Abstract
Background
There are several options for proximal humerus reconstruction in young children after resection of a malignant tumor and no one technique has been definitively shown to be superior to others, leaving the decision to surgeon and patient choice. Claviculo pro humeri (CPH) is a biologic reconstruction of the proximal humerus using the patient’s ipsilateral clavicle as a rotational osseous flap. CPH represents a potential option for this complicated clinical problem in very young children, but little is known about it because the indications for its use are so uncommon.
Questions/purposes
The purposes of this study were to (1) assess the oncologic outcomes of CPH at a minimum of 2 years in a small series of patients; (2) elicit the complications associated with this procedure; and (3) show the Musculoskeletal Tumor Society (MSTS) functional score of these patients.
Methods
Four patients (average age, 5 years 11 months; range, 4 years 5 months to 8 years 9 months at the time of surgery) were treated with CPH for reconstruction after resection of a proximal humerus sarcoma; this represented all of the patients treated with this approach for this problem between January 2008 and April 2011 at one institution. During this period, the general indications for using CPH were the need to reconstruct a proximal humerus defect in a child younger than 10 years of age. During this time, CPH was used for all patients treated for proximal humerus sarcomas meeting these criteria. Patient demographics, diagnosis, tumor size and extent, operative details, radiographs and MRIs, complications, and functional outcomes were assessed.
Results
All are alive with no evidence of disease at a minimum followup of 31 months (average, 43 months; range, 31–58 months). Two patients developed nonunion and underwent revision surgery. Osseous union and a stable neoshoulder articulation were ultimately obtained in all patients. Limited shoulder motion was the only functional deficit noted with forward elevation ranging between 30° and 90°. MSTS functional scores were excellent with a range of 87% to 90%.
Conclusions
This is a rarely used procedure in North America but we achieved functional limb salvage in all four patients. Consistent with prior literature, nonunion was the major complication in this series. The two nonunions were successfully treated without interruption of chemotherapy or significant bone graft donor site morbidity. Based on these results, the authors suggest that this procedure is a reasonable reconstruction option to consider after proximal humerus resection in patients younger than 10 years of age. Further followup will be required to assess long-term results and to determine how this procedure compares with the alternatives.
Level of Evidence
Level IV, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
Reconstruction of the proximal humerus in young children poses unique challenges because of the relative instability of the shoulder, the frequent involvement of the rotator cuff and axillary nerve that usually are resected to obtain adequate margins, and the reported higher proportion of cases requiring extraarticular resection relative to other joints [1, 12]. The best method of proximal humerus reconstruction in young children after oncologic resection has not been elucidated. Multiple reconstruction options have been advocated including osteoarticular allografts, endoprostheses, allograft-prosthetic composites (APCs), resection arthroplasties, arthrodeses using allograft bone, and vascularized fibulas. Each method has unique advantages and disadvantages (Table 1). Few address the issue of progressive limb length inequality, which invariably results from proximal humerus resections in the very young. Osteoarticular allograft reconstruction of the proximal humerus remains a preferred option at some centers. The reported results in young children are very limited. Muscolo et al. [8] reported the largest series of bulk allografts in patients aged 10 years or younger. Unfortunately, only one of the 22 reported cases was an osteoarticular proximal humerus. Similarly, a series of 16 osteoarticular proximal humerus allografts by Getty and Peabody contained no patients younger than 10 years of age [3]. Data on APC reconstructions of the proximal humerus in young children are also very limited. The largest APC series (36 cases) in the literature by Abdeen et al. had a median patient age of 23 years [1]. The number of patients younger than 10 years of age was not specified. Notably, the only case of instability in their entire series occurred in a 9 year old.
Table 1.
