Abstract
This study examines concentrations of volatile organic compounds (VOCs) measured inside and outside of 162 residences in southeast Michigan, U.S.A. Nested analyses apportioned four sources of variation: city, residence, season, and measurement uncertainty. Indoor measurements were dominated by seasonal and residence effects, accounting for 50 and 31%, respectively, of the total variance. Contributions from measurement uncertainty (<20%) and city effects (<10%) were small. For outdoor measurements, season, city and measurement variation accounted for 43, 29 and 27% of variance, respectively, while residence location had negligible impact (<2%). These results show that, to obtain representative estimates of indoor concentrations, measurements in multiple seasons are required. In contrast, outdoor VOC concentrations can use multi-seasonal measurements at centralized locations. Error models showed that uncertainties at low concentrations might obscure effects of other factors. Variance component analyses can be used to interpret existing measurements, design effective exposure studies, and determine whether the instrumentation and protocols are satisfactory.
Keywords: Volatile organic compound, Indoor air, Variance component, Uncertainty, Nested analysis
1. Introduction
Measurements of air pollutant concentrations can vary widely in both outdoor and indoor environments. Outdoors, levels of volatile organic compounds (VOCs) show both long- and short-term trends, seasonal and diurnal patterns (McCarthy et al., 2007), and spatial differences across industrial, urban, suburban and rural gradients (Jia et al., 2008a; Logue et al., 2010; Miller et al., 2009). Indoors, concentrations depend on season (Schlink et al., 2004), the presence of strong indoor sources such as an attached garage (Batterman et al., 2007), mothballs, air fresheners, dry cleaned clothing (D’Souza et al., 2009), personal activities such as smoking (Baek and Jenkins, 2004), among many other factors. Exposure assessment guidance has explicitly indicated that two sources of variation should be addressed: variability and uncertainty (Paustenbach, 2000; US EPA, 1992). Variability, a property of nature, is the true heterogeneity across people, place or time, and is not reducible with more or better information. For VOCs, variability is caused by spatial and temporal changes in emission sources, meteorological parameters, ventilation rates, and other factors. Uncertainty is a lack of knowledge about the underlying state (Frey and Rhodes,1996), and includes both random and systematic errors in field sampling and laboratory analyses. Generally, such errors can be reduced using additional measurements and more accurate instrumentation.
Quantitative information regarding the variability and uncertainty of indoor and outdoor air pollutants is incompletely documented, despite its importance in study design and in identifying exposure and concentration determinants (Spicer et al., 1996). Previous studies investigating VOC determinants using linear regression models, ANOVAs, mixed models and other techniques have rarely accounted for measurement uncertainties, e.g., typically replicates or repeated measurements are simply averaged. Variability can be represented using cumulative distributions, and uncertainty can be estimated using confidence intervals derived using boot-strapping, maximum likelihood and Bayesian methods (Cullen and Frey, 1999). However, few studies have attempted to quantify or partition sources of variability. More typically, only a simple, post-hoc and qualitative caveat regarding uncertainties is stated.
Random effects models have long been applied to differentiate variance into component parts for evaluating their importance and effects (Storm, 1962). These techniques have been applied occasionally to ambient air quality data (Bortnick and Stetzer, 2002; Rappaport and Kupper, 2004; Spicer et al., 1996). No study has apportioned the sources of variation for VOC concentrations measured indoors, where the public spends most of their time. The usual variance estimate (s2) is biased if variance components are ignored (Chou, 2006). Two-way crossed, nested or mixed models are required to estimate the relative sizes of spatial, temporal and measurement components of the total variance. From a practical perspective, such analyses are impeded by simple study designs, small sample sizes, a lack of repeated samples, a small number of target VOC species, and analytical sensitivities that do not detect low concentrations.
From 2004 to 2005, a large exposure assessment campaign monitored indoor and outdoor VOCs in industrial, urban and suburban communities in southeast Michigan. By taking advantage of the study’s nested design, this paper apportions the variability of indoor and outdoor measurements to spatial (between-city and between-residence), temporal (seasonal), and measurement uncertainty components, and identifies significant contributors and determinants of VOC concentrations.
2. Methods
2.1. Sample collection
VOC monitoring was conducted in three cities in southeast Michigan, U.S.A.: Ann Arbor, a suburban community; Ypsilanti, an urban community; and Dearborn, an industrial/urban community. A total of 162 homes were recruited, 65, 35 and 62 in Ann Arbor, Ypsilanti and Dearborn, respectively. Each home was visited twice from June 2004 to June 2005: in summer 2004 and winter 2004 in Ann Arbor and Ypsilanti, and in fall 2004 and spring 2005 in Dearborn. In the second round of sampling, 31 homes dropped out and 46 homes were newly recruited to compensate for the loss. In each visit, passive VOC samplers were deployed in the living room and at an outdoor location near the home for 3–4 weekdays. Samples were not collected at 18 outdoor sites due to weather issues or the lack of an appropriate location. Samples were collected at 2–10 homes on consecutive weeks. Typically, four blank samples were collected weekly, two each at the beginning and end of the week. Indoor and outdoor samples were collected in duplicate using identical methods, as described below. Overall, 1043 VOC samples at 162 indoor sites and 144 outdoor sites were collected (488, 455 and 100 indoor, outdoor and blank samples, respectively). Indoor and outdoor temperature and relative humidity were also measured at each site using Hobo HO8 data loggers (Onset Corp., Bourne, MA), and a walkthrough investigation was conducted at each house to identify factors that might affect VOC concentrations, e.g., the location of the residence, building characteristics, number of occupants, and smoking activities. The design, recruitment, and many findings in the study have been detailed elsewhere (Jia et al., 2008a,b).
2.2. Sampling and analytical methods for VOCs
Passive VOC samples were collected directly in thermal desorption adsorbent tubes (Scientific Instrument Services, Inc., Ringoes, NJ). Details of the method and its performance have been described elsewhere (Jia et al., 2007, 2006). After sampling, tubes were transported to the laboratory and analyzed within 5 days using an automated thermal desorption system (Scientific Instrument Services, Inc., Ringoes, NJ) followed by gas chromatography/mass spectrometry (GC/MS, Agilent 6890/5973, Santa Clara, CA) for 96 target VOCs. A special analytical strategy was applied for duplicate samples: one tube was analyzed in MS scan mode, and one in selected ion monitoring (SIM) mode, in order to take advantage of the high sensitivity of the SIM mode (Jia et al., 2006). These methods obtained equivalent results, although SIM mode obtained lower method detection limits (MDLs) of 0.003–0.27 μgm−3, depending on the compound, compared to MDLs of 0.012–0.49 μgm−3 attained by scan mode. Details of the quality assurance/quality control (QA/QC) for sample collection, transportation, storage, handling and analysis are described elsewhere (Jia et al., 2006; Peng and Batterman, 2000).
