Abstract
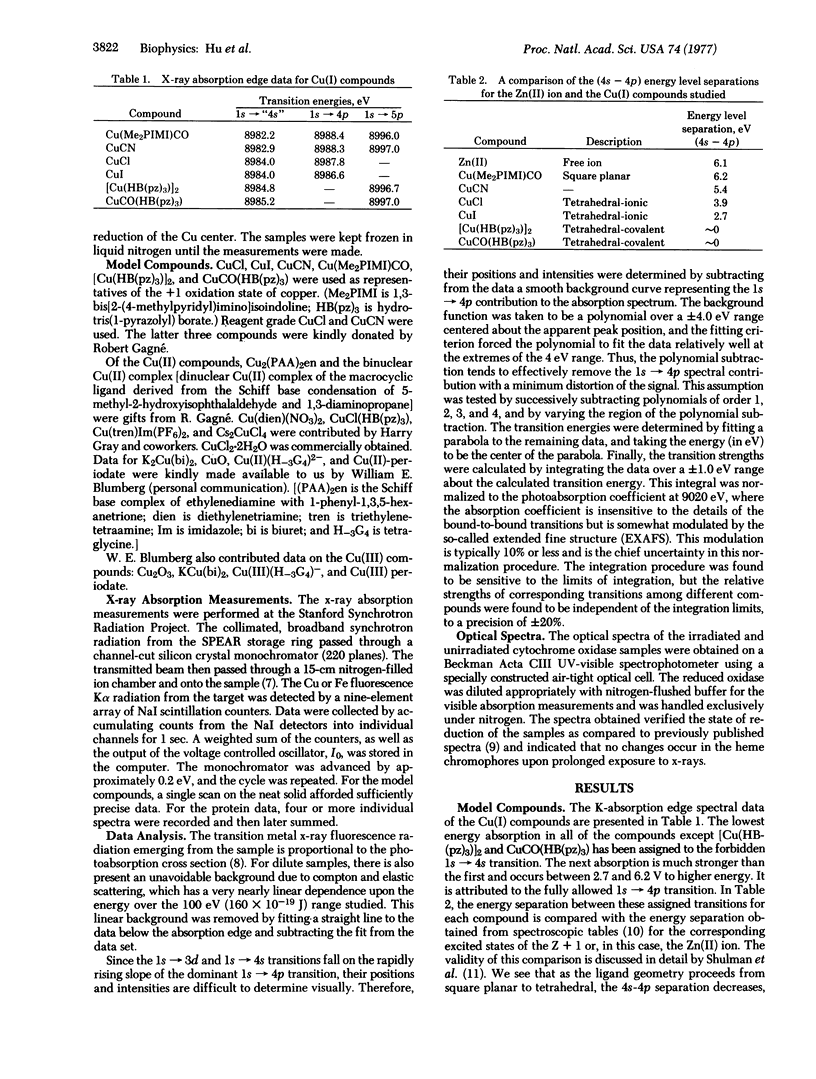
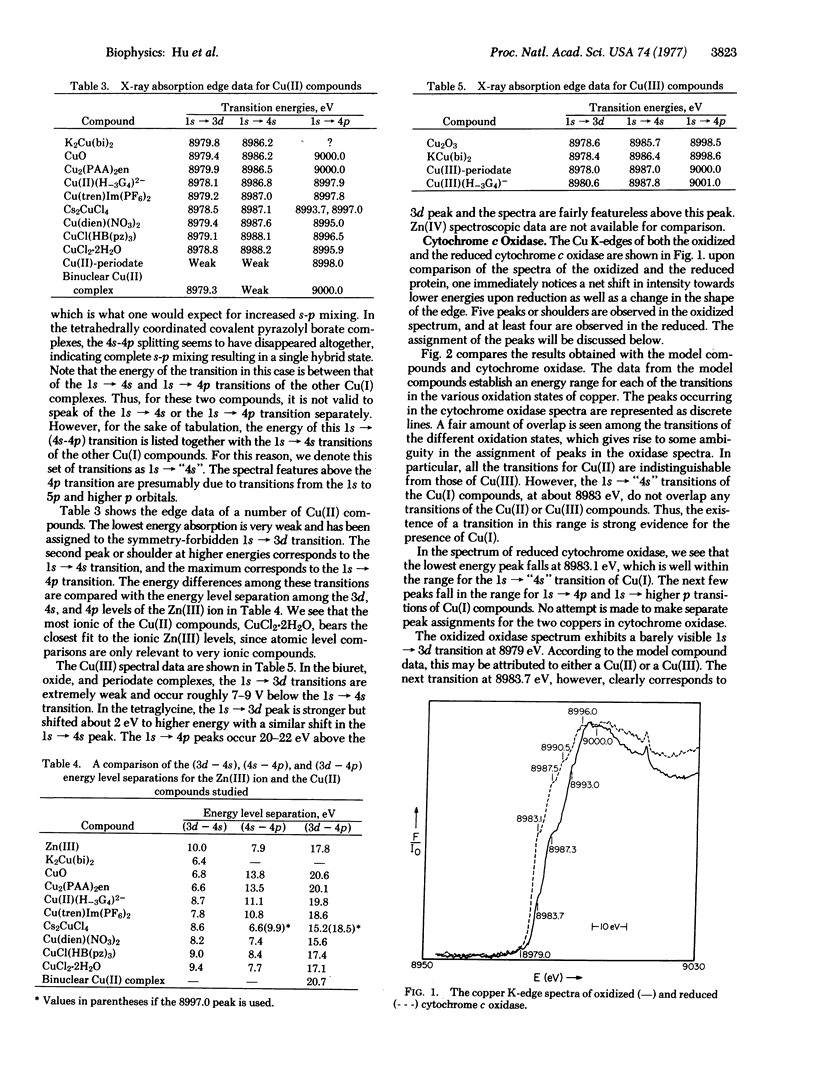
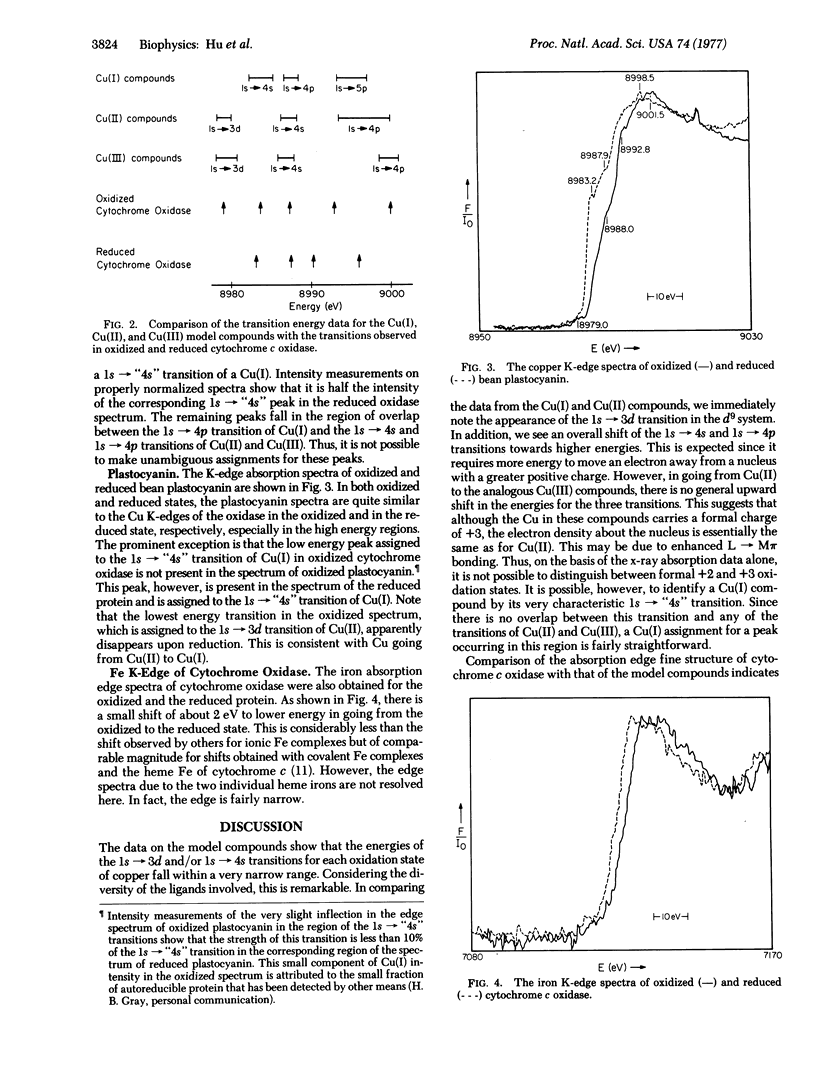
The x-ray absorption edge spectra of the Cu and Fe-centers in oxidized and reduced cytochrome c oxidase (ferrocytochrome c:oxygen oxidoreductase: EC 1.9.3.1) have been obtained using synchrotron radiation from the SPEAR storage ring at the Stanford Linear Accelerator Center. In addition, oxidized and reduced plastocyanin as well as a number of model copper compounds in various oxidation states were also examined. A comparison of the absorption edge fine structure of cytochrome oxidase with those of the models indicates that one of the two coopers in the oxidized protein is in the +1 oxidation state. Upon reduction of the protein with dithionite, the second copper becomes Cu(I). The shift in the Fe K-edge of cytochrome oxidase upon reduction is small (about 2 e V or 3 times 10(-19 J) and is comparable to that previously observed for the reduction of the heme iron of cytochrome c.