Abstract
Background
Hip arthroscopy is now commonly used to treat hip pain and pathology, including osteoarthritis (OA). Despite this, little is known about the effect of hip arthroscopy on outcomes of pain and function and progression to total hip arthroplasty (THA) in hip OA.
Questions/purposes
This systematic review aimed to (1) determine pain and function outcomes after hip arthroscopy in people with hip OA; (2) compare the outcome after hip arthroscopy between people with and without hip OA; and (3) report the likelihood of progression to THA in patients with hip OA after hip arthroscopy.
Methods
This review was conducted in accordance with the PRISMA statement. The Downs and Black checklist was used for quality appraisal. Studies scoring positively on at least 50% of items were included in final analyses. Standardized mean differences (SMDs) were calculated where possible or study conclusions are presented.
Results
Twenty-two studies were included in the final analyses. Methodological quality and followup time varied widely. Moderate to large SMDs were reported for people with and without hip OA; however, the positive effects of the intervention were smaller for people with hip OA. Greater severity of hip OA and older age each predicted more rapid progression to THA.
Conclusions
Patients with hip OA report positive outcomes from hip arthroscopy, although observed positive effects may be inflated as a result of methodological limitations of the included studies. Patients with hip OA had inferior results compared with those who did not. Chondropathy severity and patient age were associated with a higher risk and more rapid progression to THA. High-quality comparative studies are required to confirm the effects of hip arthroscopy on symptoms and structural change in people with hip OA.
Electronic supplementary material
The online version of this article (doi:10.1007/s11999-014-3943-9) contains supplementary material, which is available to authorized users.
Introduction
Hip osteoarthritis (OA) is characterized by pain and reduced physical function [2] and is a major burden to society. Between 10 [33] and 19 million [15] people in the United States are affected by hip OA, and the associated costs of arthritic disease exceed USD 24 billion annually in Australia [3]. Given the prevalence and healthcare costs related to hip OA, a focus of surgical intervention is shifting to preventive, minimally invasive arthroscopic techniques [39, 40] in people with early hip OA and coexisting pathology (eg, labral tears, femoroacetabular impingement [FAI]) [14, 29, 46]. This shift is driven by suggestions that the presence of FAI and/or labral pathology is strongly associated with an increased risk of hip OA and THA in later life [1, 42]; and hip arthroscopy in people with painful FAI and/or labral pathology may reduce future development of hip OA [41]. However, there is little evidence supporting the notion that undergoing arthroscopy can suppress the development of hip OA and the need for THA in later life [41]. Given the frequency with which hip arthroscopic surgery is now performed in people with early hip OA [39, 40], it is important to understand the implications of such procedures on symptoms and functional ability.
Recent systematic reviews report worse outcomes in those with coexisting radiographic hip OA or cartilage disease identified during hip arthroscopic surgery for labral pathology [7] and FAI [29, 41] compared with those without degenerative change. However, no systematic review has evaluated outcomes for combinations of various pathologies. Moreover, previous systematic reviews have excluded studies in which hip arthroscopy is specifically performed for hip OA [29] and, hence, the effects of hip arthroscopy for those with hip OA is not known. Finally, the effect of hip arthroscopy on progression to THA and factors associated with progression to THA in people with hip OA have not been reviewed.
This systematic review addresses these gaps in the literature with the aims to (1) determine pain and physical function outcomes after hip arthroscopy in people with hip OA; (2) compare outcome after hip arthroscopy between people with and without hip OA; and (3) report the likelihood of progression to THA in people with hip OA after hip arthroscopy.
Materials and Methods
The systematic review protocol was developed in accordance with the PRISMA statement [34]. Literature search criteria and methods were proposed and agreed on by two authors (JLK, KMC) and were established a priori to minimize selection bias.
Eligibility Criteria
Studies were eligible for inclusion if they used participants aged ≥ 17 years who had undergone hip arthroscopy as a primary intervention for hip OA (defined as chondropathy at the time of surgery or radiographic hip OA on preoperative scans). Studies following participants for at least 3 months, using patient-reported outcome measures of pain and/or function, reporting prevalence of THA, or time to THA were included. All quantitative study designs were considered, including randomized controlled trials (RCTs) and prospective or retrospective approaches (minimum Level IV evidence) [47]. Studies were excluded if they performed open surgeries as the primary intervention; did not include hip OA within the results or failed to specify the surgical procedure performed; were case series with less than 10 participants; published abstracts; nonpeer-reviewed; or published in a language other than English.
Search Strategy
A comprehensive, reproducible search strategy was performed on the following databases between January 1990 and March 2014: Scopus, Medline, CINAHL, PubMed, Ausport, SportsDiscus, PEDro, PsychINFO, and Google scholar (Appendix 1 [Supplemental materials are available with the online version of CORR®.]). January 1990 was selected as the earliest retrieval record as a result of the paucity of literature on hip arthroscopic surgery before this date [29]. Reference lists of appropriate studies were manually searched for relevant papers.
Titles and abstracts were screened for relevant studies by two independent reviewers (JLK, DM). Any disagreements regarding inclusion were resolved by an independent arbitrator (KMC). Full text versions of identified papers were then retrieved for final eligibility screening by a single reviewer (JLK).
Quality Evaluation
The Downs and Black checklist [4, 13] was used to appraise the methodological quality of included studies (see Appendix 2 for checklist [Supplemental materials are available with the online version of CORR®.]). This has adequate reliability and validity for assessing nonrandomized studies [12, 13, 49]. The original 27 items were modified to 17 items following the exclusion of criteria 9, 13, 14, 17, 19, 22, 23, 24, 26, and 27, which were not applicable for nonrandomized studies [4]. Included studies were rated by three independent reviewers (JLK, NJC, ALH) blinded to author, affiliations, and publishing journal. Any disagreements among reviewers were discussed in a consensus meeting and an independent arbitrator (KMC) used when consensus could not be met. Studies scoring positively on at least 50% of items (nine or more items) were considered to have a sufficiently low risk of bias and were included in subsequent analyses [29].
Data Management and Statistical Analysis
Eligible papers were grouped where possible based on (1) whether a between-group comparison (hip OA versus no hip OA) was undertaken; and (2) where likelihood of subsequent progression to THA was reported. Interrater agreement on the included Downs and Black criteria was evaluated using the κ statistic [23]. Data from included studies were extracted by one reviewer (JLK). Population characteristics (age, sex, type and description of hip OA, duration of symptoms) and details of outcome measures, length of followup, and surgical intervention undertaken were collated. Standardised mean differences (SMDs) were calculated to determine the magnitude of the effect of the intervention within groups or between groups and were calculated as the mean difference between preoperative and followup measures divided by the within-group preoperative SD [47]. SMD magnitude was interpreted as: ≥ 0.8 large effect; 0.5 to 0.79 moderate effect; and 0.2 to 0.49 weak effect [10, 11, 47]. Ninety-five percent confidence intervals for SMDs were calculated [5] using central t-distribution, where the 95% confidence interval = SMD ± 1.96 × SE of the SMD. Where SMDs could not be calculated, study conclusions were presented. All statistical analyses were conducted using SPSS software Version 21.0 (SPSS Inc, Chicago, IL, USA).
Results
Search Strategy
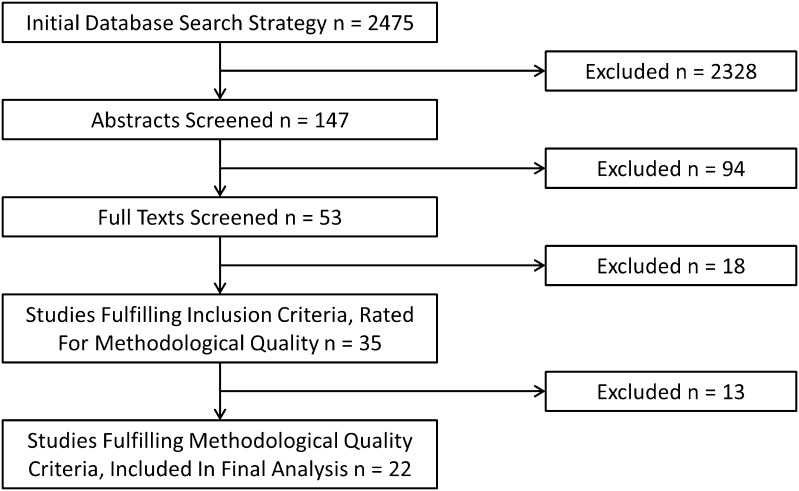
One hundred thirty-nine studies were identified for evaluation beyond title level and 53 papers for full-text evaluation, following which 18 studies were excluded (Fig. 1). Thirty-five studies fulfilled all eligibility criteria and underwent critical appraisal.
