Abstract
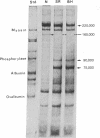
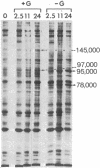
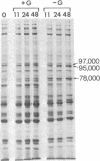
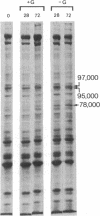
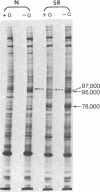
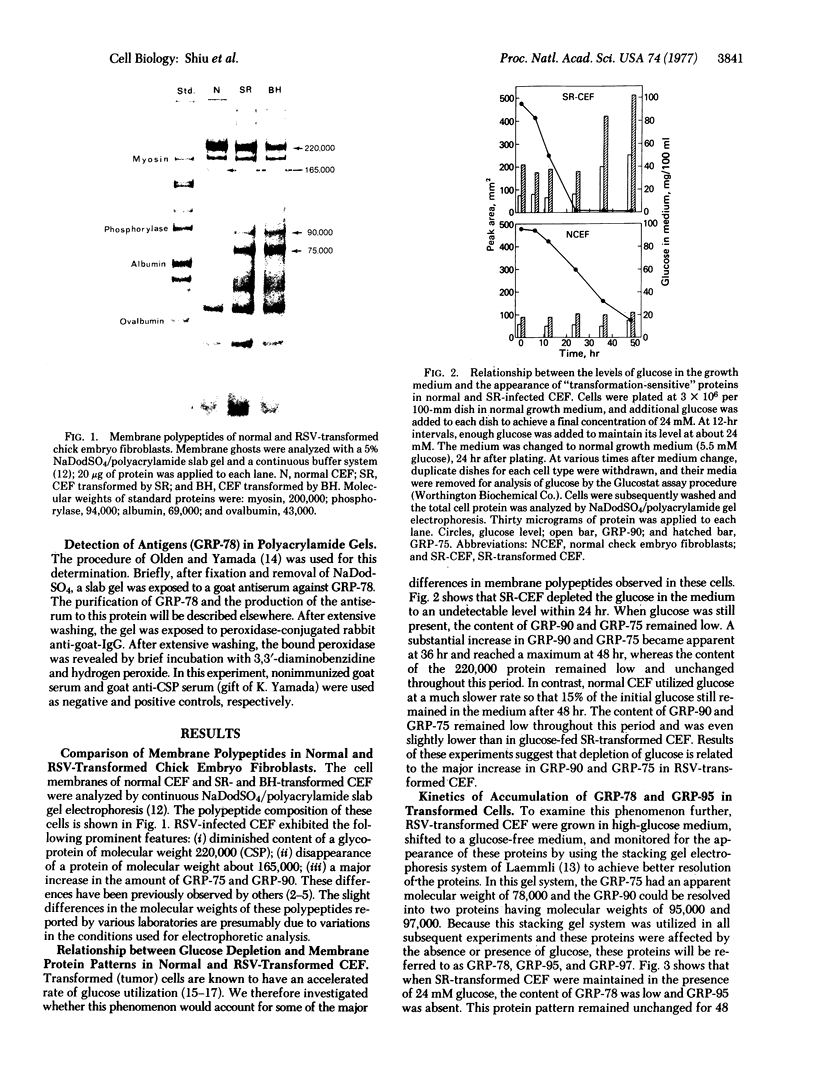
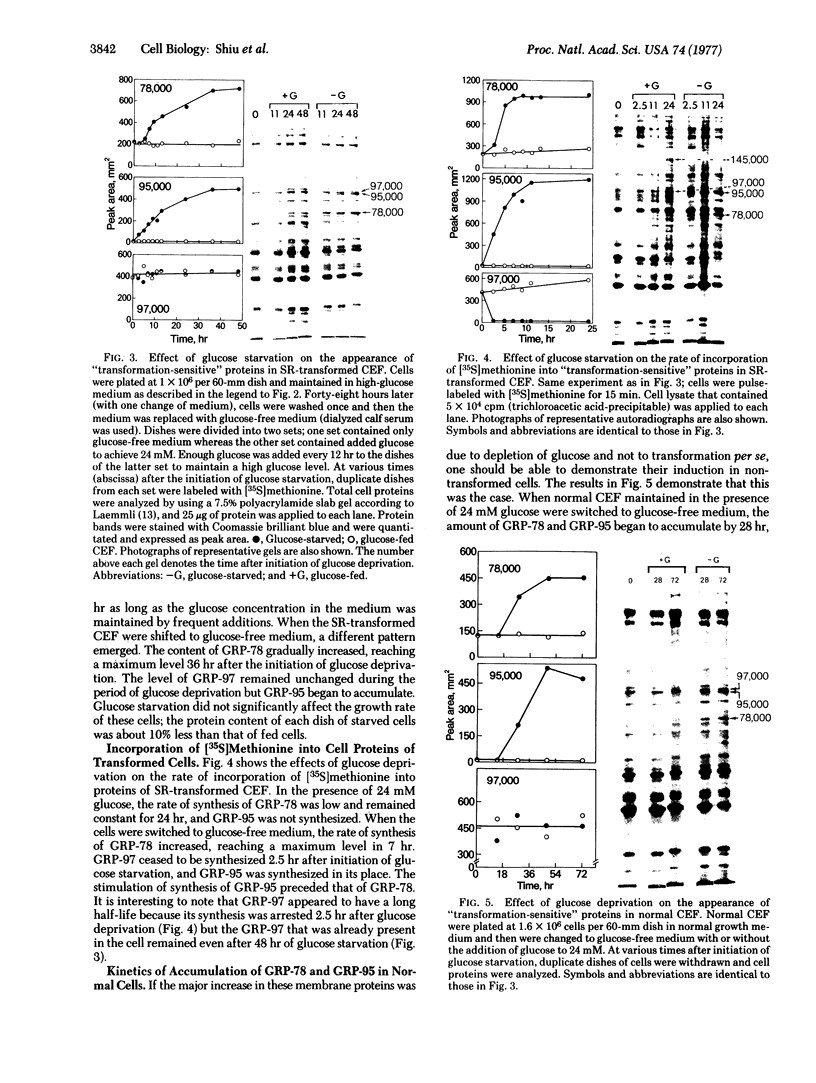
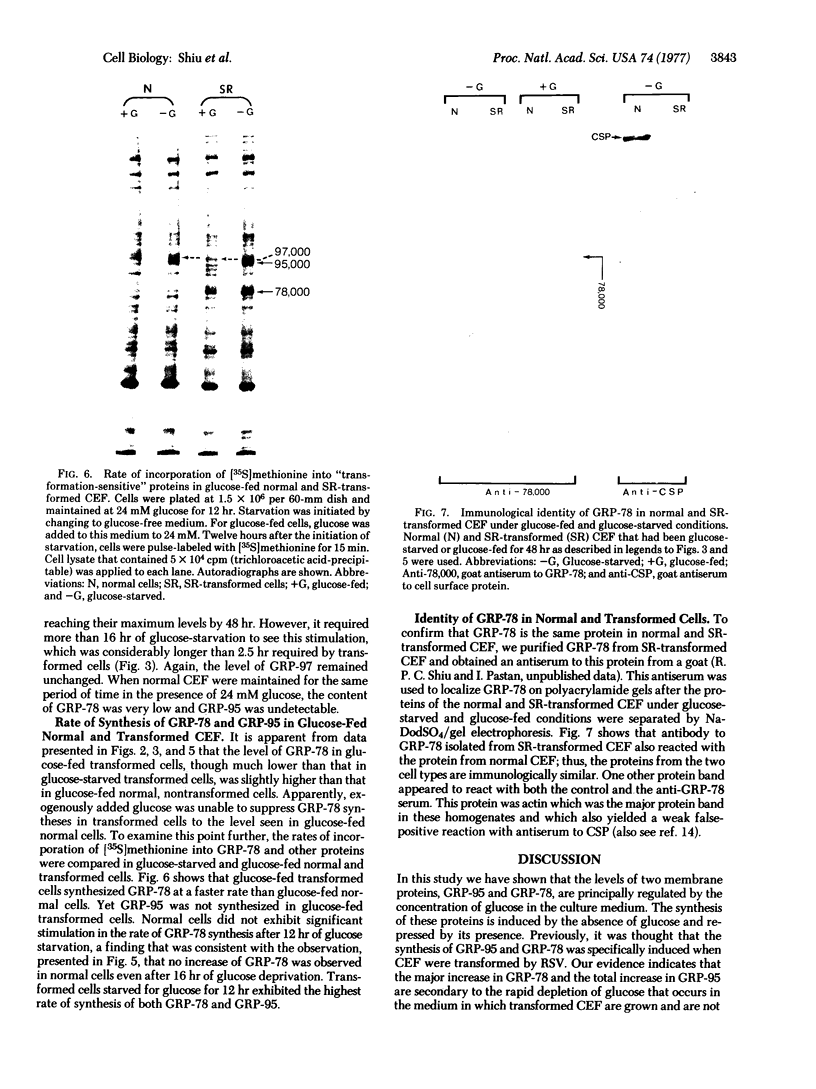
Chick embryo fibroblasts transformed by Rous sarcoma virus have an increased content of two membrane proteins of molecular weights 78,000 and 95,000. The increased content of the 95,000-dalton protein and the principal increase in the content of the 78,000-dalton protein are not an early consequence of cell transformation but instead are secondary to the rapid depletion of glucose from the growth medium of transformed cells. When glucose is maintained at high levels in the growth medium of transformed cells, the synthesis of the 95,000-dalton protein is arrested and that of the 78,000-dalton protein is markedly suppressed. Upon removal of glucose from the growth medium of normal cells, these proteins increase to levels comparable to those of transformed cells. Because the amount of these two proteins is influenced by the presence or absence of glucose, we suggest they be referred to as "glucose-regulated proteins." GRP-78 and GRP-95. These proteins may have an important role in regulating the utilization of glucose in cultured cells.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bader J. P. Temperature-dependent transformation of cells infected with a mutant of Bryan Rous sarcoma virus. J Virol. 