Orthocare Innovations, Oklahoma City, OK, USA) above the ankle of one limb for 1 week after gait laboratory testing to assess mean number of strides/day. Data were included for analysis if the patient recorded data for at least 3 weekdays and 2 weekend days. Patients were provided with a postage paid envelope to return the monitor to the laboratory.
Variables, Outcome Measures, Data Sources, and Bias
Oxygen consumption and strength data were collected during a single visit to the gait laboratory. Parking was provided for study participation but no other compensation was provided. Step watch data were collected for a 1-week period after the gait laboratory visit. Attempts to control for selection bias were made by mailing recruitment letters to all patients with endoprosthetic reconstructions for primary bone sarcomas who met inclusion criteria. Attempts to decrease observer bias were made by having a physical therapist, rather than the operating surgeon, collect all data including the completion of the MSTS evaluation.
Statistical Analysis and Study Size
All data were initially evaluated for normality. Because the data did not approximate a Gaussian distribution (validated by the Shapiro-Wilk test and visual inspection), nonparametric statistical tests were deemed appropriate. Kruskal-Wallis tests to compare outcome measures across groups were performed with post hoc analysis using Wilcoxon rank-sum test. Comparative statistics were performed using a commercially available statistics program (JMP Prov10 Statistical Software Package; SAS Institute, Cary, NC, USA). A post hoc power analysis was performed using bootstrap resampling methods (R Project for Statistical Computing, Vienna, Austria) with alpha set to 0.05 and beta set to 0.2. This indicated that our current sample size of eight per group would have 70% power to detect a 25% increase in O2 cost and 97.5% power to detect a 30% increase in O2 cost.
Demographics and Description of the Study Population
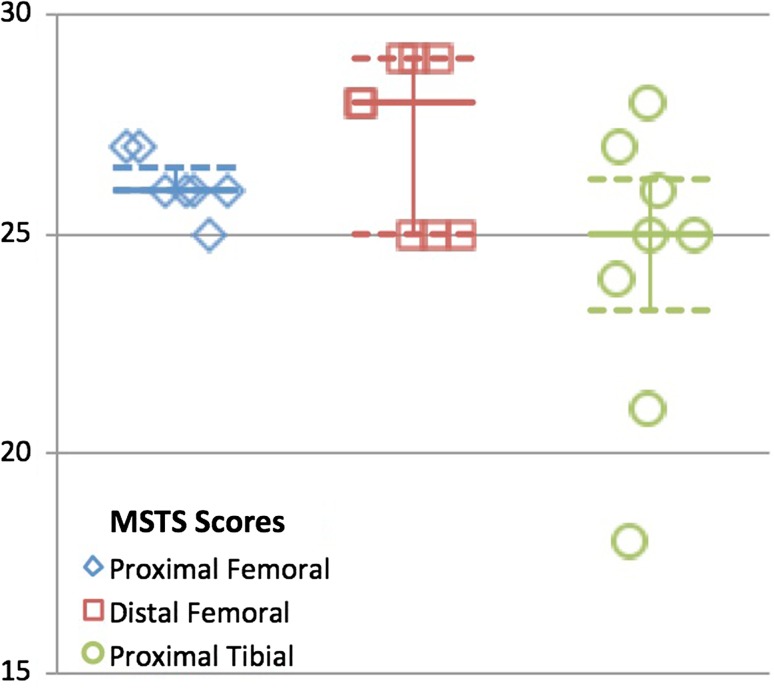
The mean age for all patients in this study was 37.0 years old (range, 18.3–63.6 years). The mean time from surgery to gait analysis was 13.2 years (range, 2.5–28.2 years). For patients with proximal femoral replacements, the mean time since surgery was 12.4 years (range, 3.6–21.6 years), for patients with distal femoral replacements, the mean was 12.0 years (range, 2.7–28.2 years), and for patients with proximal tibia replacements, the mean was 15.3 years (range, 2.5–26.3 years) (Table 1). All patients ambulated without walking aids and MSTS scores reflect overall good function: proximal femoral replacements = 26.1 (range, 25–27), distal femoral replacements = 27.3 (range, 25–29), and proximal tibia replacements = 24.3 (range, 18–28) (Fig. 1).
Table 1.