Inherent differences of reconstruction techniques used after proximal humerus resection in the skeletally immature
| Procedure | Donor morbid | Nonunion | Microsurgical skill | Loosening | Growth |
|---|---|---|---|---|---|
| Claviculo pro humeri | Local Only | Yes | No | No | Yes, some |
| Osteoarticular allograft | No | Yes | No | No | No |
| Vascularized proximal fibula epiphysis | Yes | Yes | Yes | No | Yes |
| Allograft prosthetic composite | No | Yes | No | Yes | No |
| Endoprosthesis | No | No | No | Yes | No |
| Resection arthroplasty | No | No | No | No | No |
| Arthrodesis allograft | No | Yes | No | No | No |
| Arthrodesis autograft | Yes | Yes | No | No | No |
The claviculo pro humeri (CPH) procedure was originally described as a treatment for congenital upper limb deficiency [13]. It was later modified for the treatment of shoulder girdle resections resulting from oncologic resections of sarcomas [16]. The CPH procedure is a biologic reconstruction of the proximal humerus using the patient’s ipsilateral clavicle. It does not require microsurgical anastomoses or distant donor tissue. Unlike any of the previously mentioned procedures, CPH maintains inherent stability of the transposed bone segment through retention of the acromioclavicular ligaments. Native blood supply to the lateral portion of the clavicle is also maintained through the thoracoacromial trunk. These advantages make CPH an attractive option, especially in young children. This technique has been described by other centers in Europe [12], Asia [9, 11, 14, 15], and Australia [6], but only a small number of cases has been reported. The number of patients who might be candidates for this procedure is low; therefore, assessment of feasibility and safety of the procedure by reporting more cases is important, because even at a busy tumor center, it is unlikely that any one group—or even a multicenter study—will ever accrue a large series.
We therefore sought to (1) assess the oncologic outcomes of CPH at a minimum of 2 years in a small series of patients; (2) elicit the complications associated with this procedure; and (3) show the Musculoskeletal Tumor Society (MSTS) functional score of these patients.
Patients and Methods
Inclusion Criteria, Surgical Indications, and Followup
This study was a chart review of all patients treated with the CPH approach at one center for reconstruction after resection of proximal humerus sarcoma. Between January 2008 and April 2011, we performed four CPH reconstructions in four patients (average age, 5 years 11 months; range, 4 years 5 months to 8 years 9 months at the time of surgery) after resections of proximal humerus sarcomas. Three of these patients underwent the procedure for osteosarcoma and one for Ewing’s sarcoma. Three patients are right hand-dominant and one is left hand-dominant. During this period, the general indications for using CPH in this setting were resection of the proximal humerus for sarcoma in a child younger than 10 years of age. During this time, CPH was used for all patients treated for proximal humerus sarcomas who were younger than 10 years of age. The clinical records of those four patients were reviewed in detail. Institutional review board approval of the study institution was obtained. Patient demographics, diagnosis, tumor size and extent, operative details, radiographs and MRIs, complications, and functional outcomes were assessed. Detailed review of complications requiring reoperation was undertaken in an effort to elucidate potential areas for improvement. Functional outcomes were assessed at each postoperative visit using the MSTS 1993 scoring system [2].
Surgical Technique
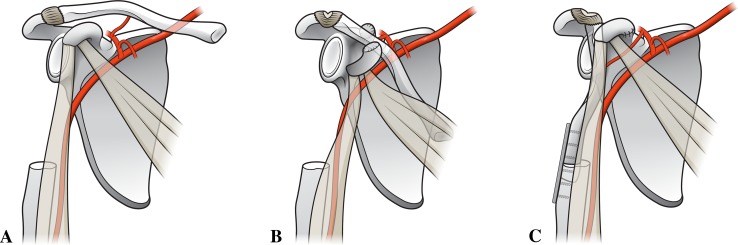
After neoadjuvant chemotherapy and preoperative staging, all patients underwent wide resection of the primary tumor using a transdeltoid approach incorporating the biopsy site and extending anterolaterally along the humerus. All resections were intraarticular; however, two of the four required sacrifice of the axillary nerve and most of the deltoid to obtain a wide surgical margin (Fig. 1A). Surgical margins were negative in all cases. The average resection length was 13.9 cm. The reconstructive phase started with extension of the incision medially along the ipsilateral clavicle. Great care was taken to preserve the clavicular and acromial branches of the thoracoacromial trunk, which supply the lateral aspect of the clavicle. Periosteum of the clavicle was preserved with the bone to improve viability of the rotated segment. The clavicle was osteotomized just lateral to the sternoclavicular joint. The coracoid process was osteotomized at its base to permit mobilization of the clavicular segment in all cases. Detachment of the sternocleidomastoid, pectoralis major, trapezius, and subclavius muscles permitted mobilization of the clavicle while maintaining the intact acromioclavicular ligaments and thoracoacromial branches laterally (Fig. 1B). In two cases, preoperative planning indicated that 75% of the humerus required resection. A nonvascularized fibular autograft was harvested from the ipsilateral leg and used to augment the length of the clavicle segment. Internal fixation of the clavicle to the humerus or clavicle to fibula to humerus junctions was performed with a small-fragment reconstruction plate (Figs. 1C, 2). Suture repair of the coracoid process fragment was performed at the time of closure (Fig. 1C). No rotation or free flaps were required for closure. A sling and swath was used for 6 weeks of postoperative immobilization. Shoulder and elbow ROM were then initiated under the guidance of a pediatric physical therapist. Adjuvant chemotherapy was initiated within 2 weeks of surgery in all patients.