1972 Aug;10(2):267–276. doi: 10.1128/jvi.10.2.267-276.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L. B., Gallimore P. H., McDougall J. K. Correlation between tumor induction and the large external transformation sensitive protein on the cell surface. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3570–3574. doi: 10.1073/pnas.73.10.3570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatanaka M. Transport of sugars in tumor cell membranes. Biochim Biophys Acta. 1974 Apr 29;355(1):77–104. doi: 10.1016/0304-419x(74)90008-0. [DOI] [PubMed] [Google Scholar]
- Isaka T., Yoshida M., Owada M., Toyoshima K. Alterations in membrane polypeptides of chick embryo fibroblasts induced by transformation with avian sarcoma viruses. Virology. 1975 May;65(1):226–237. doi: 10.1016/0042-6822(75)90023-9. [DOI] [PubMed] [Google Scholar]
- Kletzien R. F., Perdue J. F. Induction of sugar transport in chick embryo fibroblasts by hexose starvation. Evidence for transcriptional regulation of transport. J Biol Chem. 1975 Jan 25;250(2):593–600. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee S. G., Lipmann F. Isolation from normal and Rous sarcoma virus-transformed chicken fibroblasts of a factor that binds glucose and stimulates its transport. Proc Natl Acad Sci U S A. 1977 Jan;74(1):163–167. doi: 10.1073/pnas.74.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martineau R., Kohlbacher M., Shaw S. N., Amos H. Enhancement of hexose entry into chick fibroblasts by starvation: differential effect on galactose and glucose. