Abstract
Background
Corticosteroids are a common, short-term, local antiinflammatory and analgesic for treating patients with musculoskeletal disorders. Studies have shown the deleterious effects of corticosteroids on chondrocytes, suggesting a potentiation of degenerative joint disease. Mesenchymal stem cells (MSCs) are the direct progenitors of chondrocytes and other musculoskeletal tissue. Additionally, they serve an important antiinflammatory role, which can combat the chronic inflammatory state that mediates degenerative joint disease. Little is known about how corticosteroids interact with this regenerative and reparative cell population.
Questions/purposes
We asked: (1) Are corticosteroids cytotoxic to MSCs in a dose–response fashion? (2) Is there a differential effect in the level of cytotoxicity to MSCs between commercially available corticosteroid preparations?
Methods
Human MSCs were isolated and cultured from periarticular adipose tissue obtained from 20 patients undergoing primary THA. MSCs were exposed for 60 minutes to one of four commonly used corticosteroid preparations: betamethasone sodium phosphate-betamethasone acetate (6 mg/mL), dexamethasone sodium phosphate (4 mg/mL), methylprednisolone (40 mg/mL), or triamcinolone acetonide (40 mg/mL). Among the four preparations (treatment groups), cells were exposed to increasing concentrations of drugs according to the following titrations of the commercially available preparation: 0.0 (control solution of 1X phosphate buffered saline), 3.125, 6.25, 12.5, 25, 50, 75, and 100 % (undiluted commercial product). Cells were allowed to recover in standard culture media for 24 hours. After the recovery period, cell viability was measured using -(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) tetrazolium dye-based cellular viability assay and live-dead cell fluorescent staining. For the MTS assay, measurements were quantified in units of optical density (OD). ANOVA was performed at every experimental steroid concentration. When this global test was statistically significant, all pairwise comparisons were performed at that concentration with p values adjusted by the Tukey method to guard against Type I error.
Results
Exposure to corticosteroids decreased MSC viability in a curvilinear dose–response pattern. For betamethasone, the mean MTS OD at 0% steroid concentration was 1.03 (SD, 0.12) and decreased to 0.00 (SD, 0.00) at 25% steroid concentration. For dexamethasone, the mean MTS OD at 0% steroid concentration was 1.00 (SD, 0.07) and decreased to 0.00 (SD, 0.01) at 100% steroid concentration. For methylprednisolone, the mean MTS OD at 0% steroid concentration was 1.03 (SD, 0.09) and decreased to 0.00 (SD, 0.00) at 100% steroid concentration. For triamcinolone, the mean MTS OD at 0% steroid concentration was 1.02 (SD, 0.09) and decreased to 0.00 (SD, 0.00) at 75% steroid concentration. There were large differences among commercially available preparations, and these differences were present at every concentration. In general, dexamethasone was most gentle on MSCs (average OD by steroid concentration: 0% = 1.00; 3.125% = 0.86; 6.25% = 0.74; 12.5% = 0.53; 25% = 0.30; 50% = 0.20; 75% = 0.09; 100% = 0.00, triamcinolone and methylprednisolone were intermediate (triamcinolone average OD by steroid concentration: 0% = 1.02; 3.125% = 0.82; 6.25% = 0.64; 12.5% = 0.45; 25% = 0.18; 50% = 0.03; 75% = 0.00; 100% = 0.00; methylprednisolone average OD by steroid concentration: 0% = 1.03; 3.125% = 0.74; 6.25% = 0.54; 12.5% = 0.31; 25% = 0.12; 50% = 0.01; 75% = 0.00; 100% = 0.00), and betamethasone was most toxic (average OD by steroid concentration: 0% = 1.03; 3.125% = 0.74; 6.25% = 0.27; 12.5% = 0.02; 25% = 0.00; 50% = 0.00; 75% = 0.00; 100% = 0.00). ANOVA testing showed p values less than 0.0001 at every tested concentration (with the exception of the 0% control solution; p = 0.204) with subsequent pairwise comparisons supporting the relationships described above. The outcomes were maintained after stratifying by age, sex, or indication for THA (osteoarthritis versus avascular necrosis).
Conclusions
Commonly used intraarticular corticosteroids had a dose-dependent, profound, and differential effect on MSCs in this in vitro model, with betamethasone being the most toxic. Further studies are needed to assess if the in vitro effects of these agents translate into similar in vivo outcomes.
Clinical Relevance
Corticosteroids frequently are used by physicians to reduce inflammation in patients with musculoskeletal disorders, but these agents may hinder MSCs’ innate regenerative capacity in exchange for temporary analgesia. Our study suggests that choosing dexamethasone may result in less harmful effects when compared with other injectable steroids.
Electronic supplementary material
The online version of this article (doi:10.1007/s11999-014-3925-y) contains supplementary material, which is available to authorized users.
Introduction
Osteoarthritis is a painful, degenerative joint disease that affects more than 27 million Americans yearly [11]. The debilitating pain associated with osteoarthritis often is resistant to many nonoperative therapeutic modalities with time [2, 3]. Orthopaedic surgeons, rheumatologists, and primary care physicians commonly use corticosteroid injections to treat joint pain resulting from osteoarthritis [1, 14, 18, 22]. Corticosteroids generally are effective in providing short-term relief, but these agents rarely achieve sustained results for patients. Additionally, deleterious effects of corticosteroid treatment on chondrocytes have been observed [8, 15, 21]. Current recommendations limit most patients to a maximum of four corticosteroid injections per year in a given joint [26].
Although much has been written about steroid effects on chondrocytes [5, 13, 16, 19, 24], little is known regarding their effect on mesenchymal stem cells (MSCs). MSCs are multipotent progenitor cells with regenerative and repair potential found throughout human body tissue, including bone marrow, adipose, periosteum, and synovial fluid [9]. MSCs are capable of differentiating into mature cellular phenotypes, including bone, cartilage, muscle, fat, and tendon. They play a critical role in the natural healing process of intraarticular disorders, as they have been observed to proliferate in response to intraarticular damage [12, 14, 20]. The effect of injectable corticosteroids on human MSCs is relevant for soft tissue healing during the immediate postoperative period and in the potentiation of cartilage damage during the course of nonoperative treatment regimens.