2.3. Statistical analyses
A total of 7 indoor and 18 outdoor samples that were contaminated or that failed in laboratory analyses were removed. The detection frequency was calculated for each VOC as the percentage of measurements with concentrations exceeding the MDL. Descriptive statistics were calculated on the basis of the home, i.e., duplicate and repeated samples were averaged for each home. Groups of VOCs that originated from common sources were identified using factor analyses, log-transformed data, varimax rotation and eigenvalues exceeding 0.85. The total target VOC concentration (ΣVOC) was calculated as the sum of the concentrations of all detected VOCs.
A nested design was used that included multiple sampling units (residences) for each experimental unit (city), and repeated measurements for each sampling unit. Sources of variation for the concentration of compound Y may be described as:
| (1) |
where Yijkl = the measured concentration of compound Y for the lth replicate sample collected at the kth visit to the jth residence in the ith city; μY = the true overall mean concentration across all the data; Ci = the deviation from the overall mean due to the effect of the ith city; R(i)j = the deviation from a city-specific mean due to the effect of residence; S(ij)k = the deviation from a residence-specific mean due to the effect of reoccurring visits; and E(ijk)l = the deviation from a sample-specific mean due to the effect of replicate sampling and analysis. Since measurements were taken in two seasons at each home, S(ij)k reflects seasonal variation. The total variability of the concentration, Var(Yijkl), is then decomposed to four components:
| (2) |
where and = spatial variability calculated as the variances between cities and between residences, respectively, = seasonal variability calculated as the variance between two seasons, and = measurement uncertainty calculated as the variance between replicates. The variance of the mean Ȳ is estimated as (Chou, 2006):
| (3) |
The procedures and algorithms to estimate variance components, elucidated previously (Chou, 2006; Storm, 1962), were computed using the MIXED and NESTED procedures in SAS (v9.1.3, SAS Institute, Cary, NC, USA). Effects were denoted in either the MODEL or RANDOM statement using parentheses. For example, Residence (City) means residences nested within cities; Season (Residence City) and Replicate (Season Residence City) denote second and third levels of nesting, respectively. Variance components were expressed as percentages of the total variance for each compound.
Initial analyses examined city effects on indoor and outdoor VOC levels, i.e., how VOCs varied across a gradient of industrialization/urbanization levels. Thus, city was considered a fixed effect that belonged to the MODEL statement, and other effects were in the RANDOM statement. Random effects models assume normality, thus VOC concentrations were log-transformed as they were log-normally distributed. Variances were apportioned for 33 indoor and 24 outdoor VOCs detected in over 50% of homes in order to maintain sufficient sample size, and also on ΣVOC. n-Heptane and n-octane were excluded because they were not measured during the first round of sampling. Variances of VOC groups were decomposed using factor scores and the same nested analyses. Data and results were organized in Microsoft Excel 2003.
3. Results
A total of 58 and 50 VOCs were detected in indoor and outdoor environments, respectively. Descriptive statistics and the variance components for selected indoor and outdoor measurements are summarized in Tables 1 and 2, respectively. Concentrations, emission sources, and other factors influencing the concentrations have been described previously (Jia et al., 2008a,b).
Table 1.
Descriptive statistics and variance components of indoor VOC concentrations. DF = detection frequency; SD = standard deviation. = between-city variation; = between-residence variation; = seasonal variation; = measurement uncertainty. Descriptive statistics were computed by averaging replicate and repeated samples in each home, thus the sample size (n = 159) differs from that used in the variance component analysis (n = 481).
| VOCs | DF (%) | Descriptive statistics (n = 159)
|
Variance Components (n = 481)
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean (μg m−3) | SD (μg m−3) | Median (μg m−3) | Max (μg m−3) | (%) | (%) | (%) | (%) | ||
| Aromatic compounds | |||||||||
| Benzene | 100 | 2.9 | 4.8 | 1.2 | 33.7 | 0.0 | 30.3 | 67.6 | 2.1 |
| Toluene | 100 | 16.2 | 26.5 | 7.2 | 197.3 | 4.2 | 53.2 | 41.5 | 1.1 |
| Ethylbenzene | 100 | 2.2 | 4.2 | 1.1 | 40.6 | 2.9 | 38.0 | 57.6 | 1.5 |
| p,m-Xylene | 100 | 7.9 | 16.4 | 3.3 | 161.3 | 2.2 | 45.0 | 51.6 | 1.3 |
| o-Xylene | 100 | 2.4 | 4.1 | 1.2 | 37.0 | 3.3 | 44.1 | 51.0 | 1.7 |
| Styrene | 100 | 0.5 | 0.8 | 0.3 | 6.6 | 8.4 | 21.4 | 64.5 | 5.6 |
| 1,2,4-Trimethylbenzene | 100 | 3.1 | 6.6 | 1.3 | 54.4 | 7.9 | 45.5 | 44.9 | 1.7 |
| 1,3,5-Trimethylbenzene | 100 | 0.9 | 1.8 | 0.4 | 15.5 | 8.4 | 40.2 | 47.2 | 4.2 |
| 1,2,3-Trimethylbenzene | 100 | 1.0 | 2.9 | 0.4 | 32.5 | 9.6 | 36.4 | 50.0 | 4.0 |
