Abstract
BACKGROUND/OBJECTIVES
Perilla frutescens Britton leaves are a commonly consumed vegetable in different Asian countries including Korea. Cancer is a major cause of human death worldwide. The aim of the current study was to investigate the inhibitory effects of ethanol extract of perilla leaf (PLE) against important characteristics of cancer cells, including unrestricted growth, resisted apoptosis, and activated metastasis, using human cancer cells.
MATERIALS/METHODS
Two human cancer cell lines were used in this study, HCT116 colorectal carcinoma cells and H1299 non-small cell lung carcinoma cells. Assays using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide were performed for measurement of cell growth. Soft agar and wound healing assays were performed to determine colony formation and cell migration, respectively. Nuclear staining and cell cycle analysis were performed for assessment of apoptosis. Fibronectin-coated plates were used to determine cell adhesion.
RESULTS
Treatment of HCT116 and H1299 cells with PLE resulted in dose-dependent inhibition of growth by 52-92% (at the concentrations of 87.5, 175, and 350 µg/ml) and completely abolished the colony formation in soft agar (at the concentration of 350 µg/ml). Treatment with PLE at the 350 µg/ml concentration resulted in change of the nucleus morphology and significantly increased sub-G1 cell population in both cells, indicating its apoptosis-inducing activity. PLE at the concentration range of 87.5 to 350 µg/ml was also effective in inhibiting the migration of H1299 cells (by 52-58%) and adhesion of both HCT116 and H1299 cells (by 25-46%).
CONCLUSIONS
These results indicate that PLE exerts anti-cancer activities against colon and lung cancers in vitro. Further studies are needed in order to determine whether similar effects are reproduced in vivo.
Keywords: Perilla leaf, cancer cell, cell growth, migration, adhesion
INTRODUCTION
Cancer is a major cause of human death worldwide. The colorectal and lung cancer burdens are among the highest in terms of their incidence and mortality [1]. Thus, prevention of these types of cancers has significant implication in public health. Adequate consumption of fruits and vegetables has been inversely associated with the risk of several cancers, particularly digestive and respiratory cancers [2]. Diverse phytochemicals found in abundance in fruits and vegetables have been shown to possess cancer-preventive properties [3]. However, there has been insufficient evidence that any one single phytochemical can account for the cancer-preventive activities of plant-based foods in humans [3]. Therefore, evaluation of the anticancer-activities of plant-based foods as a complex mixture containing different phytochemicals together with other constituents would be important.
Perilla frutescens Britton leaves have a unique aroma and are commonly consumed in different Asian countries, including Korea [4]. Perilla leaf extract (PLE) has been reported to possess anti-oxidant [5] and anti-inflammatory activities [6,7] as well as alleviating activities against hepatotoxicity [8], allergy [9,10,11], and obesity [12]. PLE has also been reported to inhibit the growth of human hepatoma and leukemia cells [13,14] and skin tumor formation in mice [15]. Several activities have been attributed to the constituents, including luteolin [15,16,17], rosmarinic acid [11,18,19,20,21], and triterpene acids [22].
The most fundamental characteristics of cancer cells involve their capabilities to sustain unrestricted growth, resist apoptosis, and activate invasion and metastasis [23]. However, there are few studies systematically reporting on the effect of PLE against such characteristics of human cancer cells. The aim of the current study was to investigate the effects of PLE on the growth, apoptosis, migration, and adhesion of cancer cells using human colon and lung cancer cell lines.
MATERIALS AND METHODS
Preparation of perilla leaf extract
Perilla leaves were purchased from a local retail store (Cheongju, Korea). The leaf samples were washed, ground (MU-3000, TC Angel, Seoul, Korea), freeze-dried (PH1316, IshinBioBase, Yangju, Korea), and then extracted with 10-fold volume of 70% ethanol at room temperature for 4 h. The extraction solution was then centrifuged at 3,000 × g for 3 min (A320101, Gyrozen, Daejeon, Korea), and the supernatant was collected. The ethanol solvent in the supernatant was removed using a vacuum evaporator (NB-503CIR, N-bioteck, Bucheon, Korea). The dried extract was weighed and stored at -70℃ for further analysis. The extraction yield was 17.6 ± 2.3% on a dry weight basis.
Cell culture
HCT116 human colon cancer cells and H1299 human lung cancer cells were purchased from Korean Cell Line Bank (Seoul, Korea). HCT116 and H1299 cells were maintained in Macoy's and RPMI medium (Gibco Co., Rockville, MD, USA), respectively, containing 10% fetal bovine serum (FBS; Thermo Scientific, Logan, UT, US) and 100 units/mL penicillin/0.1 mg/mL streptomycin (Welgene Inc., Daegu, Korea). Cells were cultured at 37℃ in 95% humidity and 5% CO2. The extracts were dissolved in dimethyl sulfoxide (DMSO; Biosesang Inc., Seongnam, Korea) and further diluted using the corresponding cell culture medium immediately before treatment of the cells. The final concentration of DMSO in the culture medium was less than 0.2% (v/v).
Cell viability assay
Cell viability was determined by the 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide (MTT; Sigma-Aldrich, St, Louis, MO, US) assay [24]. HCT116 and H1299 cells (5 × 103 cells/well) were seeded in 96-well plates (Corning Inc., New York, NY, USA). After 24 h, cells were treated with 0, 87.5, 175, and 350 µg/ml concentrations of PLE in serum free media for 72 and 96 h. The media was replaced by fresh media containing 0.5 mg/mL of MTT. After incubation for 4 h at 37℃, MTT-containing media were aspirated, and the reduced formazan dye on the bottom of the well was dissolved by addition of DMSO. After gentle mixing, the absorbance was measured at 540 nm using a plate reader (Bio-Rad Laboratories, Hercules, CA, US).