We therefore asked the following questions: (1) Are corticosteroids cytotoxic to MSCs in a dose–response fashion? (2) Is there a differential effect in the level of cytotoxicity to MSCs between commercially available corticosteroid preparations? Secondarily, we aimed to explore whether there are differences in viability based on source disorder type (osteoarthritis versus avascular necrosis), patient sex, and age.
Materials and Methods
Adipose Digestion
Following institutional review board approval, 2-g periarticular adipose tissue was obtained from the surgical incision of 20 patients undergoing primary THA for osteoarthritis or avascular necrosis (Table 1). Adipose tissue was processed as previously described [6]. Briefly, adipose tissue was minced with a surgical scalpel and incubated in 0.01% collagenase type I (Sigma Aldrich, St Louis, MO, USA) for 90 minutes. The digested adipose tissue then was centrifuged, washed, and strained to separate tissue debris; then incubated in red blood cell lysis buffer (Stem Cell Technologies, Vancouver, BC, Canada). The derived solution was centrifuged, supernatant removed, and the pellet resuspended in expansion media containing advanced minimum essential medium (aMEM) with 10% fetal bovine serum (FBS), 100 µ/mL penicillin, 100 g/mL streptomycin, and 2 mol/L-glutamine (Invitrogen, Carlsbad, CA, USA).
Table 1.
Demographic summary of patient information
| Patient number | Sex | Age (years) | Indication for THA |
|---|---|---|---|
| 1 | Female | 54 | Osteoarthritis |
| 2 | Female | 40 | Osteoarthritis |
| 3 | Male | 56 | Avascular necrosis |
| 4 | Female | 48 | Avascular necrosis |
| 5 | Female | 52 | Osteoarthritis |
| 6 | Male | 58 | Avascular necrosis |
| 7 | Female | 53 | Osteoarthritis |
| 8 | Female | 54 | Osteoarthritis |
| 9 | Male | 39 | Osteoarthritis |
| 10 | Female | 46 | Osteoarthritis |
| 11 | Female | 39 | Osteoarthritis |
| 12 | Male | 35 | Osteoarthritis |
| 13 | Male | 26 | Avascular necrosis |
| 14 | Male | 38 | Avascular necrosis |
| 15 | Male | 56 | Osteoarthritis |
| 16 | Male | 34 | Osteoarthritis |
| 17 | Female | 58 | Avascular necrosis |
| 18 | Female | 49 | Osteoarthritis |
| 19 | Male | 54 | Avascular necrosis |
| 20 | Male | 51 | Osteoarthritis |
MSC Culture
Cells were grown at 37°C in a humidified 5% CO2 incubator for 24 hours. Next, nonadherent cells were removed from the culture dishes and expansion media were changed every 48 to 72 hours. Cells were expanded until they reached 90% confluence, at which time they were trypsinized and expanded until the third passage. Cells then were seeded in triplicate on 96-well plates at a density of 2500 cells per well and cultured for 72 hours in expansion medium. During this growth period, cell cultures achieved 90% confluence, after which time they were exposed to experimental treatment.
Immunophenotypic Characterization of Cells
In addition to seeding cells on 96-well plates for experimental treatment after the third passage in cell culture, a subset of cells was attained for immunophenotypic analysis. Trypsinized cells were washed in medium and centrifuged for 5 minutes at 1700 revolutions per minute to create a cell pellet. The cell pellet then was resuspended in 100 μL of 1× phosphate-buffered saline (PBS) (Corning Inc, Manassas, VA, USA) and removed for immunophenotypic analysis. Each cell preparation was stained with the following monoclonal antibodies: mouse anti-human CD34-APC (clone MOPC-21), CD14-FITC (clone G155-178), CD45-PE-Cy7 (clone MOPC-21) (all from BD Biosciences, San Jose, CA, USA), CD105-PE (clone SN6), and CD90-PerCP-Cy5.5 (clone 5E10) (both from eBioscience, San Diego, CA, USA). Analysis was performed using an LSR II flow cytometer, and the subsequent data were analyzed with Cell Quest Pro software (both from BD Biosciences).
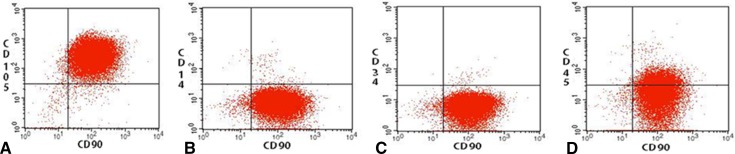
Immunophenotypic analysis showed a high proportion of cells that were CD 90 and CD 105 positive, while simultaneously being CD 14, CD 34, and CD 45 negative (Fig. 1). The combination of cell markers is consistent with MSC phenotype and was representative of the entire patient cohort.
Fig. 1A–D.
MSCs were isolated in culture from periarticular adipose tissue. After the third passage, cells in culture were analyzed with phenotypic immunostaining. The diagram shows that relatively pure populations of (A) CD90/105 positive, (B) CD90 positive, CD14 negative, (C) CD90 positive, CD34 negative, and (D) CD90 positive, CD45 negative cells were isolated, which is consistent with the MSC phenotype.
Treatment Groups
MSC cultures were treated with one of four corticosteroids commonly used in clinical practice: betamethasone sodium phosphate-betamethasone acetate 6 mg/mL (American Regent Inc, Shirley, NY, USA); dexamethasone sodium phosphate 4 mg/mL (APP Pharmaceuticals LLC, Shaumburg, IL, USA); methylprednisolone (Depo-Medrol®) 40 mg/mL (Pfizer Inc, New York, NY, USA); and triamcinolone acetonide (Kenalog®) 40 mg/mL (Bristol-Myers Squibb, Princeton, NJ, USA).
