Abstract
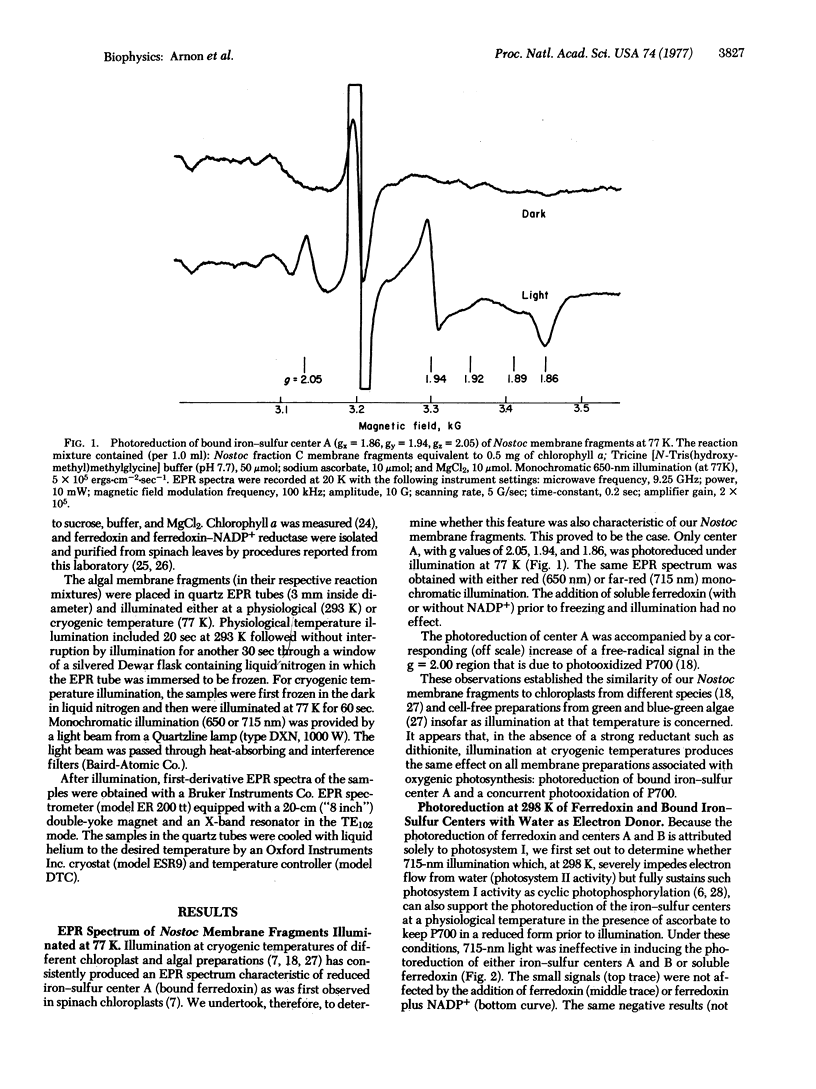
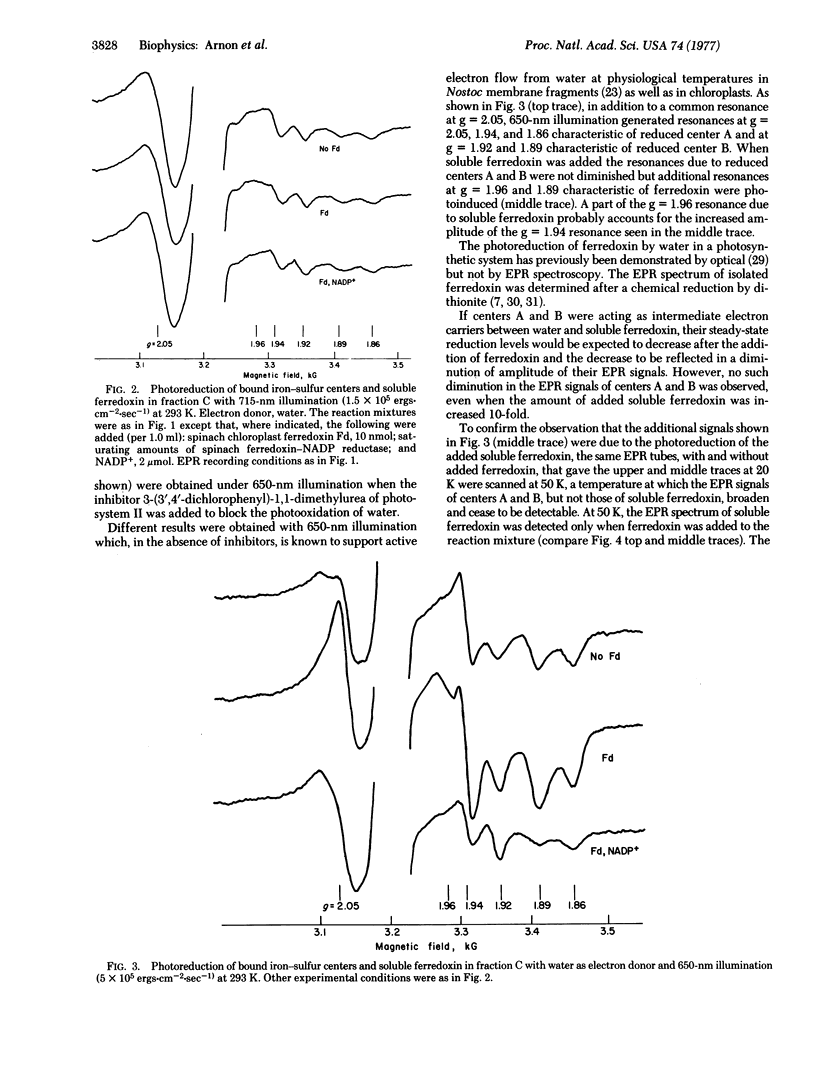
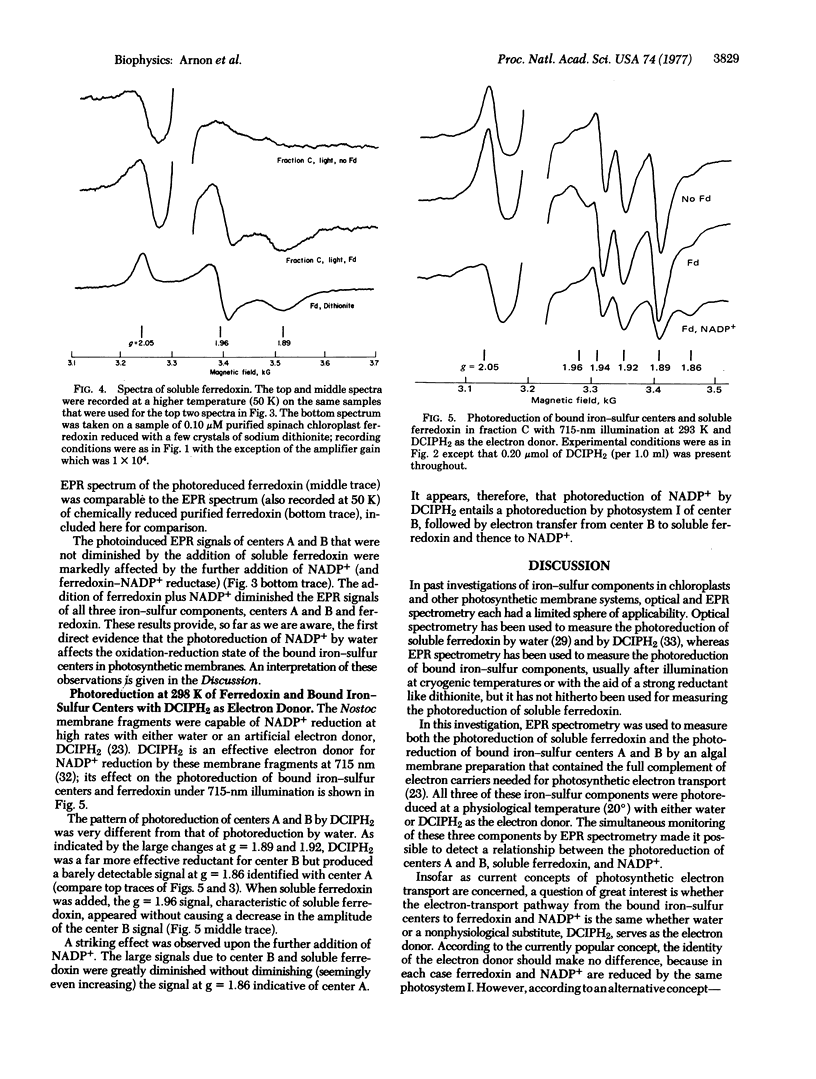
Electron paramagnetic resonance spectrometry was used to investigate, at physiological temperatures, light-induced electron transport from membrane-bound iron-sulfur components (bound ferredoxin) to soluble ferredoxin and NADP+ in membrane fragments (from the blue-green alga, Nostoc muscorum) that had high rates of electron transport from water to NADP+ and from an artificial electron donor, reduced dichlorophenolindophenol (DCIPH2) to NADP+. Illumination at 20° resulted in the photoreduction of membrane-bound iron-sulfur centers A and B. Photoreduction by water gave electron paramagnetic resonance signals of both centers A and B; photoreduction by DCIPH2 was found to generate a strong electron paramagnetic signal of only center B.
When water was the reductant, the addition and photoreduction of soluble ferredoxin generated additional signals characteristics of soluble ferredoxin without causing a decrease in the amplitude of the signals due to centers A and B. The further addition of NADP+ (and its photoreduction) greatly diminished signals due to the bound iron-sulfur centers and to soluble ferredoxin. An outflow of electrons from center B to soluble ferredoxin and NADP+ was particularly pronounced when DCIPH2 was the reductant. These observations provide the first evidence for a light-induced electron transport between membrane-bound iron-sulfur centers and ferredoxin-NADP+. The relationship of these observations to current concepts of photosynthetic electron transport is discussed.
Keywords: photosynthesis, reducing power, electron carriers
