SUMMARY
Mice deficient in the nuclear hormone receptor RORγt have defective development of thymocytes, lymphoid organs, Th17 cells and type 3 innate lymphoid cells. RORγt binds to oxysterols derived from cholesterol catabolism but it is not clear whether these are its natural ligands. Here, we show that sterol lipids are necessary and sufficient to drive RORγt-dependent transcription. We combined overexpression, RNA interference and genetic deletion of metabolic enzymes to study RORγ-dependent transcription. Our results are consistent with the RORγt ligand(s) being a cholesterol biosynthetic intermediate (CBI) downstream of lanosterol and upstream of zymosterol. Analysis of lipids bound to RORγ identified molecules with molecular weights consistent with CBIs. Furthermore, CBIs stabilized the RORγ ligand-binding domain and induced co-activator recruitment. Genetic deletion of metabolic enzymes upstream of the RORγt-ligand(s) affected the development of lymph nodes and Th17 cells. Our data suggest that CBIs play a role in lymphocyte development potentially through regulation of RORγt.
INTRODUCTION
Nuclear hormone receptors (NHRs) are transcription factors that direct a wide range of developmental, reproductive, and immune response programs. NHRs share a common modular structure comprised of a DNA binding domain (DBD) at the N-terminus and a ligand binding domain (LBD) at the C-terminus. LBD-ligand interaction is required for the transactivation of most NHRs and several classes of small lipophilic molecules such as hormones, vitamins, steroids, retinoids and fatty acids have been identified as NHR ligands (Huang et al., 2010). The identification of natural ligands for orphan NHRs is an important step in understanding how these receptors are regulated by dietary factors or endogenous metabolites.
RORγ (NR1F3) is broadly expressed in human and mouse tissues (Hirose et al., 1994; Medvedev et al., 1996; Ortiz et al., 1995). RORγt is the isoform of RORγ that is expressed in lymphoid tissues and is essential for the development of thymocytes, lymph nodes (Kurebayashi et al., 2000; Sun et al., 2000), gut-associated lymphoid tissues (GALT) (Eberl and Littman, 2004) and Th17 cells (Ivanov et al., 2006), and a subset of innate lymphoid cells. Co-crystallization and in-solution binding experiments have identified compounds that can bind to recombinant ROR molecules. The closely-related RORα was co-crystallized with cholesterol and cholesterol sulfate (Kallen et al., 2004; Kallen et al., 2002) and inhibition of the cholesterol biosynthetic pathway with lovastatin downregulated RORα transcriptional activity (Kallen et al., 2002). RORβ formed crystals with either stearate (Stehlin et al., 2001) or all-trans retinoic acid (ATRA) (Stehlin-Gaon et al., 2003). Structural studies show that RORs have relatively large ligand-binding pockets (>700 Å3) which could accommodate a variety of different ligands. Indeed, RORγ binds to and forms crystals with oxysterols (Jin et al., 2010; Wang et al., 2010a; Wang et al., 2010b) and vitamin D derivatives (Slominski et al., 2014) whereas RORβ can co-crystalize with fatty acids and retinoids (Stehlin-Gaon et al., 2003; Stehlin et al., 2001) which are unrelated to cholesterol. In addition, RORγ has been co-crystallized with various antagonists or inverse agonists with conformations that differ markedly from cholesterol. The biological relevance of various compounds reported to bind to the RORs remains unclear.
Cholesterol biosynthesis is a complex process that involves more than 20 enzymes and biosynthetic steps (Nes, 2011). These can be classified into a few basic sub-processes: acetate is converted into squalene oxide which is then cyclized into lanosterol, and lanosterol is converted into cholesterol (Bloch, 1965). How this pathway regulates the activity of lymphoid cells is still an open question. We have investigated the role of sterol lipids in the regulation of RORγ transcriptional activity. Using biochemical and genetic tools, we demonstrated that in mammalian cells the RORγ ligand maps to a step in the cholesterol biosynthetic pathway that is downstream of lanosterol and upstream of 4α-methyl-cholesta-8,24-dien-3-one. Binding of one intermediate metabolite, 4α-carboxy, 4β-methyl-zymosterol (4ACD8) to the RORγ LBD resulted in co-activator peptide recruitment, which was consistent with the structure of LBD-4ACD8 co-crystals. Mutations in enzymes of the cholesterol biosynthesis pathway abrogated the development of RORγt-dependent lymph node anlagen and the differentiation of Th17 cells. Our results thus suggest that cholesterol biosynthetic intermediates regulate RORγt-dependent immune system development and lymphoid functions.