Assay for colony formation in soft agar
Anchorage-independent growth was determined by the colony formation assay in soft agar [25]. HCT116 (6 × 104 cells/well) and H1299 (8 × 103 cells/well) cells were mixed with 2 ml of culture media containing 0.3% agar (DC Chemical Co., Seoul, Korea) and then overlaid on 1 ml of 0.6% agar in 6-cm culture dishes (Corning Inc.). Cells were treated with 350 µg/ml of PLE in serum complete media for 21 days, changing the media twice a week. After three weeks, colonies were visualized by staining with 0.05% crystal violet for 1 h. The number of colonies was then counted at four randomly selected points in each well under phase contrast time-lapse microscopy (Primo Vert, Carl Zeiss, Oberkochen, Germany) at a magnification of 100 fold. The images were captured using iSolution Lite software (IMT i-solution, Burnarby, Canada).
Staining of cell nucleus
DNA staining was performed using 4',6-diamidino-2-phenylindole dihydrochloride (DAPI, Sigma-Aldrich) for morphological observation of apoptotic cells [26]. HCT116 (7 × 103 cells/well) and H1299 (6 × 103 cells/well) cells were seeded on 8-well chamber slides (Corning Inc.). After 24 h, cells were treated with PLE at 0, 87.5, 175, and 350 µg/ml in serum free media for 24 h. Cells were fixed using 4% formaldehyde (Sigma-Aldrich) for 15 min at room temperature, followed by incubation with DAPI. The nuclear morphology was observed using confocal laser scanning microscopy at a magnification of 200 fold (MRC-1024, Bio-Rad Laboratories). Apoptotic cells were recognized by condensed, fragmented, or degraded nuclei [27,28]. DAPI staining intensity was determined using Image-J software (NIH, Bethesda, MD, USA).
Cell cycle analysis
Cell cycle was determined as previously described [25] with a slight modification. Briefly, HCT116 and H1299 cells (3 × 105 cells/well) were seeded in 10-cm culture dishes (Corning Inc.). After 24 h, cells were synchronized for another 24 h in serum free media. Cells were then treated with PLE at 350 µg/ml in serum complete media for 24 h (in the case of H1299) or 72 h (in the case of HCT116). Adherent cells were detached by brief trypsinization (0.25% trypsin-EDTA; Sigma-Aldrich). Cell pellets were washed with ice-cold PBS and then re-suspended in 70% ethanol overnight for fixation. After centrifugation at 3,000 × g, the supernatant was removed, and cells were incubated with PBS containing 1 µg/ml RNAse (Sigma-Aldrich) and 50 µg/ml propidium iodine (Sigma-Aldrich) for 20 min at room temperature. Single-cell suspension was generated by gentle pipetting. Cell cycle analysis was performed using a flow cytometer (FACS Calibur-S System, BD Biosciences, Heidelberg, Germany), and data were processed using Cell Quest Pro software (BD Biosciences).
Cell migration assay
Cell migration was determined using the scratch wound healing assay [29]. HCT116 and H1299 cells (5 × 103 cells/well) were seeded on 6-well plates (Corning Inc.). When the cells reached approximately 80% confluence, a wound was generated by scratching the cell monolayer using a pipette tip and then washed with PBS for removal of cell debris. Cells were maintained with serum free media containing PLE at 0, 87.5, 175, and 350 µg/ml and allowed to migrate into the wound area for 24 h. Images of the wound area were captured using iSolution Lite software (IMT i-solution, Burnarby, Canada). The average wound widths at 0 h and 24 h time points were quantified using Image-J software (NIH), and the wound closure during the time interval was calculated by subtracting the width at 24 h time point from the width at 0 h time point.
Cell adhesion assay
Cell adhesion was determined as previously described [30] with a slight modification. Briefly, 96-well plates were precoated with fibronectin (1 µg/ml, Sigma-Aldrich) in Hank's buffer (Welgene Inc.) for 2 h at 37℃, washed with media containing 0.1% bovine serum albumin (BSA, Sigma-Aldrich) twice, and then blocked with 0.5% BSA for 1 h at 37℃. HCT116 and H1299 cells were suspended in either PLE-containing or DMSO-containing serum complete media and were then plated into 96-well plates (1 × 103 cells/well). After 1 h, floating non-adherent cells in the media were aspirated. Adherent cells were stained with 0.2% crystal violet for 10 min at room temperature, washed with PBS twice, dissolved with 1% sodium dodecyl sulfate (Sigma-Aldrich), and then quantified by reading the absorbance at 540 nm using a plate reader (Bio-Rad Laboratories).
Statistical analyses
SPSS software (version 12.0; SPSS Inc., Chicago, IL, USA) was used for statistical analyses. Student's t-test was used for a simple comparison between two groups. One-way ANOVA combined with Tukey's as a post-hoc test was used for comparisons among multiple groups. Regression analysis was used for assessment of a dose-response relationship. A probability value of P < 0.05 was considered significant.