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARNON D. I., TSUJIMOTO H. Y., MCSWAIN B. D. ROLE OF FERREDOXIN IN PHOTOSYNTHETIC PRODUCTION OF OXYGEN AND PHOSPHORYLATION BY CHLOROPLASTS. Proc Natl Acad Sci U S A. 1964 Jun;51:1274–1282. doi: 10.1073/pnas.51.6.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnon D. I., Chain R. K. Regulation of ferredoxin-catalyzed photosynthetic phosphorylations. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4961–4965. doi: 10.1073/pnas.72.12.4961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnon D. I., McSwain B. D., Tsujimoto H. Y., Wada K. Photochemical activity and components of membrane preparations from blue-green algae. I. Coexistence of two photosystems in relation to chlorophyll a and removal of phycocyanin. Biochim Biophys Acta. 1974 Aug 23;357(2):231–245. doi: 10.1016/0005-2728(74)90063-2. [DOI] [PubMed] [Google Scholar]
- Arnon D. I. The light reactions of photosynthesis. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2883–2892. doi: 10.1073/pnas.68.11.2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bearden A. J., Malkin R. Chloroplast photosynthesis: the reaction center of photosystem I. Brookhaven Symp Biol. 1976 Jun 7;(28):247–266. [PubMed] [Google Scholar]
- Bearden A. J., Malkin R. Correlation of reaction-center chlorophyll (P-700) oxidation and bound iron-sulfur protein photoreduction in chloroplast photosystem I at low temperatures. Biochim Biophys Acta. 1976 Jun 8;430(3):538–547. doi: 10.1016/0005-2728(76)90029-3. [DOI] [PubMed] [Google Scholar]
- Bearden A. J., Malkin R. Primary photochemical reactions in chloroplast photosynthesis. Q Rev Biophys. 1974 May;7(2):131–177. doi: 10.1017/s0033583500001396. [DOI] [PubMed] [Google Scholar]
- Bearden A. J., Malkin R. Quantitative EPR studies of the primary reaction of photosystem I in chloroplasts. Biochim Biophys Acta. 1972 Dec 14;283(3):456–468. doi: 10.1016/0005-2728(72)90262-9. [DOI] [PubMed] [Google Scholar]