The FDA has approved all corticosteroids used in our investigation. However, these agents were used only on tissue samples in the laboratory and not for direct patient care for the purpose of clinical research. The cell cultures were allocated in eight replicate wells per corticosteroid and exposed to increasing concentrations of drugs according to the following titrations of the commercially available preparation: (1) 0.0% (control group of 1× PBS); (2) 3.125%; (3) 6.25%; (4) 12.5%; (5) 25%; (6) 50%; (7) 75%; and (8) 100% (undiluted commercial product). All dilutions were created with 1× PBS, which yielded 32 treatment solutions (four corticosteroids at eight concentrations), and triplicate analyses were performed for each of the 20 patient samples. Preliminary studies in our laboratory showed that 60 minutes of PBS exposure followed by 24 hours of recovery in aMEM-10% FBS media did not adversely affect cell viability compared with cells continuously exposed to aMEM-10% FBS media for the same time (data not shown). In addition, PBS has been used successfully as a control solution and dilution solvent for similar studies of drug-induced cytotoxicity [4, 7]. Conversions of steroid concentration for each of the 32 treatment solutions were made to units of mg/mL and molarity for comparison (Table 2).
Table 2.
Concentration of corticosteroid for each treatment group
| Corticosteroid | 0.0% Concentration | 3.125% Concentration | 6.25% Concentration | 12.5% Concentration | 25% Concentration | 50% Concentration | 75% Concentration | 100% Concentration |
|---|---|---|---|---|---|---|---|---|
| Betamethasone | 0 mg/mL; 0 mol/L | 0.188 mg/mL; 0.381 mol/L | 0.375 mg/mL; 0.763 mol/L | 0.75 mg/mL; 1.525 mol/L | 1.5 mg/mL; 3.05 mol/L | 3 mg/mL; 6.1 mol/L | 4.5 mg/mL; 9.15 mol/L | 6 mg/mL; 12.2 mol/L |
| Dexamethasone | 0 mg/mL; 0 mol/L | 0.125 mg/mL; 0.244 mol/L | 0.25 mg/mL; 0.488 mol/L | 0.5 mg/mL; 0.975 mol/L | 1 mg/mL; 1.95 mol/L | 2 mg/mL; 3.9 mol/L | 3 mg/mL; 5.85 mol/L | 4 mg/mL; 7.8 mol/L |
| Methylprednisolone | 0 mg/mL; 0 mol/L | 1.25 mg/mL; 3.338 mol/L | 2.5 mg/mL; 6.675 mol/L | 5 mg/mL; 13.35 mol/L | 10 mg/mL; 26.7 mol/L | 20 mg/mL; 53.4 mol/L | 30 mg/mL; 80.1 mol/L | 40 mg/mL; 106.8 mol/L |
| Triamcinolone | 0 mg/mL; 0 mol/L | 1.25 mg/mL; 2.88 mol/L | 2.5 mg/mL; 5.76 mol/L | 5 mg/mL; 11.51 mol/L | 10 mg/mL; 23.03 mol/L | 20 mg/mL; 46.05 mol/L | 30 mg/mL; 69.08 mol/L | 40 mg/mL; 92.1 mol/L |
The expansion media were aspirated and 200 μL of the suitable treatment solution was added to each well. The cells then were incubated at 37°C in a humidified 5% CO2 incubator for 60 minutes. They then were washed gently four times with 1× PBS solution, followed by replacement of expansion media in each well. Cells were incubated at 37°C in a humidified 5% CO2 incubator until MSC viability was measured 24 hours after treatment.
Assessment of Viability
Cellular Viability Assay
Once the cells reached 90% confluence, cellular viability was measured using the -(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) Cell Viability Assay (Promega Corporation, Madison, WI, USA). Briefly, the aMEM-10% FBS media were aspirated, and the remaining cells were washed with PBS. A 5:1 concentration of aMEM-10% FBS media and MTS substrate were added to the wells and incubated for 4 hours. At the end of the incubation, mitochondrial activity of the remaining cells was measured via standard light absorbance, and cellular viability was quantified in units of optical density (OD) for each group.
Live–dead Staining
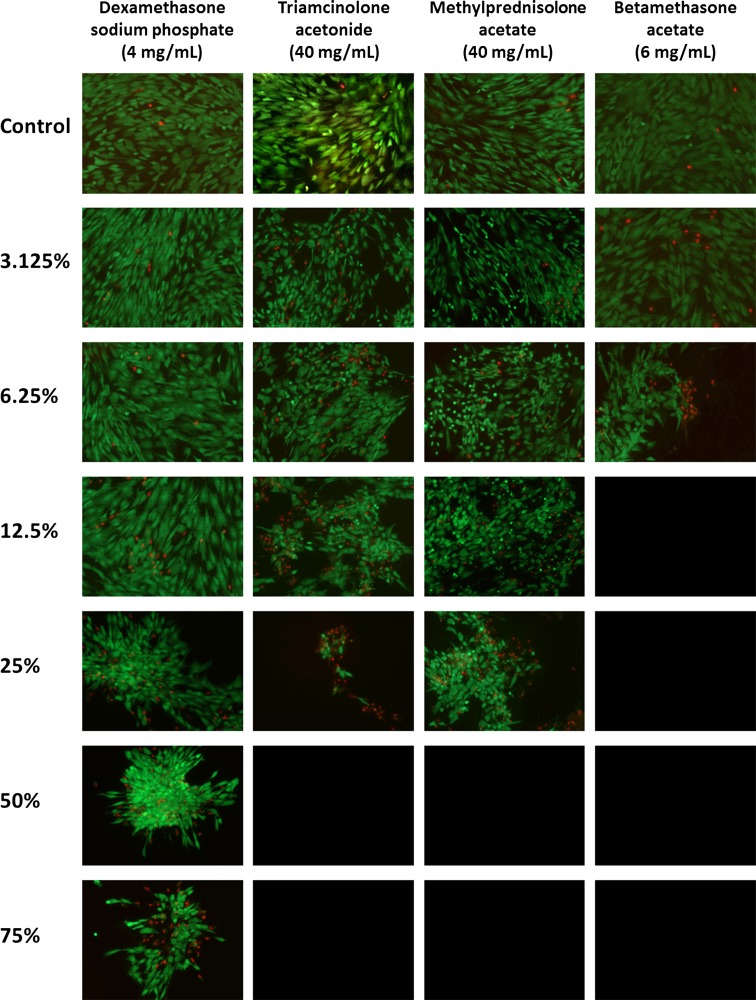
MSCs from one randomly chosen tissue donor (Patient 6) were exposed to each treatment condition in a preliminary set of experiments to determine cell viability by two different methods: MTS assays and live–dead cell staining (Fig. 2). Three replicates were performed for both of the techniques at each respective condition. Live–Dead Cell Viability Assays (Life Technologies, Carlsbad, CA, USA) provide qualitative and quantitative information regarding cell viability. Performance of the live-dead cell study allowed for correlation with the MTS assay to establish the level of validity for the MTS construct. Additionally, the assay provided images for visual confirmation of the cytotoxicity profile for each corticosteroid at all of the tested concentrations.