Fig. 1A–C.
Illustration of the CPH procedure demonstrates (A) resection of the proximal humerus, (B) rotation of the clavicle with maintained distal blood supply and ligamentous support, and (C) fixation of the clavicle to the residual humerus and repair of the coracoid process, which is released to facilitate rotation of the flap. Illustrations by Medical Graphics & Photography, University of Utah School of Medicine.
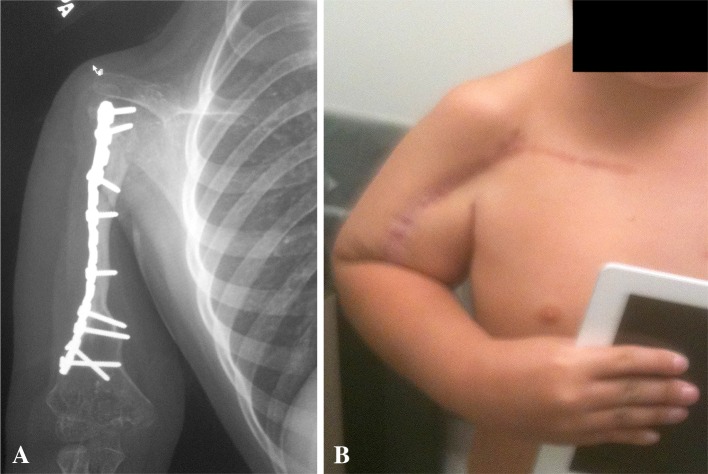
Fig. 2A–B.
Radiograph (A) and clinical photograph (B) show a CPH reconstruction.
Results
Oncologic Outcomes
All patients resumed chemotherapy within 2 weeks of surgery, and none required a delay at any point in their adjuvant chemotherapy treatment as a result of surgical complications. All patients are alive without evidence of local or distant recurrence at a mean of 50.8 months (range, 39–66 months) after surgery.
Complications
The two patients who were reconstructed with simultaneous nonvascularized fibular intercalary autografts as a result of the insufficient length of the clavicle experienced nonunions of the autograft fibula segments at the proximal junction site. Both nonunions were atrophic, and the internal fixation plates fractured in both cases. One of the nonunions was first treated with repeat nonvascularized fibular autografting. This patient again experienced atrophic nonunion and plate failure. The patient was then treated with onlay vascularized fibular shaft strut grafting using the contralateral fibulae. The second patient experiencing nonunion was treated with onlay vascularized fibular shaft strut grafting without an intervening nonvascularized procedure and healed. There were no surgical or functional complications at any of the fibular donor sites, and both humeral revisions achieved union without further complications. None of the four patients developed a surgical site or deep infection.
Functional Outcome (MSTS scores)
MSTS functional scores were excellent with a range of 87% to 90%. The only MSTS subcategories with significant deficits were hand positioning and lifting. All children were able to abduct to at least 45° and forward flex to a minimum of 60°. Internal rotation to across the chest to reach the other arm was uniformly able to be performed. Children are able to groom themselves and mange personal hygiene without any difficulties. Use of the hand and elbow was entirely normal. Once radiographic evidence of bony union was established, children were not restricted from any activity. No patient has shoulder instability or prominent hardware. Emotional acceptance of the patients and their families was excellent, and all would recommend the procedure to others.