RESULTS
Effect of PLE on growth and colony formation of human cancer cells
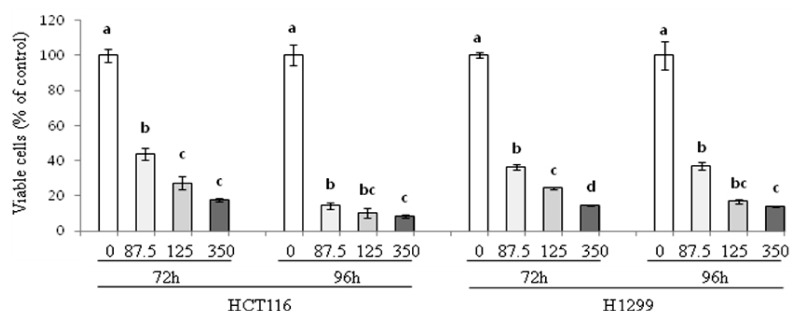
We first investigated the effect of PLE on the growth of human colon and lung cancer cells. As shown in Fig. 1, treatment of HCT116 cells with PLE at the concentration of 87.5, 175, and 350 µg/ml for 72 h resulted in significantly inhibited growth by 52-82%, with an estimated IC50 value of 76 µg/ml. Longer treatment of HCT116 cells with PLE (96 h) resulted in even greater growth inhibition by 86-92% (IC50 value of ~50 µg/ml). H1299 cancer cells were comparably susceptible to PLE; treatment with PLE for 72 h and 96 h inhibited growth by 63-86% (IC50 value of ~67 µg/ml). In both HCT116 and H1299 cells, the growth inhibitory effects of PLE were increased with its increasing concentrations, showing a significant quadratic dose-response relationship (R2 = 0.89-0.97, P < 0.001 by regression analysis).
Fig. 1.
Effect of PLE on growth of human colon and lung cancer cells. HCT116 and H1299 cells were treated with PLE at the concentrations of 0, 87.5, 175, and 350 µg/ml for 72 h and 96 h. Viable cells were quantified by the MTT assay, and data are shown as mean ± SE of 4-8 determinations. Different letters (a-d) indicate statistical differences among different concentrations of PLE at a specific time point by Tukey's test (P < 0.05).
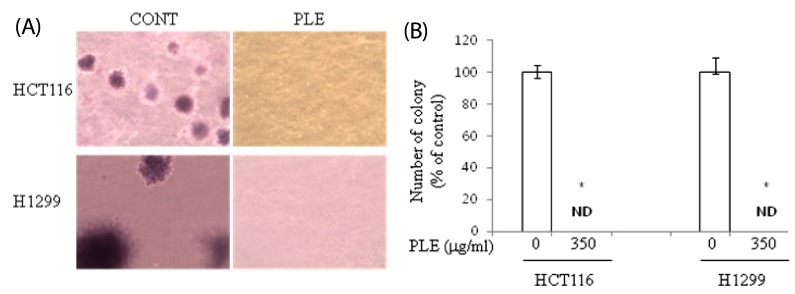
We next investigated the effect of PLE on anchorage-independent growth of human colon and lung cancer cells in a typical soft-agar assay. After the incubation of PLE in soft agar for 21 days, a substantial number of colonies formed in both HCT116 and H1299 cells, indicating their capability of anchorage-independent growth. Colonies of the control H1299 cells were found to be much larger than those of the control HCT116 cells (Fig. 2A). However, in both HCT116 and H1299 cells, treatment with PLE at the concentration of 350 µg/ml resulted in complete inhibition of colony formation; only a few cells, not colonies, were observed in PLE-treated cells (Fig. 2A & B).
Fig. 2.
Effect of PLE on colony formation of human colon and lung cancer cells in soft agar. HCT116 and H1299 cells on agar were treated with PLE at the concentrations of 0 or 350 µg/ml for 21 days (A). Colonies were stained with crystal violet, and the representative area is shown (B). The number of colonies was counted in four randomly selected points in each well under phase contrast time-lapse microscopy (×100). Asterisks indicate statistical differences between untreated control and PLE-treated cells by two-tailed student t-test (P < 0.05). ND: not detected.
Effects of PLE on nuclear morphology and cell cycle distribution in human cancer cells
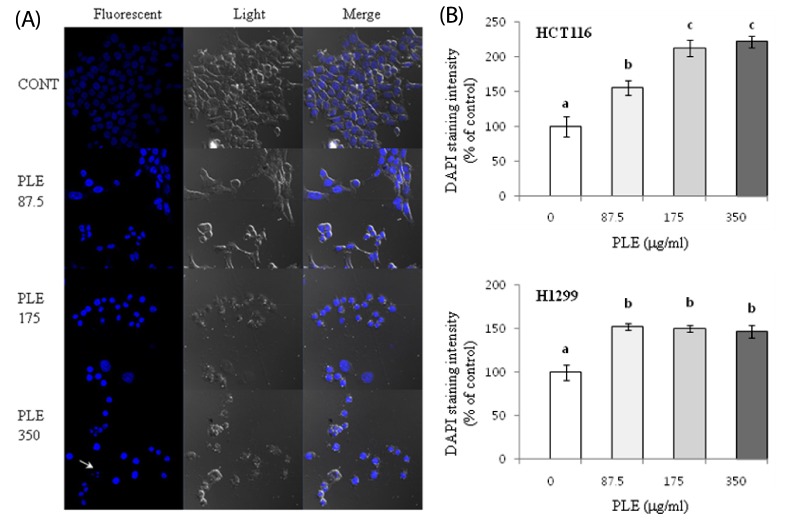
We then determined the apoptosis-inducing activities of PLE after treatment in HCT116 and H1299 cells for 24 h. Cell nuclei were visualized by DNA staining with the fluorescent dye DAPI in order to observe morphological evidence of apoptosis. Control HCT116 (data not shown) and H1299 cells (Fig. 3A) presented homogeneous and symmetric shape of nucleus. PLE-treated cells, however exhibited heterogeneous and condensed nuclear chromatin. The phenomenon was more pronounced as the concentration of PLE was increased (Fig. 3A). In particular, H1299 cells treated with PLE at the highest concentration (350 µg/ml) showed nuclear fragmentation (indicated with the arrow in Fig. 3A). The DAPI staining intensity of apoptotic cells is known to be increased due to their increased membrane permeability [31]. Treatment with PLE at the concentrations of 87.5, 175, and 350 µg/ml resulted in increased DAPI staining intensity to 156-221% of control in HCT116 cells and to 146-152% of the control in H1299 cells (Fig. 3A & B).