Fig. 2.
Dexamethasone yields the least cytotoxic damage to human MSCs. Fluorescence imaging of the live–dead cell viability assay measured the survival of human MSCs 24 hours after a 60-minute treatment with 1× PBS solution (control), or one of the following corticosteroids: dexamethasone (4 mg/mL), triamcinolone (40 mg/mL), methylprednisolone (40 mg/mL), or betamethasone (6 mg/mL). The corticosteroids were applied in a scaled titration, including 3.125%, 6.25%, 12.5%, 25%, 50%, 75%, and 100% of the concentration in the commercially available preparations. Live cells are stained with calcein-AM (green) and dead cells with EthD-1 (red)(Original magnification, ×10).
To perform the live-dead cell viability assay, expansion media were aspirated from each well, and 200 μL of a 1:5000 dilution of ethidium homodimer-1 and calcein AM was added to each well. Cells were incubated for 25 minutes at room temperature. The staining solution was aspirated and 200 μL of 1× PBS was added to each well. Cells were observed using a laser scanning confocal microscope (Carl Zeiss Microscopy, Thornwood, NY, USA) with resultant images acquired at ×10 magnification. MSC viability was measured by observing the confocal images in Photoshop (Adobe Systems, San Jose, CA, USA). In the imaging program, a grid was overlaid to facilitate independent counts by three investigators (CCW, MTH, SPW). Calcein-stained live cells displayed green fluorescence and ethidium homodimer-1 dead cells emitted a red fluorescence. For each control group (0% corticosteroid in 1× PBS), calcein-stained live cells and ethidium-stained dead cells were counted to tabulate the percentage of live cells for each treatment. The total number of cells counted in the control group was used as the standard total cell count for the remaining treatments. In the remaining cases, the number of calcein-stained live cells was counted and divided by the standard total cell count to arrive at a percentage of live cells for each treatment condition. The calculation was performed in this manner to account for dead cells that had been lost in the washouts and media changes.
Correlation of Cellular Viability and Live-dead Cell Assays
Linear regression between the average percentage of live cells (live–dead fluorescent staining) and mean OD (cellular viability assay) yielded an r2 value of 0.90. Given the strength of the correlation, the cellular viability assay was used exclusively to assess viability for all patients.
Statistical Analysis
All data are reported as mean (SD) for continuous variables and count (percentage) for discrete variables, unless noted otherwise. The primary outcome was the mean OD, as noted previously. As described above, samples from each patient were tested with four different steroids, each at eight different concentrations. Therefore, the analysis was conducted using ANOVA with repeated measures to properly account for the within-patient correlation. Because an interaction was observed between the steroid and concentration effects, the final analysis involved performing separate one-factor ANOVA models with repeated measures to compare the effect of the four steroids separately at each level of concentration. When the global F-test for steroids was statistically significant, pairwise comparisons were performed to identify which steroids were significantly different from each other; to guard against the increased Type I error rate attributable to multiple comparisons, the p values for the pairwise comparisons were adjusted using the Tukey method [25]. The data for one patient were used in an exploratory analysis. Specifically, the association of live cell counts (expressed as a percentage) with the OD was examined using linear regression. All statistical tests were two-sided and p values less than 0.05 were considered significant. All analyses were conducted using SAS version 9.2 (SAS Institute, Inc, Cary, NC, USA), and R version 3.0.1 (R Development Core Team [2011]. http://www.R-project.org/).
Results
Dose-dependent Effects of Corticosteroids on MSC Viability
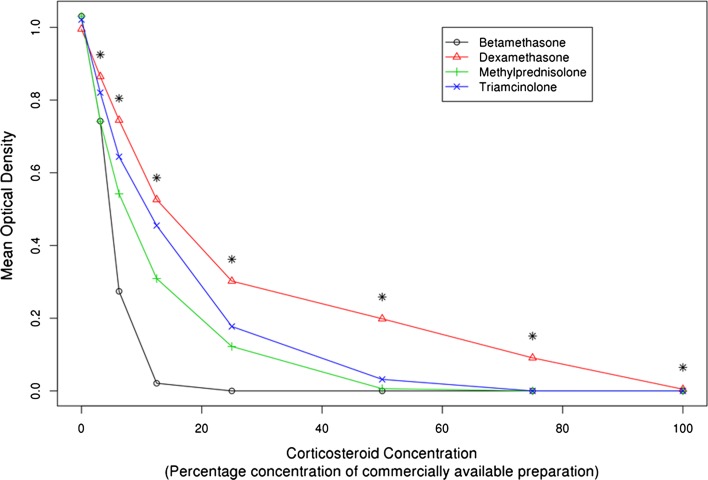
As the concentration of steroid exposure increased, cell viability decreased in a consistent curvilinear dose-response pattern (Figs. 2 and 3). For betamethasone, the mean MTS OD at 0% steroid concentration was 1.03 (SD, 0.12) and decreased to 0.00 (SD, 0.00) at 25% steroid concentration. For dexamethasone, the mean MTS OD at 0% steroid concentration was 1.00 (SD, 0.07) and decreased to 0.00 (SD, 0.01) at 100% steroid concentration. For methylprednisolone, the mean MTS OD at 0% steroid concentration was 1.03 (SD, 0.09) and decreased to 0.00 (SD, 0.00) at 100% steroid concentration. For triamcinolone, the mean MTS OD at 0% steroid concentration was 1.02 (SD, 0.09) and decreased to 0.00 (SD, 0.00) at 75% steroid concentration.