Discussion
The best method of proximal humerus reconstruction in young children after oncologic resection has not been elucidated. Multiple reconstruction options have been advocated with each having unique advantages and disadvantages. The number of patients who might be candidates for this procedure is low; therefore, assessment of feasibility and safety of the procedure by reporting more cases is important, because even at a busy tumor center, it is unlikely that any one group—or even a multicenter study—will ever accrue a large series. We describe the CPH procedure and its suitability for young pediatric patients with a sarcoma of the proximal humerus. All four patients had local control of their tumor and achieved functional limb salvage with a painless, stable neohumeri and excellent hand, wrist, and elbow function. However, two of the four patients developed nonunions, and both underwent further surgery for this.
A major limitation of this report is the small size of the series and lack of a direct comparison cohort. As a result of the rarity of proximal humerus sarcomas in young children, this limitation is true of all series addressing this problem. The followup of these patients was too short to determine how this reconstruction will work when the patient reaches skeletal maturity and how durable it will be in adulthood. Late complications related to the abnormal shoulder articulation may become more apparent with longer followup. We acknowledge that MSTS scores that take into account occupational considerations may not be the ideal outcome measure in this young age group, but it is commonly used to assess outcomes after tumor surgery. We also have no comparison group to demonstrate whether this procedure is superior to any other option.
In our small number of patients, we were able to achieve adequate local control and all of our patients have survived to date.
We had no local recurrences to date in these patients. This suggests that this is a safe procedure from an oncologic point of view although the sample size is limited. Because we only studied four patients, we cannot directly compare our local recurrence with that in the literature.
Two of the four patients experienced nonunions, and both patients underwent further surgery to treat this problem. Both nonunions were ultimately salvaged by onlay vascularized fibular autografts to achieve union. Prior CPH series (Table 2) reported similar results with high proportions of the patients achieving limb salvage and good function, but reoperations were common to treat nonunions and other complications. The largest series of which we are aware, that of Rodl et al., reported 15 CPH procedures with 10 patients undergoing a total of 15 reoperations [12]. Notably, the Rodl et al. study found that the patients undergoing CPH had higher final MSTS scores than achieved by patients undergoing allograft and prosthesis procedures performed at the same institution, despite the patients undergoing CPH being younger, having a higher reoperation rate, and requiring a higher percentage of extraarticular resections. Kitagawa et al. reported two reoperations among seven CPH cases, one for local recurrence and the other for rupture of the acromioclavicular ligaments with associated instability [6]. The present study is the only CPH series restricted to children, which we believe further potentiated the nonunion risk. Size mismatch makes fixation of the clavicle to the residual humerus difficult in all cases but is exacerbated in children because of their small bone size, which precludes the use of standard implants and classic fixation principles. Small-fragment reconstruction plates were used in all of our cases, and plate fracture occurred with the two nonunions. The series with the lowest proportion of patients experiencing nonunions [14] included adult cases in which stouter dynamic compression plates were used for osteosynthesis. Rodl et al. noted similar problems with pediatric fixation and subsequently developed a custom plate to better address the size mismatch problem [12]. The largest series of proximal humerus oncologic resections and reconstructions in young patients consists of 54 patients younger than 13 years of age collected over 30 years at a single institution [7]. In their series, only five of 25 endoprosthesis cases were in patients younger than 10 years of age and two of these cases had early aseptic loosening. Their series included 11 vascularized proximal fibula epiphyseal transplants in patients younger than 10 years of age. Complications in the 11 patients included three fractures requiring surgery, one severe infection requiring graft removal, and three failures of longitudinal growth. Only one pediatric proximal humerus case was included in the series of eight (the second largest) vascularized proximal fibula epiphysis by Onoda et al. [10]. That patient had a successful transplant without undergoing reoperation. Donor site morbidity with vascularized proximal fibula transplantation may be significant because motor branches of the deep peroneal nerve often require transaction and repair to harvest the graft [5]. Limb length inequality is of greater concern in the young pediatric population, although we did not assess it in these patients. Continued growth of the unaffected contralateral side will increase the inequality with time. The lateral clavicular physis contributes some longitudinal growth but probably not enough to compensate for the sacrificed proximal humeral physis. Autogenous nonvascularized fibula grafts were used to increase the length of the constructs in the two nonunion cases reported here. We avoided use of vascularized fibular autografts at the index procedure in these patients as a result of concerns of increasing the operative time, surgical complexity, and risk of early complications. It might be reasonable to consider vascularized onlay fibular strut grafting in cases requiring resection of greater than 75% of the diaphysis. Although there is no evidence clearly showing that the addition of a pediatric microvascular surgery procedure to an already complex oncologic case will increase the risk of early complications, intuition and general knowledge of the relationship between increased operative time and complications suggest this is a valid concern. It has been shown that a delay in resumption of chemotherapy for any reason, may adversely affect prognosis [4].
