Abstract
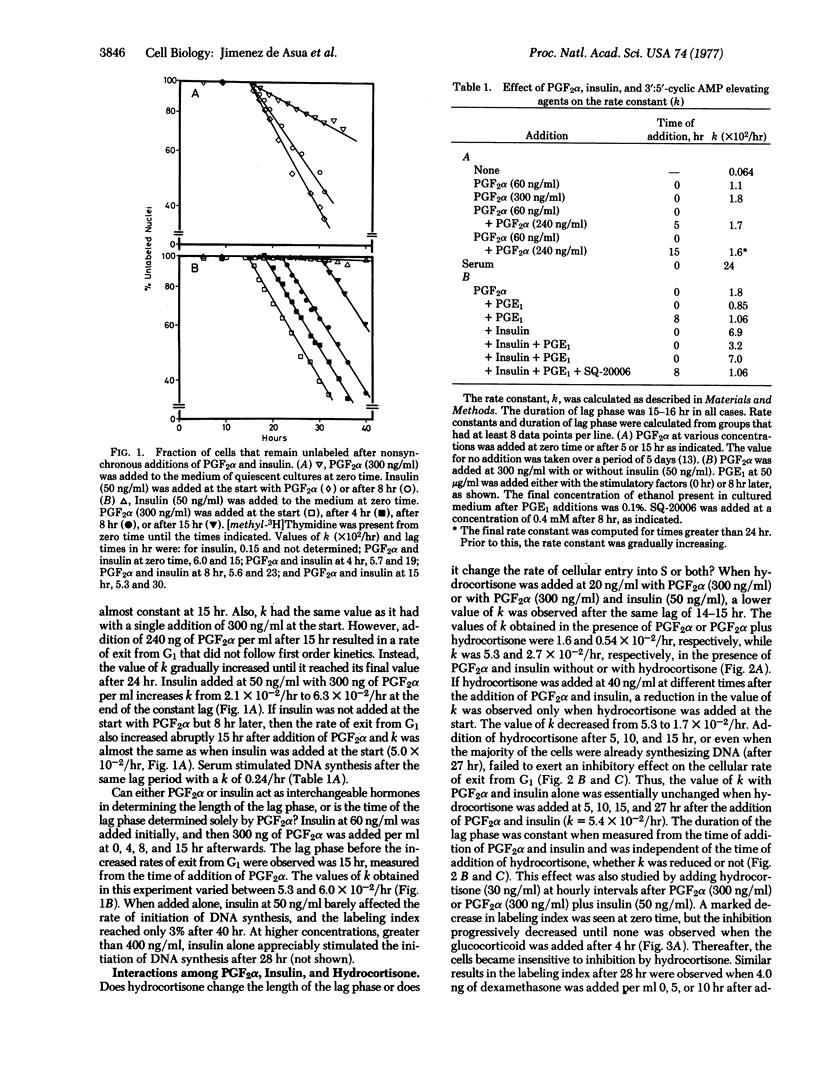
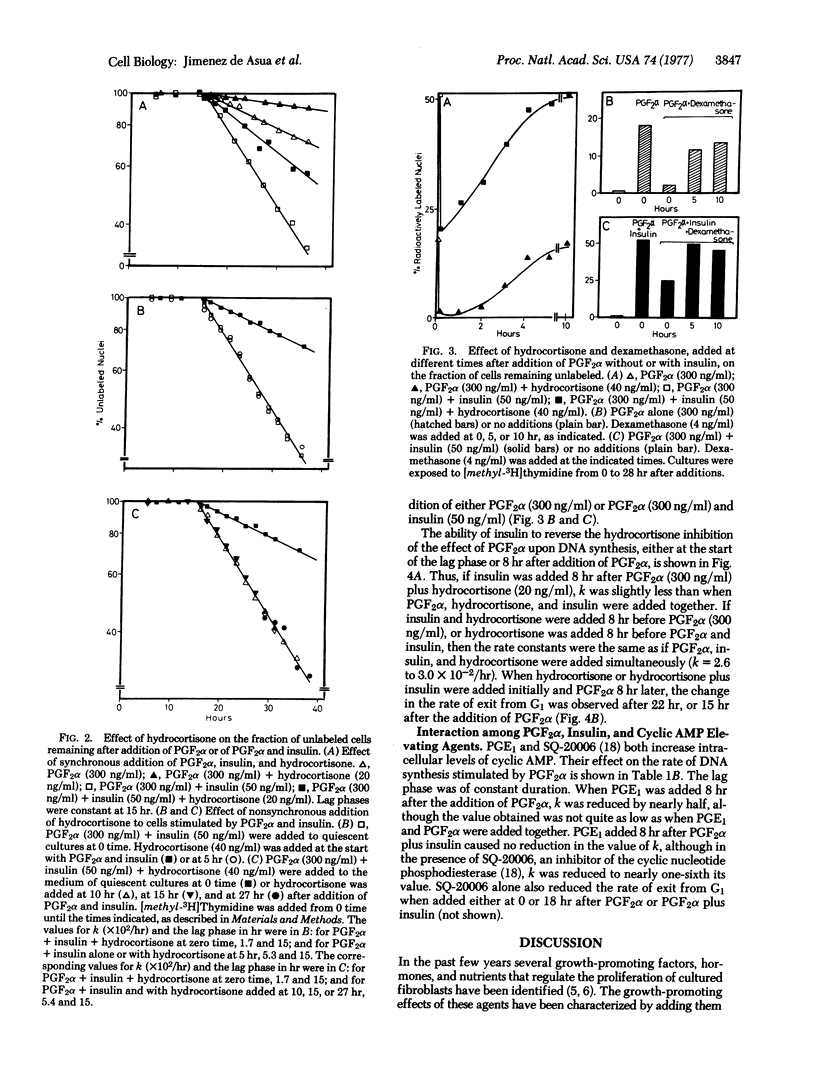
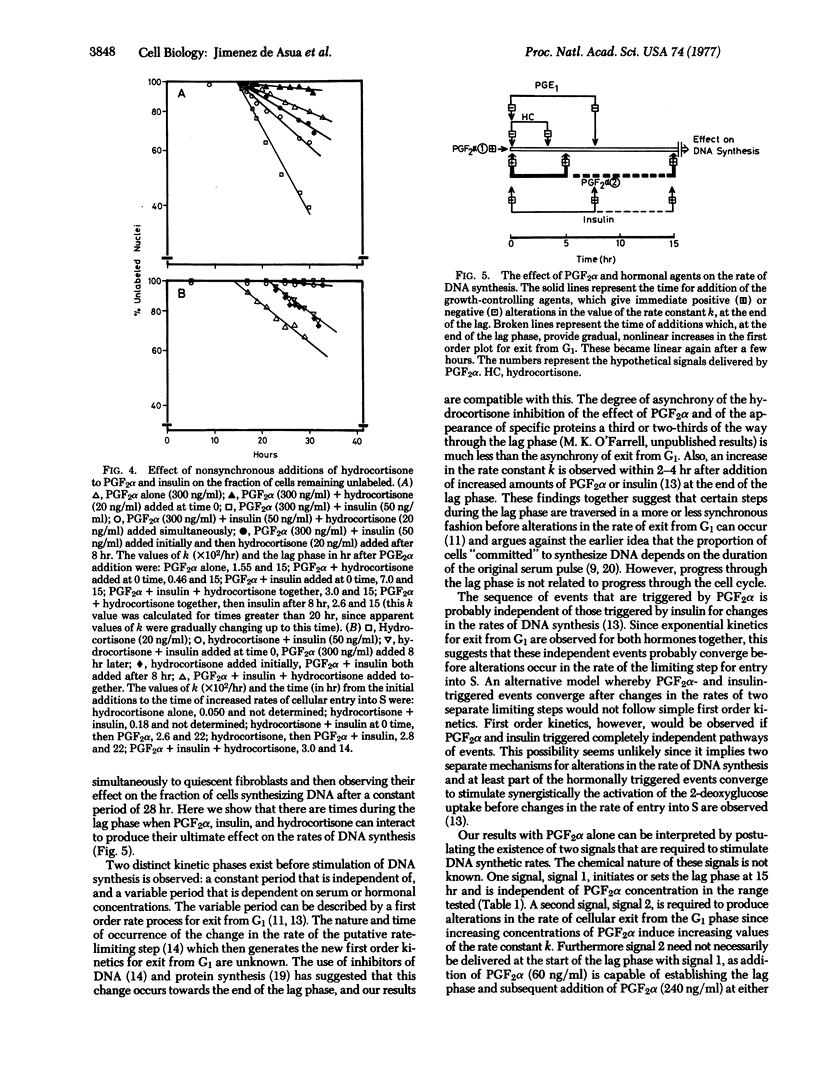
After addition of prostaglandin F2alpha (PGF2alpha) and insulin to quiescent cultured 3T3 fibroblasts a constant lag phase of 15 hr occurs before an increased rate of cellular exit from G1 is observed. The latter process follows first order kinetics, which can be quantified by a rate constant k. The temporal relationship of the interactions of PGF2 alpha, insulin, and hydrocortisone to produce alterations in the rate of exit from G1 was investigated. PGF2alpha establishes a constant lag phase and produces alterations in the rate constant k. These two effects can be partially separated by adding two concentrations of PGF2alpha at different times. Insulin fails to establish the lag phase but can stimulate the effect of PGF2alpha when added at any time after PGF2alpha. Hydrocortisone reduces the value of k when added between 0 and 3 hr after PGF2alpha. These results show that the lag phase can beseparated into temporal regions during which hormones can interact to produce changes in the rate of cellular exit from G1.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bombik B. M., Baserga R. Increased RNA synthesis in nuclear monolayers of WI-38 cells stimulated to proliferate. Proc Natl Acad Sci U S A. 1974 May;71(5):2038–2042. doi: 10.1073/pnas.71.5.2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks R. F. Regulation of fibroblast cell cycle by serum. Nature. 1976 Mar 18;260(5548):248–250. doi: 10.1038/260248a0. [DOI] [PubMed] [Google Scholar]
- Chasin M., Harris D. N., Phillips M. B., Hess S. M. 1-Ethyl-4-(isopropylidenehydrazino)-1H-pyrazolo-(3,4-b)-pyridine-5-carboxylic acid, ethyl ester, hydrochloride (SQ 20009)--a potent new inhibitor of cyclic 3',5'-nucleotide phosphodiesterases. Biochem Pharmacol. 1972 Sep 15;21(18):2443–2450. doi: 10.1016/0006-2952(72)90414-5. [DOI] [PubMed] [Google Scholar]