Fig. 3.
Differential dose-response cytotoxicity to human MSCs with four commonly used corticosteroids is shown in the graph. Mean optical density of the MTS tetrazolium dye-based cell viability assay measured the survival of human MSCs 24 hours after a 60-minute treatment with 1× PBS solution (control), or one of the following corticosteroids: dexamethasone (4 mg/mL), triamcinolone (40 mg/mL), methylprednisolone (40 mg/mL), or betamethasone (6 mg/mL). The corticosteroids were applied in a scaled titration including 3.125%, 6.25%, 12.5%, 25%, 50%, 75%, or 100% of the concentration in the commercially available preparations. *Table 3 shows specific pairwise comparisons.
Cytotoxicity of Different Corticosteroid Preparations
In general, dexamethasone was most gentle on the MSCs, triamcinolone and methylprednisolone were intermediate, and betamethasone was most toxic (Figs. 2 and 3). Dexamethasone resulted in the following average OD by steroid concentration: 0% = 1.00; 3.125% = 0.86; 6.25% = 0.74; 12.5% = 0.53; 25% = 0.30; 50% = 0.20; 75% = 0.09; 100% = 0.00 (Table 3). Triamcinolone resulted in the following average OD by steroid concentration: 0% = 1.02; 3.125% = 0.82; 6.25% = 0.64; 12.5% = 0.45; 25% = 0.18; 50% = 0.03; 75% = 0.00; 100% = 0.00) (Table 3). Methylprednisolone resulted in the following average OD by steroid concentration: 0% = 1.03; 3.125% = 0.74; 6.25% = 0.54; 12.5% = 0.31; 25% = 0.12; 50% = 0.01; 75% = 0.00; 100% = 0.00) (Table 3). Betamethasone resulted in the following average OD by steroid concentration: 0% = 1.03; 3.125% = 0.74; 6.25% = 0.27; 12.5% = 0.02; 25% = 0.00; 50% = 0.00; 75% = 0.00; 100% = 0.00) (Table 3). ANOVA testing showed p values less than 0.0001 at every tested concentration with the exception of the 0% control (p = 0.204). Pairwise comparisons between triamcinolone, methylprednisolone, and betamethasone showed differences at the various concentrations, but generally followed the patterns noted above (Table 3). The mean difference in OD, with 95% CIs and p values, for all pairwise comparisons was determined at each concentration level (Table 3).
Table 3.
Effect of steroid by dilution, overall
| Dilution | Steroid | Mean | SD | p value* | Pairwise comparisons† |
|---|---|---|---|---|---|
| 0.00% | Beta | 1.03 | 0.12 | 0.204 | Beta vs Dex (DMOD = 0.035; 95% CI, −0.014–0.085) |
| Dex | 1.00 | 0.07 | Depo vs Beta (DMOD = 0; 95% CI, −0.05–0.05) | ||
| Depo | 1.03 | 0.09 | Beta vs Kenalog (DMOD = 0.009; 95% CI, −0.04–0.059) | ||
| Kenalog | 1.02 | 0.09 | Depo vs Dex (DMOD = 0.036; 95% CI, −0.014–0.085) | ||
| Kenalog vs Dex (DMOD = 0.026; 95% CI, −0.024–0.076) | |||||
| Depo vs Kenalog (DMOD = 0.01; 95% CI, −0.04–0.059) | |||||
| 3.125% | Beta | 0.74 | 0.12 | < 0.0001 | Dex vs Beta (DMOD = 0.123; 95% CI, 0.066–0.179; p < 0.001) |
| Dex | 0.86 | 0.09 | Beta vs Depo (DMOD = 0.001; 95% CI, −0.055–0.057; p > 0.999) | ||
| Depo | 0.74 | 0.08 | Kenalog vs Beta (DMOD = 0.078; 95% CI, 0.022–0.135; p = 0.003) | ||
| Kenalog | 0.82 | 0.08 | Dex vs Depo (DMOD = 0.124; 95% CI, 0.068–0.18; p < 0.001) | ||
| Dex vs Kenalog (DMOD = 0.044; 95% CI, −0.012–0.101; p = 0.17) | |||||
| Kenalog vs Depo (DMOD = 0.08; 95% CI, 0.023–0.136; p = 0.002) | |||||
| 6.25% | Beta | 0.27 | 0.11 | < 0.0001 | Dex vs Beta (DMOD = 0.471; 95% CI, 0.388–0.553; p < 0.001) |
| Dex | 0.74 | 0.14 | Depo vs Beta (DMOD = 0.268; 95% CI, 0.186–0.351; p < 0.001) | ||
| Depo | 0.54 | 0.11 | Kenalog vs Beta (DMOD = 0.37; 95% CI, 0.288–0.453; p < 0.001) | ||
| Kenalog | 0.64 | 0.10 | Dex vs Depo (DMOD = 0.203; 95% CI, 0.12–0.285; p < 0.001) | ||
| Dex vs Kenalog (DMOD = 0.101; 95% CI, 0.018–0.183; p = 0.011) | |||||
| Kenalog vs Depo (DMOD = 0.102; 95% CI, 0.02–0.184; p = 0.01) | |||||
| 12.5% | Beta | 0.02 | 0.03 | < 0.0001 | Dex vs Beta (DMOD = 0.505; 95% CI, 0.427–0.583; p < 0.001) |
| Dex | 0.53 | 0.12 | Depo vs Beta (DMOD = 0.288; 95% CI, 0.21–0.366; p < 0.001) | ||
| Depo | 0.31 | 0.11 | Kenalog vs Beta (DMOD = 0.434; 95% CI, 0.356–0.512; p < 0.001) | ||
| Kenalog | 0.45 | 0.01 | Dex vs Depo (DMOD = 0.217; 95% CI, 0.139–0.295; p < 0.001) | ||
| Dex vs Kenalog (DMOD = 0.071; 95% CI, −0.006–0.149; p = 0.083) | |||||
| Kenalog vs Depo (DMOD = 0.146; 95% CI, 0.068–0.224; p < 0.001) | |||||
| 25.0% | Beta | 0.00 | 0.00 | < 0.0001 | Dex vs Beta (DMOD = 0.302; 95% CI, 0.246–0.359; p < 0.001) |
| Dex | 0.30 | 0.08 | Depo vs Beta (DMOD = 0.122; 95% CI, 0.066–0.179; p < 0.001) | ||
| Depo | 0.12 | 0.06 | Kenalog vs Beta (DMOD = 0.177; 95% CI, 0.121–0.234; p < 0.001) | ||
| Kenalog | 0.18 | 0.11 | Dex vs Depo (DMOD = 0.18; 95% CI, 0.123–0.236; p < 0.001) | ||
| Dex vs Kenalog (DMOD = 0.125; 95% CI, 0.068–0.181; p < 0.001) | |||||