- Bearden A. J., Malkin R. The bound ferredoxin of chloroplasts: a role as the primary electron acceptor of photosystem I. Biochem Biophys Res Commun. 1972 Feb 16;46(3):1299–1305. doi: 10.1016/s0006-291x(72)80116-5. [DOI] [PubMed] [Google Scholar]
- Chain R. K., Arnon D. I. Quantum efficiency of photosynthetic energy conversion. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3377–3381. doi: 10.1073/pnas.74.8.3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E. H., Cammack R. Properties of the primary electron acceptor complex of photosystem I in the blue green alga Chlorogloea fritschii. Biochem Biophys Res Commun. 1976 Feb 23;68(4):1212–1218. doi: 10.1016/0006-291x(76)90326-0. [DOI] [PubMed] [Google Scholar]
- Evans M. C., Reeves S. G., Cammack R. Determination of the oxidation-reduction potential of the bound iron-sulphur proteins of the primary electron acceptor complex of photosystem I in spinach chloroplasts. FEBS Lett. 1974 Dec 1;49(1):111–114. doi: 10.1016/0014-5793(74)80644-7. [DOI] [PubMed] [Google Scholar]
- Evans M. C., Reeves S. G., Telfer A. The detection of a bound ferredoxin in the photosynthetic lamellae of blue-green algae and other oxygen evolving photosynthetic organisms. Biochem Biophys Res Commun. 1973 Apr 2;51(3):593–596. doi: 10.1016/0006-291x(73)91355-7. [DOI] [PubMed] [Google Scholar]
- Evans M. C., Reeves S. G., Telfer A. The detection of a bound ferredoxin in the photosynthetic lamellae of blue-green algae and other oxygen evolving photosynthetic organisms. Biochem Biophys Res Commun. 1973 Apr 2;51(3):593–596. doi: 10.1016/0006-291x(73)91355-7. [DOI] [PubMed] [Google Scholar]
- Evans M. C., Sihra C. K., Cammack R. The properties of the primary electron acceptor in the Photosystem I reaction centre of spinach chloroplasts and its interaction with P700 and the bound ferredoxin in various oxidation-reduction states. Biochem J. 1976 Jul 15;158(1):71–77. doi: 10.1042/bj1580071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans M. C., Telfer A., Lord A. V. Evidence for the role of a bound ferredoxin as the primary electron acceptor of photosystem I in spinach chloroplasts. Biochim Biophys Acta. 1972 Jun 23;267(3):530–537. doi: 10.1016/0005-2728(72)90181-8. [DOI] [PubMed] [Google Scholar]
