Abstract
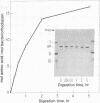
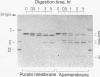
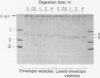
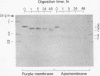
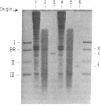
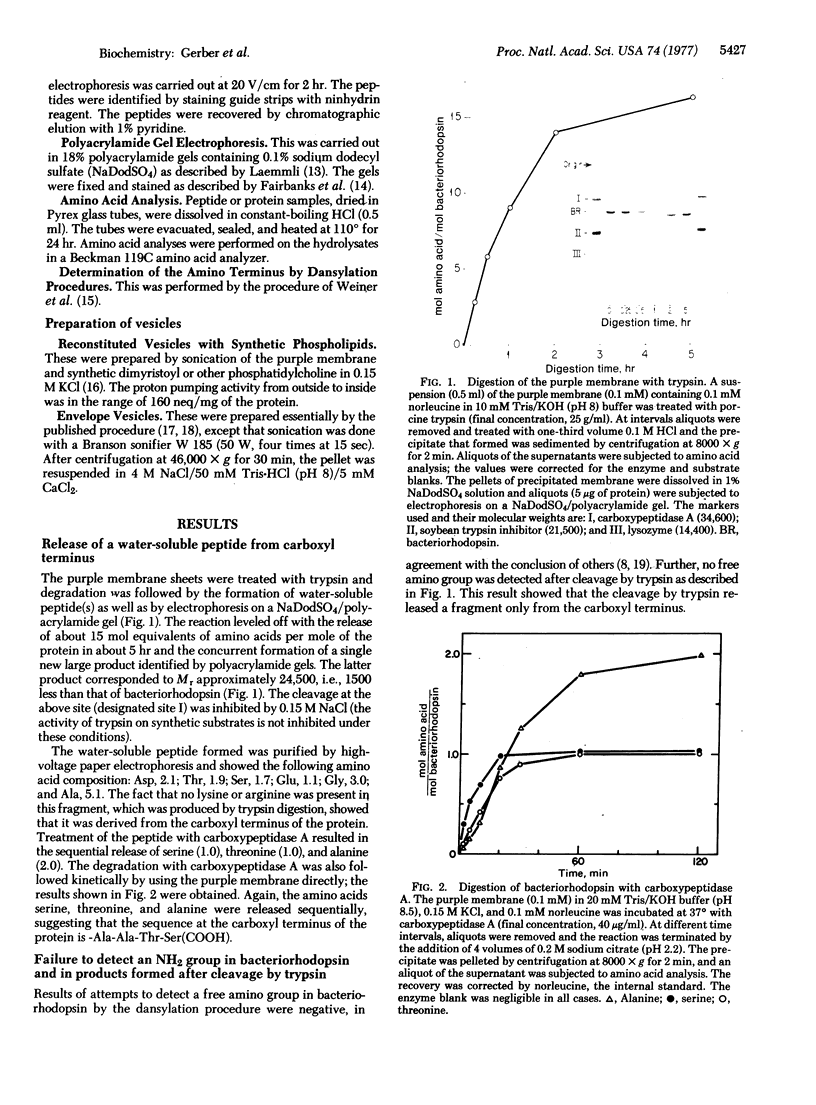
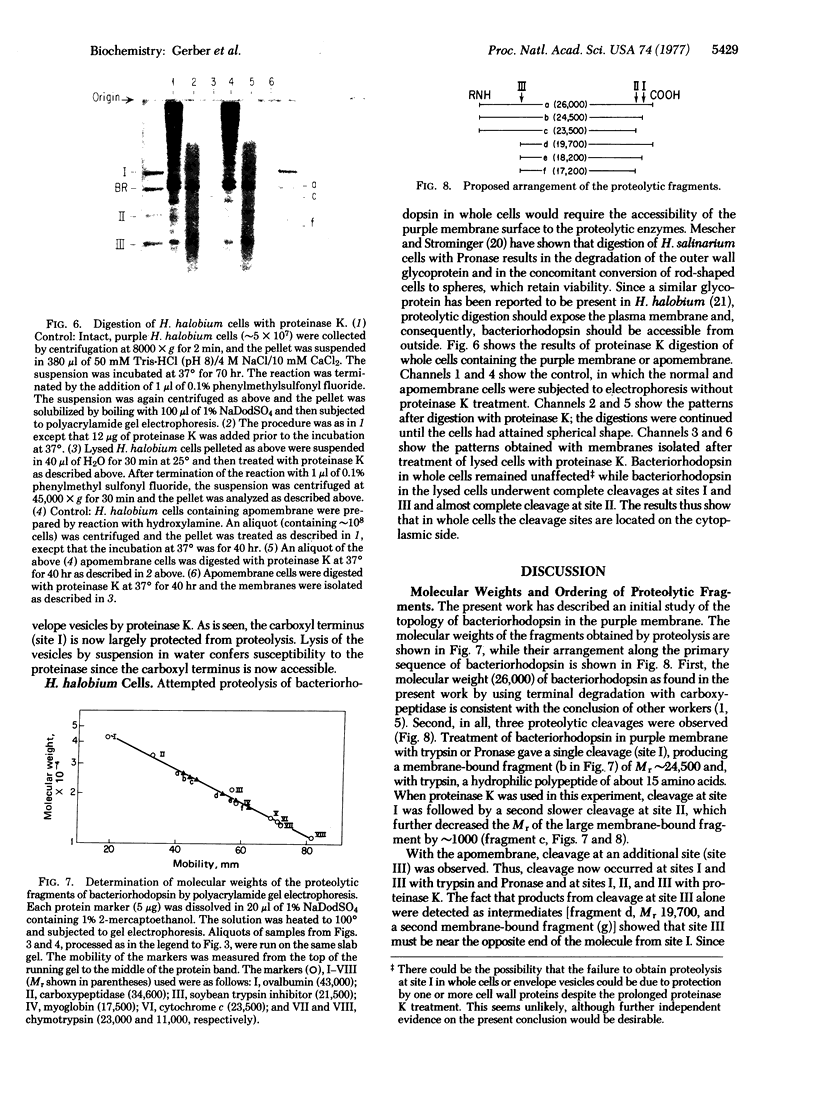
The orientation of bacteriorhodopsin in the purple membrane of Halobacterium halobium has been studied by proteolytic degradation of purple membrane sheets, reconstituted vesicles, and whole cells, with the following results: (i) Bacteriorhodopsin in purple membrane sheets is cleaved at a single site by Pronase or trypsin; a polypeptide segment of about 15 amino acids is lost from the carboxyl end. Carboxypeptidase A sequentially releases amino acids from the carboxyl end; the tetrapeptide sequence -Ala-Ala-Thr-Ser(COOH) was tentatively deduced for this terminus. (ii) The apomembrane, which lacks retinal, undergoes a second cleavage with trypsin releasing a fragment of approximately 6300 molecular weight from the amino terminus. (iii) Vesicles reconstituted from the purple membrane sheets and synthetic lecithins, in which the direction of proton pumping is opposite to that in the whole cells, have the carboxyl terminus of bacteriorhodopsin accessible to proteolysis. (iv) In envelope vesicles, which largely pump protons in the same direction as the whole cells, the carboxyl terminus is largely protected against proteolysis. (v) Treatment of whole cells with proteinase K hydrolyzes the cell wall proteins but has no effect on acteriorhodopsin. However, the same treatment after lysis of the cells results in degradation of the hydrophilic region at the carboxyl terminus. The results show that the carboxyl terminus as well as the additional cleavage site near the amino terminus observed in apomembrane are on the cytoplasmic side of the purple membrane.