| 4-Ethyl toluene | 98 | 4.0 | 6.1 | 2.3 | 57.4 | 10.0 | 33.5 | 54.1 | 2.5 |
| 2-Ethyl toluene | 100 | 0.8 | 1.7 | 0.4 | 14.0 | 9.0 | 37.9 | 50.3 | 2.8 |
| Isopropylbenzene | 99 | 0.2 | 0.3 | 0.1 | 2.3 | 6.0 | 35.8 | 45.7 | 12.5 |
| n-Propylbenzene | 100 | 0.6 | 1.2 | 0.3 | 10.6 | 6.1 | 40.5 | 48.0 | 5.4 |
| p-Isopropyltoluene | 100 | 1.7 | 2.4 | 1.0 | 19.2 | 22.2 | 14.6 | 56.8 | 6.4 |
| n-Butylbenzene | 96 | 0.2 | 1.0 | 0.1 | 12.6 | 10.1 | 27.5 | 40.4 | 22.0 |
| Naphthalene | 100 | 3.5 | 11.1 | 0.8 | 91.7 | 0.0 | 61.7 | 25.0 | 13.3 |
| Chlorinated compounds | |||||||||
| Chloroform | 97 | 0.7 | 1.5 | 0.4 | 14.6 | 38.2 | 17.0 | 24.6 | 20.2 |
| Tetrachloroethene | 98 | 1.0 | 2.8 | 0.4 | 27.8 | 13.4 | 36.5 | 27.8 | 22.2 |
| Carbon tetrachloride | 100 | 1.0 | 0.5 | 0.9 | 4.2 | 3.0 | 0.0 | 58.9 | 38.1 |
| 1,4-Dichlorobenzene | 90 | 4.2 | 20.8 | 0.2 | 184.9 | 9.2 | 53.1 | 27.3 | 10.4 |
| Aliphatic compounds | |||||||||
| Methyl cyclohexane | 99 | 1.0 | 1.7 | 0.5 | 12.7 | 12.8 | 28.3 | 46.6 | 12.3 |
| n-Nonane | 99 | 3.3 | 14.1 | 0.6 | 154.2 | 3.5 | 6.5 | 63.6 | 26.4 |
| n-Decane | 99 | 8.2 | 52.2 | 0.8 | 621.7 | 4.5 | 7.7 | 75.5 | 12.3 |
| n-Undecane | 99 | 8.0 | 65.9 | 0.7 | 828.5 | 2.8 | 15.9 | 69.1 | 12.2 |
| n-Dodecane | 99 | 2.6 | 20.0 | 0.5 | 252.5 | 5.3 | 25.0 | 53.1 | 16.6 |
| n-Tridecane | 93 | 1.1 | 3.3 | 0.3 | 37.3 | 14.4 | 28.3 | 42.6 | 14.6 |
| n-Tetradecane | 99 | 1.4 | 2.5 | 0.7 | 20.2 | 10.5 | 34.4 | 42.1 | 13.0 |
| n-Pentadecane | 99 | 0.8 | 1.1 | 0.5 | 10.3 | 6.9 | 22.9 | 55.5 | 14.7 |
| n-Hexadecane | 99 | 0.6 | 0.6 | 0.4 | 4.2 | 4.5 | 21.6 | 50.7 | 23.2 |
| n-Heptadecane | 99 | 0.4 | 0.4 | 0.3 | 2.8 | 4.2 | 13.0 | 54.7 | 28.1 |
| Terpenoid compounds | |||||||||
| α-Pinene | 100 | 9.2 | 17.3 | 3.4 | 106.6 | 2.1 | 51.0 | 45.5 | 1.4 |
| d-Limonene | 100 | 27.6 | 35.1 | 18.1 | 258.5 | 11.1 | 19.6 | 66.5 | 2.8 |
Table 2.
Descriptive statistics and variance components of outdoor VOC concentrations. Otherwise as Table 1.
| VOCs | DF (%) | Descriptive statistics (n = 144)
|
Variance Components (n = 437)
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean (μg m−3) | SD (μg m−3) | Median (μg m−3) | Max (μg m−3) | (%) | (%) | (%) | (%) | ||
| Aromatic compounds | |||||||||
| Benzene | 100 | 1.2 | 1.3 | 1.0 | 15.2 | 10.9 | 0.0 | 82.2 | 6.9 |
| Toluene | 100 | 3.3 | 9.6 | 1.8 | 114.2 | 37.6 | 0.0 | 56.0 | 6.4 |
| Ethylbenzene | 100 | 0.7 | 2.7 | 0.3 | 31.9 | 38.3 | 0.0 | 55.3 | 6.4 |
| p,m-Xylene | 100 | 3.0 | 13.5 | 1.3 | 162.4 | 41.5 | 1.0 | 51.6 | 5.9 |
| o-Xylene | 100 | 0.9 | 3.9 | 0.4 | 47.3 | 41.3 | 0.0 | 49.9 | 8.8 |
| Styrene | 92 | 0.0 | 0.0 | 0.0 | 0.3 | 19.2 | 0.0 | 53.6 | 27.2 |
| 1,2,4-Trimethylbenzene | 99 | 0.9 | 3.2 | 0.4 | 38.0 | 44.4 | 0.0 | 45.8 | 9.8 |
| 1,3,5-Trimethylbenzene | 99 | 0.3 | 0.9 | 0.1 | 10.2 | 45.1 | 3.4 | 37.8 | 13.7 |
| 1,2,3-Trimethylbenzene | 99 | 0.2 | 0.5 | 0.1 | 5.6 | 45.4 | 0.0 | 35.3 | 19.2 |
| 4-Ethyl toluene | 98 | 1.0 | 3.5 | 0.5 | 41.6 | 35.0 | 0.6 | 45.7 | 18.7 |
| 2-Ethyl toluene | 99 | 0.2 | 0.6 | 0.1 | 6.6 | 42.6 | 0.0 | 42.3 | 15.1 |
| Isopropylbenzene | 97 | 0.0 | 0.1 | 0.0 | 0.9 | 35.2 | 0.6 | 27.4 | 36.8 |
| n-Propylbenzene | 98 | 0.1 | 0.5 | 0.1 | 5.5 | 44.1 | 1.4 | 31.5 | 22.9 |
| p-Isopropyltoluene | 95 | 0.0 | 0.1 | 0.0 | 1.0 | 31.9 | 0.0 | 50.5 | 17.6 |
| Naphthalene | 94 | 0.3 | 0.6 | 0.2 | 6.0 | 5.6 | 0.0 | 49.6 | 44.9 |
| Chlorinated compounds | |||||||||
| Tetrachloroethene | 97 | 0.3 | 0.3 | 0.2 | 2.0 | 28.8 | 5.3 | 6.0 | 59.9 |
| Carbon tetrachloride | 100 | 1.0 | 0.3 | 1.0 | 2.2 | 2.9 | 0.0 | 58.6 | 38.5 |
| 1,4-Dichlorobenzene | 92 | 0.1 | 0.2 | 0.0 | 1.7 | 17.4 | 13.6 | 14.4 | 54.6 |
| Aliphatic compounds | |||||||||
| Methyl cyclohexane | 97 | 0.2 | 0.5 | 0.1 | 6.4 | 25.8 | 0.0 | 29.2 | 45.1 |
| n-Nonane | 83 | 0.2 | 0.4 | 0.1 | 3.5 | 11.4 | 0.0 | 45.3 | 43.3 |
| n-Decane | 89 | 0.2 | 0.3 | 0.1 | 2.9 | 20.4 | 1.5 | 39.8 | 38.3 |
| Terpenoid compounds | |||||||||
| α-Pinene | 99 | 0.2 | 0.2 | 0.1 | 1.5 | 0.0 | 0.0 | 64.5 | 35.5 |
| d-Limonene | 97 | 0.4 | 0.9 | 0.1 | 8.4 | 32.8 | 0.0 | 11.8 | 55.4 |
3.1. Variance components of indoor VOCs
Indoor VOC concentrations varied mostly due to seasonal and between-residence (local spatial) effects, which respectively explained an average of 50 ± 13% and 31 ± 15% of the total variance, depending on the VOC. The variance components reflect specific VOC sources, e.g., carbon tetrachloride is ubiquitous in the atmosphere at a stable concentration, so its spatial variation was minimal. The sizable between-residence variation arose from differences in house types (e.g., houses with and without attached garages) and indoor emission sources (e.g., pesticide use, indoor tobacco smoking) across the homes recruited into the study (Jia et al., 2008b). Similarly large variation in indoor VOC concentrations among homes has been reported elsewhere (Sexton et al., 2004; Zhu et al., 2005). Relatively few studies have characterized temporal variability of VOC levels in residences, although large seasonal variation has been shown in apartments (Rehwagen et al., 2003). (70% of residences studied here were single family homes.)