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3407–3411. doi: 10.1073/pnas.69.11.3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakata Y., Bader J. P. Studies on the fixation and development of cellular transformation by Rous sarcoma virus. Virology. 1968 Nov;36(3):401–410. doi: 10.1016/0042-6822(68)90165-7. [DOI] [PubMed] [Google Scholar]
- Olden K., Yamada K. M. Direct detection of antigens in sodium dodecyl sulfate-polyacrylamide gels. Anal Biochem. 1977 Apr;78(2):483–490. doi: 10.1016/0003-2697(77)90108-7. [DOI] [PubMed] [Google Scholar]
- Robbins P. W. Comparisons of major cell-surface proteins of normal and transformed cells. Am J Clin Pathol. 1975 May;63(5):671–676. doi: 10.1093/ajcp/63.5.671. [DOI] [PubMed] [Google Scholar]
- Stone K. R., Smith R. E., Joklik W. K. Changes in membrane polypeptides that occur when chick embryo fibroblasts and NRK cells are transformed with avian sarcoma viruses. Virology. 1974 Mar;58(1):86–100. doi: 10.1016/0042-6822(74)90143-3. [DOI] [PubMed] [Google Scholar]
- Yamada K. M., Weston J. A. The synthesis, turnover, and artificial restoration of a major cell surface glycoprotein. Cell. 1975 May;5(1):75–81. doi: 10.1016/0092-8674(75)90094-x. [DOI] [PubMed] [Google Scholar]
- Yamada K. M., Yamada S. S., Pastan I. Cell surface protein partially restores morphology, adhesiveness, and contact inhibition of movement to transformed fibroblasts. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1217–1221. doi: 10.1073/pnas.73.4.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]