Table 2.
Summary of all reported claviculo pro humeri results
| Study | Year | Number of patients | Age at surgery (years) | Extraarticular | Number of revised patients | Total reoperations | MSTS score |
|---|---|---|---|---|---|---|---|
| Rodl et al. [12] | 2002 | 15 | 18 | 10 | 10 | 15 | 82 |
| Tsukushi et al. [14] | 2006 | 7 | 36 | 4 | 1 | 1 | 69 |
| Kitagawa et al. [6] | 2007 | 7 | 29 | 7 | 2 | 2 | N/R |
| Nishida et al. [9] | 2008 | 2 | 16 | 2 | 1 | 1 | 80 |
| Current study | 2014 | 4 | 6 | 0 | 2 | 3 | 88 |
MSTS = Musculoskeletal Tumor Society; N/R = not reported.
We found that MSTS scores were excellent in this small cohort. Limited shoulder ROM as would be expected after rotator cuff resection was identified. We believe this functional deficit results from the requisite rotator cuff resection and not the specific reconstruction technique. As this patient cohort matures, they may develop greater functional disability and body image concerns relative to their peers. Further followup of this cohort into late adolescence and adulthood will be necessary to assess whether these issues or other unexpected problems arise over time.
We believe that CPH is a reasonable reconstruction option that the surgeon and family can consider and compare it with other options in young children requiring oncologic reconstruction of the proximal humerus. Its apparent lack of early donor site morbidity and relative technical simplicity have led us to prefer this to vascularized epiphyseal transplantation. However, we acknowledge that less morbid and less technically demanding vascularized strut autografting was ultimately required in two of our patients. More studies are needed to compare this directly with other options such as allografts or prostheses, and longer followup is needed for all of these reconstruction options. Consistent with other studies on CPH, nonunion was found to be the most common complication. Followup of these patients as they reach skeletal maturity and adulthood will be necessary to fully assess outcomes of this procedure.