- De Asua L. J., Clingan D., Rudland P. S. Initiation of cell proliferation in cultured mouse fibroblasts by prostaglandin F2alpha. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2724–2728. doi: 10.1073/pnas.72.7.2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulbecco R., Elkington J. Conditions limiting multiplication of fibroblastic and epithelial cells in dense cultures. Nature. 1973 Nov 23;246(5430):197–199. doi: 10.1038/246197a0. [DOI] [PubMed] [Google Scholar]
- Holley R. W. Control of growth of mammalian cells in cell culture. Nature. 1975 Dec 11;258(5535):487–490. doi: 10.1038/258487a0. [DOI] [PubMed] [Google Scholar]
- Jimenez de Asua L., Carr B., Clingan D., Rudland P. Specific glucocorticoid inhibition of growth promoting effects of prostaglandin F2alpha on 3T3 cells. Nature. 1977 Feb 3;265(5593):450–452. doi: 10.1038/265450a0. [DOI] [PubMed] [Google Scholar]
- Jimenez de Asua L., O'Farrell M., Bennett D., Clingan D., Rugland P. Interaction of two hormones and their effect on observed rate of initiation of DNA synthesis in 3T3 cells. Nature. 1977 Jan 13;265(5590):151–153. doi: 10.1038/265151a0. [DOI] [PubMed] [Google Scholar]
- Maganiello V., Vaughan M. Prostaglandin E 1 effects on adenosine 3':5'-cyclic monophosphate concentration and phosphodiesterase activity in fibroblasts (mouse L cells-tissue culture-enzyme kinetics-prostaglandin homologues). Proc Natl Acad Sci U S A. 1972 Jan;69(1):269–273. doi: 10.1073/pnas.69.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller G. C. Biochemical events in the animal cell cycle. Fed Proc. 1969 Nov-Dec;28(6):1780–1789. [PubMed] [Google Scholar]