Fig. 3.
Effect of PLE on nuclear morphology of human colon and lung cancer cells. Cells were treated with 0, 87.5, 175, and 350 µg/ml of PLE for 24 h (A). Nuclear morphology was observed by DAPI staining, and a representative area of H1299 cells is shown. The arrow indicates the nuclear fragmentation (B). DAPI staining intensity was quantified using Image J software and presented as % of control (mean ± SE). Different letters (a-c) indicate statistical differences among different concentrations by Tukey's test (P < 0.05).
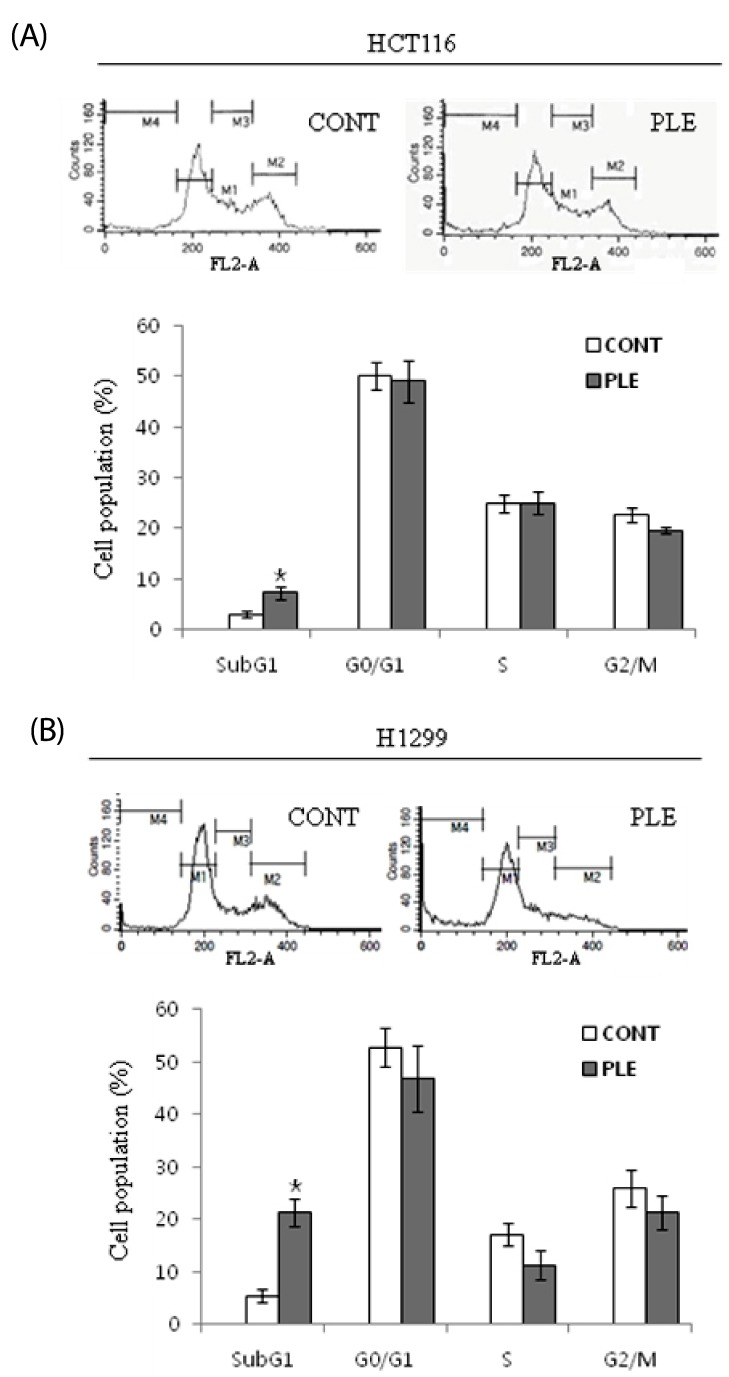
To confirm the apoptosis-inducing activities and further characterize the growth inhibitory effect of PLE, cell cycle analyses were performed on HCT116 and H1299 cells after treatment with PLE at the concentration of 350 µg/ml. As shown in Fig. 4, treatment with PLE resulted in significantly increased sub-G1 cell population to 2.4-fold of control in HCT116 cells (at 72 h; P < 0.05) and 7.4-fold of control in H1299 cells (at 24 h; P < 0.05). However, treatment with PLE did not result in any significant changes in cell population at G0-G1, S, and G2-M phases.