Fig. 1.
Summary of the search strategy results is shown.
Methodological Quality
Initial overall agreement between the independent raters was substantial (κ = 0.687) (agreement on 480 of 595 [81%] items) [31]. Consensus was reached on all 115 remaining items following discussion. Methodological quality scores of the 35 included studies varied from 1 [45] to 15 [44] out of 17 points (mean 9.4 [SD 3.0]) (Appendix 3 [Supplemental materials are available with the online version of CORR®.]). Twenty-two papers received a score of 9 or more points and were included in further analyses, whereas the remaining 13 studies were excluded. All included papers were Level IV evidence (case series), with the exception of three studies, which were Level III (case-control studies) evidence [14, 16, 37].
Participants
Participant characteristics were heterogeneous in the 22 studies. Sample sizes ranged from 13 [26] to 560 [14] participants and the percentage of women ranged from 20% [21] to 65% [35]. Mean age across all studies was 40 years (range, 29 [38] to 58 years [22]). All studies defined hip degenerative disease as chondral pathology seen at arthroscopy and/or preoperative hip degenerative changes observed on radiographs. In 18 studies reporting symptom duration before surgery, the mean duration was 27 months (range, 3 months [25] to 13 years [36]).
Outcome Scores
Several patient-reported outcome measures were reported. The modified Harris hip score (mHHS) was most frequently used (13 studies) and is reliable in hip arthroscopy populations, although it demonstrates ceiling effects and poor content validity [30]. The Non-arthritic Hip Score (NAHS) was used in eight studies. It has reported reliability and validity after hip arthroscopy, although its responsiveness is unknown [53]. The Hip Outcome Score (HOS) was used by two studies. Whereas the HOS has good reliability and responsiveness, it has reduced content validity and a ceiling effect in a hip arthroscopy population [30]. Two papers used the WOMAC and pain visual analog scale, although their psychometric properties in a hip arthroscopy population are unknown [29]. One study used a measure that lacked demonstrated psychometric properties, asking participants to rate their pain on a 3-point Likert scale (better, same, worse) [22].
Outcomes of Hip Arthroscopy for Hip Osteoarthritis
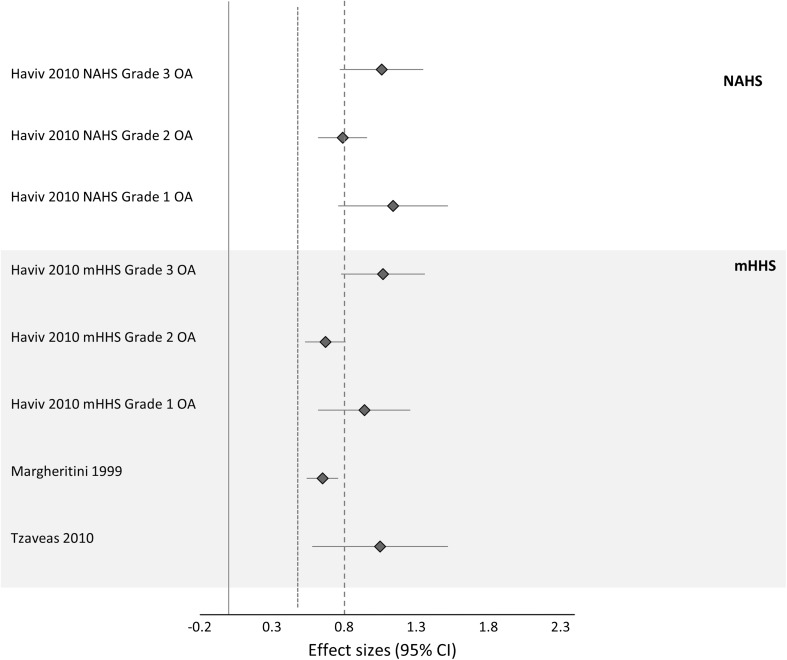
Of the 22 studies, 15 examined pre/post outcomes for hip arthroscopy in people with hip OA without comparison to a control group (Table 1). The mean ± SD methodological quality score of these 15 papers was 11 ± 2 (range, 9–13 points) out of a possible 15 points. Nine papers prospectively followed participants [8, 9, 16, 22, 24, 29, 46, 51, 55], whereas six were retrospective; no studies used a RCT design. Eleven studies defined hip OA based on chondral pathology at surgery [8, 9, 16, 21, 24, 26, 36, 37, 46, 51, 55], whereas six [16, 20–22, 24, 46] classified subjects based on preoperative radiographic disease in combination with articular cartilage disease observed at the time of surgery. Surgical intervention for hip degenerative disease included débridement of chondropathy, microfracture, use of fibrin adhesive, synovectomy, and removal of loose bodies. Corrective surgery for FAI included femoral and acetabular osteoplasty. Followup ranged from 12 months [55] to 13 years [36]. SMDs could be calculated for three studies [21, 35, 55] (Fig. 2).
Table 1.
Summary of included studies evaluating effect of hip arthroscopy on hip osteoarthritis
| Study (first author, year) | Study characteristics | Sample characteristics | Effect size at followup | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total score using modified Downs and Black appraisal | Arthroscopic intervention for OA pathology | Classification of OA pathology | Sample size | Sex | Age (years)* | Duration of symptoms (months)† | Followup (months)* | Outcome | (95% CI) | Study conclusions (where effect size = ID) | |
| Byrd (2000) [9] | 10 | Chondral débridement | Chondral injury | 38 | 17 F; 18 M | 38 | 21 (1–156) | 24 | mHHS | ID | Labrum only mHHS preoperative 63; postoperative 94; improved 31 points Labrum and chondral injury mHHS preoperative 51; postoperative 69; improved 18 points |
| Byrd (2011) [8] | 12 | Chondral débridement, microfracture (n = 18) | Chondral damage Outerbridge I–IV | 100 | 33 F; 67 M | 34 | 30 | 24 | mHHS | ID | All patients mHHS preoperative 65; postoperative 87; improved 22 points (p < 0.001) Microfracture mHHS pre NR; post NR; improved 21 points |
| Fontana (2012) [16] | 13 | Group A Autologous chondrocyte transfer (n = 15) Group B Débridement (n = 15) |
Mild OA on radiograph (Tönnis 1–2) Chondropathy Outerbridge ≥ 3 |
30 | Group A 9 F; 6 M Group B 9 F; 6 M |
Group A 40.7 Group B 42.3 |
NR | 74 | HHS | ID | Group A HHS preoperative 48; postoperative 88; improved 40 points Group B HHS preoperative 46; postoperative 56; improved 8 points |
| Haviv (2010) [21] | 11 | RF ablation; débridement; microfracture (n = 29) | Preoperative radiograph < Tönnis Grade 3 Outerbridge 2–4 |
166 | 34 F; 132 M | 37 | NR | 22 | mHHS NAHS |
mHHS Grade 1 0.94 (0.63–1.25) mHHS Grade 2 0.67 (0.52–0.81) mHHS Grade 3 1.07 (0.78–1.36) NAHS Grade 1 1.14 (0.76–1.52) NAHS Grade 2 0.79 (0.62–0.96) NAHS Grade 3 1.06 (0.77–1.35) |
|
| Haviv (2011) [20] | 11 | RF ablation, débridement | Preoperative radiograph Tönnis Grade 0 only Outerbridge 2–4 |
81 | 61 F; 20 M | 44 | 12 weeks | 3 years | mHHS NAHS |
ID | Preoperative mHHS 61, NAHS 56; postoperative mHHS 80, NAHS 78; improved 19 and 22 points (p = 0.001) Pearson correlation coefficient chondropathy and synovitis to clinical outcome r = 0.45, p < 0.05 |
| Helenius (2001) [22] | 11 | Débridement | Preoperative radiographic changes Outerbridge 0–4 |
68 | 42 F; 26 M | 58 | 39 | 1.3 years | Pain “same, better, or worse?” | ID | Outerbridge 0–89% patients better Outerbridge 1–84% patients better Outerbridge 2–73% patients better Outerbridge 3–43% patients better |
