Abstract
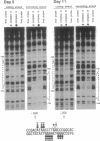
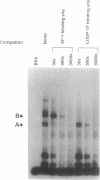
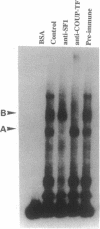
The peptide hormone oxytocin is highly expressed in the hypothalamus within only a small number of magnocellular neurons. However, it is also expressed in a much larger number of cells in the bovine corpus luteum at high levels in an estrous cycle-dependent manner. By using nuclear extracts from this tissue for in vitro binding studies, two protein complexes have been shown to bind to a common site in the bovine oxytocin promoter. One of these proteins has been identified as the bovine homologue of the chicken ovalbumin upstream promoter transcription factor (COUP-TF). The second protein is here characterized as the bovine homologue of a tissue-specific transcription factor, steroidogenic factor 1 (SF-1). The relative expression of these two factors during luteal development correlates with the level of luteal oxytocin gene expression, with SF-1 being the factor binding to the promoter of the oxytocin gene when this promoter is activated. Cotransfection experiments using the murine testicular cell line TM4 show that SF-1 can stimulate the expression of a transfected oxytocin gene, suggesting that SF-1 may be involved in upregulation of the oxytocin gene in vivo, possibly by transducing a stimulatory signal to the RNA polymerase.
Full text
PDF
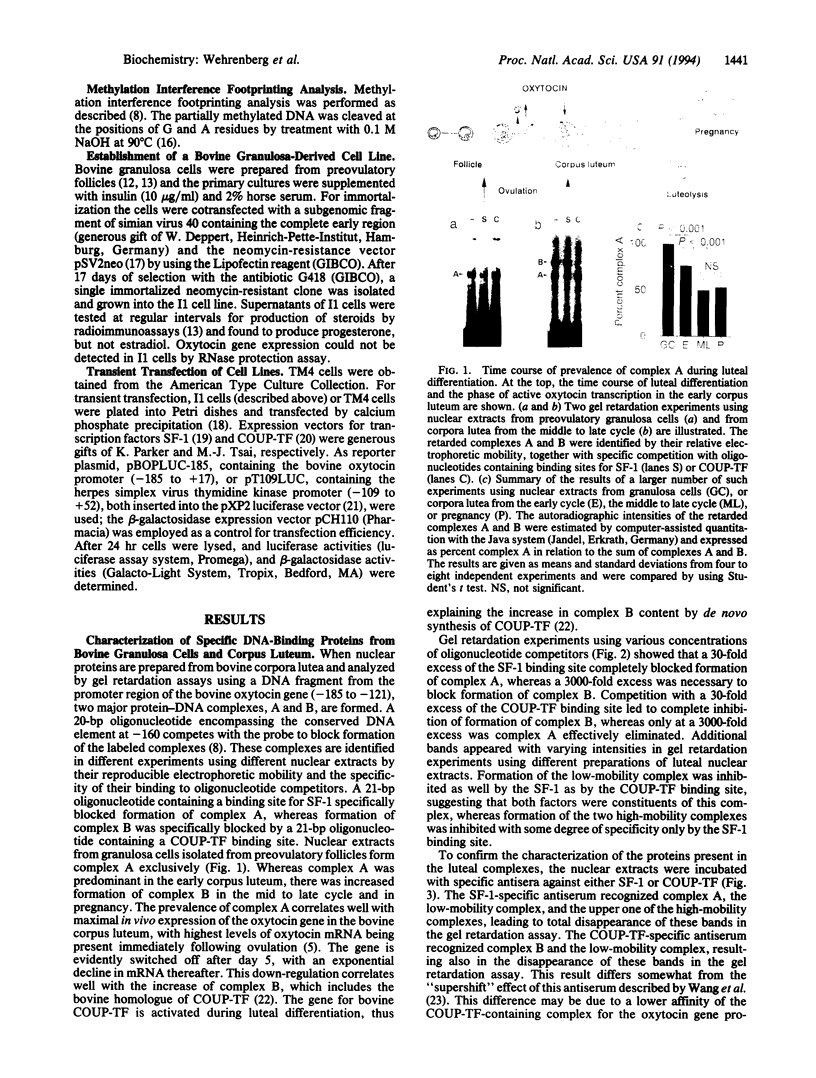
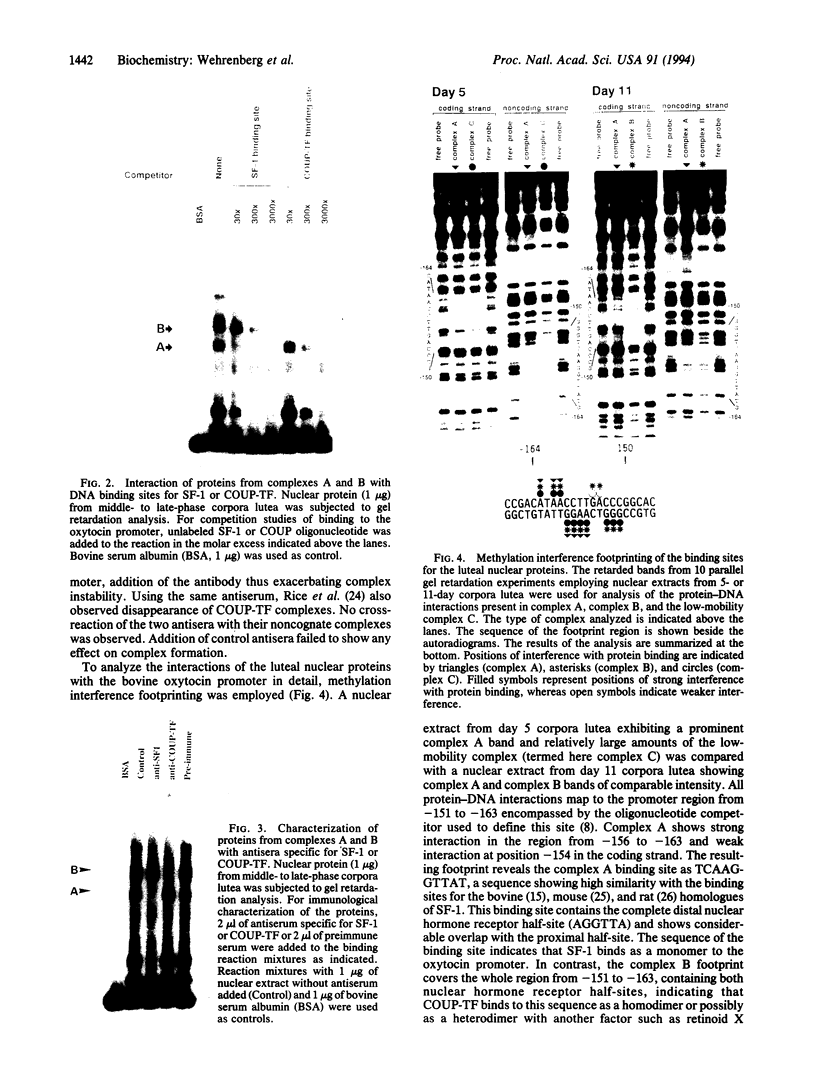
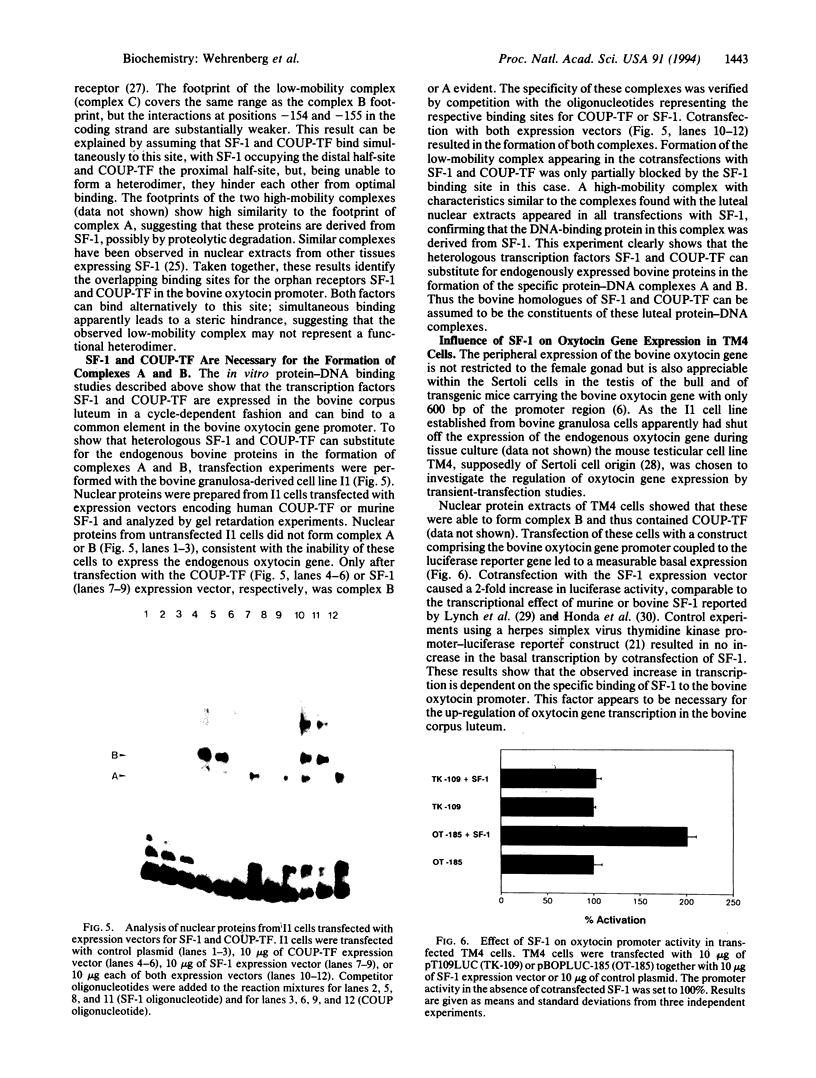

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adan R. A., Cox J. J., van Kats J. P., Burbach J. P. Thyroid hormone regulates the oxytocin gene. J Biol Chem. 1992 Feb 25;267(6):3771–3777. [PubMed] [Google Scholar]