Patient demographics
| Demographic | Proximal femoral replacements (N = 7) | Distal femoral replacements (N = 8) | Proximal tibia replacements (N = 7) |
|---|---|---|---|
| Current age (years) | 42.4 (25.0–63.0) | 35.2 (20.0–56.0) | 32.0 (18.0–42.0) |
| Time since surgery (years) | 12.4 (3.6–21.6) | 12.0 (2.7–28.2) | 15.3 (2.5–26.3) |
| Sex (percent male) | 29% | 44% | 38% |
| Height (cm) | 169 (163–180) | 170 (158–180) | 167 (156–174) |
| Weight (kg) | 67.2 (55.9–84.0) | 73.6 (49.9–96.8) | 78.5 (62.3–96.4) |
| BMI (kg/m2) | 23.4 (20.3–29.1) | 25.2 (20.1–30.6) | 27.9 (23.8–35.0) |
Values are means with ranges in parentheses; BMI = body mass index.
Fig. 1.
Graph demonstrating MSTS scores for each reconstruction group. Median is identified with the solid line and 25th and 75th percentiles are identified with the dashed line.
Accounting for All Patients and Study Subjects
Letters inviting patients to participate in the study were sent to all patients who met the eligibility criteria (N = 69). Twenty-four patients (seven with proximal femoral replacements, nine with distal femoral replacements, and eight with proximal tibia replacements) volunteered and signed an informed consent. Although all 24 patients were offered StepWatch Activity Monitors™ to wear at home, 18 patients (five proximal femoral replacements, six distal femoral replacements, and seven proximal tibia replacements) provided sufficient data to be included in this portion of the study, prospectively defined as readings from at least 3 weekday and 2 weekend day time intervals.