Keywords: purple membrane, apomembrane, retinal, proton pump, reconstituted vesicles
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bridgen J., Walker I. D. Photoreceptor protein from the purple membrane of Halobacterium halobium. Molecular weight and retinal binding site. Biochemistry. 1976 Feb 24;15(4):792–798. doi: 10.1021/bi00649a010. [DOI] [PubMed] [Google Scholar]
- Danon A., Stoeckenius W. Photophosphorylation in Halobacterium halobium. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1234–1238. doi: 10.1073/pnas.71.4.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Henderson R., Unwin P. N. Three-dimensional model of purple membrane obtained by electron microscopy. Nature. 1975 Sep 4;257(5521):28–32. doi: 10.1038/257028a0. [DOI] [PubMed] [Google Scholar]
- Hsia J. C., Wong P. T., MacLennan D. H. Salt-dependent conformational changes in the cell membrane of Halobacterium salinarium. Biochem Biophys Res Commun. 1971 Apr 2;43(1):88–93. doi: 10.1016/s0006-291x(71)80090-6. [DOI] [PubMed] [Google Scholar]
- Kanner B. I., Racker E. Light-dependent proton and rubidium translocation in membrane vesicles from Halobacterium halobium. Biochem Biophys Res Commun. 1975 Jan 2;64(3):1054–1061. doi: 10.1016/0006-291x(75)90154-0. [DOI] [PubMed] [Google Scholar]
- Keefer L. M., Bradshaw R. A. Structural studies on Halobacterium halobium bacteriorhodopsin. Fed Proc. 1977 May;36(6):1799–1804. [PubMed] [Google Scholar]
- Kushwaha S. C., Kates M., Martin W. G. Characterization and composition of the purple and red membrane from Halobacterium cutirubrum;. Can J Biochem. 1975 Mar;53(3):284–292. doi: 10.1139/o75-040. [DOI] [PubMed] [Google Scholar]
- Kushwaha S. C., Kates M., Stoeckenius W. Comparison of purple membrane from Halobacterium cutirubrum and Halobacterium halabium. Biochim Biophys Acta. 1976 Apr 5;426(4):703–710. doi: 10.1016/0005-2736(76)90135-8. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- MacDonald R. E., Lanyi L. K. Light-induced leucine transport in Halobacterium halobium envelope vesicles: a chemiosmotic system. Biochemistry. 1975 Jul;14(13):2882–2889. doi: 10.1021/bi00684a014. [DOI] [PubMed] [Google Scholar]
- Mescher M. F., Strominger J. L. Structural (shape-maintaining) role of the cell surface glycoprotein of Halobacterium salinarium. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2687–2691. doi: 10.1073/pnas.73.8.2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oesterhelt D. Bacteriorhodopsin as an example of a light-driven proton pump. Angew Chem Int Ed Engl. 1976 Jan;15(1):17–24. doi: 10.1002/anie.197600171. [DOI] [PubMed] [Google Scholar]
- Oesterhelt D., Schuhmann L., Gruber H. Light-dependent reaction of bacteriorhodopsin with hydroxylamine in cell suspensions of Halobacterium halobium: demonstration of an apo-membrane. FEBS Lett. 1974 Aug 30;44(3):257–261. doi: 10.1016/0014-5793(74)81152-x. [DOI] [PubMed] [Google Scholar]
- Oesterhelt D., Stoeckenius W. Functions of a new photoreceptor membrane. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2853–2857. doi: 10.1073/pnas.70.10.2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oesterhelt D., Stoeckenius W. Rhodopsin-like protein from the purple membrane of Halobacterium halobium. Nat New Biol. 1971 Sep 29;233(39):149–152. doi: 10.1038/newbio233149a0. [DOI] [PubMed] [Google Scholar]
- Racker E., Stoeckenius W. Reconstitution of purple membrane vesicles catalyzing light-driven proton uptake and adenosine triphosphate formation. J Biol Chem. 1974 Jan 25;249(2):662–663. [PubMed] [Google Scholar]
- Smith W. P., Tai P. C., Thompson R. C., Davis B. D. Extracellular labeling of nascent polypeptides traversing the membrane of Escherichia coli. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2830–2834. doi: 10.1073/pnas.74.7.2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita M., Marchesi V. T. Amino-acid sequence and oligosaccharide attachment sites of human erythrocyte glycophorin. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2964–2968. doi: 10.1073/pnas.72.8.2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner A. M., Platt T., Weber K. Amino-terminal sequence analysis of proteins purified on a nanomole scale by gel electrophoresis. J Biol Chem. 1972 May 25;247(10):3242–3251. [PubMed] [Google Scholar]
- Wickner W. Asymmetric orientation of phage M13 coat protein in Escherichia coli cytoplasmic membranes and in synthetic lipid vesicles. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1159–1163. doi: 10.1073/pnas.73.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildenauer D., Khorana H. G. The preparation of lipid-depleted bacteriorhodopsin. Biochim Biophys Acta. 1977 Apr 18;466(2):315–324. doi: 10.1016/0005-2736(77)90227-9. [DOI] [PubMed] [Google Scholar]