- Andrews N. C., Faller D. V. A rapid micropreparation technique for extraction of DNA-binding proteins from limiting numbers of mammalian cells. Nucleic Acids Res. 1991 May 11;19(9):2499–2499. doi: 10.1093/nar/19.9.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrodin T. J., Marks M. S., Ozato K., Linney E., Lazar M. A. Heterodimerization among thyroid hormone receptor, retinoic acid receptor, retinoid X receptor, chicken ovalbumin upstream promoter transcription factor, and an endogenous liver protein. Mol Endocrinol. 1992 Sep;6(9):1468–1478. doi: 10.1210/mend.6.9.1331778. [DOI] [PubMed] [Google Scholar]
- Cooney A. J., Tsai S. Y., O'Malley B. W., Tsai M. J. Chicken ovalbumin upstream promoter transcription factor (COUP-TF) dimers bind to different GGTCA response elements, allowing COUP-TF to repress hormonal induction of the vitamin D3, thyroid hormone, and retinoic acid receptors. Mol Cell Biol. 1992 Sep;12(9):4153–4163. doi: 10.1128/mcb.12.9.4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick S. L., Richards J. S. cis-acting elements of the rat aromatase promoter required for cyclic adenosine 3',5'-monophosphate induction in ovarian granulosa cells and constitutive expression in R2C Leydig cells. Mol Endocrinol. 1993 Mar;7(3):341–354. doi: 10.1210/mend.7.3.8387157. [DOI] [PubMed] [Google Scholar]
- Hara Y., Battey J., Gainer H. Structure of mouse vasopressin and oxytocin genes. Brain Res Mol Brain Res. 1990 Oct;8(4):319–324. doi: 10.1016/0169-328x(90)90045-f. [DOI] [PubMed] [Google Scholar]
- Holtorf A. P., Furuya K., Ivell R., McArdle C. A. Oxytocin production and oxytocin messenger ribonucleic acid levels in bovine granulosa cells are regulated by insulin and insulin-like growth factor-I: dependence on developmental status of the ovarian follicle. Endocrinology. 1989 Nov;125(5):2612–2620. doi: 10.1210/endo-125-5-2612. [DOI] [PubMed] [Google Scholar]