Results
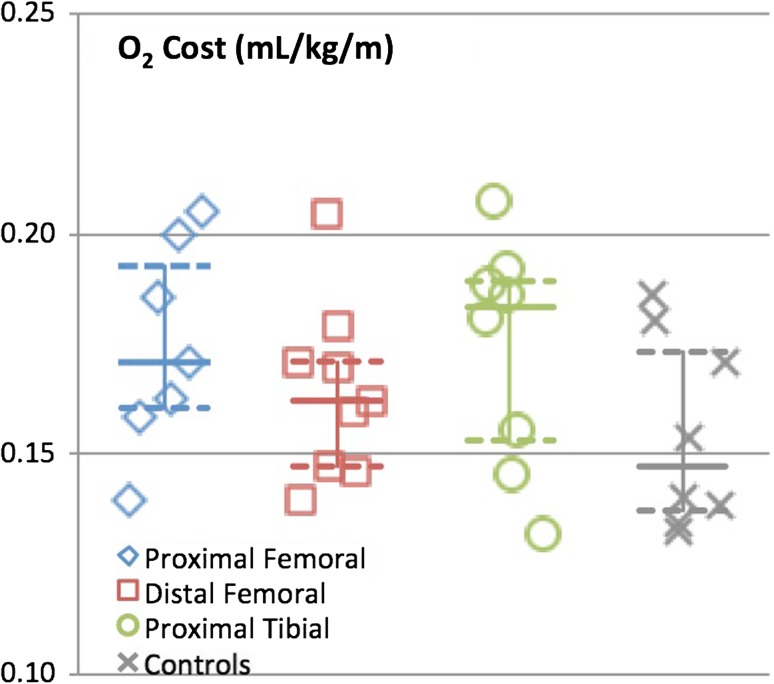
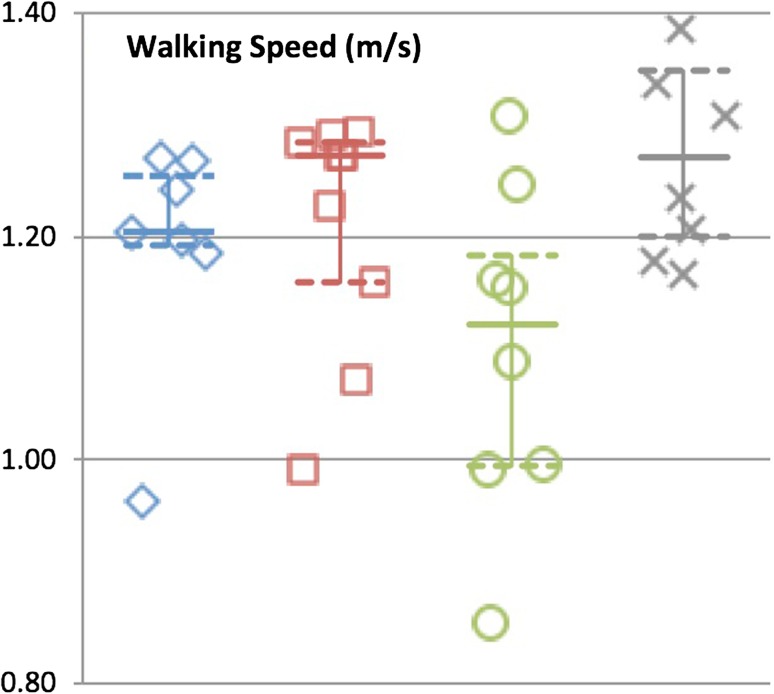
Median O2 cost during gait was 0.17 mL/kg/m (0.16–0.20), 0.16 mL/kg/m (0.15–0.18), and 0.18 mL/kg/m (0.15–0.19) for patients with proximal femoral replacements, distal femoral replacements, and proximal tibia replacements, respectively. With the numbers available, there was no difference between any of the groups and controls (0.15 mL/kg/m [0.14–0.18], p = 0.21) (Fig. 2). Median gait speeds were 1.20 m/s (1.19–1.27), 1.27 (1.12–1.29), and 1.12 (0.99–1.23) for patients with proximal femoral replacements, distal femoral replacements, and proximal tibia replacements, respectively. With the numbers available, there was no difference between any of the groups and the controls (1.27 [1.19–1.38], p = 0.08) (Fig. 3).
Fig. 2.
Graph demonstrating oxygen consumption for each reconstruction group and control subjects. Median is identified with the solid line and 25th and 75th percentiles are identified with the dashed line.
Fig. 3.
Graph demonstrating gait velocity for each reconstruction group and control subjects. Median is identified with the solid line and 25th and 75th percentiles are identified with the dashed line.
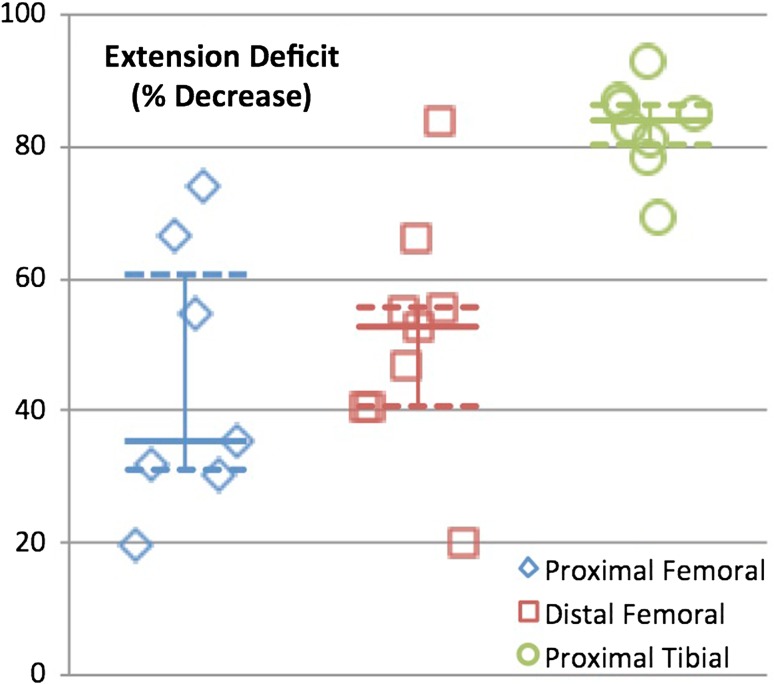
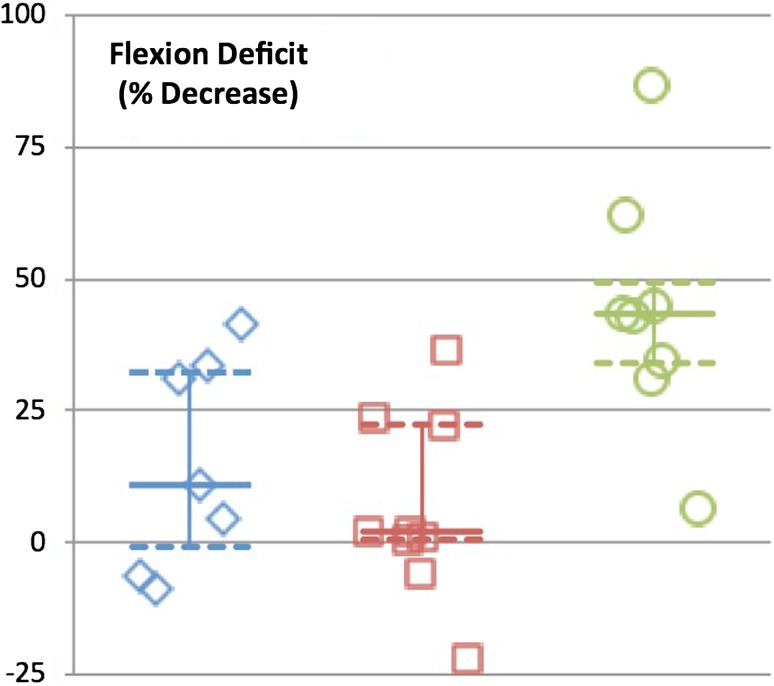