| Kenalog vs Depo (DMOD = 0.055; 95% CI, −0.001–0.112; p = 0.059) | |||||
| 50.0% | Beta | 0.00 | 0.00 | < 0.0001 | Dex vs Beta (DMOD = 0.198; 95% CI, 0.167–0.23; p < 0.001) |
| Dex | 0.20 | 0.06 | Depo vs Beta (DMOD = 0.006; 95% CI, −0.025–0.037; p = 0.953) | ||
| Depo | 0.01 | 0.02 | Kenalog vs Beta (DMOD = 0.032; 95% CI, 0.001–0.063; p = 0.045) | ||
| Kenalog | 0.03 | 0.05 | Dex vs Depo (DMOD = 0.192; 95% CI, 0.161–0.223; p < 0.001) | ||
| Dex vs Kenalog (DMOD = 0.167; 95% CI, 0.135–0.198; p < 0.001) | |||||
| Kenalog vs Depo (DMOD = 0.026; 95% CI, −0.006–0.057; p = 0.145) | |||||
| 75.0% | Beta | 0.00 | 0.00 | < 0.0001 | Dex vs Beta (DMOD = 0.091; 95% CI, 0.066–0.116]; p < 0.001) |
| Dex | 0.09 | 0.06 | Depo vs Beta (DMOD = 0; 95% CI, −0.025–0.025; p > 0.999) | ||
| Depo | 0.00 | 0.00 | Beta vs Kenalog (DMOD = 0; 95% CI, −0.025–0.025; p > 0.999) | ||
| Kenalog | 0.00 | 0.00 | Dex vs Depo (DMOD = 0.091; 95% CI, 0.066–0.116; p < 0.001) | ||
| Dex vs Kenalog (DMOD = 0.091; 95% CI, 0.066–0.116; p < 0.001) | |||||
| Depo vs Kenalog (DMOD = 0; 95% CI, −0.025–0.025; p > 0.999) | |||||
| 100.0% | Beta | 0.00 | 0.00 | < 0.0001 | Dex vs Beta (DMOD = 0.005; 95% CI, 0–0.009; p = 0.024) |
| Dex | 0.00 | 0.01 | Beta vs Depo (DMOD = 0; 95% CI, −0.004–0.004; p > 0.999) | ||
| Depo | 0.00 | 0.00 | Beta vs Kenalog (DMOD = 0; 95% CI, −0.004–0.004; p > 0.999) | ||
| Kenalog | 0.00 | 0.00 | Dex vs Depo (DMOD = 0.005; 95% CI, 0–0.009; p = 0.024) | ||
| Dex vs Kenalog (DMOD = 0.005; 95% CI, 0–0.009; p = 0.024) | |||||
| Depo vs Kenalog (DMOD = 0; 95% CI, −0.004–0.004; p > 0.999) |
Beta = betamethasone; Dex = dexamethasone; Depo = methylprednisolone; Kenalog = triamcinolone; DMOD = difference in mean optical density; *p value of the four-way ANOVA; †pairwise comparisons performed only when the overall ANOVA test was statistically significant (p < 0.05). Pairwise comparison p values were adjusted with the Tukey method to guard against Type I error.
Other Findings
When accounting for surgical indication, there were few differences in cell viability between those with osteoarthritis (n = 13) compared with avascular necrosis (n = 7) at the various concentrations of corticosteroids (Tables 4 and 5; Supplemental material is available with the online version of CORR®). Furthermore, adjusting for patient sex (women, n = 10; men, n = 10) or a binary analysis of age (younger than 50 years, n = 10; older than 50 years, n = 10) did not alter statistical significance of outcomes (Tables 6 and 7; Supplemental material is available with the online version of CORR®).
Discussion
Osteoarthritis has a growing incidence and prevalence [10, 23]. Current clinical guidelines recommend the use of intraarticular corticosteroids for treatment of moderate-to-severe joint pain associated with osteoarthritis [14]. Although effective for short-term pain relief, consideration of the effect of these cytotoxic treatments on progression of joint damage and postoperative tissue healing rates is needed. Studies have shown that corticosteroids have a negative effect on chondrocytes [8, 15, 21] and that local anesthetics have a cytotoxic, yet differential, effect on MSC viability [4, 17]. However, little is known regarding the cytotoxic effects of these injections on reparative MSCs. Owing to the important role MSCs play in tissue regeneration and the intraarticular healing cascade [14], the cytotoxic effect could have significant clinical ramifications. We aimed to determine if there is a dose-dependent cytotoxic effect of corticosteroids, and if this effect varies between different drug preparations.
Our findings must be taken in light of several limitations. First, the nature of an in vitro experiment may not recapitulate the in vivo intraarticular microenvironment and translate to a similar clinical result. Furthermore, it is difficult to reproduce the true intraarticular concentration of corticosteroids owing to the varying degrees of effusions and pharmacokinetic idiosyncrasies of the intraarticular microenvironment. Additional studies are needed to determine if the immediate and profound cytotoxic in vitro effects of corticosteroids translate into in vivo intraarticular toxicities. Another limitation is that our experimental model was performed using titrations of commercially available corticosteroid preparations. An alternative approach would have been to standardize the concentrations by milligrams per milliliter or by molarity (Table 2). The rationale behind our titration system was to most closely mimic clinical practice, which commonly uses dilutions of commercially available corticosteroids.