| Horisberger (2010) [24] | 9 | Débridement, microfracture | Outerbridge ≥ 2, preoperative radiograph Tönnis ≥ 1 | 20 | 4 F; 16 M | 47 | NR | 3 years | NAHS | ID | 10 patients underwent THA at followup and did not complete NAHS Preoperative NAHS in remaining patients 47; postoperative NAHS 78; improved 31 points (p = 0.004) |
| Ilizaliturri (2007) [26] | 11 | Débridement, microfracture | Chondropathy secondary to pediatric hip disorder | 13 | 7 F; 6 M | 31 | 30 | WOMAC | ID | Preoperative WOMAC 78 points; postoperative WOMAC 87 points; improved 9 points (p < 0.001) | |
| Kamath (2009) [27] | 11 | Labral and chondral débridement | Outerbridge ≥ 1 | 52 | 32 F; 20 M | 42 | 22 | 58 | mHHS | ID | All patients mHHS pre 56.8; postoperative 80.4; improved 23.6 points; no significant effect on outcome for chondral pathology (p > 0.05) |
| Margheritini (1999) [35] | 9 | Débridement, removal loose bodies, synovectomy | OA diagnosed by radiograph, MRI, or arthroscopy (not specific) | 133 | 87 F; 46 M | 42 | NR | 18 | mHHS | 0.65 (0.54–0.76) | |
| McCarthy (2011) [36] | 13 | Resection, microfracture, removal loose bodies | Outerbridge 0 Outerbridge 1–2 Outerbridge 3–4 |
111 | 64 F; 47 M | 39 | NR | 13 years | NAHS | ID | NAHS followup only for non-THA hips (n = 62) 87 points Overall 10-year survivorship (patients not having THA) 63% Outerbridge Grade 1–2 10-year survivorship 80% Outerbridge Grade 3–4 10-year survivorship 12% OR needing THA 3.6 times if age > 40 years OR needing THA 20 times if acetabular Outerbridge 3–4 OR needing THA 58 times if femoral Outerbridge 3–4 |
| McCormick (2012) [37] | 13 | Débridement | Group 1 Outerbridge ≤ 3 nonarthritic (n = 80) Group 2 Outerbridge 4 arthritic (n = 45) |
125 | 75 F; 50 M | 43 < 40 years (n = 47); ≥ 40 years (n = 78) | NR | 4.2 years | mHHS mHHS HOS |
ID | Overall postoperative mHHS 84 points; postoperative HOS ADL 87 points; HOS sport 72 points Age < 40 years–39 people mHHS > 80; 8 people mHHS < 80 Age ≥ 40 years–32 people mHHS > 80; 46 people mHHS < 80 Group 1 Outerbridge ≤ 3 52 people mHHS > 80; 28 people mHHS < 80 Group 2 Outerbridge 4 19 people mHHS > 80; 26 people mHHS < 80 OR poor outcome 2.5 times if Outerbridge 4; 7 times if aged ≥ 40 years |
| Philippon (2009) [46] | 12 | Chondroplasty, microfracture | Joint space measured on preoperative radiograph Outerbridge 1–2 (n = 74)–mild Outerbridge 3 (n = 29)–moderate Outerbridge 4 (n = 9)–poor |
112 | 62 F; 50 M | 41 | 34 | 2.3 | mHHS (8) HOS ADL (5) HOS sport (6) NAHS |
ID | Preoperative mHHS 58; postoperative mHHS 84 points; improved 26 points Preoperative HOS ADL 70; postoperative HOS ADL 88; improved 18 points Preoperative HOS SP 43; postoperative 69 points; improved 26 points Preoperative NAHS 66; postoperative 81 points; improved 15 points 10 of 112 patients progressed to THA Postoperative mHHS joint space < 2.0 mm = 68 points; joint space ≥ 2.0 mm = 87 points (p = 0.002) Postoperative mHHS cartilage change mild = 87; moderate = 79; poor = 62 (p = 0.011) OR progressing to THA 39 times if preoperative joint space < 2.0 mm |
| Streich (2009) [51] | 12 | Chondral débridement | Outerbridge 0–1 (n = 30) Outerbridge 2–4 (n = 20) |
50 | 29 F; 21 M | 33 | NR | 34 | mHHS | ID | All patients preoperative mHHS 60; postoperative mHHS 72; improved 12 points Outerbridge 0–1 preoperative mHHS 63; postoperative mHHS 87; improved 24 points Outerbridge 2–4 preoperative mHHS 55; postoperative mHHS 52; worsened 3 points |
| Tzaveas (2010) [55] | 9 | Fibrin adhesive to chondral flap | Acetabular chondral flap delamination | 19 | 14 F; 5 M | 36 | NR | 12 | mHHS | 1.05 (0.58–1.52) | |
* Mean (SD); †range; OA = osteoarthritis; CI = confidence interval; RF = radiofrequency; mHHS = modified Harris hip score range 0–100 points, 100 points = best possible outcome; VAS = visual analog scale score range 0–10 points, 0 points = best possible outcome; HHS = Harris hip score range 0–100 points, 100 points = best possible outcome; NAHS = Non-arthritic Hip Score score range 0–100 points, 100 points = best possible outcome; WOMAC score range 0–100 points, 100 points = best possible outcome; HOS = Hip Outcome Score; ADL = activities of daily living; F = female; M = male; ID = insufficient data; Tönnis = preoperative grading of osteoarthritis using Tönnis scale; Outerbridge = intraoperative grading of chondral injury; NR = not reported; NS = not specific.
Fig. 2.
Effect sizes (studies that reported sufficient data) of hip arthroscopy for hip OA are shown. A positive CI denotes a significant effect; a dashed line represents large effect size > 0.8; and a dotted line represents moderate effect size > 0.5.
Chondral Débridement
Seven studies examined the effects of chondral débridement as an isolated intervention [9, 20, 22, 27, 35, 37, 51]. Within-subject SMDs could be calculated for one study only. Margheritini and Villar [35] reported a significant moderate SMD of 0.65 (95% confidence interval [CI], 0.54–0.76) on the mHHS at 18 months after surgery. McCormick et al. [37] showed that the likelihood of a poor outcome at 4 years was 2.5 times greater if chondral disease equaled Outerbridge Grade IV. Similarly, Streich and Schmitt [52] reported an improvement in pain and function of 24 points on the mHHS for the less severe OA group (Outerbridge 0–1), which was not observed in the severe OA (Outerbridge > 1) group.
Microfracture and Chondral Débridement
Of the six studies that examined the effect of microfracture [8, 20, 24, 26, 36, 46], five included chondral débridement during corrective surgery for FAI. Moderate to large within-group SMDs were determined ranged from 0.67 (95% CI, 0.52–0.81) (mHHS, Outerbridge Grade II) to 1.14 (0.76–1.52) (NAHS, Outerbridge Grade I) at 22 months followup for a single study [21]. SMDs could not be calculated for the remaining studies as a result of insufficient data, and their reported results were inconsistent. Major improvements were reported by three studies [46]. Two studies reported 40% to 50% of patients had converted to THA at (3–10 years) followup [24, 36].
Fibrin Adhesive for Chondral Flaps
The one study examining outcomes after the use of fibrin adhesive to repair acetabular chondral flaps reported a large SMD (1.05 [0.58–1.52]) for mHHS at 12 months [55].