Strength deficits about the knee were expressed as percent deficits compared with the contralateral limb. Extension deficits were noted in all groups but were significantly greater in the proximal tibia group (proximal femoral replacements = 35.4% [30–66], distal femoral replacements = 52.6% [41–61], proximal tibia replacements = 84.0% [79–87], p = 0.001) (Fig. 4). Significant knee flexion strength deficits were found for the proximal tibia replacement group only (proximal femoral replacement = 10.9% [−6.1 to 33], distal femoral replacement = 2.1% [−2.7 to 23], proximal tibia replacement = 43% [32–58], p = 0.006) (Fig. 5).
Fig. 4.
Graph demonstrating strength deficit of extension for each reconstruction group. Median is identified with the solid line and 25th and 75th percentiles are identified with the dashed line.
Fig. 5.
Graph demonstrating strength deficit of flexion for each reconstruction group. Median is identified with the solid line and 25th and 75th percentiles are identified with the dashed line.
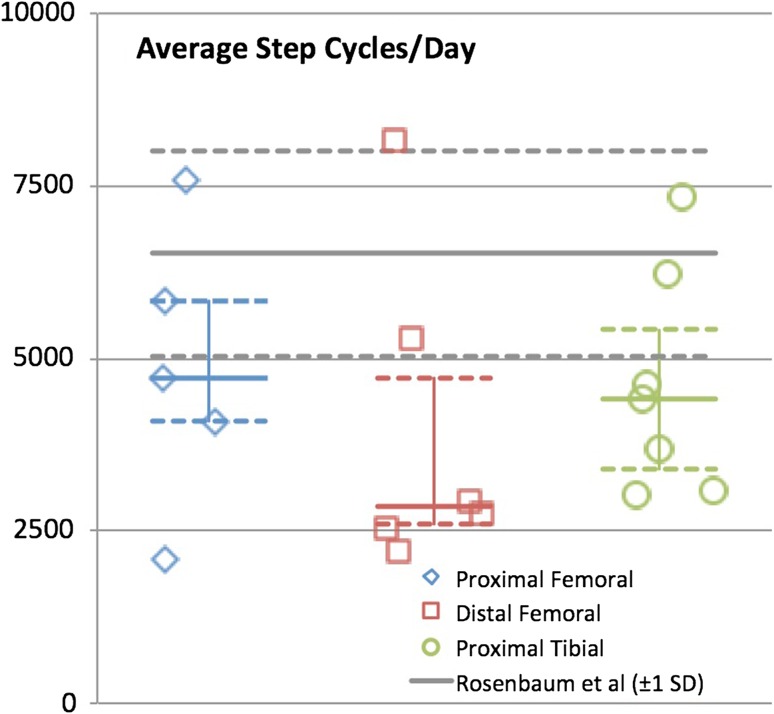
With the numbers available, there were no differences in mean number of strides per day at home and in the community among the different reconstructions (proximal femoral replacements = 4709 strides/day [3094–6696], distal femoral replacements = 2854 [2461–6015], and proximal tibia replacements = 4411 [3093–6215], p = 0.53) (Fig. 6).
Fig. 6.
Graph demonstrating average strides per day for each reconstruction group. Median is identified with the solid line and 25th and 75th percentiles are identified with the dashed line. Norms ± 1 SD from Rosenbaum et al. [11] are shown for comparison.