Between-city variation ( ) contributed under 10% of the total variance for most VOCs. Generally, values were similar to or smaller than measurement variances, indicating that city effects were small, and that indoor concentrations were determined primarily by indoor sources. Neighborhoods also had only small effects on indoor VOC concentrations in three communities in Minnesota, MN (Sexton et al., 2004). Still, between-city variation can arise due to systematic spatial differences in VOC use or emission patterns (Ohura et al., 2009). In the present study, for example, the notably high of chloroform (38%) likely resulted from regional differences in water disinfection practices: Ypsilanti and Dearborn rely on chlorine (which produces chloroform as a byproduct), while Ann Arbor uses ozone.
Measurement uncertainty accounted for only a small portion (average of 11 ± 10%) of the total variance and depended on the type and concentration of VOC, e.g., contributions were generally within 5%, 25%, 20% and 5% for aromatic, chlorinated, aliphatic and terpenoid compounds, respectively. Measurement uncertainty primarily depends on GC/MS method performance and detection limits, and relative uncertainties generally increase at low concentrations (Bortnick and Stetzer, 2002; Jia et al., 2006; Le et al., 2007). In the present study, this is shown by negative correlations between measurement uncertainties and mean (r = −0.51) and median (r = −0.62) concentrations of each VOC (Fig. 1A). Median concentrations of chlorinated and aliphatic compounds were low, mostly below 1 μgm−3, and agreement within 25% was considered acceptable (US EPA, 1999). Carbon tetrachloride showed the highest uncertainty, a result of co-elution with benzene in GC/MS analysis (Jia et al., 2006). Overall, measurement uncertainty was considered to be low, and the sampling and analytical methods were appropriate for the indoor application.
Fig. 1.
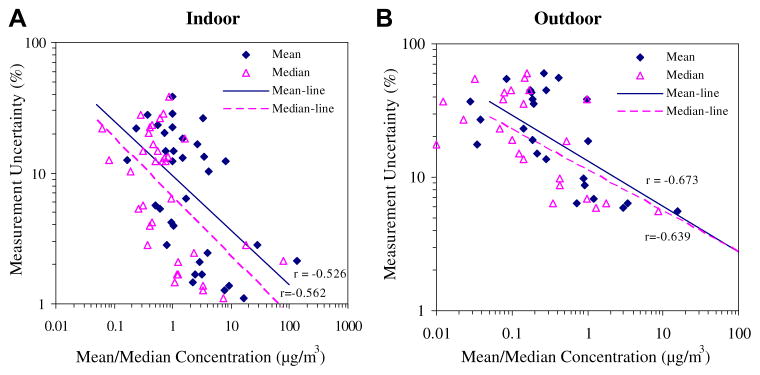
Relationship between measurement uncertainty and indoor/outdoor VOC concentrations. Each point represents a different VOC. A. Indoor. B. Outdoor.
As mentioned, when available in a study, duplicate measurements typically have been averaged. The effect of this practice did not significantly change and for most compounds, but increased, a result of nesting replicate samples within season (Supplemental Table 1). Thus, ignoring measurement uncertainty has the effect of increasing the apparent seasonal variability, especially for compounds with comparable to or larger than variance contributions from other sources.
3.2. Variance components of outdoor VOCs
Seasonal differences accounted for the largest variance component (average of 43 ± 17%) of the outdoor measurements. Especially large seasonal effects were seen for benzene (82%), reflecting annual cycles in emissions, photochemistry and meteorology, and for α-pinene (65%), which is emitted mainly from plants during the growing season (Kim, 2001). The seasonal variability of tetrachloroethene, an industrial and dry-cleaning solvent, varied by only 6% due to season, possibly because measurements were uncertain or because emission sources were stable. Many VOCs had higher concentrations in the cooler seasons. Similar seasonal effects have been reported for ambient VOC measurements elsewhere in the U.S. (McCarthy et al., 2007).
Between-city variation contributed 29 ± 15% of the total variance, second to the seasonal variation. Neighborhood effects were expected due to distinct differences in the level of urbanization and industrialization in the study cities, e.g., fugitive, point and mobile source VOC emissions were significantly higher in Dearborn than the two other cities (Jia et al., 2008a). However, between-city variance was very small for naphthalene (5.6%), carbon tetrachloride (2.9%), and α-pinene (0.0%). Again, the ubiquitous compound carbon tetrachloride shows low variability. Any neighborhood effects for α-pinene were overshadowed by large seasonal changes. For naphthalene, our previous review indicated only modest variation among urban settings, although indoor levels vary considerably (Jia and Batterman, 2010).
Outdoor measurement uncertainty averaged 27 ± 18%, considerably higher than found indoors. Uncertainties were below 10% for the aromatic VOCs measured at higher concentrations, e.g., benzene, toluene, ethylbenzene and xylene (BTEX) and 1,2,4-trimethylbenzene. Median concentrations of most other VOCs were below 0.2 μg/m3, and were often close to MDLs. As seen with the indoor measurements, relative uncertainties increased at low concentrations (Fig. 1B), and averaging duplicate measurements tended to increase , the variance component attributed to season ( and did not differ significantly). However, due to the larger uncertainties, shifts were larger than seen indoors, e.g., variance components for chlorinated compounds, alkanes and terpenes shifted by 36–60% (Supplemental Table 2). As seen for indoor samples, seasonal effects can be exaggerated if replicate samples are averaged, a result of nesting replicates within season.