Autologous Chondrocyte Transfer
Fontana et al. [16] compared the effects of autologous chondrocyte transfer (ACT) and chondral débridement in participants with mild hip OA on preoperative radiographs (Tönnis Grade I–II) and cartilage disease classified as Outerbridge Grade III to IV that was greater than 2 cm2 in area. Those who received ACT had significantly (p < 0.001) greater improvements in mHHS (40 points) at 74 months followup compared with the chondral débridement group (8 points) [16].
Comparison of Outcomes of Hip Arthroscopy Between People With and Without Hip Osteoarthritis
Five studies directly compared hip arthroscopy outcomes between people with and without hip OA (Table 2). Three studies were prospective in design [14, 44, 50] with a mean ± SD quality score of 12 ± 2 (range, 9–15). Hip OA was classified based on chondropathy severity at surgery in two studies [14, 44] and preoperative radiographic changes using the Tönnis grading system [54] in three studies [45–47].
Table 2.
Results of comparison of effects of hip arthroscopy OA versus no OA
| Study (first author, year) | Study characteristics | Sample characteristics | Effect size at followup | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total score using modified Downs and Black appraisal [maximum17 points] | Arthroscopic intervention for OA pathology | Classification of OA pathology | FAI (Y/N; type) | Sample size (OA subsample) | Sex | Age (years)* | Duration of symptoms (months) † | Follow up (months)† | Outcome | (95% CI) | Study conclusions (where effect size = ID) | Difference between groups reported | |
| Egerton (2013) [14] | 13 | Débridement, microfracture | Chondral damage Outerbridge ≥ 1 | N | 560 (108) | 277 F; 283 M | 42 | NR | 12 | mHHS NAHS |
mHHS no OA 0.82 (0.75–0.90) mHHS OA 0.62 (0.50–0.73) NAHS no OA 0.89 (0.81–0.97) NAHS OA 0.44 (0.35–0.52) |
mHHS p = 0.001 NAHS p < 0.001 |
|
| Gedouin (2010) [17] | 10 | Débridement, microfracture | Preoperative radiograph Tönnis Grade 0 or 1 | N | 110 (45) | 32 F; 78 M | 31 | 10 | 15 | WOMAC | WOMAC no OA 1.23 (0.93–1.53) WOMAC OA 0.95 (0.67–1.22) |
p < 0.001 | |
| Larson (2011) [32] | 13 | Débridement, microfracture | Group FAI–no joint space narrowing Tönnis 0 to 1 (n = 154) Group FAI OA–joint space narrowing Tönnis > 1 (n = 56) |
Y (cam, pincer, and mixed) | 227 (56) | Group FAI 81 F; 88 M Group FAI OA 13 F; 43 M |
Group FAI 32 Group FAI OA 45 |
NR | 27 | mHHS SF-12 VAS |
ID | Group FAI preoperative mHHS 63; postoperative mHHS 88; improved 25 points (p < 0.001) Group FAI OA preoperative mHHS 60; postoperative mHHS 67 points; improved 7 points (p = 0.986) Predictors of lower mHHS were joint space narrowing (p = 0.001); greater duration of symptoms (p = 0.014); higher grade chondropathy preoperative MRI (p = 0..019) |
P < 0.001 |
| Palmer (2012) [44] | 15 | Chondroplasty, microfracture | Outerbridge 0–3 (n = 157) Outerbridge 4 (n = 44) Chondral defects < 1.5 cm2 (n = 201) |
Y (cam, pincer, and mixed) | 201 (201) | 102 F; 99M | 40 | 59 | 46 | NAHS | ID | 13 of 201 patients progressed to THA Outerbridge 0–3 NAHS improved 23 points (p < 0.001) Outerbridge 4 NAHS improved 25 points (p < 0.001) Pincer FAI NAHS improved 16 points; no pincer FAI improved 23 points (p < 0.01) |
p = 0.48 |
| Stahelin (2008) [50] | 9 | Débridement, microfracture | Preoperative radiograph Tönnis 0 (n = 14); Tönnis 1 (n = 5); Tönnis 2 (n = 3) | All patients cam FAI | 22 (8) | 7 F; 15 M | 42 | 6 | NAHS VAS |
NAHS Tönnis 0 1.55 (0.74–2.36) NAHS Tönnis 1–2 0.92 (0.28–1.55) VAS Tönnis 0 2.26 (1.08–3.45) VAS Tönnis 1–2 1.63 (0.50–2.75) |
p < 0.05 | ||
* Mean (SD); †range; OA = osteoarthritis; FAI = femoroacetabular impingement; Y = yes; N = no; CI = confidence interval; mHHS = modified Harris hip score range 0–100 points, 100 points = best possible outcome; VAS = visual analog scale score range 0–10 points, 0 points = best possible outcome; HHS = Harris hip score range 0–100 points, 100 points = best possible outcome; NAHS = Non-arthritic Hip Score score range 0–100 points, 100 points = best possible outcome; WOMAC score range 0–100 points, 100 points = best possible outcome; HOS = Hip Outcome Score; ADL = activities of daily living; F = female; M = male; ID = insufficient data; Tönnis = preoperative grading of osteoarthritis using Tönnis scale; Outerbridge = intraoperative grading of chondral injury; NR = not reported; NS = not specific.
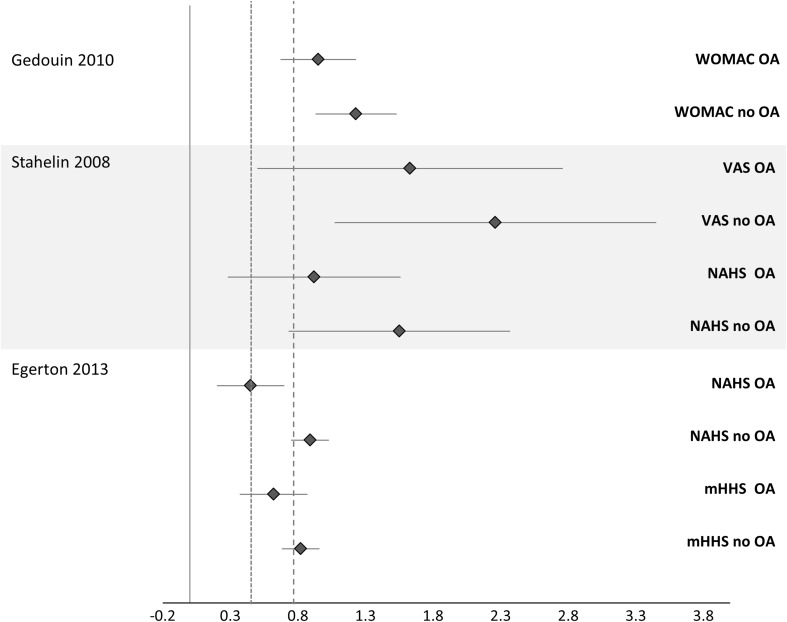
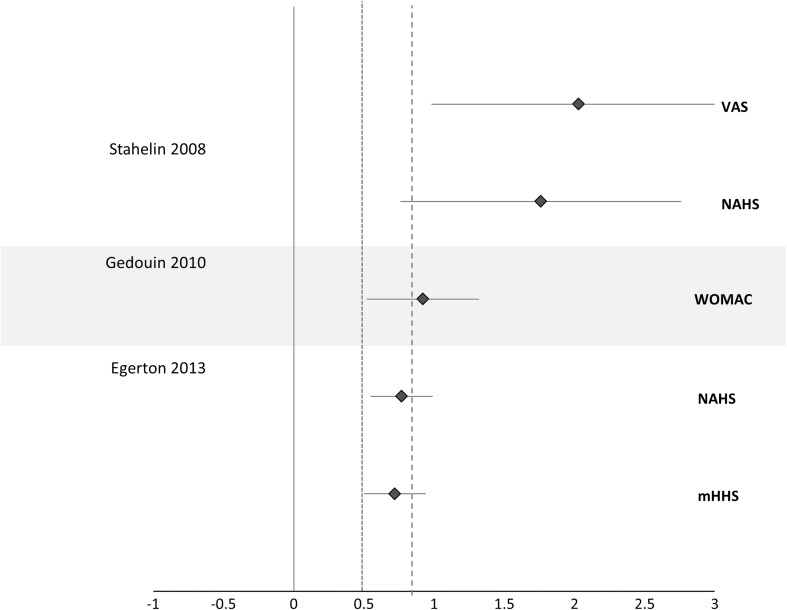
SMDs could be calculated for three papers [14, 17, 50] to determine within-group effects (Fig. 3) and between-group effects (Fig. 4). Larger within-group SMDs (range, 0.82–2.36) were observed for those having arthroscopy without OA than for those with OA (range, 0.44–1.63) (Fig. 3) [14]. When between-group SMDs were calculated for the three studies, each study demonstrated moderate to large SMDs between the groups for the patient-reported outcomes, favoring the no hip OA group (Fig. 4).