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aasa R., Albracht P. J., Falk K. E., Lanne B., Vänngard T. EPR signals from cytochrome c oxidase. Biochim Biophys Acta. 1976 Feb 13;422(2):260–272. doi: 10.1016/0005-2744(76)90137-6. [DOI] [PubMed] [Google Scholar]
- BEINERT H., GRIFFITHS D. E., WHARTON D. C., SANDS R. H. Properties of the copper associated with cytochrome oxidase as studied by paramagnetic resonance spectroscopy. J Biol Chem. 1962 Jul;237:2337–2346. [PubMed] [Google Scholar]
- Hartzell C. R., Beinert H. Oxido-reductive titrations of cytochrome c oxidase followed by EPR spectroscopy. Biochim Biophys Acta. 1976 Feb 16;423(2):323–338. doi: 10.1016/0005-2728(76)90189-4. [DOI] [PubMed] [Google Scholar]
- Hartzell C. R., Hansen R. E., Beinert H. Electron carriers of cytochrome oxidase detectable by electron paramagnetic resonance and their relationship to those traditionally recognized in this enzyme. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2477–2481. doi: 10.1073/pnas.70.9.2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heineman W. R., Kuwana T., Hartzell C. R. Indirect electrochemical titration of beef heart cytochrome c oxidase. Biochem Biophys Res Commun. 1972 Oct 6;49(1):1–8. doi: 10.1016/0006-291x(72)90001-0. [DOI] [PubMed] [Google Scholar]
- Kuboyama M., Yong F. C., King T. E. Studies on cytochrome oxidase. 8. Preparation and some properties of cardiac cytochrome oxidase. J Biol Chem. 1972 Oct 25;247(20):6375–6383. [PubMed] [Google Scholar]
- Peisach J., Blumberg W. E. Structural implications derived from the analysis of electron paramagnetic resonance spectra of natural and artificial copper proteins. Arch Biochem Biophys. 1974 Dec;165(2):691–708. doi: 10.1016/0003-9861(74)90298-7. [DOI] [PubMed] [Google Scholar]
- Shulman G. R., Yafet Y., Eisenberger P., Blumberg W. E. Observations and interpretation of x-ray absorption edges in iron compounds and proteins. Proc Natl Acad Sci U S A. 1976 May;73(5):1384–1388. doi: 10.1073/pnas.73.5.1384. [DOI] [PMC free article] [PubMed] [Google Scholar]