- Honda S., Morohashi K., Nomura M., Takeya H., Kitajima M., Omura T. Ad4BP regulating steroidogenic P-450 gene is a member of steroid hormone receptor superfamily. J Biol Chem. 1993 Apr 5;268(10):7494–7502. [PubMed] [Google Scholar]
- Ikeda Y., Lala D. S., Luo X., Kim E., Moisan M. P., Parker K. L. Characterization of the mouse FTZ-F1 gene, which encodes a key regulator of steroid hydroxylase gene expression. Mol Endocrinol. 1993 Jul;7(7):852–860. doi: 10.1210/mend.7.7.8413309. [DOI] [PubMed] [Google Scholar]
- Ivell R., Brackett K. H., Fields M. J., Richter D. Ovulation triggers oxytocin gene expression in the bovine ovary. FEBS Lett. 1985 Oct 14;190(2):263–267. doi: 10.1016/0014-5793(85)81296-5. [DOI] [PubMed] [Google Scholar]
- Ivell R., Hunt N., Abend N., Brackman B., Nollmeyer D., Lamsa J. C., McCracken J. A. Structure and ovarian expression of the oxytocin gene in sheep. Reprod Fertil Dev. 1990;2(6):703–711. doi: 10.1071/rd9900703. [DOI] [PubMed] [Google Scholar]
- Kliewer S. A., Umesono K., Heyman R. A., Mangelsdorf D. J., Dyck J. A., Evans R. M. Retinoid X receptor-COUP-TF interactions modulate retinoic acid signaling. Proc Natl Acad Sci U S A. 1992 Feb 15;89(4):1448–1452. doi: 10.1073/pnas.89.4.1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lala D. S., Rice D. A., Parker K. L. Steroidogenic factor I, a key regulator of steroidogenic enzyme expression, is the mouse homolog of fushi tarazu-factor I. Mol Endocrinol. 1992 Aug;6(8):1249–1258. doi: 10.1210/mend.6.8.1406703. [DOI] [PubMed] [Google Scholar]
- Luck M. R., Jungclas B. Catecholamines and ascorbic acid as stimulators of bovine ovarian oxytocin secretion. J Endocrinol. 1987 Sep;114(3):423–430. doi: 10.1677/joe.0.1140423. [DOI] [PubMed] [Google Scholar]