Outdoor measurements of most VOCs had negligible between-residence variation ( ) (generally less than 2%), a strong contrast to indoor measurements. 1,4-Dichlorobenzene showed a relatively high (13.6%), possibly reflecting off-label uses as a pest repellent in yards near the ambient samples (Jia and Batterman, 2010). Overall, VOC concentrations within each community were quite homogeneous.
3.3. Variance components of VOC groups
The factor analysis of the indoor VOC measurements identified seven groups (Supplemental Table 3): Group 1 consisted of aromatics, all components of gasoline; Group 2 included heavy alkanes (C13–C17) emitted from paints and adhesives; Group 3 contained C9–C12 alkanes possibly from water-based adhesives; Group 4 was characterized by terpenes found in liquid cleaners and disinfectants; Group 5 had two chlorinated compounds mainly emitted from deodorants; Group 6 was composed of naphthalene and 1,4-dichlorobenzene, both of which are constituents of moth repellents; and Group 7 was dominated by benzene and carbon tetrachloride, which are ubiquitous compounds in the atmosphere. The variance components differed by group (Fig. 2). Seasonal variability accounted for most of the variance for groups 1, 3, 4 and 6. The between-residence variance fraction exceeded 50% for Groups 1 and 5, showing the importance of indoor sources of aromatics and chloroform. Measurement uncertainty was smallest for Groups 1, 4 and 5 (aromatics, terpenes, and chlorinated compounds), and <20% for all groups. The city effect was negligible except for Group 7, which is understandable since the total variance is small for these ubiquitous compounds. Variance components for ΣVOC (last bar in Fig. 2A) reflect the average across all compounds.
Fig. 2.
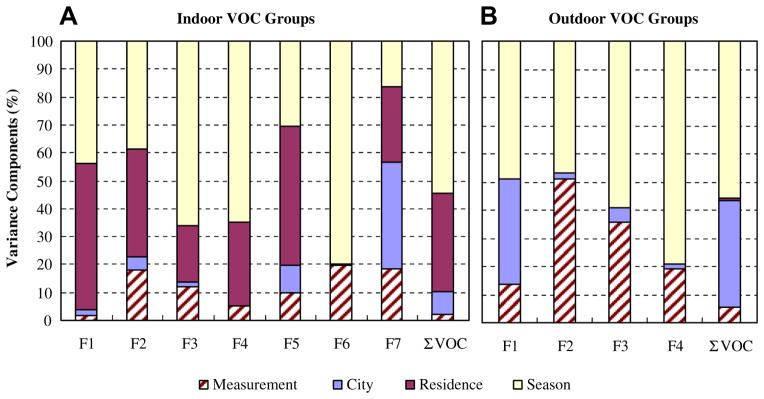
Variance components of VOC groups in indoor and outdoor air.
In outdoor air, four groups were formed (Supplemental Table 4): Group 1 encompassed aromatic compounds emitted as gasoline vapor and in vehicle exhaust; Group 2 was a mixture of aromatics, light hexanes and tetrachloroethene, which may arise from pesticides and combustion sources; Group 3 consisted of terpenes and p-isopropyltoluene, both emitted by plants (Howard, 1997); and Group 4 again had the two ubiquitous compounds, benzene and carbon tetrachloride. Variance components were dominated by seasonal variation, followed by measurement uncertainty (Fig. 2B). The neighborhood effect was meaningful only for Group 1 (aromatics), probably due to the industrial and mobile emission sources in Dearborn (Jia et al., 2008a). Between-residence variation was negligible, indicating that VOC levels were homogeneous within cities. Measurement uncertainty was large for Groups 2 and 3 (up to 50%). The variance breakdown for ΣVOC reflects those of Group 1 because aromatic compounds were the dominant constituents.
Results of factor analyses depend on the number of factors selected and the specific data set, thus the VOC groups and identifications are study-specific. However, variance components using groups have two advantages: they may better represent the variability of emission sources (as compared to individual compounds); and they summarize results for related VOCs. Overall, variance components for the groups were similar to results shown earlier for individual VOCs.
3.4. Measurement uncertainty models
As mentioned, measurement uncertainty and concentration were highly correlated (Fig. 1). Linear regression models were fitted to predict the average relative error of each VOC species, derived from the duplicate measurements, as a function of median or average concentration. Models for indoor and outdoor measurements were similar, and thus data were pooled, which yielded the following:
| (4) |
| (5) |
where = the measurement uncertainty (in %) and mean and median = mean and median concentrations (μg/m3), respectively. These models include an irreducible error term (the intercept), and an error that is proportional to the measured concentration (the slope). Using eqs. (4) and (5), the predicted relative uncertainties are 10.6 and 7.4% for mean and median concentrations of 1.0 μgm−3, respectively, and 28% and 20% at 0.1 μgm−3. Guidance for VOC measurements requires replicate precision under 25% (US EPA, 1999). While these criteria were achieved for many VOCs, especially in indoor settings in this study, improvements are needed for VOCs found at low concentrations. These models are study- and method-specific, and they may not represent errors for some VOCs that co-elute or have other issues. Still, the approach is transferable and allows study-specific estimates of measurement uncertainty.
4. Discussion
Concentrations of VOCs and other pollutants reflect emissions, environmental factors that disperse or remove pollutants, e.g., mixing, wind speed, temperature and humidity, and measurement uncertainty. The variance component analyses show the relative importance of spatial, temporal and measurement uncertainties, which have significant implications for interpreting existing measurements, identifying factors that affect variability, and informing the design of future studies designed to meet specific goals.
Nested study designs are not uncommon. As air pollution examples, the RIOPA study conducted indoor, outdoor and personal sampling in ~300 nonsmoking homes in three cities during 1999–2000 (Weisel et al., 2005); Sexton et al. (2004) collected indoor, outdoor and personal samples in three communities in Minneapolis/St. Paul metropolitan area in 1999; and the TEACH project collected personal, indoor home, and outdoor home samples in New York and Los Angles in 1999–2000 (Sax et al., 2006). Each of these studies collected samples in at least two seasons. However, variance analyses for indoor VOCs in these or other studies have not been reported.
