Abstract
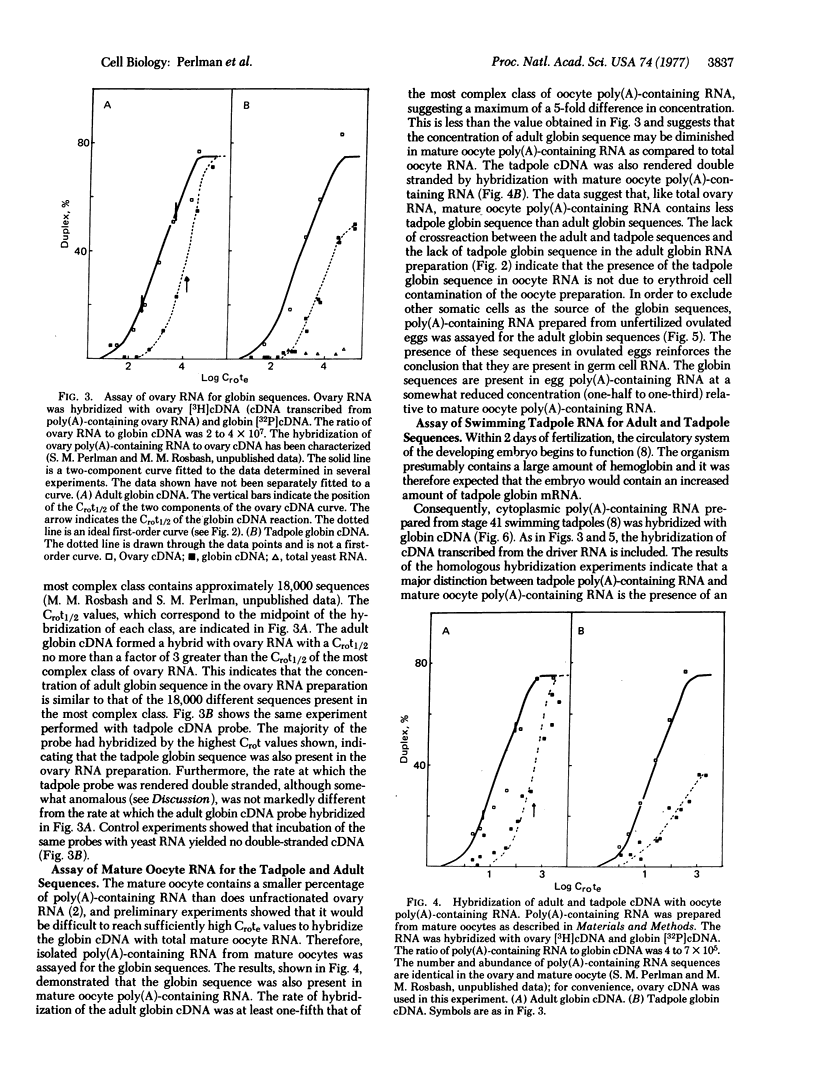
Complementary DNA transcribed from adult Xenopus laevis globin mRNA was used to assay ovary RNA from Xenopus for the presence of globin sequences by RNA·cDNA hybridization. These sequences are present at approximately the same concentration as the majority of poly(A)-containing ovary sequences. The sequences are also found at approximately 200,000 copies per cell in poly(A)-containing RNA extracted from mature oocytes.