Footnotes
Each author certifies that he or she, or a member of his or her immediate family, has no funding or commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research ® editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
References
- 1.Abdeen A, Hoang BH, Athanasian EA, Morris CD, Boland PJ, Healey JH. Allograft-prosthesis composite reconstruction of the proximal part of the humerus: functional outcome and survivorship. J Bone Joint Surg Am. 2009;91:2406–2415. doi: 10.2106/JBJS.H.00815. [DOI] [PubMed] [Google Scholar]
- 2.Enneking WF, Dunham W, Gebhardt MC, Malawar M, Pritchard DJ. A system for the functional evaluation of reconstructive procedures after surgical treatment of tumors of the musculoskeletal system. Clin Orthop Relat Res. 1993;286:241–246. [PubMed] [Google Scholar]
- 3.Getty PJ, Peabody TD. Complications and functional outcomes of reconstruction with an osteoarticular allograft after intra-articular resection of the proximal aspect of the humerus. J Bone Joint Surg Am. 1999;81:1138–1146. doi: 10.2106/00004623-199908000-00009. [DOI] [PubMed] [Google Scholar]
- 4.Imran H, Enders F, Krailo M, Sim F, Okuno S, Hawkins D, Neglia J, Randall RL, Womer R, Mascarenhas L, Arndt CA. Effect of time to resumption of chemotherapy after definitive surgery on prognosis for non-metastatic osteosarcoma. J Bone Joint Surg Am. 2009;91:604–612. doi: 10.2106/JBJS.H.00449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Innocenti M, Delcroix L, Romano GF. Epiphyseal transplant: harvesting technique of the proximal fibula based on the anterior tibial artery. Microsurgery. 2005;25:284–292. doi: 10.1002/micr.20130. [DOI] [PubMed] [Google Scholar]
- 6.Kitagawa Y, Thai DM, Choong PF. Reconstructions of the shoulder following tumour resection. J Orthop Surg. 2007;15:201–206. doi: 10.1177/230949900701500216. [DOI] [PubMed] [Google Scholar]
- 7.Manfrini M, Tiwari A, Ham J, Colangeli M, Mercuri M. Evolution of surgical treatment for sarcomas of proximal humerus in children: retrospective review at a single institute over 30 years. J Pediatr Orthop. 2011;31:56–64. doi: 10.1097/BPO.0b013e318202c223. [DOI] [PubMed] [Google Scholar]
- 8.Muscolo DL, Ayerza MA, Aponte-Tinao L, Farfalli G. Allograft reconstruction after sarcoma resection in children younger than 10 years old. Clin Orthop Relat Res. 2008;466:1856–1862. doi: 10.1007/s11999-008-0303-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nishida Y, Tsukushi S, Yamada Y, Kamei Y, Toriyama K, Ishiguro N. Reconstruction of the proximal humerus after extensive extraarticular resection for osteosarcoma: a report of two cases with clavicula pro humero reconstruction. Oncol Rep. 2008;20:1105–1109. [PubMed] [Google Scholar]
- 10.Onoda S, Sakuraba M, Asano T, Miyamoto S, Beppu Y, Chuman H, Kawai A, Nakatani F, Kimata Y. Use of vascularized free fibular head grafts for upper limb oncologic reconstruction. Plast Reconstr Surg. 2011;127:1244–1253. doi: 10.1097/PRS.0b013e318205f34b. [DOI] [PubMed] [Google Scholar]
- 11.Ozaki T, Hashizume H, Kunisada T, Kawai A, Nishida K, Sugihara S, Inoue H. Reconstruction of the proximal humerus with the clavicle after tumor resection: a case report. Clin Orthop Relat Res. 2001;385:170–175. doi: 10.1097/00003086-200104000-00026. [DOI] [PubMed] [Google Scholar]
- 12.Rodl RW, Gosheger G, Gebert C, Lindner N, Ozaki T, Winkelmann W. Reconstruction of the proximal humerus after wide resection of tumours. J Bone Joint Surg Br. 2002;84:1004–1008. doi: 10.1302/0301-620X.84B7.12989. [DOI] [PubMed] [Google Scholar]
- 13.Sulamaa M. Upper extremity phocomelia. A contribution to its operative treatment. Clin Pediatr. 1963;2:251–257. doi: 10.1177/000992286300200511. [DOI] [PubMed] [Google Scholar]
- 14.Tsukushi S, Nishida Y, Takahashi M, Ishiguro N. Clavicula pro humero reconstruction after wide resection of the proximal humerus. Clin Orthop Relat Res. 2006;447:132–137. doi: 10.1097/01.blo.0000201169.80011.ff. [DOI] [PubMed] [Google Scholar]
- 15.Tsukushi S, Nishida Y, Yamada Y, Hosono K, Ishiguro N. Vascularized clavicular rotation graft for revised shoulder arthrodesis after tumor resection of the proximal humerus: a case report. J Shoulder Elbow Surg. 2009;18:e13–e16. doi: 10.1016/j.jse.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 16.Winkelmann WW. [Clavicula pro humero—a new surgical method for malignant tumors of the proximal humerus] [in German] Z Orthop Ihre Grenzgeb. 1992;130:197–201. doi: 10.1055/s-2008-1040138. [DOI] [PubMed] [Google Scholar]