Fig. 4.
Effect of PLE on cell cycle distribution in human colon and lung cancer cells. Cells were treated with 350 µg/ml concentration of PLE for 72 h (in the case of HCT116; A) and 24 h (in the case of H1299; B). Cells were stained with PI, and the cell population (%) at sub-G1, G0-G1, S, and M-G2 phase was analyzed. Representative cell cycle distribution is shown in the upper panel, and data are shown as mean ± SE of three determinations in the lower panel. Asterisks indicate statistical differences between untreated control and PLE-treated cells by two-tailed student t-test (P < 0.05).
Effect of PLE on migration in human colon cancer cells
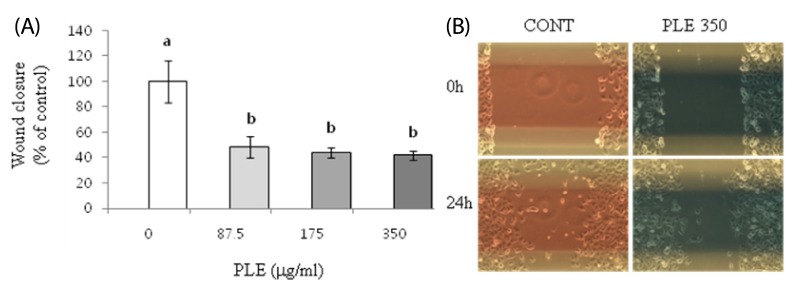
We evaluated the effects of PLE on migration of human cancer cells in a wound healing assay. Following introduction of a wound, cells were treated for 24 h with PLE at 87.5, 175, and 350 µg/ml concentrations since the treatment did not significantly affected the cell growth at the 24 h time point (data not shown). Treatment of H1299 cells with PLE at the concentrations of 87.5, 175, and 350 µg/ml resulted in significantly reduced wound closure by 52-58% (Fig. 5A & B). HCT116 cells, however, were not responsive to PLE; treatment with 87.5, 175, and 350 µg/ml of PLE for 24 h did not cause any significant changes in migration of HCT116 cells (data not shown).
Fig. 5.
Effect of PLE on migration in human lung cancer cells. H1299 cells were treated with 0, 87.5, 175, and 350 µg/ml of PLE for 24 h. (A) The width of wound was quantified using Image-J software, and the wound closure during the 24 h time interval is shown as mean ± SE of 4-5 determinations. Different letters (a-c) indicate statistical differences among different concentrations by Tukey's test (P < 0.05). (B) Representative wound area of H1299 cells before and after 24 h treatment with PLE at the concentration of 350 µg/ml is shown.
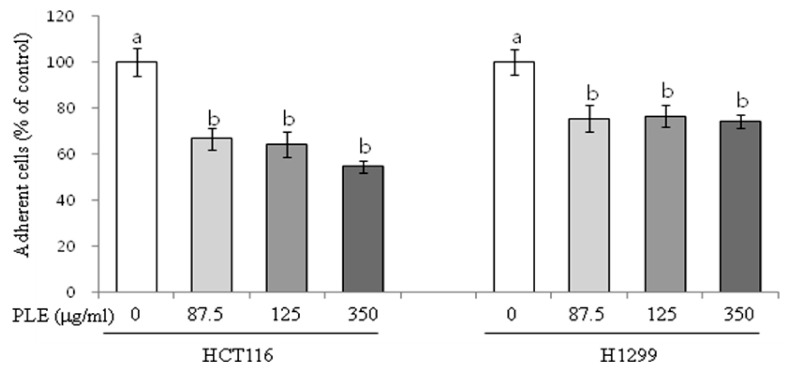
Effect of PLE on cell adhesion in human colon cancer cells
Treatment of HCT116 cells with PLE at 87.5, 175, and 350 µg/ml concentrations inhibited the cell adhesion to the fibronectin-coated bottom of the culture plate by 34-46% (P < 0.05, Fig. 6). H1299 cells were slightly less responsive to PLE than HCT116 cells in inhibiting the adhesion; treatment with PLE at 87.5, 175, and 350 µg/ml was effective in inhibiting cell adhesion by 25-26% (P < 0.05).
Fig. 6.
Effect of PLE on adhesion in human colon and lung cancer cells. HCT116 and H1299 cells were suspended with 0, 87.5, 175, and 350 µg/ml concentration of PLE and plated into a fibronectin (1 µg/ml)-coated 96-well plate. After 2 h, adherent cells were stained with crystal violet, dissolved with sodium dodecyl sulfate, and then quantified by reading the absorbance at 540 nm using a plate reader. Data are shown as mean ± SE of four determinations. Different letters (a-b) indicate statistical differences among different concentrations by Tukey's test (P < 0.05).
DISCUSSION
Sustaining unrestricted growth, resisting apoptosis, and activating metastasis are the most fundamental characteristics of cancer cells [23]. The aim of the current study was to investigate the inhibitory effects of PLE against such fundamental characteristics of cancer cells in vitro. Two human cancer cell lines were used in this study, HCT116 colorectal carcinoma cells and H1299 non-small cell lung carcinoma cells. Both have been reported to have highly proliferative and metastatic propensities [32,33] and are therefore used extensively for screening a variety of potential anti-proliferative and anti-metastatic compounds [29,34,35,36].