Variance component analyses have been demonstrated for several outdoor measurement programs. At six sites in Columbus, OH, temporal variability was predominant, and spatial variability contributed under 20% of the total variability (Spicer et al., 1996). Similarly, variability was mostly driven by the temporal component in an analysis of Urban Air Toxics Monitoring Program (UATMP) data (Bortnick and Stetzer, 2002). Rappaport and Kupper (2004) partitioned the spatial variation into within-city (10.2%) and between-site (15.7%) effects, and showed smaller neighborhood effect but higher heterogeneity within communities. Monitoring in Mexico City showed that monitoring at a centrally located sampling site could represent outdoor residential levels (Serrano-Trespalacios et al., 2004). Our results largely confirm these findings, and also indicate the benefit of placing monitoring sites in different types of communities. The three cities studied in the present study differed with respect to level of industrialization, urbanization, income and other respects, thus, our conclusions of neighborhood effects may not apply to communities that are more homogeneous with respect to building types and indoor and outdoor emission sources.
4.1. Measurement uncertainty
Uncertainties associated with sampling and analysis can be significant, and large measurement errors can mask the true spatial and temporal variation. The present study measured a wider range of VOC species than found in most other studies, and used two analytical methods that varied in sensitivity, which may have slightly compromised reproducibility. Still, measurement uncertainties remained comparable to or smaller than those reported elsewhere. As examples: the OVM passive samplers used in RIOPA gave a relative standard deviation (RSD) up to 42% (Weisel et al., 2005); VOC data using canisters had RSDs averaging 35–38% though many compounds exceeded 50% (Le et al., 2007); and the Radiello® diffusive sampler showed RSDs of 10–15% for BTEX compounds (Bruno et al., 2005). Measurement uncertainty was below 25% of the total variance for most VOCs, but over 30% for chlorinated compounds (Spicer et al., 1996). A detailed study of ambient VOCs indicated the importance of analytical errors, which ranged up to 44% (Bortnick and Stetzer, 2002).
The quantitative measurement error models, eqs. (4) and (5), can be used for several purposes. These include evaluating precision objectives required for Quality Assurance Project Plans (US EPA, 2001); providing “model-based” uncertainty estimates for source apportionment models such as positive matrix factorization (US EPA, 2008) and chemical mass balance (Christensen and Gunst, 2004); assisting in the imputation of missing data (Le et al., 2007); and developing probability distributions to compare with air quality standards (Curran and Suggs, 1986).
4.2. Implications for study design
Nested analysis of variance models facilitate the ability to make valid and precise inferences, e.g., identifying the factors affecting concentration measurements, and testing the equality of the means at each level. Possibly the most important application is to design more efficient future experiments (Storm, 1962). This includes determining sample sizes and frequencies, optimizing monitor deployments, allocating resources to minimize uncertainties, evaluating the performance of measurement methods, and improving sampling precision. Variance estimates can be used to determine the sample size needed to attain a precision goal, i.e., n = t2s2/e2, where n = required sample size; s2 = sample variance; e2 = acceptable level of error; and t = t value with a d.f. = n − 1. To obtain a precise and representative sample mean Ȳ, we want to minimize its variance (Eq. (3)). As temporal variability ( ) generally had the largest variance component for both indoor and outdoor VOC measurements, the most effective way to reduce uncertainty is to increase the number of samples at the same site. Although this appears counterintuitive, sampling in two (or possible more) seasons may give more information than sampling at additional homes or outdoor sites. Next, for indoor air studies, it may be most effective to increase the number of homes (n) studied, since is relatively large. For ambient VOCs, the next step would be to increase the number of communities monitored, since is large. These broad conclusions are tempered in that they are derived from a study examining only three communities in one region, and geographical differences in smoking rates, house/garage configuration and other factors can cause differences. However, the literature supports these findings.
The high levels of VOCs found indoors suggest that it is best to increase the number of samples covering multiple seasons and residences, rather than to deploy more sophisticated and expensive measurement methods. If replicate variation is negligible, then the number of replicates could be reduced. These steps would increase statistical power more than additional measurements at a smaller number of residences. For outdoor VOCs, the small between-site variation but significant between-city effects found in this study suggest that central monitoring stations can be representative of community exposure. Strategies to reduce uncertainties at these low concentrations include the use of more advanced monitoring techniques, more replicates, and possibly longer sampling periods (for passive measurements).
Nested variance analyses are well-suited to evaluate spatial and temporal variability in large datasets. They can play an important evaluative role in programs aimed at collecting pollutant data with higher temporal and spatial resolution (US EPA, 2010).
4.3. Implications for exploring indoor-outdoor relationship
In this work, indoor and outdoor measurements were not merged into a larger model since indoor concentrations of VOCs typically far exceed outdoor levels (Guo et al., 2004). Still, variance component analyses provide an alternate or complementary method to analyze indoor/outdoor relationships and emission sources, e.g., supplementing the widely used indoor/outdoor ratio. If the spatial variation is small indoors and outdoors, the compound is ubiquitous, e.g., carbon tetrachloride. Indoors, large indicates compounds with principally indoor sources, e.g., most aromatics and terpenes. Large but small suggests distinct usage patterns, e.g., chlorine/ozone as tap water disinfectants, or primarily outdoor-sourced compounds, for example, particulate matter (PM) (Meng et al., 2005) and some (5–7 ring) polycyclic aromatic hydrocarbons (PAHs) (Naumova et al., 2002). In ambient air, variance analyses can identify compounds arising from indoor, industrial and other source types since such compounds are likely to have significant spatial variation, e.g., dichlorobenzene.
4.4. Limitations
The present analysis has several limitations. Measurement uncertainty was limited to duplicate precision, and additional replicates might provide better estimates. The thermal desorption analysis did not allow multiple analyses of the same sample, and thus sampling and analytical errors could not be separated. Other sources of measurement uncertainty include sample preservation, transportation, storage and instrumental fluctuations. Although instrumental analyses are typically more precise, Bortnick and Stetzer (2002) showed that analytical errors sometimes exceeded sampling errors. At a given site, samples were collected in only two seasons, and only one period in each season was sampled. Sample sizes were not balanced among three cities, and seasonally repeated sampling was not available for a subset of residences, which may reduce the power of the analyses. We set residence, season and replicate as random variables, but season was not strictly random. Finally, analyses were computed using log-transformed data to meet the normality assumption required by nested analysis, so care should be taken when using or interpreting the variances.