RESULTS
RORγ has broad specificity for sterol lipids in insect cells
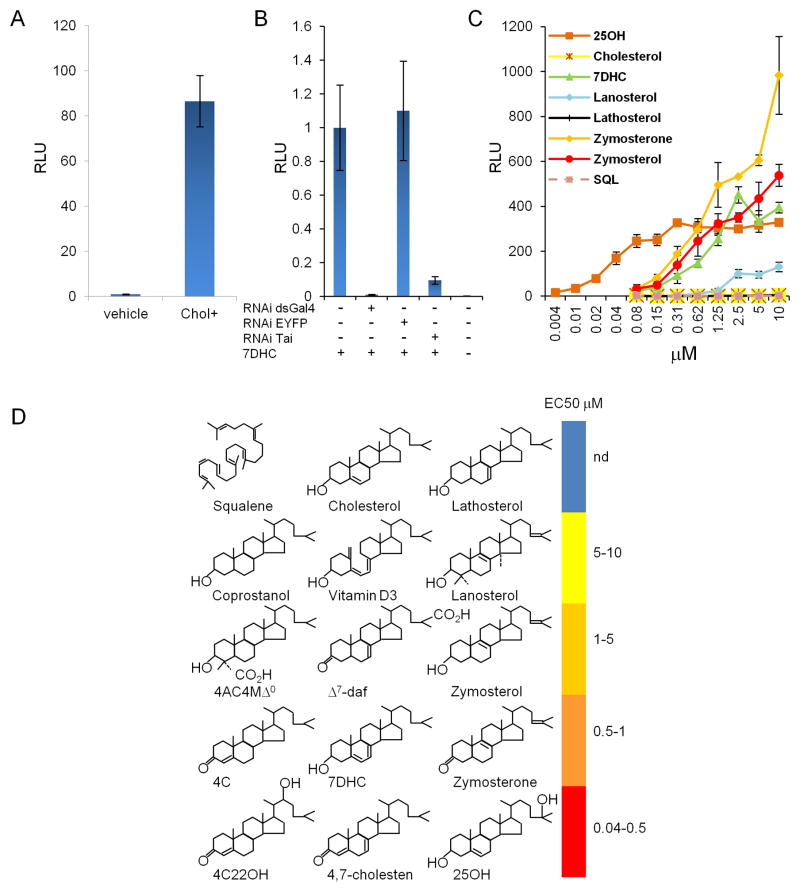
To investigate the nature of RORγ ligand, we employed an insect cell-based RORγ reporter system (Huh et al., 2011). Insects are auxotrophic for polyunsaturated fatty acids, retinoids and sterols and obtain these factors from dietary sources (Cooke and Sang, 1970). However, some insect cells can grow in lipid-depleted media (Silberkang et al., 1983), and we developed a lipid-free chemically-defined medium (CDM) for either the short-term maintenance of Drosophila melanogaster S2 cells or the continuous culture of Kc167 cells (see Supplementary Methods). Insect cells grown in media with fetal calf serum (FCS) displayed strong RORγ transcriptional activity (Huh et al., 2011). However, there was no activity in lipid-free CDM, although activity was restored in cholesterol-supplemented CDM (Figure 1A). Thus RORγ reporter activity in insect cells is dependent on sterol lipids. The NHR transcriptional machinery of insect cells maintained in lipid-free medium functioned in a conventional coactivator-dependent manner. The dsRNA knockdown of taiman, the Drosophila NHR coactivator (Bai et al., 2000), abrogated RORγ reporter activity in Kc167 cells grown in sterol-supplemented CDM (Figure 1B). Moreover, culture in lipid-free medium did not affect the specificity of other NHRs, including dafachronic acid (Daf12) (Motola et al., 2006) and ecdysone receptors (EcR) (Koelle et al., 1991) (Figure S1A,B). However, in contrast to the Daf12 and EcR receptors, which are highly selective for their cognate ligands, RORγ exhibited broad specificity for a range of sterols but not for other lipids (Figure 1C,D).
Figure 1. RORγ reporter activity induced by sterols in insect cells.
(A) Cholesterol is necessary and sufficient to promote RORγ reporter activity in insect cells. D.m. Kc167 cells were grown in continuous cultures in CDM with or without cholesterol and were transiently transfected with RORγ-gal4/UAS firefly luciferase reporter. Representative experiment of n=3. (B) RORγ reporter activity in insect cells grown in CDM is dependent on the coactivator taiman. Kc167 cells were transfected with RORγ reporter described in (A) plus dsRNA. Cells were incubated overnight with 10 μM 7DHC prior to measurement of luciferase activity. RNAi dsGal4 inhibits expression of the RORγ-gal4 fusion protein, RNAi EYFP is negative control and RNAi Tai is specific for the D.m. coactivator taiman. Representative experiment of n=10. (C) CBIs induce RORγ reporter activity in Kc cells maintained in sterol-free CDM. Kc cells were transiently transfected with RORγ-gal4 as described in (A). 25OH (25-hydroxycholesterol), 7DHC (7-dehydrocholesterol), and SQL (squalene). (D) Structure-activity relationships (SAR) of sterols that promote RORγ reporter activity in insect cells. Shown are one non-cyclic and 8 sterol backbones based on the position of the internal double bond. The color bar on the right depicts the range (nd to 0.04 μM) as EC50 of RORγ reporter activity induced by each compound. We tested 78 sterols and 46 were bioactive. Compounds (n=120) used as negative controls included 101 non-sterol compounds such as PUFAs, fatty acids, phospholipids, glycerolipids, amino acid derivatives and retinoids. nd: no activity detected. 4C (4-cholest-4-en-3-one), 4C22OH (4-cholest-4-en-3-one-22-ol), 4,7-cholesten (cholest-4,7-dien-3-one), 4AC4MΔ0 (4α-carboxy, 4β-methyl-cholesta-3-ol), Δ7-daf (Δ7-dafachronic acid). Compound nomenclature: common sterols are labeled by abbreviation and common name i.e, 7DHC is 7-dehydrocholesterol, for rare compounds an abbreviation followed by standard nomenclature for sterol lipids (Nes, 2011) is provided, for example 4AC4MΔ0 (4α-carboxy, 4β-methyl-cholestan-3-ol).The results are a summary of n=33 experiments. Each compound was tested at least twice in technical triplicates. Error bars are standard deviations.
