Abstract
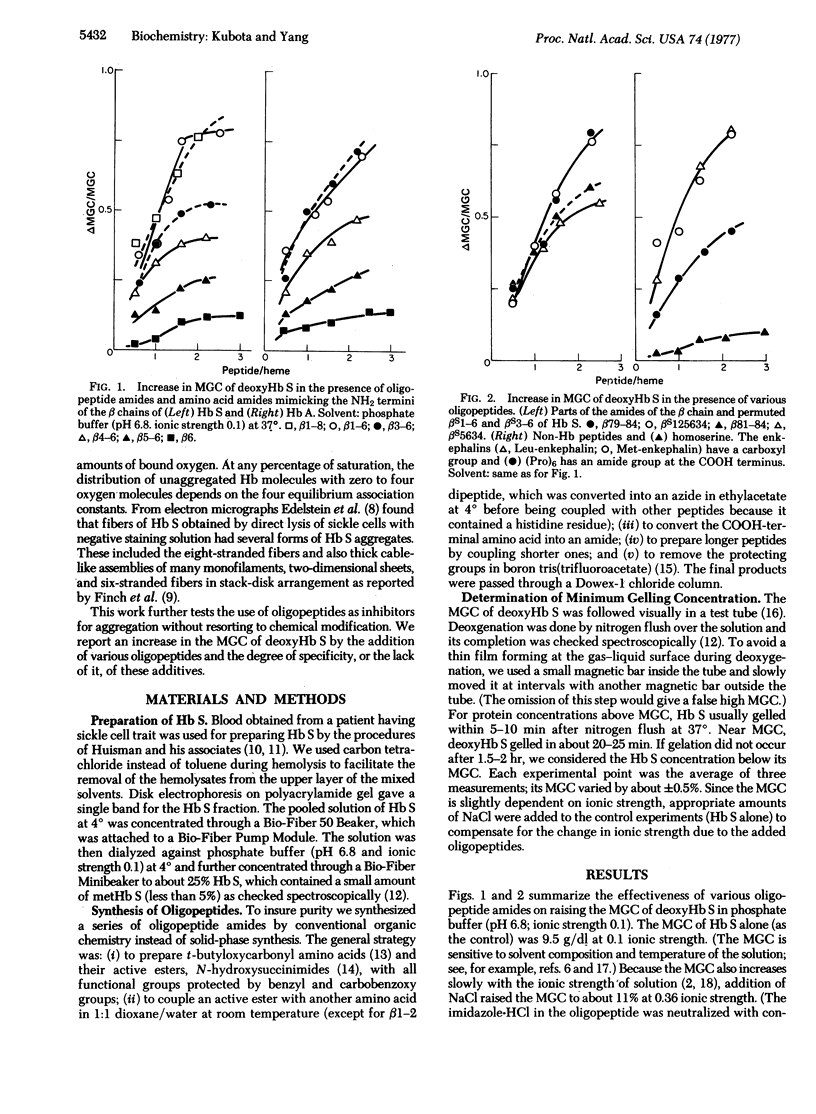
Oligopeptides that mimic segments of the amino acid sequence of hemoglobin S at potential contact sites can be used to inhibit aggregation. These oligopeptide inhibitors raise the minimum gelling concentration of deoxyhemoglobin S so that chemical modification does not have to be used. The hexapeptide amides of both betaS 1-6 which is believed to be one of the contact areas among aggregates, and betaA 1-6 of hemoglobin A increase the minimum gelling concentration by more than 70%. The hexapeptide amide beta79-84 behaves like beta1-6 (beta being betaS or betaA). Shorter oligopeptides, such as betaS3-6, are less effective as an inhibitor but longer ones, such as betaS1-8, are no more effective than beta1-6. Permutations of the sequence, such as betaS125634, do not alter the percent increase in the minimum gelling concentration. Leu- and Met-enkephalin increase the minimum gelling concentration just as beta1-6 does, but (Pro)6 is not very effective. Thus, the use of complementary oligopeptides as inhibitors is extended to include certain "flexible" peptides, which can adapt themselves to interfere with the molecular contacts and thereby gelation of deoxyhemoglobin S.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames B. N., Ames G. F., Young J. D., Tsuchiya D., Lecocq J. Illicit transport: the oligopeptide permease. Proc Natl Acad Sci U S A. 1973 Feb;70(2):456–458. doi: 10.1073/pnas.70.2.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodemann H., Passow H. Factors controlling the resealing of the membrane of human erythrocyte ghosts after hypotonic hemolysis. J Membr Biol. 1972;8(1):1–26. doi: 10.1007/BF01868092. [DOI] [PubMed] [Google Scholar]
- Briehl R. W., Ewert S. Effects of pH, 2,3-diphosphoglycerate and salts on gelation of sickle cell deoxyhemoglobin. J Mol Biol. 1973 Nov 5;80(3):445–458. doi: 10.1016/0022-2836(73)90415-4. [DOI] [PubMed] [Google Scholar]
- Clark M. R., Shohet S. B. Hybrid erythrocytes for membrane studies in sickle cell disease. Blood. 1976 Jan;47(1):121–131. [PubMed] [Google Scholar]
- Finch J. T., Perutz M. F., Bertles J. F., Döbler J. Structure of sickled erythrocytes and of sickle-cell hemoglobin fibers. Proc Natl Acad Sci U S A. 1973 Mar;70(3):718–722. doi: 10.1073/pnas.70.3.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregoriadis G. The carrier potential of liposomes in biology and medicine (first of two parts). N Engl J Med. 1976 Sep 23;295(13):704–710. doi: 10.1056/NEJM197609232951305. [DOI] [PubMed] [Google Scholar]