The results of our study indicated that PLE has strong growth-inhibitory activities in both HCT116 and H1299 cells (Fig. 1). This is in agreement with previous reports on the potent anti-proliferative effects of PLE at a range of concentrations (50-500 µg/ml) in human hepatoma [14] and leukemia cells [13]. Our results also showed that treatment with PLE virtually nullified the colony formation of both HCT116 and H1299 cells (Fig. 2). Since the anchorage independent growth assay is regarded as an in vitro test for tumorigenesis in vivo [37], the results of our study suggest that PLE may reduce the tumorigenicity of these cells; further research is needed. Apoptosis is a process of programmed cell death for removal of unwanted cells, and the disregulation of apoptosis may lead to malignant cell growth [27]. Thus, restoration of apoptosis in cancer cells is regarded as an effective strategy for cancer prevention and treatment [23,27]. Our results indicated that PLE effectively induced apoptosis, as shown by the alteration of nuclear morphology, the increase of DAPI staining intensity, and the accumulation of cell population in sub-G1 phase (Fig. 3 & 4). These findings suggest that the growth-inhibitory activities of PLE observed in the current study may be, at least in part, due to the induction of apoptosis by PLE. These results are consistent with those of previous studies showing apoptosis-inducing effects of PLE in human hepatoma [14] and leukemia cells [13]. In hepatoma cells, the apoptosis-inducing activities have been associated with down-regulation of Bcl-2 and up-regulation of caspases [14].
Metastasis is a multistep process where cancer cells leave the site of the primary lesion, pass through the circulatory system, and establish a secondary tumor at a new distant organ site [23]. Metastasis contributes to over 90% of human cancer mortality [23], therefore, anti-metastatic properties of a wide variety of compounds have been under extensive investigation [38]. Migration of cancer cells and adhesion of migrating cells to secondary organ sites are prominent events in the metastatic cascade [23]. Our results showed that PLE inhibited migration in H1299 cells and adhesion in both HCT116 and H1299 cell lines (Fig. 5 & 6). To the best of our knowledge, this is the first study reporting such inhibitory activities of PLE in human cancer cells. We can rule out the possibility that the inhibition of migration and adhesion by PLE were due to inhibition of cell growth since inhibition of migration and adhesion was observed at earlier time points (2-24 h; Fig. 5 & 6) where no inhibition of cell growth by PLE was evident (data not shown). Different responsiveness to PLE in inhibiting migration was observed in the two different cell lines (Fig. 5). We observed that H1299 cells were rapidly moved compared to HCT116 (data not shown), and such cell type specificity might contribute to the different responses.
Several constituents of perilla leaves have been shown to possess anti-cancer activities. Luteolin, a common flavonoid that exists in many types of plants including perilla leaves, has been shown to inhibit cell growth and metastasis but induced apoptosis in different types of cancer cells, modulating different cell signaling pathways [39]. Rosmarinic acid has been suggested as an active constituent for the anti-carcinogenic activity of perilla leaves in a mouse skin papilloma model, and the activities have been associated with its anti-inflammatory and antioxidant activities [19]. Isoegomaketone isolated from perilla leaves has been reported to inhibit tumor formation of hepatoma cells in a xenograft model, in conjunction with blocking phosphoinositide-3-kinase signaling [40]. However, clarification is needed with regard to whether inhibitory activities of PLE against different characteristics of cancer cells found in the current study are attributed to a single compound or different compounds in combination.
In summary, PLE inhibited growth, anchorage-independent colony formation, and adhesion in both human colon and lung cancer cells as well as migration in human lung cancer cells, indicating the anti-cancer activities of PLE in vitro. Whether or not similar inhibitory effects of PLE can be reproduced in relevant animal models and finally humans still needs to be determined. More studies are needed in order to better understand the detailed mechanism of the inhibitory action of PLE against colon and lung cancers.
Footnotes
This work was supported by the National Research Foundation of Korea grant funded by the Korean government (No. 2010-0025311).