Discussion
Background and Rationale
Objective assessment of long-term functional outcomes after endoprosthetic reconstructions of bony deficits after tumor removal is increasingly important because oncologic outcomes continue to improve. Objective functional data will be of value to provide a framework from which patients can generate reasonable expectations for function once recovery is complete. This serves both to provide the patient with meaningful expectations for function as well as a concrete, tangible window into “life beyond cancer.” Additionally, objective functional data points are comparable across reconstruction techniques and among different orthopaedic oncology centers. As more centers adopt objective outcome metrics, surgeons will be provided a means to compare different surgical techniques, rehabilitation protocols, and implantable devices. In this study, at a mean 13.2 years (range, 2.5–28.2 years) from their endoprosthetic reconstruction, patients with proximal femoral replacements, distal femoral replacements, and proximal tibia replacements all walked efficiently. Patients with proximal tibia replacements had more muscle weakness about the knee, but all groups remained similarly active at home and in the community.
Limitations
This study had a number of limitations. First, there is a potential selection bias in that patients more content with their results may be more likely to volunteer to return for functional assessment. Additionally, there is a high degree of variability in the required muscle resection in these procedures because some tumors have dramatically larger soft tissue components. This variability makes comparison difficult and patients with more extensive resections, who would presumably be less functional, may be less likely to return for analysis, thereby skewing results toward the positive. With only 24 of 69 invited patients returning for analysis, further study is needed to determine the applicability of the data. Prospective functional outcome assessment is necessary to determine if these conclusions are representative of all patients. Second, actual functional outcomes are clearly more complex than oxygen consumption measurements and strength testing in a controlled environment. One may reasonably ask whether these patients have dramatic deficits that would be uncovered by testing on hills, steps, or other, more “real-world” environments. One would expect that the dramatic weakness about the knee noted in patients with proximal tibia replacements would have affected speed and efficiency in stair walking or on uneven ground where quad weakness cannot be masked by knee hyperextension. The implementation of a Step Watch Activity Monitor™ assessment was added to the study to provide some data from the patient’s actual life. The step monitor data in this study also suffered from small numbers, because only 18 patients wore the monitor over a sufficient timeframe to meet inclusion criteria. Endoprosthetic reconstructions for bony tumors are rare and a larger prospective trial in which objective functional outcomes are embedded in the postoperative protocol is likely necessary to power a study that could overcome this limitation. Despite these limitations, we believe the study provides important information for patients as to outcomes, even if selection bias and small size skew the data toward optimal outcomes. The results of this study suggest patients can achieve function comparable to a THA and that is a powerful message to patients surrounded by uncertainty and fear. The study also lays out an easily reproducible method for other institutions to evaluate their endoprosthesis patients, potentially paving the way for comparative studies evaluating outcomes, surgical techniques, rehabilitation protocols, and orthopaedic implants.
Previous functional assessments of endoprosthetic reconstructions found greater disability in gait in terms of oxygen cost and gait velocity than in the present study [2, 4, 9, 10, 13]. Kawai et al. [9] reported a cohort of proximal femoral replacements in which the mean energy cost of free walking was 141% of that for control subjects (0.24 versus 0.17 mL/m/kg) and gait velocity was significantly decreased (63.9 versus 80.6 m/s). These authors reported similar findings for a separate study of distal femoral reconstructions: an increase in oxygen consumption of 35% and decrease in walking speed of 21% compared with control subjects [10]. Cammisa et al. [4] described a cohort of distal femoral replacement endoprosthetic reconstructions with an increase in oxygen consumption of 44% and a decrease in walking speed of 14%. Rompen et al. [13] performed gait analysis on a cohort including all femoral reconstructions–proximal femoral replacements (N = 3), distal femoral replacements (N = 12), and total femoral endoprostheses (N = 3)–and found a less dramatic deficit in velocity as patients had a decrease of 12% as compared with that of control patients. The current study shows O2 cost to be minimally increased in any group and the gait speed to be only reduced for patients with proximal tibia replacements. These differences have several potential explanations: first, the exclusion of patients who were less than 2 years postlimb salvage in this study was intended to achieve a picture of stability after rehabilitation from their operation. It is our anecdotal experience that patients continue to improve functionally out to 2 years and the aforementioned studies did not use this exclusion criterion. Shorter followup could explain the greater deficits. Also, it should be noted that the previous studies used a Douglas-bag collection technique, whereas the current study uses a portable breath-by-breath pulmonary exchange unit. It is unlikely that this is a cause for significant disparity, however, because both have been demonstrated to be precise and accurate measurement tools [1] and internal control patients were used to account for equipment differences.