Cytotoxic effects of corticosteroids on MSCs are dose-dependent. Previously, the cytotoxic effect of corticosteroids on chondrocytes was established in human and animal models [5, 13, 16, 19, 24]. Studies have shown that repeated use of intraarticular methylprednisolone led to increased release of cellular degradation products from articular cartilage [19]. Similarly, in our study, as the concentration of corticosteroid was increased for each of the four preparations, cell viability decreased in a curvilinear manner. Our study is the first, to our knowledge, that investigates the MSC toxicity profile of corticosteroids in their clinically available form. A previous study showed that higher doses of intraarticular corticosteroid injection produce abnormal changes in the cytoplasm and nucleus of chondrocytes, leading to cell degeneration [16]. Accordingly, in our in vitro study, corticosteroid preparations in their undiluted form resulted in no MSCs surviving after treatment. Cell culture damage was documented even with the lowest tested titration at 3.125% of the original clinical preparation. Our findings on dose-dependent cytotoxicity in MSCs concur with previous findings of corticosteroid toxicity in chondrocytes.
Each drug preparation was cytotoxic to MSCs in a dose-responsive manner, yet the level of severity differed greatly among groups. Betamethasone showed the largest degree of injury to MSCs compared with dexamethasone, methylprednisolone, and triamcinolone. Similarly, previous in vitro studies in human chondrocytes showed higher chrondrotoxicity with betamethasone compared with triamcinolone acetonide [8]. One investigation showed that betamethasone alone exerted minor toxic effects on chondrocytes whereas the coupling of betamethasone with its common inactive ingredient, benzalkonium chloride, resulted in greater than 99% of articular chondrocyte death [7]. Elucidating the effect of benzalkonium chloride in addition to other inactive ingredients on MSCs deserves further investigation. Additionally, studies have shown that local anesthetics have a differential effect on the viability of MSCs with ropivacaine being least injurious to cells [4, 17, 21]. The differential toxicity of local anesthetics is similar to the variable effect of corticosteroids on MSCs. These results may allow clinicians to choose an ideal combination of local anesthetic and corticosteroid with a lower toxicity profile for intraarticular injections.
Corticosteroids had a profound cytotoxic effect on MSCs in this in vitro model. Although all preparations were injurious to MSC viability in a curvilinear dose-response pattern, dexamethasone was associated with the smallest degree of harm, whereas betamethasone had the most pronounced toxicity to the MSCs. The findings of our in vitro investigation shed light on the importance of clinical investigations examining the modulatory effect of the intraarticular microenvironment and whether any measures potentially can offer cytoprotection for MSCs exposed to corticosteroids. The differential cytotoxicity shown by our investigation suggests that certain preparations could mitigate the side-effect profile of corticosteroid use. Young patients and those without imminent need for reconstructive procedures may benefit from this consideration. Our study findings give providers and patients another element to consider when deciding to use intraarticular corticosteroids, in addition to other factors, such as duration of efficacy, cost, and risk of local side effects.
Electronic supplementary material
Acknowledgments
We thank Dirk R. Larson MS (Mayo Clinic Department of Biomedical Statistics and Informatics) for assistance with statistical analysis, Ruben Crespo-Diaz PhD (Mayo Clinic Center for Regenerative Medicine) for support with experimental techniques, German Norambuena MD (Mayo Clinic Department of Orthopedic Surgery) for thoughtful discussion and critique of the material, and Paul Stalboerger for aid in study logistics.
Footnotes
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research.
Funding for this study was provided by an Accelerated Regenerative Medicine Research Grant from the Department of Regenerative Medicine of the Mayo Clinic, Rochester, MN, USA.
The institution of one or more of the authors (RJS) has received, during the study period, funding from Biomet Inc (Warsaw, IN, USA). One of the authors certifies that he (RJS), or a member of his immediate family, has received or may receive payments or benefits, during the study period, an amount of 10,000 USD to 100,000 USD from Biomet Inc (Warsaw, IN, USA).
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
References
- 1.Bellamy N, Campbell J, Robinson V, Gee T, Bourne R, Wells G. Intraarticular corticosteroid for treatment of osteoarthritis of the knee. Cochrane Database Syst Rev. 2006:CD005328. [DOI] [PubMed]
- 2.Bjordal JM, Johnson MI, Lopes-Martins RA, Bogen B, Chow R, Ljunggren AE. Short-term efficacy of physical interventions in osteoarthritic knee pain: a systematic review and meta-analysis of randomised placebo-controlled trials. BMC Musculoskelet Disord. 2007;8:51. doi: 10.1186/1471-2474-8-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bjordal JM, Klovning A, Ljunggren AE, Slordal L. Short-term efficacy of pharmacotherapeutic interventions in osteoarthritic knee pain: a meta-analysis of randomised placebo-controlled trials. Eur J Pain. 2007;11:125–138. doi: 10.1016/j.ejpain.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 4.Breu A, Eckl S, Zink W, Kujat R, Angele P. Cytotoxicity of local anesthetics on human mesenchymal stem cells in vitro. Arthroscopy. 2013;29:1676–1684. doi: 10.1016/j.arthro.2013.06.018. [DOI] [PubMed] [Google Scholar]