Fig. 3.
Effect sizes (studies that reported sufficient data) compare outcomes between people with and without hip OA. A positive CI favors the postoperative time point; a dashed line represents large effect size > 0.8; and a dotted line represents moderate effect size > 0.5. VAS = visual analog scale.
Fig. 4.
Forest plot shows the between-group effect sizes (people with and without hip OA). A positive CI favors the no OA group; a dashed line represents large effect size > 0.8; and a dotted line represents moderate effect size > 0.5 favoring the no OA groups.
Likelihood of Progression to THA in People With Osteoarthritis Who Undergo Hip Arthroscopy
Nine studies reported progression to THA as an outcome measure (Table 3). The mean modified Downs and Black score was 11 ± 2 points. The mean time to THA progression ranged from 7 months [35] to 4.8 years [36]. Older age was identified as a risk factor for progression to THA in two of the three studies that measured it as a risk factor [19, 36] (Table 3). McCarthy et al. [36] observed that people aged older than 40 years were 3.6 times more likely to progress to THA. Haviv and O’Donnell [19] observed that in patients with chondropathy graded as Outerbridge IV, older age was associated with a likelihood of a more rapid progression to THA (Pearson’s correlation coefficient [r] = −0.34).
Table 3.
Summary of included studies evaluating risk of progression to THA in people with hip OA undergoing hip arthroscopy
| Study (first author, year) | Total score using modified Downs and Black appraisal (maximum 17 points) | Intervention for OA pathology | Classification of OA pathology | Sample size | Sex | Age (years)* | Duration symptom | Followup time to THA (mean) | Study conclusions regarding THA risk |
|---|---|---|---|---|---|---|---|---|---|
| Gedouin (2010) [18] | 10 | Débridement, microfracture | Preoperative radiograph Tönnis Grade 0 or 1 | 110 (45) | 32 F; 78 M | 31 | 10 months | 1 year | 5 of 110 patients progressed to THA |
| Haviv (2010) [19] | 10 | Débridement; microfracture | All patients–Outerbridge 4 Mild = < 30% acetabulum Moderate = > 30% acetabulum Severe = also femoral head |
90 | 55 F; 35 M | 55 | NR | Mild–2.2 years Moderate–1.2 years Severe–1.1 years Overall–1.5 years |
Pearson’s correlation Age versus time to THA r = −0.34 Severity cartilage change versus time to THA r = −0.35 If age < 55 years, time to THA 1.9 years If age > 55 years, time to THA 1.2 years (p = 0.004) |
| Horisberger (2010) [24] | 9 | Débridement, microfracture | Outerbridge ≥ 2, preoperative radiograph Tönnis ≥ 1 | 20 | 4 F; 16 M | 47 | NR | 1.4 years | 10/20 patients underwent THA Tönnis ≤ 2 less likely to progress to THA than Tönnis > 2 (p = 0.03) Age and Outerbridge score did not affect likelihood of progression to THA |
| Larson (2011) [32] | 13 | Débridement, microfracture | Group FAI–no joint space narrowing Tönnis 0 to 1 (n = 154) Group FAI OA–joint space narrowing Tönnis >1 (n = 56) |
227 (56) | Group FAI 81 F; 88 M Group FAI OA 13 F; 43 M |
Group FAI 32 Group FAI OA 45 |
NR | 3 years | 1 of 154 progressed to THA in Group FAI 20 of 56 progressed to THA in Group FAI OA Greater JSN predicted increased THA rate (p = 0.014) Greater duration symptoms predicted increased THA rate (p = 0.015) |
| Margheritini (1999) [35] | 9 | Débridement, removal loose bodies, synovectomy | OA diagnosed by radiograph, MRI, or arthroscopy (not specific) | 133 | 87 F; 46 M | 42 | NR | 7 months | 21 of 133 progressed to THA |
| McCarthy (2011) [36] | 13 | Resection, microfracture, removal loose bodies | Outerbridge 0 Outerbridge 1–2 Outerbridge 3–4 |
111 | 64 F; 47 M | 39 | NR | 4.8 years | Overall 10-year survivorship (not having THA) 63% Outerbridge Grade 1–2 10 year survivorship 80% Outerbridge Grade 3–4 10 year survivorship 12% OR needing THA 3.6 times if age > 40 years OR needing THA 20 times if acetabular Outerbridge 3–4 OR needing THA 58 times if femoral Outerbridge 3–4 Overall probability of THA in 10 years if Outerbridge 3–4 = 99% regardless of age |
| Palmer (2012) [44] | 15 | Chondroplasty, microfracture | Outerbridge 0–3 (n = 157) Outerbridge 4 (n = 44) Chondral defects < 1.5 cm2 (n = 201) |
201 (201) | 102 F; 99 M | 40 | 59 months | 46 months | 12/201 patients progressed to THA Significantly more patients with Outerbridge 4 progressed to THA (p = 0.03) |
| Philippon (2009) [46] | 12 | Chondroplasty, microfracture | Joint space measured on preoperative radiograph Outerbridge 1–2 (n = 74)–mild Outerbridge 3 (n = 29)–moderate Outerbridge 4 (n = 9)–poor |
112 | 62 F; 50 M | 41 | 34 months | 16 months | 10 of 112 patients progressed to THA OR progressing to THA 39 times if preoperative joint space < 2.0 mm |
| Wilkin (2014) [57] | 9 | Débridement | Tönnis grading on radiograph | 41 | 31 F; 10 M | 52 | NR | 21.3 months | 6 of 41 patients progressed to THA |
* Mean (SD); OA = osteoarthritis; FAI = femoroacetabular impingement; F = female; M = male; Tönnis = preoperative grading of osteoarthritis using Tönnis scale; Outerbridge = intraoperative grading of chondral injury; NR = not reported.
More severe chondral disease increased the risk of THA in all papers that evaluated it as a risk factor. The likelihood of progression was 20 times greater with acetabular chondral disease (Outerbridge Grades III and IV) and 58 times greater with femoral chondral disease (Outerbridge Grades III and IV) [36], whereas greater severity of chondropathy was correlated with increased THA risk (r = −0.35) [19]. Horisberger et al. [24] also reported that time from arthroscopy to THA was 1.2 years in a cohort (mean age, 47 years; 20% women) with Outerbridge Grade II to IV chondropathy. Similarly, the likelihood of conversion to THA was 39 times greater if preoperative radiographic joint space was < 2 mm [46]. Larson et al. [32] reported that joint space narrowing (JSN) (p = 0.014) and longer duration of symptoms (p = 0.015) predicted higher likelihood of conversion to THA.
Adverse Events
Adverse events were reported in six studies [8, 16, 27, 32, 44, 50]. Collectively, 27 of 435 participants (6%) experienced an adverse event, including transient pudendal nerve (n = 5), lateral femoral cutaneous nerve (n = 6), and sciatic nerve hypoesthesia (n = 4); heterotrophic bone formation (n = 5); deep vein thrombosis (n = 1); and postoperative infection (n = 6).
Discussion
This systematic review evaluated outcomes after hip arthroscopy in people with and without hip OA and examined the risk of progression to THA. Included studies demonstrated considerable variability in methodological quality, sample size, definition of hip OA, surgical intervention, and followup times, which limited opportunities for meta-analysis. Our findings suggest that in people with hip OA, hip arthroscopy has a moderate to large effect, indicating an improvement of patient-reported outcomes after hip arthroscopy, although positive effects may be inflated as a result of methodological limitations of the included studies. However, the between-group effect sizes imply that people with no OA have a substantially larger positive benefit in pain and function after hip arthroscopy than those with hip OA. The average time to THA was 2 years (range, 7 months to 5 years) after hip arthroscopy in those with hip OA. Increasing age and severity of hip OA were associated with a higher likelihood and more rapid progression to THA. Notably, these findings were consistent across a range of surgical procedures.