5. Conclusions
Temporal, spatial and measurement variability is inherent in environmental measurements, and this variability must be investigated to determine whether study goals and data quality objectives are being met. This study is unique in using a nested design to apportion the variances of both indoor and outdoor VOC concentrations. Outdoor concentrations were predominantly governed by season and neighborhood effects, and measurements had moderate reproducibility, suggesting the benefit of additional replicates or more precise methods. Spatial variability within a community was negligible, suggesting that a centralized monitoring site could provide representative results. These results generally agreed with the few studies performed previously for outdoor VOC measurements. Our extension of variance decomposition analyses to indoor air quality applications and to additional VOC species showed that indoor measurements were highly reproducible, that seasonal variation was significant, and that between-city variation was negligible. Thus, indoor VOC levels were dominated by indoor sources and not the penetration of outdoor pollutants. Individual compounds and VOC groups, determined using factor analysis, showed generally similar results. Variance component analyses are valuable for interpreting existing datasets, designing future studies, and allocating resources for environmental sampling.
Supplementary Material
Acknowledgments
This three cities study was funded by the American Chemistry Council (Grant 2401). The University of Memphis Faculty Research Grant (FRG 220871) helped to support the statistical analysis. Portions of the research described in this article was conducted under contract to the Health Effects Institute (HEI), an organization jointly funded by the United States Environmental Protection Agency (EPA) (Assistance Award No. R-82811201) and certain motor vehicle and engine manufacturers. The contents of this article do not necessarily reflect the views of HEI, or its sponsors, nor do they necessarily reflect the views and policies of the EPA or motor vehicle and engine manufacturers. The authors thank Christopher Godwin and Gina Reinhold for assistance with the field work, and Dr. Xinhua Yu for his comments on statistical analyses.
Appendix. Supplementary data
Supplementary data associated with this article can be found in the online version, at doi:10.1016/j.envpol.2011.09.024.
References
- Baek SO, Jenkins RA. Characterization of trace organic compounds associated with aged and diluted sidestream tobacco smoke in a controlled atmosphere – volatile organic compounds and polycyclic aromatic hydrocarbons. Atmospheric Environment. 2004;38:6583–6599. [Google Scholar]
- Batterman S, Jia CR, Hatzivasilis G. Migration of volatile organic compounds from attached garages to residences: a major exposure source. Environmental Research. 2007;104:224–240. doi: 10.1016/j.envres.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Bortnick SM, Stetzer SL. Sources of variability in ambient air toxics monitoring data. Atmospheric Environment. 2002;36:1783–1791. [Google Scholar]
- Bruno P, Caputi M, Caselli M, de Gennaro G, de Rienzo M. Reliability of a BTEX radial diffusive sampler for thermal desorption: field measurements. Atmospheric Environment. 2005;39:1347–1355. [Google Scholar]
- Chou CJ. Assessing spatial, temporal, and analytical variation of ground-water chemistry in a large nuclear complex, USA. Environmental Monitoring and Assessment. 2006;119:571–598. doi: 10.1007/s10661-005-9044-1. [DOI] [PubMed] [Google Scholar]
- Christensen WF, Gunst RF. Measurement error models in chemical mass balance analysis of air quality data. Atmospheric Environment. 2004;38:733–744. [Google Scholar]
- Cullen AC, Frey HC. Probabilistic Techniques in Exposure Assessment: A Handbook for Dealing with Variability and Uncertainty in Models and Inputs. Society for Risk Analysis; New York, NY: 1999. [Google Scholar]
- Curran TC, Suggs JC. Effects of measurement uncertainty on air-quality summary statistics. Atmospheric Environment. 1986;20:571–576. [Google Scholar]
- D’Souza JC, Jia CR, Mukherjee B, Batterman S. Ethnicity, housing and personal factors as determinants of VOC exposures. Atmospheric Environment. 2009;43:2884–2892. [Google Scholar]
- Frey HC, Rhodes DS. Characterizing, simulating, and analyzing variability and uncertainty: an illustration of methods using an air toxics emissions example. Human and Ecological Risk Assessment. 1996;2:762–797. [Google Scholar]
- Guo H, Lee SC, Chan LY, Li WM. Risk assessment of exposure to volatile organic compounds in different indoor environments. Environmental Research. 2004;94:57–66. doi: 10.1016/s0013-9351(03)00035-5. [DOI] [PubMed] [Google Scholar]
- Howard PH. Handbook of Environmental Fate and Exposure Data for Organic Chemicals. V. CRC Press Inc; Chelsea, MI: 1997. [Google Scholar]
- Jia C, Batterman S, Godwin C. Continuous, intermittent and passive sampling of airborne VOCs. Journal of Environmental Monitoring. 2007;9:1220–1230. doi: 10.1039/b708119g. [DOI] [PubMed] [Google Scholar]
- Jia C, Batterman S, Godwin C. VOCs in industrial, urban and suburban neighborhoods, Part 1: Indoor and outdoor concentrations, variation, and risk drivers. Atmospheric Environment. 2008a;42:2083–2100. [Google Scholar]
- Jia C, Batterman S, Godwin C. VOCs in industrial, urban and suburban neighborhoods, Part 2: Factors affecting indoor and outdoor concentrations. Atmospheric Environment. 2008b;42:2101–2116. [Google Scholar]
- Jia CR, Batterman S. A critical review of naphthalene sources and exposures relevant to indoor and outdoor air. International Journal of Environmental Research and Public Health. 2010;7:2903–2939. doi: 10.3390/ijerph7072903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia CR, Batterman S, Chernyak S. Development and comparison of methods using MS scan and selective ion monitoring modes for a wide range of airborne VOCs. Journal of Environmental Monitoring. 2006;8:1029–1042. doi: 10.1039/b607042f. [DOI] [PubMed] [Google Scholar]