Mapping the RORγ ligand biosynthetic pathway
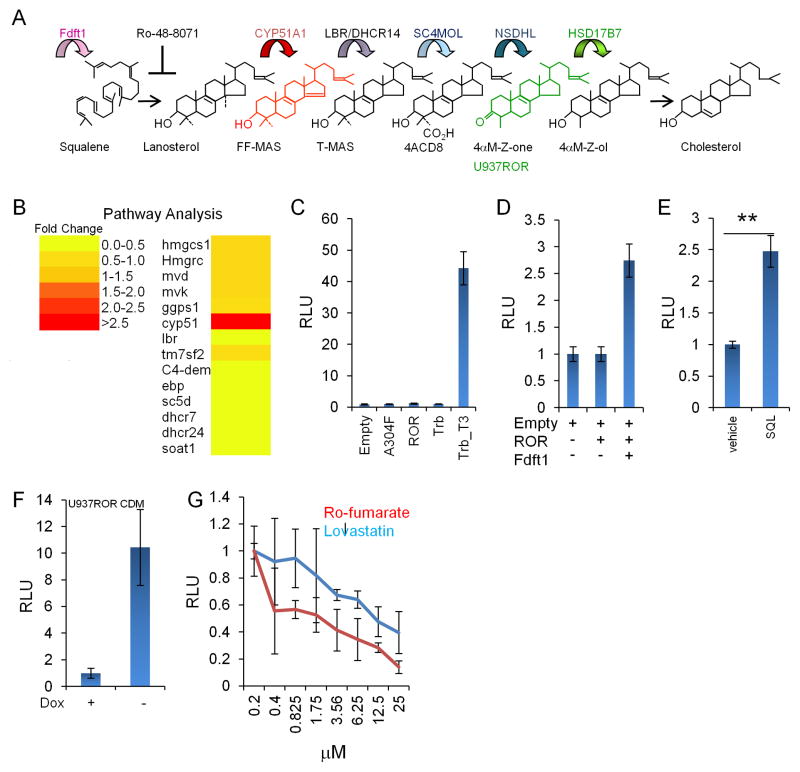
In mammalian cells we used three types of RORγ reporters (Figure S1C). RORγ reporter activity is ubiquitous in mammalian cells that are maintained in CDM (Figure S2A), supporting the hypothesis that the ligand for RORγ is a common basal metabolite. Essential metabolic pathways that are conserved between mammals and insects (Table S1) can be excluded as a source of the RORγ ligand because only sterols rescued RORγ reporter activity in insect cells grown in minimal CDM. We transfected mammalian cells with a RORγ reporter and probed multiple metabolic pathways by either the addition of 387 common metabolites to cells or by co-transfection with 78 basal metabolic enzymes found in mammalian cells (Tables S2 and S3). We found that only enzymes of the cholesterol biosynthetic pathway modulated RORγ reporter activity (Figure 2A,B). RORγ activity was lost in mammalian cells that cannot synthesize sterol lipids such as the squalene synthase (Fdft1)-deficient cell line SXLT (Saher et al., 2009) (Figure 2A,C). Complementation of SXLT cells with Fdft1 cDNA or media with soluble squalene (Figure S2E), the product of Fdft1, restored RORγ reporter activity (Figure 2D,E). We examined the enzymes downstream of Fdft1 and found that cholesterol auxotroph U937 cells deficient in HSD17B7 (Figure S2B,C) (Billheimer et al., 1987) had RORγ reporter activity comparable to that of cholesterol-sufficient cells (Figure 2F and S2D). Reintroduction of HSD17B7 in U937ROR cells rescued the cholesterol dependency of these cells and reduced RORγ reporter activity by 90% (Figure S2F). Furthermore, RORγ reporter activity in U937 cells was reduced by sterol biosynthetic inhibitors lovastatin and Ro 48-8071 fumarate but not by removal of cholesterol from synthetic media (Figure 2G, S2G). These observations support the hypothesis that RORγ reporter activity in U937ROR is dependent on endogenous sterol biosynthesis and not on exogenous cholesterol in FCS. Our results suggest that the endogenous RORγ ligand is downstream of lanosterol and upstream of 4α-methyl-cholesta-8,24-dien-3-one, the substrate of HSD17B7 (Figure 2A).
Figure 2. RORγ reporter activity maps to the cholesterol biosynthetic pathway.
(A) Metabolic map of RORγ reporter activity. Top: enzymes in the pathway. Middle: common names of CBIs. The conventional nomenclature for these sterols is: Lanosterol (14α,4α,4β-trimethyl-cholesta-8,24-dien-3-ol), FF-MAS (4α,4β-dimethyl-cholesta-8,14,24-trien-3-ol), T-MAS (4α,4β-dimethyl-cholesta-8,24-dien-3-ol), 4ACD8 (4α–carboxy, 4β-methyl-cholesta-8,24-dien-3-ol), 4αM-Z-one (4α–methyl-cholesta-8,24-dien-3-one), 4αM-Z-ol (4α–methyl-cholesta-8,24-dien-3-ol). The intermediates in the conversion of lanosterol into FF-MAS and the formyl intermediates of 4ACD8 are not shown. (B) Heatmap of RORγ reporter activity after overexpression of cholesterol biosynthetic enzymes in HEK293T cells co-transfected with RORγ-gal4/UAS-firefly luciferase reporter. (C) RORγ reporter activity is blocked in Fdft1−/− SXLT cells transfected as described in (B). Empty vector, A304F=inactive RORγ mutant, ROR=wt RORγ, Trb=thyroid hormone receptor, Trb_T3=Trb plus 50 μM Triiodothyronine. Representative experiment of n=8. (D) Transfection of hFDFT1 can rescue RORγ reporter activity in SXLT cells. Representative experiment of n=3. (E) Supplementation of media with squalene (SQL) can partially rescue the full length RORγ reporter activity from an endogenous Il-23r enhancer element in SXLT cells. Representative experiment of n=4. Statistical analysis: two-tailed unpaired Student’s t-test. **P<0.005 with a power of 0.87 for 4 aggregated experiments. (F) tTA-off inducible RORγ reporter activity in U937ROR cells grown in cholesterol supplemented CDM after removal of doxycycline. Representative experiment of n=4. (G) RORγ reporter activity in U937ROR cells maintained in media supplemented with exogenous cholesterol is inhibited in a dose dependent fashion by cholesterol biosynthetic inhibitors lovastatin and Ro 48-8071 fumarate. Representative experiment of n=3. Experiments are technical triplicates. Error bars are standard deviations.