- Lynch J. P., Lala D. S., Peluso J. J., Luo W., Parker K. L., White B. A. Steroidogenic factor 1, an orphan nuclear receptor, regulates the expression of the rat aromatase gene in gonadal tissues. Mol Endocrinol. 1993 Jun;7(6):776–786. doi: 10.1210/mend.7.6.8395654. [DOI] [PubMed] [Google Scholar]
- Mather J. P. Establishment and characterization of two distinct mouse testicular epithelial cell lines. Biol Reprod. 1980 Aug;23(1):243–252. doi: 10.1095/biolreprod23.1.243. [DOI] [PubMed] [Google Scholar]
- Morohashi K., Honda S., Inomata Y., Handa H., Omura T. A common trans-acting factor, Ad4-binding protein, to the promoters of steroidogenic P-450s. J Biol Chem. 1992 Sep 5;267(25):17913–17919. [PubMed] [Google Scholar]
- Nordeen S. K. Luciferase reporter gene vectors for analysis of promoters and enhancers. Biotechniques. 1988 May;6(5):454–458. [PubMed] [Google Scholar]
- O'Malley B. W., Conneely O. M. Orphan receptors: in search of a unifying hypothesis for activation. Mol Endocrinol. 1992 Sep;6(9):1359–1361. doi: 10.1210/mend.6.9.1331771. [DOI] [PubMed] [Google Scholar]
- O'Malley B. W., Tsai M. J. Molecular pathways of steroid receptor action. Biol Reprod. 1992 Feb;46(2):163–167. doi: 10.1095/biolreprod46.2.163. [DOI] [PubMed] [Google Scholar]
- Power R. F., Lydon J. P., Conneely O. M., O'Malley B. W. Dopamine activation of an orphan of the steroid receptor superfamily. Science. 1991 Jun 14;252(5012):1546–1548. doi: 10.1126/science.2047861. [DOI] [PubMed] [Google Scholar]
- Rice D. A., Mouw A. R., Bogerd A. M., Parker K. L. A shared promoter element regulates the expression of three steroidogenic enzymes. Mol Endocrinol. 1991 Oct;5(10):1552–1561. doi: 10.1210/mend-5-10-1552. [DOI] [PubMed] [Google Scholar]