Deficits in knee extension strength after distal femoral replacement as compared with the contralateral limb were previously reported by Benedetti et al. [2]. This result was also observed in the present study, because patients with distal femoral replacements had a 49.8% extension strength deficit. The more dramatic strength deficits associated with proximal tibia replacements, however, have not been previously reported (82.9% extension deficit and 43% flexion deficit). A greater strength deficit about the knee could be predicted in proximal tibia replacements patients given the use of a medial gastrocnemius flap. The proximal tibia replacements have the attachment of extensor mechanism removed (tibial tubercle) and have the patellar tendon sewed into the gastrocnemius flap, leading to a suboptimal extensor level arm. The loss of the gastrocnemius as a knee flexor may contribute to the flexion weakness. These deficits are dramatic and may be of more functional significance if these patients had undergone testing that required more knee extension strength such as walking on inclines or stairs.
Data collection in a controlled laboratory has inherent drawbacks in that patient performance may represent maximal capacity rather than their typical daily performance. Mobile pedometers provide insight into the actual activity level of a patient at home, and the Step Watch Activity Monitor™ system used in this study has been well validated and used in multiple orthopaedic studies [5, 15]. However, discrepancies in methods of describing gait make comparisons to historical controls quite difficult, because some papers use step (heel strike of one foot to the other) and some use stride or gait cycle (heel strike of one foot to next heel strike of the same foot). A meta-analysis aimed at establishing normative data for steps per day was performed and suggested a mean of 9448 steps (8899–9996) [3]. Using mean steps per day rather than strides per day in this study, cohorts in this group walked no less than this historical mean (proximal femoral replacements = 9716, distal femoral replacements = 7454, proximal tibia replacements = 9258). However, when mean strides per day are used and compared with a historical norm published previously, each of our cohorts was less active than healthy control subjects and was more similar to patients after THA [14]. These studies used the same monitoring as the present study and therefore we believe this is a more appropriate control. This discrepancy shows the importance of standardizing methods of recording and reporting home activity so that direct comparisons can be made.
At a mean 13.2 years after surgery, patients with endoprosthetic reconstructions after bone tumor resections can expect relatively normal energy requirements for gait as compared with healthy adults. In this cohort, patients with endoprosthetic reconstructions walked efficiently in the laboratory and were active at home and in the community. Patients with proximal tibia replacements can expect more disability, because our participants had significantly more weakness about the knee. The functional results presented should give patients and surgeons confidence that endoprosthetic reconstructions can achieve excellent results when assessed by objective metrics. Prospective studies using these metrics would provide objective data from which legitimate comparisons among surgical techniques, rehabilitation protocols, and orthopaedic implants could be performed.
Footnotes
Each author certifies that he or she, or a member of his or her immediate family, has no funding or commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research ® editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research ® neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA-approval status, of any drug or device prior to clinical use.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
References
- 1.Bassett DR, Jr, Howley ET, Thompson DL, King GA, Strath SJ, McLaughlin JE, Parr BB. Validity of inspiratory and expiratory methods of measuring gas exchange with a computerized system. J Appl Physiol. 1985;2001(91):218–224. doi: 10.1152/jappl.2001.91.1.218. [DOI] [PubMed] [Google Scholar]