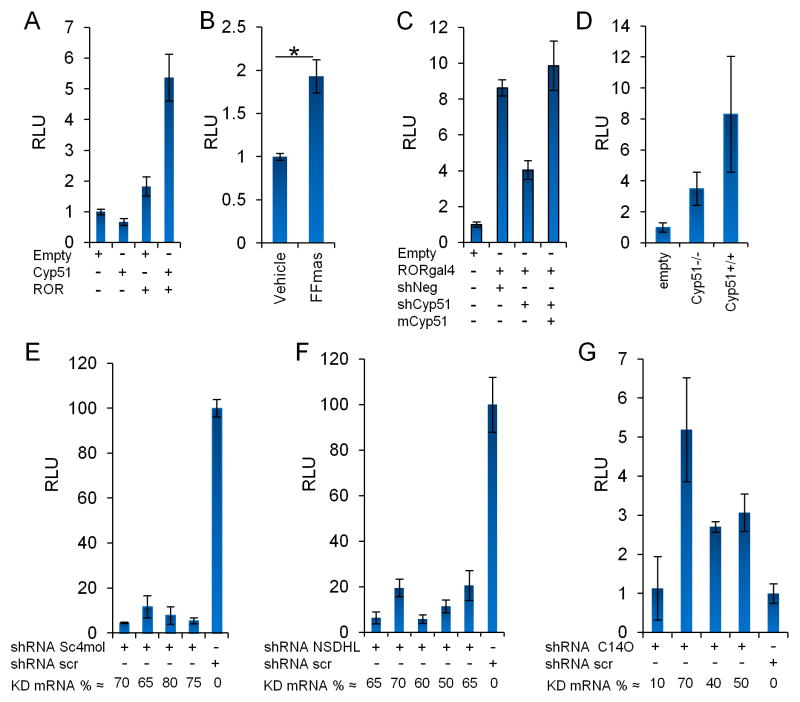
These results predicted that the five enzymes and one scaffold protein that function downstream of squalene synthase and upstream of HSD17B7 produce RORγ ligand. We carried out overexpression and shRNA knockdown experiments to further narrow down the enzymes and products that control RORγ activity. Transcriptional activity was increased only by overexpression of CYP51 (Figure 2B, 3A) or specifically by addition of FF-MAS, the product of the conversion of lanosterol by CYP51 (Figure 3B, S2H and Table S3). RORγ reporter activity in mammalian cells was reduced but not completely abolished by shRNA knockdown of human CYP51A1 or germ-line deletion of mouse Cyp51 (Figure 3C,D). These results suggest that the ligand is the product of either CYP51 or a downstream enzyme. The residual RORγ activity observed in Cyp51-deficient fibroblasts could result from the >100 fold increase in the concentration of lanosterol in Cyp51−/− cells (Keber et al., 2011). Lanosterol is the endogenous substrate of CYP51 and a RORγ ligand when given at high concentrations in insect cells (Figures 1C and 2A). Further downstream are the enzymes LBR and DHCR14, both process FF-MAS into T-MAS (Figure 2A). Thus, results of knockouts and knockdowns are difficult to interpret as these enzymes have redundant function and one single LBR allele is sufficient to maintain 20–44% of endogenous cholesterol biosynthesis (Wassif et al., 2007). The enzymes upstream of HSD17B7 are SC4MOL and NSDHL. All three enzymes are assembled into a cholesterol biosynthetic complex by the scaffold protein C14ORF1. In yeast and plants, the ortholog of C14ORF1, ERG28, tethers and contributes to the assembly of enzymatic complexes including ERG11/CYP51 and ERG25/SC4MOL, the key enzymes that were identified in our enzyme screen to regulate the synthesis of RORγ ligand (Mialoundama et al., 2013; Mo and Bard, 2005; Mo et al., 2002). In yeast, mutations in scaffold C14ORF1/ERG28 result in accumulation of intermediates of 3-keto-sterols and 4α-carboxyl sterols which are the products of SC4MOL/ERG25 and NSDHL/ERG26 suggesting a block in the last step of C4-demethylation driven by HSD17B7/ERG27 (Gachotte et al., 2001). Thus, SC4MOL and NSDHL can process substrates independently of ERG28. However, the scaffold is necessary to accelerate the processing of intermediates generated by SC4MOL and NSDHL into cholesterol. Overexpression of SC4MOL, NSDHL, and C14ORF1 had little to no effect on RORγ reporter activity (Figure 2B, Table S3). This suggests that C4-demethylation is limited by the availability of the biosynthetic precursor T-MAS, the product of LBR/DHCR14, in mammalian cells. Furthermore, the shRNA knockdown of either SC4MOL or NSDHL reduced RORγ reporter activity (Figure 3E,F), which is consistent with their involvement in the synthesis of endogenous RORγ ligand. In contrast, shRNA knockdown of C14ORF1 resulted in 2.5–5 fold increase in RORγ reporter activity (Figure 3G) which in turn is compatible with the accumulation of SC4MOL and NSDHL products in C14ORF1/ERG28 mutant yeast.
Figure 3. RORγ reporter activity maps to 4α,4β-dimethylsterols in the cholesterol biosynthetic pathway.
(A) Overexpression of Cyp51 results in increased RORγ activity in HEK293T cells. HEK293T cells were transfected as described in Figure 2B. Representative experiment of n=3. (B) FF-MAS is a weak agonist for RORγ. HEK293T Cells were transfected like A. Representative experiment of n=6 two-tailed unpaired Student’s t-test *P<0.05 power of 0.74 (6 aggregated experiments). (C) Human CYP51a1 specific shRNAs (shCyp) reduces RORγ reporter activity in HEK293T cells which was rescued by overexpression of shRNA-resistant Cyp51. Knockdown of mRNA was >80%. Representative experiment of n=3. (D). Residual RORγ reporter activity is detected in Cyp51−/− fibroblasts. Fibroblasts from Cyp51−/− embryos were immortalized and respective cell lines were transfected with the same plasmids as described in A. (E) Knockdown of SC4MOL reduced RORγ reporter activity in U937ROR cells. U937ROR cells were transfected with plasmid containing shRNA specific for SC4MOL. Cells were selected post-transfection with puromycin selected cells were cloned. Shown are the results of 4 clones compared to a scramble clone control. Representative of n=3 experiments. (F) Knockdown of NSDHL reduced RORγ reporter activity in U937ROR cells. U937ROR cells were transfected with plasmid containing shRNAs specific for NSDHL as described in D. Representative of n=2 experiments. (G). Knockdown of C14ORF1, the human homologue of 0610007P14rik results in increase of RORγ reporter activity. HEK293T cells were transfected with RORγ-gal4 driven luciferase reporter system plus plasmids containing C14ORF1 specific shRNAs from the Sigma shRNA mission library. The shRNA validation and average percentage of mRNA knockdown is shown at the bottom. Representative of n=3 experiments. Experiments are presented as averages of technical triplicates. Error bars are standard deviations.