- Hall D. O., Gibson J. F., Whatley F. R. Electron spin resonance spectra of spinach ferredoxin. Biochem Biophys Res Commun. 1966 Apr 6;23(1):81–84. doi: 10.1016/0006-291x(66)90272-5. [DOI] [PubMed] [Google Scholar]
- Ke B., Hansen R. E., Beinert H. Oxidation-reduction potentials of bound iron-sulfur proteins of photosystem I. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2941–2945. doi: 10.1073/pnas.70.10.2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkin R., Bearden A. J. Laser-flash-activated electron paramagnetic resonance studies of primary photochemical reactions in chloroplasts. Biochim Biophys Acta. 1975 Aug 11;396(2):250–259. doi: 10.1016/0005-2728(75)90039-0. [DOI] [PubMed] [Google Scholar]
- Malkin R., Bearden A. J. Primary reactions of photosynthesis: photoreduction of a bound chloroplast ferredoxin at low temperature as detected by EPR spectroscopy. Proc Natl Acad Sci U S A. 1971 Jan;68(1):16–19. doi: 10.1073/pnas.68.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh A. R., Chu M., Bolton J. R. Flash photolysis electron spin resonance studies of the electron acceptor species at low temperatures in photosystem I of spinach subchloroplast particles. Biochim Biophys Acta. 1975 Feb 17;376(2):308–314. doi: 10.1016/0005-2728(75)90023-7. [DOI] [PubMed] [Google Scholar]
- Mcintosh A. R., Bolton J. R. Electron spin resonance spectrum of species "X" which may function as the primary electron acceptor in photosystem I of green plant photosynthesis. Biochim Biophys Acta. 1976 Jun 8;430(3):555–559. [PubMed] [Google Scholar]