- Richard S., Zingg H. H. Identification of a retinoic acid response element in the human oxytocin promoter. J Biol Chem. 1991 Nov 15;266(32):21428–21433. [PubMed] [Google Scholar]
- Richard S., Zingg H. H. The human oxytocin gene promoter is regulated by estrogens. J Biol Chem. 1990 Apr 15;265(11):6098–6103. [PubMed] [Google Scholar]
- Sausville E., Carney D., Battey J. The human vasopressin gene is linked to the oxytocin gene and is selectively expressed in a cultured lung cancer cell line. J Biol Chem. 1985 Aug 25;260(18):10236–10241. [PubMed] [Google Scholar]
- Schmitz E., Mohr E., Richter D. Rat vasopressin and oxytocin genes are linked by a long interspersed repeated DNA element (LINE): sequence and transcriptional analysis of LINE. DNA Cell Biol. 1991 Mar;10(2):81–91. doi: 10.1089/dna.1991.10.81. [DOI] [PubMed] [Google Scholar]
- Schöler H. R., Gruss P. Specific interaction between enhancer-containing molecules and cellular components. Cell. 1984 Feb;36(2):403–411. doi: 10.1016/0092-8674(84)90233-2. [DOI] [PubMed] [Google Scholar]
- Tsai S. Y., Sagami I., Wang H., Tsai M. J., O'Malley B. W. Interactions between a DNA-binding transcription factor (COUP) and a non-DNA binding factor (S300-II). Cell. 1987 Aug 28;50(5):701–709. doi: 10.1016/0092-8674(87)90328-x. [DOI] [PubMed] [Google Scholar]
- Wang L. H., Tsai S. Y., Cook R. G., Beattie W. G., Tsai M. J., O'Malley B. W. COUP transcription factor is a member of the steroid receptor superfamily. Nature. 1989 Jul 13;340(6229):163–166. doi: 10.1038/340163a0. [DOI] [PubMed] [Google Scholar]
- Wehrenberg U., Ivell R., Walther N. The COUP transcription factor (COUP-TF) is directly involved in the regulation of oxytocin gene expression in luteinizing bovine granulosa cells. Biochem Biophys Res Commun. 1992 Nov 30;189(1):496–503. doi: 10.1016/0006-291x(92)91585-e. [DOI] [PubMed] [Google Scholar]