- Otten J., Johnson G. S., Pastan I. Regulation of cell growth by cyclic adenosine 3',5'-monophosphate. Effect of cell density and agents which alter cell growth on cyclic adenosine 3',5'-monophosphate levels in fibroblasts. J Biol Chem. 1972 Nov 10;247(21):7082–7087. [PubMed] [Google Scholar]
- Pardee A. B. A restriction point for control of normal animal cell proliferation. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1286–1290. doi: 10.1073/pnas.71.4.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott D. M. Regulation of cell reproduction. Cancer Res. 1968 Sep;28(9):1815–1820. [PubMed] [Google Scholar]
- Rierdan J., Brooks R. Verbal conditioning of middle and lower socioeconomic class schizophrenics. J Abnorm Psychol. 1977 Aug;86(4):369–378. doi: 10.1037//0021-843x.86.4.369. [DOI] [PubMed] [Google Scholar]
- Rudland P. S., Seifert W., Gospodarowicz D. Growth control in cultured mouse fibroblasts: induction of the pleiotypic and mitogenic responses by a purified growth factor. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2600–2604. doi: 10.1073/pnas.71.7.2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields R., Smith J. A. Cells regulate their proliferation through alterations in transition probability. J Cell Physiol. 1977 Jun;91(3):345–355. doi: 10.1002/jcp.1040910304. [DOI] [PubMed] [Google Scholar]
- TODARO G. J., GREEN H. Quantitative studies of the growth of mouse embryo cells in culture and their development into established lines. J Cell Biol. 1963 May;17:299–313. doi: 10.1083/jcb.17.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temin H. M. Stimulation by serum of multiplication of stationary chicken cells. J Cell Physiol. 1971 Oct;78(2):161–170. doi: 10.1002/jcp.1040780202. [DOI] [PubMed] [Google Scholar]