Several methodological limitations in the studies included in this review require consideration. Included studies demonstrated considerable variability in methodological quality, sample size, definition of hip OA, surgical intervention, and followup times. This variability limited opportunities for meta-analysis. There was also no opportunity to perform a sensitivity analysis given the lack of meta-analyses undertaken. The variability also restricted the direct comparisons between studies. Furthermore, the reported improvements are likely to be affected by bias, including lack of blinding of participants and surgeons, lack of randomization to a sham surgery or nonsurgical control group, and lack of reporting of characteristics of nonincluded patients, preventing the reader from determining whether the included subjects accurately represented a population of people with hip OA. These biases are difficult to overcome in surgical studies, but confidence in the reported findings is reduced by their presence. As a result of the absence of required data, SMDs could only be calculated for six studies. Overall, the methodological quality of the majority of included studies was moderate at best, which limits the robustness of findings.
We chose to include studies that examined patients aged 17 years and older. Although this age range is wide, it was used to reflect contemporary clinical practice, where findings of chondropathy are reported in this younger age group and therefore warrant evaluation. We also included studies with OA defined from preoperative radiographs and with chondropathy seen at arthroscopy. Although these definitions are different, we feel that they reflect the continuum of degenerative hip disease. Radiographic change reflects advanced OA only and does not capture degenerative joint disease in its early stages [48]. As such, both warrant inclusion in this systematic review.
When considering the effect of hip arthroscopy in people with hip OA, there were moderate to large SMDs in patient-reported outcomes observed. Thus, it appears that people with hip OA or chondropathy may derive benefits from such surgeries. However, such reported improvements are likely to be affected by biases outlined previously. Future studies examining hip arthroscopy for hip OA such as those of RCT design are required to confirm that the moderate to large positive effects reported in this systematic review are genuine and that hip arthroscopy can offer an improvement in pain and function in people with this burdensome disease. Age also appears to impact on outcomes for hip arthroscopy in people with hip OA. McCormick et al. [37] reported the likelihood of poor outcome was seven times higher in patients aged older than 40 years. It is uncertain whether this effect is the result of more advanced degenerative change or other changes in self-reported pain and function associated with older age. Physical function has been demonstrated to be associated with age in a general elderly population [6] and in people after THA [43]. It is possible that poorer outcomes in older people with hip OA may be associated with advancing age; however, this requires further investigation.
When surgical procedures for hip OA were combined with surgical correction for FAI, results were varied and appear to depend how FAI was treated. When treating patients with cam FAI only, large within-subject effect sizes [21] and large postoperative improvements [26] were reported regardless of the degree of cartilage disease at surgery. This suggests that patients with cam-type FAI have good short-term outcomes (ie, up to 30 months) regarding pain and physical function regardless of degree of chondral damage. In contrast, studies where cam and pincer FAI were combined reported a high conversion rate to THA and worse outcomes when preexisting radiographic hip OA, radiographic JSN, or coexisting cartilage disease were present [24, 46]. These findings suggest that cam and pincer FAI may affect intraarticular loads within the hip differently, resulting in varied outcomes when treated. The effect of FAI type and its surgical correction on outcomes is unclear and requires further investigation.
Emerging arthroscopic interventions such as fibrin adhesive and ACT demonstrate large SMDs and small CIs at 12 months in people with hip OA and acetabular cartilage delamination. Although these techniques show promise in patients with contained severe chondral lesions, high-quality comparative studies are required to confirm findings. Ideally, such studies should be a RCT design to minimize bias with longer-term followup. Those additional studies are particularly important given findings in the knee, where initial reports of such techniques were positive, but findings were unable to be confirmed with robust clinical trials [56].
When comparing the effects of hip arthroscopy in people with hip OA with those without hip OA, positive effects were smaller in those with hip OA. In the majority of studies, larger within-group SMDs were reported for those without hip OA compared with those with hip OA when groups were classified based on preoperative radiographic degeneration or chondral pathology of greater severity [14, 18, 50]. This finding is supported by the between-group SMDs for outcomes presented in Fig. 4, in which a substantially larger positive benefit of hip arthroscopy was reported in all three studies [14, 18, 50]. However, Palmer et al. [44], the study with the highest methodology quality score and the longest followup time of almost 4 years, did not find a difference in within-group outcomes for either group, and each group experienced significant improvement (p < 0.001). Therefore, it is possible that over a longer time period, people with hip OA and without OA will gain a similar benefit from hip arthroscopy. Moreover, those with hip OA may have a slower recovery than those without after hip arthroscopy. However, more studies with longer-term followup are required to confirm this finding.
Importantly, survivorship in avoiding THA was surprisingly low. The overall mean time to THA was 2 years in patients with hip OA who progressed to THA. In the study with the longest followup (13 years), 33% of all patients with hip OA had progressed to THA by 5 years [36]. Furthermore, the survivorship appears to differ, depending on the severity and location of cartilage disease. More severe chondropathy was a consistent predictor of progression to THA [36]. In addition, the odds of an individual requiring a THA was substantially higher if cartilage disease was femoral rather than acetabular [36]. Age also appeared to influence the likelihood of progression to THA, with people aged older than 40 years being 3.6 times more likely to progress to THA [36] and seven times more likely to have a poor outcome after hip arthroscopy [37]. However, these observations may not be independent of degenerative change severity, ability or motivation to complete postsurgical rehabilitation, or changes in self-reported pain and function associated with older age [6]. Clinicians should consider that older patients with more severe disease are more likely to require THA after arthroscopy and should consider this when undertaking postarthroscopic rehabilitation.
Guidelines for the management of hip OA highlight the importance of nonsurgical management, including education, weight management, and targeted exercise programs addressing specific physical impairments [58]. This review confirms that outcomes for hip arthroscopy are less satisfactory when OA has advanced. Although no studies have provided a direct comparison between surgical and nonsurgical OA hip interventions [29], evidence at the knee suggests that surgical treatment of OA and meniscal degeneration does not provide superior outcomes to nonsurgical interventions [28]. Thus, it is plausible that, for those with hip degenerative disease, hip arthroscopy may not provide benefits beyond those likely with a nonsurgical approach. Future comparative studies are essential to determine the efficacy of nonsurgical and surgical approaches and to differentiate the effects on outcomes in those with early versus late-stage disease.
In conclusion, this review found that patients with hip OA derive a moderate to large benefit in pain and functional outcomes from hip arthroscopy, at least in the short term, although positive effects may be inflated as a result of methodological limitations of the included studies. In addition, this benefit is smaller than that for people with less severe OA or no hip OA. This appears to be irrespective of the interventions performed. The likelihood of progression to THA after hip arthroscopy occurred on average within 2 years for those who progressed. Chondropathy severity and patient age were associated with a higher risk and more rapid progression to THA after hip arthroscopy. Notably, adverse events appear to be few and transient. However, the efficacy of hip arthroscopy compared with nonsurgical interventions for pain and function in people with hip OA is needed to be confirmed through high-quality, comparative studies.
Electronic supplementary material
Footnotes
Each author certifies that he or she, or a member of his or her immediate family, has no funding or commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research ® editors and board members are on file with the publication and can be viewed on request.
This work was performed at the University of Queensland, Brisbane, Australia.