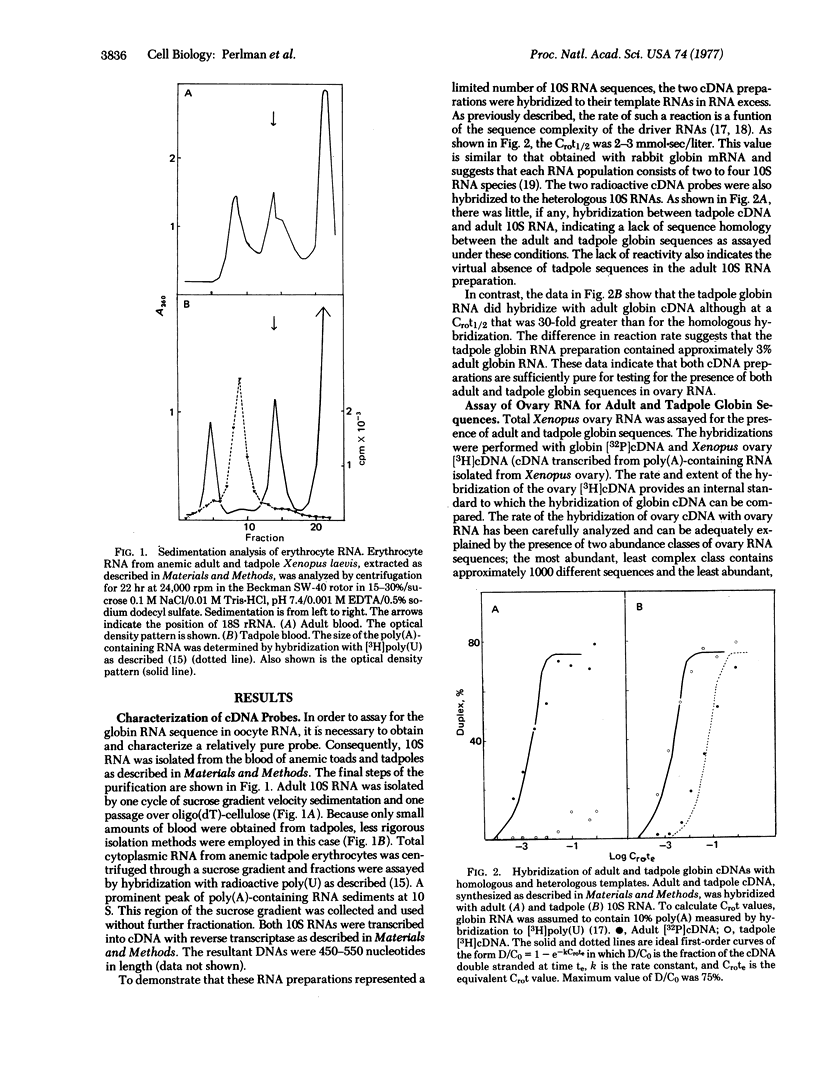
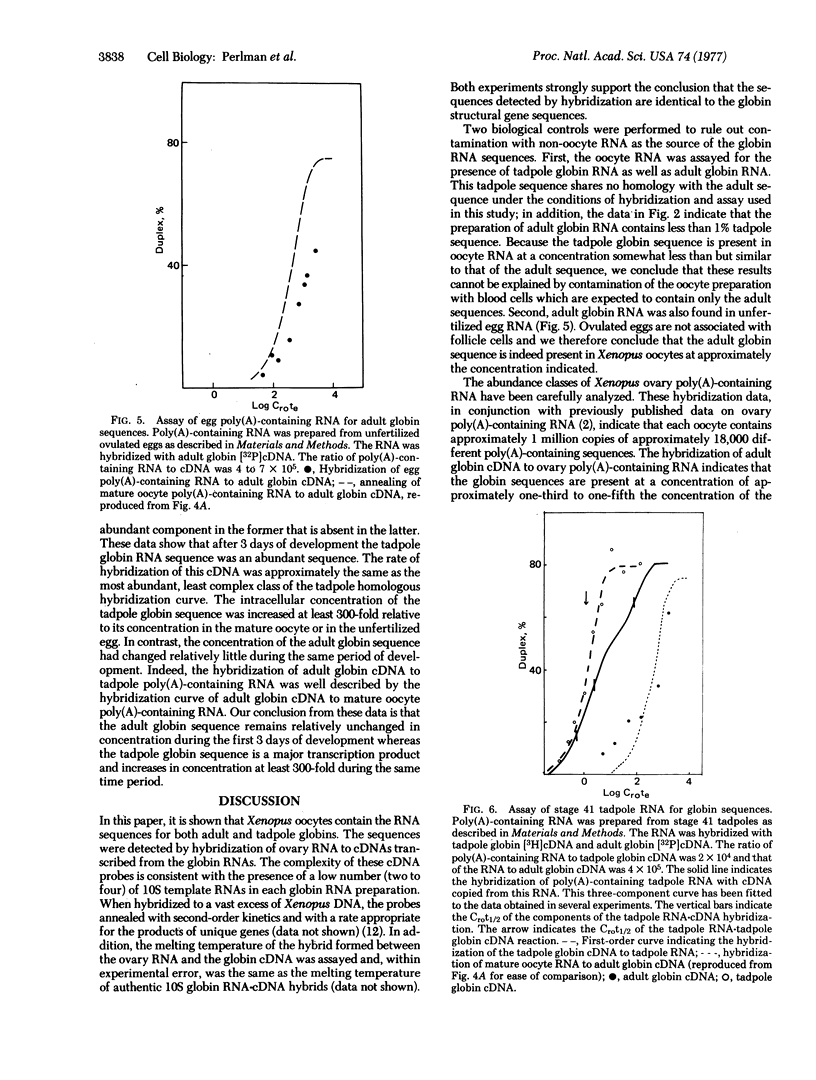
To rule out contamination of the oocytes with somatic cells, two additional experiments were performed. First, RNA isolated from ovulated unfertilized eggs, which are devoid of somatic cells, was also shown to contain the globin sequences. Second, globin mRNA was isolated from Xenopus tadpoles. Adult globin mRNA is free of the tadpole sequence and no homology was detected between adult and tadpoles globin RNA. The ovary was shown to contain tadpole globin RNA at nearly the same concentration as the adult sequences. Thus, the results cannot be explained by contamination with erythroid cells which should contain only the adult sequence.