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Surh YJ. Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer. 2003;3:768–780. doi: 10.1038/nrc1189. [DOI] [PubMed] [Google Scholar]
- 3.Miller PE, Snyder DC. Phytochemicals and cancer risk: a review of the epidemiological evidence. Nutr Clin Pract. 2012;27:599–612. doi: 10.1177/0884533612456043. [DOI] [PubMed] [Google Scholar]
- 4.Seo WH, Baek HH. Characteristic aroma-active compounds of Korean perilla (Perilla frutescens Britton) leaf. J Agric Food Chem. 2009;57:11537–11542. doi: 10.1021/jf902669d. [DOI] [PubMed] [Google Scholar]
- 5.Meng L, Lozano YF, Gaydou EM, Li B. Antioxidant activities of polyphenols extracted from Perilla frutescens varieties. Molecules. 2008;14:133–140. doi: 10.3390/molecules14010133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ueda H, Yamazaki M. Inhibition of tumor necrosis factor-alpha production by orally administering a perilla leaf extract. Biosci Biotechnol Biochem. 1997;61:1292–1295. doi: 10.1271/bbb.61.1292. [DOI] [PubMed] [Google Scholar]
- 7.Ueda H, Yamazaki M. Anti-inflammatory and anti-allergic actions by oral administration of a perilla leaf extract in mice. Biosci Biotechnol Biochem. 2001;65:1673–1675. doi: 10.1271/bbb.65.1673. [DOI] [PubMed] [Google Scholar]
- 8.Kim MK, Lee HS, Kim EJ, Won NH, Chi YM, Kim BC, Lee KW. Protective effect of aqueous extract of Perilla frutescens on tert-butyl hydroperoxide-induced oxidative hepatotoxicity in rats. Food Chem Toxicol. 2007;45:1738–1744. doi: 10.1016/j.fct.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 9.Makino T, Furuta A, Fujii H, Nakagawa T, Wakushima H, Saito K, Kano Y. Effect of oral treatment of Perilla frutescens and its constituents on type-I allergy in mice. Biol Pharm Bull. 2001;24:1206–1209. doi: 10.1248/bpb.24.1206. [DOI] [PubMed] [Google Scholar]
- 10.Liu JY, Chen YC, Lin CH, Kao SH. Perilla frutescens leaf extract inhibits mite major allergen Der p 2-induced gene expression of pro-allergic and pro-inflammatory cytokines in human bronchial epithelial cell BEAS-2B. PLoS One. 2013;8:e77458. doi: 10.1371/journal.pone.0077458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Makino T, Furuta Y, Wakushima H, Fujii H, Saito K, Kano Y. Antiallergic effect of Perilla frutescens and its active constituents. Phytother Res. 2003;17:240–243. doi: 10.1002/ptr.1115. [DOI] [PubMed] [Google Scholar]
- 12.Kim MJ, Kim HK. Perilla leaf extract ameliorates obesity and dyslipidemia induced by high-fat diet. Phytother Res. 2009;23:1685–1690. doi: 10.1002/ptr.2811. [DOI] [PubMed] [Google Scholar]
- 13.Kwak CS, Yeo EJ, Moon SC, Kim YW, Ahn HJ, Park SC. Perilla leaf, Perilla frutescens, induces apoptosis and G1 phase arrest in human leukemia HL-60 cells through the combinations of death receptor-mediated, mitochondrial, and endoplasmic reticulum stress-induced pathways. J Med Food. 2009;12:508–517. doi: 10.1089/jmf.2008.1103. [DOI] [PubMed] [Google Scholar]
- 14.Lin CS, Kuo CL, Wang JP, Cheng JS, Huang ZW, Chen CF. Growth inhibitory and apoptosis inducing effect of Perilla frutescens extract on human hepatoma HepG2 cells. J Ethnopharmacol. 2007;112:557–567. doi: 10.1016/j.jep.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 15.Ueda H, Yamazaki C, Yamazaki M. Inhibitory effect of Perilla leaf extract and luteolin on mouse skin tumor promotion. Biol Pharm Bull. 2003;26:560–563. doi: 10.1248/bpb.26.560. [DOI] [PubMed] [Google Scholar]
- 16.Jeon IH, Kim HS, Kang HJ, Lee HS, Jeong SI, Kim SJ, Jang SI. Anti-inflammatory and antipruritic effects of luteolin from Perilla (P. frutescens L.) leaves. Molecules. 2014;19:6941–6951. doi: 10.3390/molecules19066941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ueda H, Yamazaki C, Yamazaki M. Luteolin as an anti-inflammatory and anti-allergic constituent of Perilla frutescens. Biol Pharm Bull. 2002;25:1197–1202. doi: 10.1248/bpb.25.1197. [DOI] [PubMed] [Google Scholar]
- 18.Osakabe N, Yasuda A, Natsume M, Sanbongi C, Kato Y, Osawa T, Yoshikawa T. Rosmarinic acid, a major polyphenolic component of Perilla frutescens, reduces lipopolysaccharide (LPS)-induced liver injury in D-galactosamine (D-GalN)-sensitized mice. Free Radic Biol Med. 2002;33:798–806. doi: 10.1016/s0891-5849(02)00970-x. [DOI] [PubMed] [Google Scholar]
- 19.Osakabe N, Yasuda A, Natsume M, Yoshikawa T. Rosmarinic acid inhibits epidermal inflammatory responses: anticarcinogenic effect of Perilla frutescens extract in the murine two-stage skin model. Carcinogenesis. 2004;25:549–557. doi: 10.1093/carcin/bgh034. [DOI] [PubMed] [Google Scholar]
- 20.Sanbongi C, Takano H, Osakabe N, Sasa N, Natsume M, Yanagisawa R, Inoue KI, Sadakane K, Ichinose T, Yoshikawa T. Rosmarinic acid in perilla extract inhibits allergic inflammation induced by mite allergen, in a mouse model. Clin Exp Allergy. 2004;34:971–977. doi: 10.1111/j.1365-2222.2004.01979.x. [DOI] [PubMed] [Google Scholar]