- Gregoriadis G. The carrier potential of liposomes in biology and medicine (second of two parts). N Engl J Med. 1976 Sep 30;295(14):765–770. doi: 10.1056/NEJM197609302951406. [DOI] [PubMed] [Google Scholar]
- Huisman T. H., Dozy A. M. Studies on the heterogeneity of hemoglobin. IX. The use of Tris(hydroxymethyl)aminomethanehcl buffers in the anion-exchange chromatography of hemoglobins. J Chromatogr. 1965 Jul;19(1):160–169. doi: 10.1016/s0021-9673(01)99434-8. [DOI] [PubMed] [Google Scholar]
- Kang S. S., Benjamin G. Homoserine inhibition of in vitro sickling of erythrocytes. Exp Mol Pathol. 1975 Apr;22(2):220–224. doi: 10.1016/0014-4800(75)90065-9. [DOI] [PubMed] [Google Scholar]
- Kubota S., Chang C. T., Samejima T., Yang J. T. Letter: Oligopeptides as potential antiaggregation agent for proteins: hemoglobin S gel and insulin dimer. J Am Chem Soc. 1976 Apr 28;98(9):2677–2678. doi: 10.1021/ja00425a053. [DOI] [PubMed] [Google Scholar]
- Minton A. P. Relations between oxygen saturation and aggregation of sickle-cell hemoglobin. J Mol Biol. 1976 Feb 5;100(4):519–542. doi: 10.1016/s0022-2836(76)80043-5. [DOI] [PubMed] [Google Scholar]
- Pless J., Bauer W. Boron tris(trifluoroacetate) for removal of protecting groups in peptide chemistry. Angew Chem Int Ed Engl. 1973 Feb;12(2):147–148. doi: 10.1002/anie.197301471. [DOI] [PubMed] [Google Scholar]
- Poste G., Papahadjopoulos D. Lipid vesicles as carriers for introducing materials into cultured cells: influence of vesicle lipid composition on mechanism(s) of vesicle incorporation into cells. Proc Natl Acad Sci U S A. 1976 May;73(5):1603–1607. doi: 10.1073/pnas.73.5.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumen N. M. Inhibition of sickling in erythrocytes by amino acids. Blood. 1975 Jan;45(1):45–48. [PubMed] [Google Scholar]
- Sophianopoulos A. J., Sophianopoulos J. A., Grush O. C., Wilson J. S., Fales F. W. Two new antisickling agents and the use of amino acid salts. Clin Biochem. 1974 Jun;7(2):112–118. doi: 10.1016/s0009-9120(74)91160-6. [DOI] [PubMed] [Google Scholar]
- Votano J. R., Gorecki M., Rich A. Sickle hemoglobin aggregation: a new class of inhibitors. Science. 1977 Jun 10;196(4295):1216–1219. doi: 10.1126/science.870976. [DOI] [PubMed] [Google Scholar]
- Ward K. B., Wishner B. C., Lattman E. E., Love W. E. Structure of deoxyhemoglobin A crystals grown from polyethylene glycol solutions. J Mol Biol. 1975 Oct 15;98(1):161–177. doi: 10.1016/s0022-2836(75)80107-0. [DOI] [PubMed] [Google Scholar]
- Yang J. T. Intermolecular contacts of deoxyhemoblogin S: a hypothesis and search for possible anti-sickling agents. Biochem Biophys Res Commun. 1975 Mar 3;63(1):232–238. doi: 10.1016/s0006-291x(75)80034-9. [DOI] [PubMed] [Google Scholar]