Characterization of lipids bound to RORγ immunoprecipitated from mammalian cells
We next characterized, using liquid chromatography coupled to mass spectrometry (LC-MS), the lipid species co-immunoprecipitated with RORγ from mammalian cells (Figure S3A). We observed several peaks, most of which were consistent with sterol structures, likely corresponding to oxidized cholesterol and methylsterols (Figure S3B). One of the peaks, with ions of mz 459.3746 [M+H]+, corresponding to a compound with the exact mass of 458.3668 (±0.0091) Da and expected molecular formula of C30H50O3, caught our attention, as it was partially displaced by the competitive RORγ inhibitor digoxin (Huh et al., 2011) (Figure S3C) and is compatible with sterol intermediates downstream of lanosterol. We could not define the precise structure of the lipids bound to RORγ due to the limited amount of material present in the sample. Analysis of the immunoprecipitates by GC-MS showed that only one non-identifiable sterol was differentially present in RORγ immunoprecipitates. The exact mass of MW 458 suggests a hydroxylated lanosterol derivative. These could be formed by non-enzymatic or by enzymatic action in which lanosterol is converted into non-canonical CBIs through the activity of CYP51 or other enzymes in the cholesterol biosynthetic pathway (Figure S3D). We therefore synthesized several oxides of lanosterol, specifically, 4α–hydroxymethyl,4β,14α-dimethyl-cholesta-7,24-dien-3-ol and 4α–formyl,4β,14α-dimethyl-cholesta-7,24-dien-3-ol, which are metabolic precursors of the 4α–carboxylated lanosterol (Figure S3D). These compounds had no activity in the RORγ reporter assay in mammalian cells, possibly due to already high endogenous ligand-mediated activity, but showed strong activity in insect cells, with EC50 ranging from 150 nM to 500 nM. Thus, non-canonical lanosterol products as well as canonical CBIs may potentially serve as RORγ ligands. Furthermore, lipids isolated from endogenous RORγ precipitated from 3.5 × 1010 AKR1 thymoma cells show a main ion of mz 437.3855 Da which is suggestive of a Na+ adduct of a 24,25-dihydro lanosterol metabolite. While these results are not a proof, they support our data of a CBI as an endogenous physiological RORγ ligand.
Biochemical and structural properties of RORγ LBD bound to CBIs
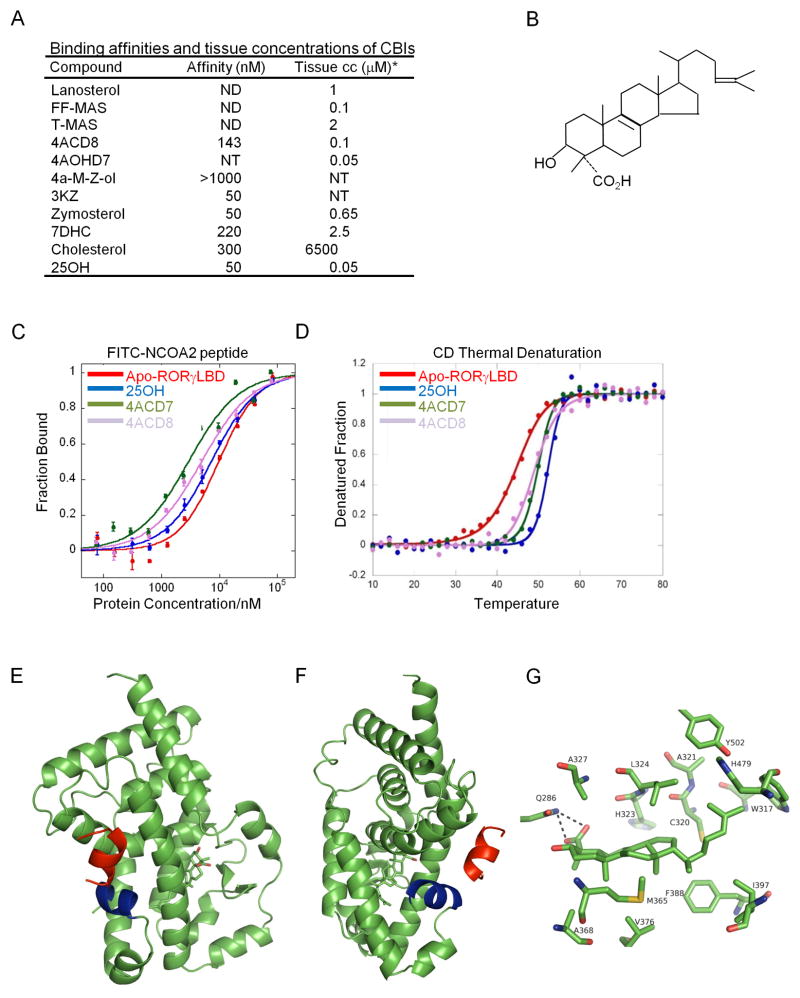
Although non-canonical products of lanosterol are plausible ligands for RORγ, the marked increase in activity observed upon overexpression of CYP51A1 led us to focus our biochemical studies on canonical cholesterol pathway intermediates downstream from lanosterol. We found that RORγ reporter activity in insect cells correlated well with binding of such compounds to the recombinant RORγ LBD. These bound ligands included CBIs that are abundant in mammalian cells (Keber et al., 2011) (Figure 4A). We investigated whether these compounds have biochemical properties similar to those of other RORγ agonists reported in the literature. We focused on 4α-carboxy, 4β-methyl-zymosterol (4ACD8) (Figure 4B), a CBI product of SC4MOL. 4ACD8 showed specific agonist activity for RORγ (Figure S4A,B) and like 25-hydroxycholesterol, 4ACD8 promoted co-activator peptide recruitment, increased the thermal stability of the RORγ LBD (Figure 4C,D) and can compete with inhibitor to restore RORγ reporter activity in mammalian cells (Figure S4C).