- 21.Takano H, Osakabe N, Sanbongi C, Yanagisawa R, Inoue K, Yasuda A, Natsume M, Baba S, Ichiishi E, Yoshikawa T. Extract of Perilla frutescens enriched for rosmarinic acid, a polyphenolic phytochemical, inhibits seasonal allergic rhinoconjunctivitis in humans. Exp Biol Med (Maywood) 2004;229:247–254. doi: 10.1177/153537020422900305. [DOI] [PubMed] [Google Scholar]
- 22.Banno N, Akihisa T, Tokuda H, Yasukawa K, Higashihara H, Ukiya M, Watanabe K, Kimura Y, Hasegawa J, Nishino H. Triterpene acids from the leaves of Perilla frutescens and their anti-inflammatory and antitumor-promoting effects. Biosci Biotechnol Biochem. 2004;68:85–90. doi: 10.1271/bbb.68.85. [DOI] [PubMed] [Google Scholar]
- 23.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 24.Lambert JD, Lu G, Lee MJ, Hu J, Ju J, Yang CS. Inhibition of lung cancer growth in mice by dietary mixed tocopherols. Mol Nutr Food Res. 2009;53:1030–1035. doi: 10.1002/mnfr.200800438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Irons R, Tsuji PA, Carlson BA, Ouyang P, Yoo MH, Xu XM, Hatfield DL, Gladyshev VN, Davis CD. Deficiency in the 15-kDa selenoprotein inhibits tumorigenicity and metastasis of colon cancer cells. Cancer Prev Res (Phila) 2010;3:630–639. doi: 10.1158/1940-6207.CAPR-10-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Toton E, Ignatowicz E, Bernard MK, Kujawski J, Rybczynska M. Evaluation of apoptotic activity of new condensed pyrazole derivatives. J Physiol Pharmacol. 2013;64:115–123. [PubMed] [Google Scholar]
- 27.Fesik SW. Promoting apoptosis as a strategy for cancer drug discovery. Nat Rev Cancer. 2005;5:876–885. doi: 10.1038/nrc1736. [DOI] [PubMed] [Google Scholar]
- 28.Florent M, Godard T, Ballet JJ, Gauduchon P, Sola B. Detection by the comet assay of apoptosis induced in lymphoid cell lines after growth factor deprivation. Cell Biol Toxicol. 1999;15:185–192. doi: 10.1023/a:1007641821779. [DOI] [PubMed] [Google Scholar]
- 29.Ju J, Kwak Y, Hao X, Yang CS. Inhibitory effects of calcium against intestinal cancer in human colon cancer cells and Apc(Min/+) mice. Nutr Res Pract. 2012;6:396–404. doi: 10.4162/nrp.2012.6.5.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matsuura N, Miyamae Y, Yamane K, Nagao Y, Hamada Y, Kawaguchi N, Katsuki T, Hirata K, Sumi S, Ishikawa H. Aged garlic extract inhibits angiogenesis and proliferation of colorectal carcinoma cells. J Nutr. 2006;136:842S–846S. doi: 10.1093/jn/136.3.842S. [DOI] [PubMed] [Google Scholar]
- 31.Hotz MA, Gong J, Traganos F, Darzynkiewicz Z. Flow cytometric detection of apoptosis: comparison of the assays of in situ DNA degradation and chromatin changes. Cytometry. 1994;15:237–244. doi: 10.1002/cyto.990150309. [DOI] [PubMed] [Google Scholar]
- 32.Li K, Zhu ZC, Liu YJ, Liu JW, Wang HT, Xiong ZQ, Shen X, Hu ZL, Zheng J. ZFX knockdown inhibits growth and migration of non-small cell lung carcinoma cell line H1299. Int J Clin Exp Pathol. 2013;6:2460–2467. [PMC free article] [PubMed] [Google Scholar]
- 33.Tang J, Zhang L, She X, Zhou G, Yu F, Xiang J, Li G. Inhibiting CD164 expression in colon cancer cell line HCT116 leads to reduced cancer cell proliferation, mobility, and metastasis in vitro and in vivo. Cancer Invest. 2012;30:380–389. doi: 10.3109/07357907.2012.666692. [DOI] [PubMed] [Google Scholar]
- 34.Dolfi SC, Yang Z, Lee MJ, Guan F, Hong J, Yang CS. Inhibitory effects of different forms of tocopherols, tocopherol phosphates, and tocopherol quinones on growth of colon cancer cells. J Agric Food Chem. 2013;61:8533–8540. doi: 10.1021/jf401076g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang X, Wang Q, Ives KL, Evers BM. Curcumin inhibits neurotensin-mediated interleukin-8 production and migration of HCT116 human colon cancer cells. Clin Cancer Res. 2006;12:5346–5355. doi: 10.1158/1078-0432.CCR-06-0968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lu G, Xiao H, Li GX, Picinich SC, Chen YK, Liu A, Lee MJ, Loy S, Yang CS. A gamma-tocopherol-rich mixture of tocopherols inhibits chemically induced lung tumorigenesis in A/J mice and xenograft tumor growth. Carcinogenesis. 2010;31:687–694. doi: 10.1093/carcin/bgp332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ballet F, Petit J, Poupon R, Darnis F. Soft agar clonogenic assay for predicting chemosensitivity of human tumor cells from malignant effusions. Biomedicine. 1981;35:177–178. [PubMed] [Google Scholar]
- 38.Shu L, Cheung KL, Khor TO, Chen C, Kong AN. Phytochemicals: cancer chemoprevention and suppression of tumor onset and metastasis. Cancer Metastasis Rev. 2010;29:483–502. doi: 10.1007/s10555-010-9239-y. [DOI] [PubMed] [Google Scholar]
- 39.Lin Y, Shi R, Wang X, Shen HM. Luteolin, a flavonoid with potential for cancer prevention and therapy. Curr Cancer Drug Targets. 2008;8:634–646. doi: 10.2174/156800908786241050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang Y, Huang X, Han J, Zheng W, Ma W. Extract of Perilla frutescens inhibits tumor proliferation of HCC via PI3K/AKT signal pathway. Afr J Tradit Complement Altern Med. 2012;10:251–257. [PMC free article] [PubMed] [Google Scholar]