The swimming tadpole, which possesses an active circulatory system, was also assayed for the tadpole and adult globin sequences. Whereas the adult sequences are present at approximately the same concentration as in the mature oocyte, the concentration of the tadpole sequences increases at least 300-fold in the first 3 days following fertilization.
Keywords: oogenesis, embryogenesis, complementary DNA, molecular hybridization
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamson E. D., Woodland H. R. Histone synthesis in early amphibian development: histone and DNA syntheses are not co-ordinated. J Mol Biol. 1974 Sep 15;88(2):263–285. doi: 10.1016/0022-2836(74)90481-1. [DOI] [PubMed] [Google Scholar]
- BARTH L. G., BARTH L. J. Differentiation of cells of the Rana pipiens gastrula in unconditioned medium. J Embryol Exp Morphol. 1959 Jun;7:210–222. [PubMed] [Google Scholar]
- Bishop J. O., Beckmann J. S., Campo M. S., Hastie N. D., Izquierdo M., Perlman S. DNA-RNA hybridization. Philos Trans R Soc Lond B Biol Sci. 1975 Nov 6;272(915):147–157. doi: 10.1098/rstb.1975.0077. [DOI] [PubMed] [Google Scholar]
- Bishop J. O. Molecular hybridization of ribonucleic acid with a large excess of deoxyribonucleic acid. Biochem J. 1972 Jan;126(1):171–185. doi: 10.1042/bj1260171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop J. O., Rosbash M. Polynucleotide sequences in eukaryotic DNA and RNA that form ribonuclease-resistant complexes with polyuridylic acid. J Mol Biol. 1974 May 5;85(1):75–86. doi: 10.1016/0022-2836(74)90130-2. [DOI] [PubMed] [Google Scholar]
- Bishop J. O. The effect of genetic complexity on the time-course of ribonucleic acid-deoxyribonucleic acid hybridization. Biochem J. 1969 Aug;113(5):805–811. doi: 10.1042/bj1130805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurdon J. B., Woodland H. R., Lingrel J. B. The translation of mammalian globin mRNA injected into fertilized eggs of Xenopus laevis I. Message stability in development. Dev Biol. 1974 Jul;39(1):125–133. doi: 10.1016/s0012-1606(74)80014-x. [DOI] [PubMed] [Google Scholar]
- Hereford L. M., Rosbash M. Number and distribution of polyadenylated RNA sequences in yeast. Cell. 1977 Mar;10(3):453–462. doi: 10.1016/0092-8674(77)90032-0. [DOI] [PubMed] [Google Scholar]
- Humphries S., Windass J., Williamson R. Mouse globin gene expression in erythroid and non-erythroid tissues. Cell. 1976 Feb;7(2):267–277. doi: 10.1016/0092-8674(76)90026-x. [DOI] [PubMed] [Google Scholar]
- KIRBY K. S. ISOLATION AND CHARACTERIZATION OF RIBOSOMAL RIBONUCLEIC ACID. Biochem J. 1965 Jul;96:266–269. doi: 10.1042/bj0960266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird C. D., McCarthy B. J. Magnitude of interspecific nucleotide sequence variability in Drosophila. Genetics. 1968 Oct;60(2):303–322. doi: 10.1093/genetics/60.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maclean N., Jurd R. D. The haemoglobins of healthy and anaemic Xenopus laevis. J Cell Sci. 1971 Sep;9(2):509–528. doi: 10.1242/jcs.9.2.509. [DOI] [PubMed] [Google Scholar]
- Penman S. RNA metabolism in the HeLa cell nucleus. J Mol Biol. 1966 May;17(1):117–130. doi: 10.1016/s0022-2836(66)80098-0. [DOI] [PubMed] [Google Scholar]
- Rosbash M., Ford P. J., Bishop J. O. Analysis of the C-value paradox by molecular hybridization. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3746–3750. doi: 10.1073/pnas.71.9.3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosbash M. Polyadenylic acid-containing RNA in Xenopus laevis oocytes. J Mol Biol. 1974 May 5;85(1):87–101. doi: 10.1016/0022-2836(74)90131-4. [DOI] [PubMed] [Google Scholar]
- Woodland H. R. Changes in the polysome content of developing Xenopus laevis embryos. Dev Biol. 1974 Sep;40(1):90–101. doi: 10.1016/0012-1606(74)90111-0. [DOI] [PubMed] [Google Scholar]