References
- 1.Agricola R, Heijboer M, Bierma-Zeinstra S, Verhaar J, Weinans H, Waarsing J. Cam-type deformities strongly predict total hip replacement within 5 years in those with early symptomatic OA: a prospective cohort study (check) Osteoarthritis Cartilage. 2012;20(Suppl 1):S203. doi: 10.1016/j.joca.2012.02.330. [DOI] [Google Scholar]
- 2.Altman R, Alarcon G, Appelrouth D, Bloch D, Borenstein D, Brandt K, Brown C, Cooke TD, Daniel W, Feldman D, Greenwald R, Hochberg M, Howell D, Ike R, Kapila P, Kaplan D, Koopman W, Marino C, McDonald E. The American College of Rheumatology criteria for the classification and reporting of osteoarthritis of the hip. Arthritis Rheum. 1991;34:505–514. doi: 10.1002/art.1780340502. [DOI] [PubMed] [Google Scholar]
- 3.Arthritis Australia. Arthritis—The Bottom Line. The Economic Impact of Arthritis in Australia. Canberra, Australia: Arthritis Australia; 2005.
- 4.Barton C, Lack S, Malliaras P, Morrissey D. Gluteal muscle activity and patellofemoral pain syndrome: a systematic review. Br J Sports Med. 2013;47:207–214. doi: 10.1136/bjsports-2012-090953. [DOI] [PubMed] [Google Scholar]
- 5.Bauer G, Kinzl L. Arthrodesis of the ankle joint [in German] Orthopade. 1996;25:158–165. doi: 10.1007/s001320050028. [DOI] [PubMed] [Google Scholar]
- 6.Beckett LA, Brock DB, Lemke JH, de Leon CFM, Guralnik JM, Fillenbaum GG, Branch LG, Wetle TT, Evans DA. Analysis of change in self-reported physical function among older persons in four population studies. Am J Epidemiol. 1996;143:766–778. doi: 10.1093/oxfordjournals.aje.a008814. [DOI] [PubMed] [Google Scholar]
- 7.Bedi A, Chen N, Robertson W, Kelly BT. The management of labral tears and femoroacetabular impingement of the hip in the young, active patient. Arthroscopy. 2008;24:1135–1145. doi: 10.1016/j.arthro.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 8.Byrd JT, Jones KM. Arthroscopic management of femoroacetabular impingement: minimum 2-year follow-up. Arthroscopy. 2011;27:1379–1388. doi: 10.1016/j.arthro.2011.05.018. [DOI] [PubMed] [Google Scholar]
- 9.Byrd JW, Jones KS. Prospective analysis of hip arthroscopy with 2-year follow-up. Arthroscopy. 2000;16:578–587. doi: 10.1053/jars.2000.7683. [DOI] [PubMed] [Google Scholar]
- 10.Cohen J. Statistical Power Analysis for the Behavioral Sciences. Hillsdale, NJ, USA: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 11.Collins N, Bisset L, McPoil T, Vicenzino B. Foot orthoses in lower limb overuse conditions: a systematic review and meta-analysis. Foot Ankle Int. 2007;28:396–412. doi: 10.3113/FAI.2007.0396. [DOI] [PubMed] [Google Scholar]
- 12.Deeks JJ, Dinnes J, D’Amico R, Sowden AJ, Sakarovitch C, Song F, Petticrew M, Altman DG. Evaluating non-randomised intervention studies. Health Technol Assess. 2003;7:iii–x, 1–173. [DOI] [PubMed]
- 13.Downs S, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52:377–384. doi: 10.1136/jech.52.6.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Egerton T, Hinman RS, Takla A, Bennell KL, O’Donnell J. Intraoperative cartilage degeneration predicts outcome 12 months after hip arthroscopy. Clin Orthop Relat Res. 2013;471:593–599. doi: 10.1007/s11999-012-2594-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Felson DT. Epidemiology of hip and knee osteoarthritis. Epidemiol Rev. 1988;10:1–28. doi: 10.1093/oxfordjournals.epirev.a036019. [DOI] [PubMed] [Google Scholar]
- 16.Fontana A, Bistolfi A, Crova M, Rosso F, Massazza G. Arthroscopic treatment of hip chondral defects: autologous chondrocyte transplantation versus simple débridement—a pilot study. Arthroscopy. 2012;28:322–329. doi: 10.1016/j.arthro.2011.08.304. [DOI] [PubMed] [Google Scholar]
- 17.Gedouin JE, Duperron D, Langlais F, Thomazeau H. Update to femoroacetabular impingement arthroscopic management. Orthop Traumatol Surg Res. 2010;96:222–227. doi: 10.1016/j.otsr.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 18.Gedouin JE, May O, Bonin N, Nogier A, Boyer T, Sadri H, Villar RN, Laude F. Assessment of arthroscopic management of femoroacetabular impingement. A prospective multicenter study. Orthop Traumatol Surg Res. 2010;96:S59–S67. [DOI] [PubMed]
- 19.Haviv B, O’Donnell J. The incidence of total hip arthroplasty after hip arthroscopy in osteoarthritic patients. Sports Med Arthrosc Rehabil Ther Technol. 2010;2:18. doi: 10.1186/1758-2555-2-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haviv B, O’Donnell J. Arthroscopic treatment for acetabular labral tears of the hip without bony dysmorphism. Am J Sports Med. 2011;39(Suppl):79S–84S. doi: 10.1177/0363546511412915. [DOI] [PubMed] [Google Scholar]
- 21.Haviv B, Singh PJ, Takla A, O’Donnell J. Arthroscopic femoral osteochondroplasty for cam lesions with isolated acetabular chondral damage. J Bone Joint Surg Br. 2010;92:629–633. doi: 10.1302/0301-620X.92B5.23667. [DOI] [PubMed] [Google Scholar]
- 22.Helenius I, Tanskanen P, Haapala J, Niskanen R, Remes V, Mokka R, Korkala O. Hip arthroscopy in osteoarthritis: a review of 68 patients. Scand J Surg. 2001;90:28–31. [PubMed] [Google Scholar]
- 23.Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Chichester, West Sussex, UK: Wiley Blackwell; 2008. [Google Scholar]
- 24.Horisberger M, Brunner A, Herzog RF. Arthroscopic treatment of femoral acetabular impingement in patients with preoperative generalized degenerative changes. Arthroscopy. 2010;26:623–629. doi: 10.1016/j.arthro.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 25.Ilizaliturri VM, Jr, Gonzalez-Gutierrez B, Gonzalez-Ugalde H, Camacho-Galindo J. Hip arthroscopy after traumatic hip dislocation. Am J Sports Med. 2011;39(Suppl):50S–57S. doi: 10.1177/0363546511411642. [DOI] [PubMed] [Google Scholar]
- 26.Ilizaliturri VM, Jr, Nossa-Barrera JM, Acosta-Rodriguez E, Camacho-Galindo J. Arthroscopic treatment of femoroacetabular impingement secondary to paediatric hip disorders. J Bone Joint Surg Br. 2007;89:1025–1030. doi: 10.1302/0301-620X.89B8.19152. [DOI] [PubMed] [Google Scholar]
- 27.Kamath AF, Componovo R, Baldwin K, Israelite CL, Nelson CL. Hip arthroscopy for labral tears: review of clinical outcomes with 4.8-year mean follow-up. Am J Sports Med. 2009;37:1721–1727. doi: 10.1177/0363546509333078. [DOI] [PubMed] [Google Scholar]
- 28.Katz JN, Brophy RH, Chaisson CE, de Chaves L, Cole BJ, Dahm DL, Donnell-Fink LA, Guermazi A, Haas AK, Jones MH, Levy BA, Mandl LA, Martin SD, Marx RG, Miniaci A, Matava MJ, Palmisano J, Reinke EK, Richardson BE, Rome BN, Safran-Norton CE, Skoniecki DJ, Solomon DH, Smith MV, Spindler KP, Stuart MJ, Wright J, Wright RW, Losina E. Surgery versus physical therapy for a meniscal tear and osteoarthritis. N Engl J Med. 2013;368:1675–1684. doi: 10.1056/NEJMoa1301408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kemp JL, Collins NJ, Makdissi M, Schache AG, Machotka Z, Crossley K. Hip arthroscopy for intra-articular pathology: a systematic review of outcomes with and without femoral osteoplasty. Br J Sports Med. 2012;46:632–643. doi: 10.1136/bjsports-2011-090428. [DOI] [PubMed] [Google Scholar]