- 2.Benedetti MG, Catani F, Donati D, Simoncini L, Giannini S. Muscle performance about the knee joint in patients who had distal femoral replacement after resection of a bone tumor. An objective study with use of gait analysis. J Bone Joint Surg Am. 2000;82:1619–1625. doi: 10.2106/00004623-200011000-00016. [DOI] [PubMed] [Google Scholar]
- 3.Bohannon RW. Number of pedometer-assessed steps taken per day by adults: a descriptive meta-analysis. Phys Ther. 2007;87:1642–1650. doi: 10.2522/ptj.20060037. [DOI] [PubMed] [Google Scholar]
- 4.Cammisa FP, Jr, Glasser DB, Otis JC, Kroll MA, Lane JM, Healey JH. The Van Nes tibial rotationplasty. A functionally viable reconstructive procedure in children who have a tumor of the distal end of the femur. J Bone Joint Surg Am. 1990;72:1541–1547. [PubMed] [Google Scholar]
- 5.Daniel J, Ziaee H, Pradhan C, Pynsent PB, McMinn DJ. Blood and urine metal ion levels in young and active patients after Birmingham hip resurfacing arthroplasty: four-year results of a prospective longitudinal study. J Bone Joint Surg Br. 2007;89:169–173. doi: 10.1302/0301-620X.89B2.18519. [DOI] [PubMed] [Google Scholar]
- 6.Enneking WF, Dunham W, Gebhardt MC, Malawar M, Pritchard DJ. A system for the functional evaluation of reconstructive procedures after surgical treatment of tumors of the musculoskeletal system. Clin Orthop Relat Res. 1993;286:241–246. [PubMed] [Google Scholar]
- 7.Griffin AM, Parsons JA, Davis AM, Bell RS, Wunder JS. Uncemented tumor endoprostheses at the knee: root causes of failure. Clin Orthop Relat Res. 2005;438:71–79. doi: 10.1097/01.blo.0000180050.27961.8a. [DOI] [PubMed] [Google Scholar]
- 8.Jaffe N, Frei E, 3rd, Traggis D, Bishop Y. Adjuvant methotrexate and citrovorum-factor treatment of osteogenic sarcoma. N Engl J Med. 1974;291:994–997. doi: 10.1056/NEJM197411072911902. [DOI] [PubMed] [Google Scholar]
- 9.Kawai A, Backus SI, Otis JC, Inoue H, Healey JH. Gait characteristics of patients after proximal femoral replacement for malignant bone tumour. J Bone Joint Surg Br. 2000;82:666–669. doi: 10.1302/0301-620X.82B5.10264. [DOI] [PubMed] [Google Scholar]
- 10.Kawai A, Lin PP, Boland PJ, Athanasian EA, Healey JH. Relationship between magnitude of resection, complication, and prosthetic survival after prosthetic knee reconstructions for distal femoral tumors. J Surg Oncol. 1999;70:109–115. doi: 10.1002/(SICI)1096-9098(199902)70:2<109::AID-JSO9>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 11.Link MP, Goorin AM, Miser AW, Green AA, Pratt CB, Belasco JB, Pritchard J, Malpas JS, Baker AR, Kirkpatrick JA, et al. The effect of adjuvant chemotherapy on relapse-free survival in patients with osteosarcoma of the extremity. N Engl J Med. 1986;314:1600–1606. doi: 10.1056/NEJM198606193142502. [DOI] [PubMed] [Google Scholar]
- 12.Meyers PA, Heller G, Vlamis V. Osteosarcoma of the extremities: chemotherapy experience at Memorial Sloan-Kettering. Cancer Treat Res. 1993;62:309–322. doi: 10.1007/978-1-4615-3518-8_37. [DOI] [PubMed] [Google Scholar]
- 13.Rompen JC, Ham SJ, Halbertsma JP, van Horn JR. Gait and function in patients with a femoral endoprosthesis after tumor resection: 18 patients evaluated 12 years after surgery. Acta Orthop Scand. 2002;73:439–446. doi: 10.1080/00016470216319. [DOI] [PubMed] [Google Scholar]
- 14.Rosenbaum D, Brandes M, Hardes J, Gosheger G, Rodl R. Physical activity levels after limb salvage surgery are not related to clinical scores-objective activity assessment in 22 patients after malignant bone tumor treatment with modular prostheses. J Surg Oncol. 2008;98:97–100. doi: 10.1002/jso.21091. [DOI] [PubMed] [Google Scholar]
- 15.Silva M, Shepherd EF, Jackson WO, Dorey FJ, Schmalzried TP. Average patient walking activity approaches 2 million cycles per year: pedometers under-record walking activity. J Arthroplasty. 2002;17:693–697. doi: 10.1054/arth.2002.32699. [DOI] [PubMed] [Google Scholar]
- 16.Wirganowicz PZ, Eckardt JJ, Dorey FJ, Eilber FR, Kabo JM. Etiology and results of tumor endoprosthesis revision surgery in 64 patients. Clin Orthop Relat Res. 1999;358:64–74. doi: 10.1097/00003086-199901000-00009. [DOI] [PubMed] [Google Scholar]
- 17.Zeegen EN, Aponte-Tinao LA, Hornicek FJ, Gebhardt MC, Mankin HJ. Survivorship analysis of 141 modular metallic endoprostheses at early followup. Clin Orthop Relat Res. 2004;420:239–250. doi: 10.1097/00003086-200403000-00034. [DOI] [PubMed] [Google Scholar]