- 5.Celeste C, Ionescu M, Robin Poole A, Laverty S. Repeated intraarticular injections of triamcinolone acetonide alter cartilage matrix metabolism measured by biomarkers in synovial fluid. J Orthop Res. 2005;23:602–610. doi: 10.1016/j.orthres.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 6.Crespo-Diaz R, Behfar A, Butler GW, Padley DJ, Sarr MG, Bartunek J, Dietz AB, Terzic A. Platelet lysate consisting of a natural repair proteome supports human mesenchymal stem cell proliferation and chromosomal stability. Cell Transplant. 2011;20:797–811. doi: 10.3727/096368910X543376. [DOI] [PubMed] [Google Scholar]
- 7.Davis D, Cyriac M, Ge D, You Z, Savoie FH. In vitro cytotoxic effects of benzalkonium chloride in corticosteroid injection suspension. J Bone Joint Surg Am. 2010;92:129–137. doi: 10.2106/JBJS.H.01561. [DOI] [PubMed] [Google Scholar]
- 8.Dragoo JL, Danial CM, Braun HJ, Pouliot MA, Kim HJ. The chondrotoxicity of single-dose corticosteroids. Knee Surg Sports Traumatol Arthrosc. 2012;20:1809–1814. doi: 10.1007/s00167-011-1820-6. [DOI] [PubMed] [Google Scholar]
- 9.Jones EA, English A, Henshaw K, Kinsey SE, Markham AF, Emery P, McGonagle D. Enumeration and phenotypic characterization of synovial fluid multipotential mesenchymal progenitor cells in inflammatory and degenerative arthritis. Arthritis Rheum. 2004;50:817–827. doi: 10.1002/art.20203. [DOI] [PubMed] [Google Scholar]
- 10.Kotlarz H, Gunnarsson CL, Fang H, Rizzo JA. Osteoarthritis and absenteeism costs: evidence from US National Survey Data. J Occup Environ Med. 2010;52:263–268. doi: 10.1097/JOM.0b013e3181cf00aa. [DOI] [PubMed] [Google Scholar]
- 11.Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, Deyo RA, Gabriel S, Hirsch R, Hochberg MC, Hunder GG, Jordan JM, Katz JN, Kremers HM, Wolfe F, National Arthritis Data Workgroup Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58:26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee DH, Sonn CH, Han SB, Oh Y, Lee KM, Lee SH. Synovial fluid CD34− CD44+ CD90+ mesenchymal stem cell levels are associated with the severity of primary knee osteoarthritis. Osteoarthritis Cartilage. 2012;20:106–109. doi: 10.1016/j.joca.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 13.Mankin HJ, Conger KA. The acute effects of intra-articular hydrocortisone on articular cartilage in rabbits. J Bone Joint Surg Am. 1966;48:1383–1388. [PubMed] [Google Scholar]
- 14.Morito T, Muneta T, Hara K, Ju YJ, Mochizuki T, Makino H, Umezawa A, Sekiya I. Synovial fluid-derived mesenchymal stem cells increase after intra-articular ligament injury in humans. Rheumatology (Oxford). 2008;47:1137–1143. doi: 10.1093/rheumatology/ken114. [DOI] [PubMed] [Google Scholar]
- 15.Nakazawa F, Matsuno H, Yudoh K, Watanabe Y, Katayama R, Kimura T. Corticosteroid treatment induces chondrocyte apoptosis in an experimental arthritis model and in chondrocyte cultures. Clin Exp Rheumatol. 2002;20:773–781. [PubMed] [Google Scholar]
- 16.Papacrhistou G [sic], Anagnostou S, Katsorhis T. The effect of intraarticular hydrocortisone injection on the articular cartilage of rabbits. Acta Orthop Scand Suppl. 1997;275:132–134. [DOI] [PubMed]
- 17.Rahnama R, Wang M, Dang AC, Kim HT, Kuo AC. Cytotoxicity of local anesthetics on human mesenchymal stem cells. J Bone Joint Surg Am. 2013;95:132–137. doi: 10.2106/JBJS.K.01291. [DOI] [PubMed] [Google Scholar]
- 18.Raynauld JP, Buckland-Wright C, Ward R, Choquette D, Haraoui B, Martel-Pelletier J, Uthman I, Khy V, Tremblay JL, Bertrand C, Pelletier JP. Safety and efficacy of long-term intraarticular steroid injections in osteoarthritis of the knee: a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2003;48:370–377. doi: 10.1002/art.10777. [DOI] [PubMed] [Google Scholar]
- 19.Robion FC, Doize B, Boure L, Marcoux M, Ionescu M, Reiner A, Poole AR, Laverty S. Use of synovial fluid markers of cartilage synthesis and turnover to study effects of repeated intra-articular administration of methylprednisolone acetate on articular cartilage in vivo. J Orthop Res. 2001;19:250–258. doi: 10.1016/S0736-0266(00)90008-1. [DOI] [PubMed] [Google Scholar]
- 20.Sekiya I, Ojima M, Suzuki S, Yamaga M, Horie M, Koga H, Tsuji K, Miyaguchi K, Ogishima S, Tanaka H, Muneta T. Human mesenchymal stem cells in synovial fluid increase in the knee with degenerated cartilage and osteoarthritis. J Orthop Res. 2012;30:943–949. doi: 10.1002/jor.22029. [DOI] [PubMed] [Google Scholar]
- 21.Seshadri V, Coyle CH, Chu CR. Lidocaine potentiates the chondrotoxicity of methylprednisolone. Arthroscopy. 2009;25:337–347. doi: 10.1016/j.arthro.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Skedros JG, Hunt KJ, Pitts TC. Variations in corticosteroid/anesthetic injections for painful shoulder conditions: comparisons among orthopaedic surgeons, rheumatologists, and physical medicine and primary-care physicians. BMC Musculoskelet Disord. 2007;8:63. doi: 10.1186/1471-2474-8-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suri P, Morgenroth DC, Hunter DJ. Epidemiology of osteoarthritis and associated comorbidities. PM R. 2012;4(5 suppl):S10–S19. doi: 10.1016/j.pmrj.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 24.Syed HM, Green L, Bianski B, Jobe CM, Wongworawat MD. Bupivacaine and triamcinolone may be toxic to human chondrocytes: a pilot study. Clin Orthop Relat Res. 2011;469:2941–2947. doi: 10.1007/s11999-011-1834-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tukey JW. The Problem of Multiple Comparisons. In Braun HI, ed. The Collected Works of John W. Tukey. Vol 8. New York, NY: Chapman & Hall; 1994:1–560.
- 26.Zhang W, Moskowitz RW, Nuki G, Abramson S, Altman RD, Arden N, Bierma-Zeinstra S, Brandt KD, Croft P, Doherty M, Dougados M, Hochberg M, Hunter DJ, Kwoh K, Lohmander LS, Tugwell P. OARSI recommendations for the management of hip and knee osteoarthritis. Part II: OARSI evidence-based, expert consensus guidelines. Osteoarthritis Cartilage. 2008;16:137–162. doi: 10.1016/j.joca.2007.12.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.