- Palmer G., Sands R. H. On the magnetic resonance of spinach ferredoxin. J Biol Chem. 1966 Jan 10;241(1):253–253. [PubMed] [Google Scholar]
- SHIN M., ARNON D. I. ENZYMIC MECHANISMS OF PYRIDINE NUCLEOTIDE REDUCTION IN CHLOROPLASTS. J Biol Chem. 1965 Mar;240:1405–1411. [PubMed] [Google Scholar]
- SHIN M., TAGAWA K., ARNON D. I. CRYSTALLIZATION OF FERREDOXIN-TPN REDUCTASE AND ITS ROLE IN THE PHOTOSYNTHETIC APPARATUS OF CHLOROPLASTS. Biochem Z. 1963;338:84–96. [PubMed] [Google Scholar]
- SHIN M., TAGAWA K., ARNON D. I. CRYSTALLIZATION OF FERREDOXIN-TPN REDUCTASE AND ITS ROLE IN THE PHOTOSYNTHETIC APPARATUS OF CHLOROPLASTS. Biochem Z. 1963;338:84–96. [PubMed] [Google Scholar]
- TAGAWA K., ARNON D. I. Ferredoxins as electron carriers in photosynthesis and in the biological production and consumption of hydrogen gas. Nature. 1962 Aug 11;195:537–543. doi: 10.1038/195537a0. [DOI] [PubMed] [Google Scholar]
- TAGAWA K., TSUJIMOTO H. Y., ARNON D. I. Role of chloroplast ferredoxin in the energy conversion process of photosynthesis. Proc Natl Acad Sci U S A. 1963 Apr;49:567–572. doi: 10.1073/pnas.49.4.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAGAWA K., TSUJIMOTO H. Y., ARNON D. I. SEPARATION BY MONOCHROMATIC LIGHT OF PHOTOSYNTHETIC PHOSPHORYLATION FROM OXYGEN EVOLUTION. Proc Natl Acad Sci U S A. 1963 Sep;50:544–549. doi: 10.1073/pnas.50.3.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujimoto H. Y., McSwain B. D., Hiyama T., Arnon D. I. Effect of NADP on light-induced cytochrome changes in membrane fragments from a blue-green alga. Biochim Biophys Acta. 1976 Feb 16;423(2):303–312. doi: 10.1016/0005-2728(76)90187-0. [DOI] [PubMed] [Google Scholar]
- WHATLEY F. R., TAGAWA K., ARNON D. I. Separation of the light and dark reactions in electron transfer during photosynthesis. Proc Natl Acad Sci U S A. 1963 Feb 15;49:266–270. doi: 10.1073/pnas.49.2.266. [DOI] [PMC free article] [PubMed] [Google Scholar]