- 30.Kemp JL, Collins NJ, Roos EM, Crossley KM. Psychometric properties of patient-reported outcome measures for hip arthroscopy. Am J Sports Med. 2013;41:2065–2073. doi: 10.1177/0363546513494173. [DOI] [PubMed] [Google Scholar]
- 31.Landis JR, Koch GG. Agreement methods for categorical data. Biometrics. 1977;33:159–174. doi: 10.2307/2529310. [DOI] [PubMed] [Google Scholar]
- 32.Larson CM, Giveans MR, Taylor M. Does arthroscopic FAI correction improve function with radiographic arthritis? Clin Orthop Relat Res. 2011;469:1667–1676. doi: 10.1007/s11999-010-1741-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lawrence RC, Helmick CG, Arnett FC, Deyo RA, Felson DT, Giannini EH, Heyse SP, Hirsch R, Hochberg MC, Hunder GG, Liang MH, Pillemer SR, Steen VD, Wolfe F. Estimates of the prevalence of arthritis and selected musculoskeletal disorders in the United States. Arthritis Rheum. 1998;41:778–799. doi: 10.1002/1529-0131(199805)41:5<778::AID-ART4>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 34.Liberati A, Tetzlaff, Mulrow, Gøtzsche The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6:1–28. doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Margheritini F, Villar RN. The efficacy of arthroscopy in the treatment of hip osteoarthritis. Chir Organi Mov. 1999;84:257–261. [PubMed] [Google Scholar]
- 36.McCarthy JC, Jarrett BT, Ojeifo O, Lee JA, Bragdon CR. What factors influence long-term survivorship after hip arthroscopy? Clin Orthop Relat Res. 2011;469:362–371. doi: 10.1007/s11999-010-1559-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McCormick F, Nwachukwu BU, Alpaugh K, Martin SD. Predictors of hip arthroscopy outcomes for labral tears at minimum 2-year follow-up: the influence of age and arthritis. Arthroscopy. 2012;28:1359–1364. doi: 10.1016/j.arthro.2012.04.059. [DOI] [PubMed] [Google Scholar]
- 38.McDonald JE, Herzog MM, Philippon MJ. Return to play after hip arthroscopy with microfracture in elite athletes. Arthroscopy. 2014;29:330–335. doi: 10.1016/j.arthro.2012.08.028. [DOI] [PubMed] [Google Scholar]
- 39.Millenium Research Group. Millennium research group report 2009. October 26, 2009. Available at: http://www.bio-medicine.org/medicine-news-1/Hip-Arthroscopy-Procedures-to-Soar-Through-2013-51045-1/. Accessed November 10, 2011.
- 40.Montgomery SR, Ngo SS, Hobson T, Nguyen S, Alluri R, Wang JC, Hame SL. Trends and demographics in hip arthroscopy in the United States. Arthroscopy. 2013;29:661–665. doi: 10.1016/j.arthro.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 41.Ng VY, Arora N, Best TM, Xueliang P, Ellis TJ. Efficacy of surgery for femoroacetabular impingement: a systematic review. Am J Sports Med. 2010;38:2337–2345. doi: 10.1177/0363546510365530. [DOI] [PubMed] [Google Scholar]
- 42.Nicholls AS, Kiran A, Pollard TCB, Hart DJ, Arden CPA, Gill T, Murray DW, Carr AJ, Arden NK. The association between hip morphology parameters and nineteen-year risk of end-stage osteoarthritis of the hip: a nested case-control study. Arthritis Rheum. 2011;63:3392–3400. doi: 10.1002/art.30523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nilsdotter AK, Lohmander LS. Age and waiting time as predictors of outcome after total hip replacement for osteoarthritis. Rheumatology. 2002;41:1261–1267. doi: 10.1093/rheumatology/41.11.1261. [DOI] [PubMed] [Google Scholar]
- 44.Palmer DH, Ganesh V, Comfort T, Tatman P. Midterm outcomes in patients with cam femoroacetabular impingement treated arthroscopically. Arthroscopy. 2012;28:1671–1681. doi: 10.1016/j.arthro.2012.04.154. [DOI] [PubMed] [Google Scholar]
- 45.Parvizi J, Bican O, Bender B, Mortazavi SMJ, Purtill JJ, Erickson J, Peters C. Arthroscopy for labral tears in patients with developmental dysplasia of the hip: a cautionary note. J Arthroplasty. 2009;24:110–113. doi: 10.1016/j.arth.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 46.Philippon MJ, Briggs KK, Yen Y, Kuppersmith DA. Outcomes following hip arthroscopy for femoroacetabular impingement with associated chondrolabral dysfunction: minimum two-year follow-up. J Bone Joint Surg Br. 2009;91:16–23. doi: 10.1302/0301-620X.91B1.21329. [DOI] [PubMed] [Google Scholar]
- 47.Portney L, Watkins M. Foundations of Clinical Research-Applications to Clinical Practice. Upper Saddle River, NJ, USA: Pearson Education; 2009. [Google Scholar]
- 48.Pritzker KP, Gay S, Jimenez SA, Ostergaard K, Pelletier JP, Revell PA, Salter D, van den Berg WB. Osteoarthritis cartilage histopathology: grading and staging. Osteoarthritis Cartilage. 2006;14:13–29. doi: 10.1016/j.joca.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 49.Saunders LD, Soomro GM, Buckingham J, Jamtvedt G, Raina P. Assessing the methodological quality of nonrandomized intervention studies. West J Nurs Res. 2003;25:223–237. doi: 10.1177/0193945902250039. [DOI] [PubMed] [Google Scholar]
- 50.Stahelin L, Stahelin T, Jolles BM, Herzog RF. Arthroscopic offset restoration in femoroacetabular cam impingement: accuracy and early clinical outcome. Arthroscopy. 2008;24(51–57):e51. doi: 10.1016/j.arthro.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 51.Streich NA, Gotterbarm T, Barie A, Schmitt H. Prognostic value of chondral defects on the outcome after arthroscopic treatment of acetabular labral tears. Knee Surg Sports Traumatol Arthrosc. 2009;17:1257–1263. doi: 10.1007/s00167-009-0833-x. [DOI] [PubMed] [Google Scholar]
- 52.Streich NA, Schmitt H. Does a cartilage defect affect the result of partial acetabular labrum resection? Sports Orthopedics and Traumatology. 2008;23:260–263. [Google Scholar]
- 53.Tijssen M, van Cingel R, van Melick N, de Visser E. Patient-Reported Outcome questionnaires for hip arthroscopy: a systematic review of the psychometric evidence. BMC Musculoskelet Disord. 2011;12:117–124. doi: 10.1186/1471-2474-12-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tönnis D, Heinecke A. Acetabular and femoral anteversion: relationship with osteoarthritis of the hip. J Bone Joint Surg Am. 1999;81:1747–1770. doi: 10.2106/00004623-199912000-00014. [DOI] [PubMed] [Google Scholar]
- 55.Tzaveas AP, Villar RN. Arthroscopic repair of acetabular chondral delamination with fibrin adhesive. Hip Int. 2010;20:115–119. doi: 10.1177/112070001002000117. [DOI] [PubMed] [Google Scholar]
- 56.Vasiliadis HS, Wasiak J. Autologous chondrocyte implantation for full thickness articular cartilage defects of the knee. Cochrane Database Syst Rev. 2010;10:CD003323. [DOI] [PMC free article] [PubMed]
- 57.Wilkin G, March G, Beaulé P. Arthroscopic acetabular labral débridement in patients forty-five years of age or older has minimal benefit for pain of function. J Bone Joint Surg Am. 2014;96:113–118. doi: 10.2106/JBJS.L.01710. [DOI] [PubMed] [Google Scholar]
- 58.Zhang W, Nuki G, Moskowitz RW, Abramson S, Altman RD, Arden NK, Bierma-Zeinstra S, Brandt KD, Croft P, Doherty M, Dougados M, Hochberg M, Hunter DJ, Kwoh K, Lohmander LS, Tugwell P. OARSI recommendations for the management of hip and knee osteoarthritis. Part III: changes in evidence following systematic cumulative update of research published through January 2009. Osteoarthritis Cartilage. 2010;18:476–499. doi: 10.1016/j.joca.2010.01.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.