Figure 4. Sterol lipids are RORγ ligands.
(A) CBIs abundant in mammalian cells bind RORγ with high affinity. Binding affinity for recombinant RORγ protein was defined by ability of the compound to compete with fluoresceinated 25-hydroxycholesterol or by alphascreen technology (7DHC and lanosterol). Concentrations of different CBIs in total embryonic tissue measured by mass spectrometry are derived from (Keber et al., 2011) and thymic concentration of SC4MOL products 4ACD8 and 4α-hydroxymethyl-cholest-7-en-3β-ol (4AOHD7) were defined in supplemental material and methods. The concentration of 25-hydroxycholesterol are estimates from normal mouse serum and lung tissue (Bauman et al., 2009). ND=not detected NT=not tested. (B) Drawing of 4ACD8. (C) Sterols promote the recruitment of fluoresceinated NCOA2 coactivator peptide to RORγ. NCOA2 peptide recruitment was compared between holo and apo RORγ receptor produced in bacteria using fluorescence polarization assay. (D) Sterols increase stability of RORγ in thermal denaturation assays. The rate of protein denaturation with increasing temperature was measured comparing empty receptor (apo) with ligand-bound receptor (holo forms). For (C) and (D), Apo receptor (red), holo 25-hydroxycholesterol (blue), holo 4α-carboxy-cholest-7-en-3β-ol (4ACD7) (green) and 4ACD8 (lila). (E) Ribbon drawing showing the structure of RORγ bound to the CBI 4ACD8. The RORγ LBD is depicted in green with the ligand inside containing oxygen atoms (Red). An LXXLL motif-containing coactivator peptide is shown in red and Helix 12 in blue. (F) A 180 degree rotation of (E). Graphs with measurements are representatives of n=3 experiments. Cell culture experiments are in technical triplicates and error bars are standard deviations. (G) The 4α-carboxy group of 4ACD8 forms a salt-bridge with RORγ LBD residue Q286 that is homologous to the 4ACD8 contact residue of RORα (Q289).
Reported RORα and RORγ-ligand complex structures were prepared with cholesterol-like compounds (Jin et al., 2010; Kallen et al., 2004; Kallen et al., 2002). We similarly generated crystals of RORα (Figure S4D–H) and RORγ in complex with 4ACD8. In the RORγ:4ACD8 crystal structure, Helix 12 was in an active conformation, tightly packed to the LBD surface, fostering direct interactions with the co-activator peptide (Huang et al., 2010) (Figure 4E,F). The crystal structure showed a well-ordered arrangement of the sterol backbone of 4ACD8 in the RORγ ligand-binding pocket with the carboxy group of 4ACD8 contacting amino acid residues homologous in RORα (Q289,Y290) and RORγ (Q286,L287) (Figure 4G). These residues are essential for the interaction of RORs with their ligands. In RORα, residues Q289 and Y290 interact with either the 3β-hydroxyl or the sulfate group of either cholesterol or cholesterol sulfate (Kallen et al., 2004; Kallen et al., 2002). In RORβ residues Q228 and Y229 interact with the carboxy group of stearate and ATRA (Stehlin-Gaon et al., 2003; Stehlin et al., 2001) and residues Q286 and L287 in RORγ interact with the 3β-hydroxyl group of oxysterols and the carboxy group of 4ACD8. These interactions are important for the binding affinity to RORγ because CBIs that have a 4α-methyl group instead of 4α-carboxy group, such as T-MAS, bind very weakly if at all to RORγ (Figure 4A). Taken together, these results suggest that universal and abundant CBIs are bona-fide RORγ agonists with similar properties to those of other known RORγ agonists.
RORγt-dependent developmental programs are regulated by cholesterol biosynthetic enzymes
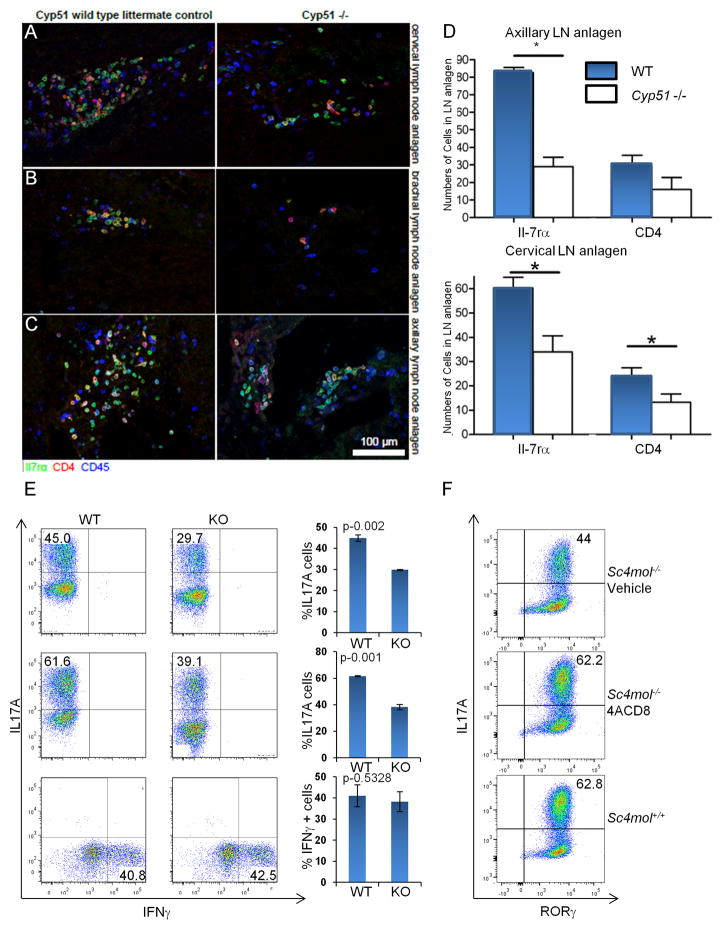
We hypothesized that if CBIs are physiological modulators of RORγ/γt activity, genetic mutations in enzymes of this pathway would mimic RORγt deficiency phenotypes. We therefore examined the development of lymphoid tissues in embryos deficient for Cyp51. We found that at E14.5 the lymph node anlagen of Cyp51−/− embryos (Keber et al., 2011) were smaller than those of wild type littermates (Figure 5A–C) and development of lymph node structures was stunted or absent. The most striking phenotype was a complete absence of brachial lymph node anlagen in 75% of Cyp51−/− embryos (Figure 5B). While the total number of hematopoietic cells in lymph node anlagen of Cyp51−/− embryos was comparable to that of wild type controls, these cells were located outside the aggregates containing CD4+ and IL7RA+ lymphoid tissue inducer (LTi) cells. The numbers of IL7RA+ and CD4+ LTi cells were reduced by approximately 50% in cervical and axillary lymph node anlagen (Figure 5D). Thus, the developmental processes regulated by RORγt were severely affected in Cyp51-deficient embryos.