- Kim JC. Factors controlling natural VOC emissions in a southeastern US pine forest. Atmospheric Environment. 2001;35:3279–3292. [Google Scholar]
- Le HQ, Batterman SA, Wahl RL. Reproducibility and imputation of air toxics data. Journal of Environmental Monitoring. 2007;9:1358–1372. doi: 10.1039/b709816b. [DOI] [PubMed] [Google Scholar]
- Logue JM, Small MJ, Stern D, Maranche J, Robinson AL. Spatial variation in ambient air toxics concentrations and health risks between industrial-influenced, urban, and rural sites. Journal of the Air & Waste Management Association. 2010;60:271–286. doi: 10.3155/1047-3289.60.3.271. [DOI] [PubMed] [Google Scholar]
- McCarthy MC, Hafner HR, Chinkin LR, Charrier JG. Temporal variability of selected air toxics in the United States. Atmospheric Environment. 2007;41:7180–7194. [Google Scholar]
- Meng QY, Turpin BJ, Korn L, Weisel CP, Morandi M, Colome S, Zhang JFJ, Stock T, Spektor D, Winer A, Zhang L, Lee JH, Giovanetti R, Cui W, Kwon J, Alimokhtari S, Shendell D, Jones J, Farrar C, Maberti S. Influence of ambient (outdoor) sources on residential indoor and personal PM2.5 concentrations: analyses of RIOPA data. Journal of Exposure Analysis and Environmental Epidemiology. 2005;15:17–28. doi: 10.1038/sj.jea.7500378. [DOI] [PubMed] [Google Scholar]
- Miller L, Xu XH, Luginaah I. Spatial variability of volatile organic compound concentrations in Sarnia, Ontario, Canada. Journal of Toxicology and Environmental Health-Part A-Current Issues. 2009;72:610–624. doi: 10.1080/15287390802706413. [DOI] [PubMed] [Google Scholar]
- Naumova YY, Eisenreich SJ, Turpin BJ, Weisel CP, Morandi MT, Colome SD, Totten LA, Stock TH, Winer AM, Alimokhtari S, Kwon J, Shendell D, Jones J, Maberti S, Wall SJ. Polycyclic aromatic hydrocarbons in the indoor and outdoor air of three cities in the US. Environmental Science & Technology. 2002;36:2552–2559. doi: 10.1021/es015727h. [DOI] [PubMed] [Google Scholar]
- Ohura T, Amagai T, Shen XY, Li SA, Zhang P, Zhu LZ. Comparative study on indoor air quality in Japan and China: characteristics of residential indoor and outdoor VOCs. Atmospheric Environment. 2009;43:6352–6359. [Google Scholar]
- Paustenbach DJ. The practice of exposure assessment: a state-of-the-art review (reprinted from principles and methods of toxicology, 4th edition, 2001) Journal of Toxicology and Environmental Health-Part B-Critical Reviews. 2000;3:179–291. doi: 10.1080/10937400050045264. [DOI] [PubMed] [Google Scholar]
- Peng CY, Batterman S. Performance evaluation of a sorbent tube sampling method using short path thermal desorption for volatile organic compounds. Journal of Environmental Monitoring. 2000;2:313–324. doi: 10.1039/b003385p. [DOI] [PubMed] [Google Scholar]
- Rappaport SM, Kupper LL. Variability of environmental exposures to volatile organic compounds. Journal of Exposure Analysis and Environmental Epidemiology. 2004;14:92–107. doi: 10.1038/sj.jea.7500309. [DOI] [PubMed] [Google Scholar]
- Rehwagen M, Schlink U, Herbarth O. Seasonal cycle of VOCs in apartments. Indoor Air. 2003;13:283–291. doi: 10.1034/j.1600-0668.2003.00206.x. [DOI] [PubMed] [Google Scholar]
- Sax SN, Bennett DH, Chillrud SN, Ross J, Kinney PL, Spengler JD. A cancer risk assessment of inner-city teenagers living in New York City and Los Angeles. Environmental Health Perspectives. 2006;114:1558–1566. doi: 10.1289/ehp.8507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlink U, Rehwagen M, Damm M, Richter M, Borte M, Herbarth O. Seasonal cycle of indoor-VOCs: comparison of apartments and cities. Atmospheric Environment. 2004;38:1181–1190. [Google Scholar]
- Serrano-Trespalacios PI, Ryan L, Spengler JD. Ambient, indoor and personal exposure relationships of volatile organic compounds in Mexico City Metropolitan Area. Journal of Exposure Analysis and Environmental Epidemiology. 2004;14:S118–S132. doi: 10.1038/sj.jea.7500366. [DOI] [PubMed] [Google Scholar]
- Sexton K, Adgate JL, Ramachandran G, Pratt GC, Mongin SJ, Stock TH, Morandi MT. Comparison of personal, indoor, and outdoor exposures to hazardous air pollutants in three urban communities. Environmental Science & Technology. 2004;38:423–430. doi: 10.1021/es030319u. [DOI] [PubMed] [Google Scholar]
- Spicer CW, Buxton BE, Holdren MW, Smith DL, Kelly TJ, Rust SW, Pate AD, Sverdrup GM, Chuang JC. Variability of hazardous air pollutants in an urban area. Atmospheric Environment. 1996;30:3443–3456. [Google Scholar]
- Storm L. Nested analysis of variance: review of methods. Metrika. 1962;5:158–183. [Google Scholar]
- US EPA. Guidelines for Exposure Assessment. U.S. Environmental Protection Agency; Washington, DC: 1992. [Google Scholar]
- US EPA. Compendium Method TO-15, Determination of Volatile Organic Compounds (VOCs) in Air Collected in Specially-Prepared Canisters and Analyzed by Gas Chromatograpy/Mass Spectrometry (GC/MS) U.S. Environmental Protection Agency; Cincinnati, OH: 1999. p. 1. [Google Scholar]
- US EPA. EPA Requirements for Quality Assurance Project Plans (EPA QA/R-5) U.S. Environmental Protection Agency; Washington, DC: 2001. [Google Scholar]
- US EPA. EPA Positive Matrix Factorization (PMF) 3.0 Fundamentals & User Guide. U.S. Environmental Protection Agency; Washington, DC: 2008. [Google Scholar]
- US EPA. Fiscal Year 2011–2015 EPA Strategic Plan. U.S. Environmental Protection Agency; Washington, DC: 2010. [Google Scholar]
- Weisel CP, Zhang JF, Turpin BJ, Morandi MT, Colome S, Stock TH, Spektor DM, Korn L, Winer A, Alimokhtari S, Kwon J, Mohan K, Harrington R, Giovanetti R, Cui W, Afshar M, Maberti S, Shendell D. Relationship of indoor, outdoor and personal air (RIOPA) study: study design, methods and quality assurance/control results. Journal of Exposure Analysis and Environmental Epidemiology. 2005;15:123–137. doi: 10.1038/sj.jea.7500379. [DOI] [PubMed] [Google Scholar]
- Zhu JP, Newhook R, Marro L, Chan CC. Selected volatile organic compounds in residential air in the city of Ottawa, Canada. Environmental Science & Technology. 2005;39:3964–3971. doi: 10.1021/es050173u. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