Figure 5. Deficiencies in the cholesterol biosynthetic pathway affect lymph node development and Th17 cell differentiation.
(A) Lti populations are reduced in cervical lymph node anlagen of day E14.5 Cyp51−/− mouse embryos. (B) Brachial lymph node anlagen are absent of day E14.5 Cyp51−/− mouse embryos. (C) Lti populations are reduced in axillary lymph node anlagen of day E14.5 Cyp51−/− mouse embryos. Embryos were obtained from time-mated pregnancies and fixed with formaldehyde. Whole embryo serial sections of wt (n=3) and Cyp51−/− (n=4) embryos were prepared and stained for IL7Rα chain (GREEN), CD4 (RED) and hematopoietic lineage marker CD45 (BLUE). (D) Quantitation of IL7Rα+ and CD4+ cells in lymph node anlagen of Cyp51−/− embryos shown in (A) and (C). Left axillary, right cervical lymph node anlagen. Data are quantified as bar graphs of averages of per cent cells per embryo. (E) Impaired Th17 cell differentiation in RORγt-cre Sc4mol-deficient naïve CD4+ T cells. FACS analysis of Th17 cells stained for IL-17A and IFNγ 72 h after polarization (top and bottom panels) and 144 h after polarization (middle panel). Each experiment compares naïve CD4 T cells sorted from Sc4mol+/+ and Sc4mol−/− animals and polarized side by side as technical triplicates. Th17 polarization, representative experiment (n=6). **P<0.005 and power 0.96 for P<0.01. Th1 polarization, representative experiment (n=3). (F) Addition of 1 μM 4ACD8 rescues Th17 cell differentiation in RORγt-cre Sc4mol−/− naïve CD4+ T cells. Cells were processed and analyzed as described in (E). Representative of (n=3) experiments with P<0.005 and power 0.99 for P<0.01. Data are quantified as bar graphs with averages (%) of differentiated cells. Error bars are standard deviations. Statistics: two tailed unpaired Student’s t-test with equal variance, power estimates are for aggregate experiments.
We next investigated whether Sc4mol, the enzyme downstream of Cyp51 that modifies the C4-methyl groups of CBIs, modulates RORγt-dependent processes. We bred Sc4molf/f mice (Skarnes et al., 2011) to CD4-cre and RORγt-cre transgenic animals. The CD4-cre transgene limits the deletion of the targeted alleles to TCRαβ T cells (Lee et al., 2001), and the RORγt-cre transgene induces the deletion of Sc4mol in all cells expressing RORγt, including T cells and LTi cells (Eberl and Littman, 2004). The excision efficiency of the Sc4mol f/f alleles in the CD4+ T cells of RORγt-cre and CD4-cre animals was above 90% (Figure S5A) and Sc4mol−/− cells failed to expand in sterol-free media. Furthermore, Sc4mol−/− cells had a 30–50% reduction in the in vitro polarization of IL-17+ CD4+ T cells when compared to wild type controls, while the differentiation of Th1 and Treg cells was unaffected (Figures 5E, S5C,D, data not shown). The Th17 polarization defect of Sc4mol−/− T cells was not due to reduced proliferation or cell death, as both Sc4mol+/+ and Sc4mol−/− cultures had similar numbers of live cells (data not shown). The Th17 polarization defect was also not due to the lack of end products of the cholesterol biosynthetic pathway as the Th17 polarization defect of Sc4mol−/− T cells could not be alleviated by cholesterol supplementation (Figure S5B) but was rescued by addition of CBIs, like 4ACD8 to media (Figure 5F, S5E). In vivo CD4-cre and RORγt-cre animals had normal lymphoid populations in their lymph nodes, thymi and gut-associated lymphoid tissues. This suggested that Sc4mol−/− cells could obtain CBIs and cholesterol for cell growth via the endocytosis of exogenous LDL (Goldstein and Brown, 1990). Alternatively, 4,4-dimethyl-sterols like FF-MAS and T-MAS, which are upstream of SC4MOL and are weak RORγ ligands at physiological concentrations, may replace the endogenous RORγ ligands in the absence of SC4MOL, as there is a 500-fold increase in their concentration in humans with hypomorphic mutations of SC4MOL (He et al., 2011). A good example of this effect is lanosterol, which at physiological concentrations (1 μM) has little to no activity in insect cells, while at higher concentrations it has significant RORγ reporter activity (Figure 1C). Thus, an increase in the intracellular concentrations of 4,4-dimethylsterols could partially compensate for the loss of physiological RORγ ligand in Sc4mol−/− cells.
DISCUSSION
In this study we combined biochemical and genetic approaches to investigate the nature of RORγ ligands. RORγ is required for the differentiation of Lin-CD45+IL7rα+ LTi cells that drive lymphoid tissue development in the fetus (Kurebayashi et al., 2000; Sun et al., 2000) and adult LTi-like cells that drive the development of lymphoid structures in the gut of adult animals (Eberl and Littman, 2004). We propose that RORγ ligands are endogenous metabolites and not compounds derived from dietary or microbial products. The depletion of RORγ ligands would be expected to phenocopy RORγ deficiency. Lymph nodes, Peyer’s patches, cryptopatches and isolated lymphoid follicles, as well as RORγt-dependent class 3 innate lymphoid cells are present in germ-free animals (Hamada et al., 2002; Kanamori et al., 1996), suggesting that microbiota are not a critical source of RORγ ligands. Germ-free mice reared for generations on chemically defined low molecular weight diets also have normal thymocyte (Vos et al., 1992) and lymph node (Wostmann et al., 1970) development. Furthermore, RORγt-dependent Th17 cells can be differentiated in minimal media containing only amino acids, vitamins and minerals, supplemented by a source of insulin and transferrin. These observations suggest that an exogenous source of ligand is not required for RORγt function, and that endogenous ligand is sufficient for most of the physiological functions of RORγt during lymphoid tissue development.
Our data suggest that the physiological ligands for RORγ are methylated CBIs or their metabolites. Until recently, CBIs were considered biosynthetic intermediates with no biological activities of their own. However, evidence is growing for roles of CBIs in functions other than the synthesis of cholesterol. The products of CYP51 (FF-MAS) and LBR/DHCR14 (T-MAS), the result of the 14α-demethylation of lanosterol, have been shown to promote meiosis (Byskov et al., 1995). Another abundant CBI, desmosterol, modulates LXR activity in atherogenic macrophages (Spann et al., 2012). These data support the hypothesis that CBIs have intrinsic biological activities that are independent of their role in cholesterol biosynthesis. Our work extends these findings and suggests that canonical CBIs such as 4ACD8 can function as endogenous ligands for RORγt. We would also like to raise the possibility that non-canonical metabolites may function as ligands for NHRs. The existence of non-canonical metabolites of lanosterol has been suggested by genetic and biochemical studies. Lanosterol was shown in vitro to be a SC4MOL substrate (Rahier et al., 2006) and lanosterol-C4α-carboxy accumulated in yeast with a mutation in ERG26/NSDHL, the enzyme downstream of SC4MOL (Gachotte et al., 1998). Furthermore, LBR/DHCR14-deficient mice accumulated 4-demethylated FF-MAS, suggesting that FF-MAS, the product of CYP51 is processed by SC4MOL and that SC4MOL can modify multiple 4,4-dimethyl-sterols in the cholesterol biosynthetic pathway (Wassif et al., 2007). Our own data indicate that non-canonical metabolites of lanosterol found in thymus can stimulate RORγ activity in insect cells and may serve as ligands for RORγt in this tissue.
The canonical CBIs exhibit high affinity for RORγ (Kd <150 nM) and are abundant within cells at intracellular concentrations ranging from 50–5000 nM. Therefore, these CBIs are advantageously placed to occupy the majority of RORγ receptors those concentration in thymocytes is projected at 3–30 nM. We estimate that more than 80% of all available RORγ would be occupied by an endogenous ligand present at 500 nM. As the function for occupancy is quite steep, further increases in receptor occupancy from 80% to 98% would require the endogenous concentration of ligand to increase from 500 nM to 5000 nM. These calculations and the fact that many sterol lipids are agonists for RORγ, could explain the moderate effect of exogenous ligands on RORγ activity in our reporter assays with mammalian cells and insect cells maintained in FCS.
Earlier publications have suggested that oxysterols can potentially serve as RORγ ligands. Some of the hydroxymethyl, formyl or carboxylated CBIs are oxysterols by definition. However, it had not been clear whether the RORγ ligand is a pre- or a post-cholesterol oxysterol. Post-cholesterol oxysterols like 25-hydroxycholesterol are products of the catabolism of cholesterol. While we observed weak but specific RORγ reporter activity with CBIs (Figure S2H,I and S4A,B), our data on oxysterols derived from cholesterol catabolism are in agreement with the observations that many function as inverse agonists of RORγ activity in mammalian cells (Wang et al., 2010a; Wang et al., 2010b), although we find that they act as agonists in insect cells (Table S2). The oxysterols 25 and 22-hydroxycholesterol can compete with inhibitor and restore RORγ transcriptional activity in mammalian cells lines (Figure S4C). Furthermore, 7α or 7β,27-dihydroxycholesterol were shown to compete with RORγ antagonist to restore transcriptional activity and direct expression of IL-17 in T cells, which suggests that under some conditions oxysterols can act as agonists in mammalian cells (Soroosh et al., 2014). However, the ability to compete out antagonists in mammalian cells is a property of several sterols including some CBIs (Figure S4C) and the recovery was not much higher than what was observed in cells without antagonists.
An outstanding question is how RORγ is regulated. The experiments in the insect system suggest that many sterols can induce an agonist conformation in RORγ. This includes many CBIs and oxysterols but not cholesterol (Figure 1C,D). Moreover, oxysterols that are inactive or have been identified as “inverse agonists” in mammalian cells, are agonists in insect cells (Table S2). Since all these ligands have the ability to induce an agonist conformation in insect cells, their differential activity in mammalian cells may depend upon post-translational modifications or protein-protein interactions that can accommodate only some of the ligands bound to RORγ. Furthermore, although our results suggest that the physiological RORγ ligand is universal in mammalian cells, it is not known whether access to such ligand regulates transcriptional activity. The intracellular location of the ligand biosynthesis machinery and RORγ may contribute to regulation, and the concentration of ligand may also be affected by the differentiation program of RORγ-expressing cells. For example, STAT3 was found bound to the promoter region of the 0610007P14Rik gene, the mouse homolog of C14ORF1 (Ciofani et al., 2012), and may regulate its levels and, hence, ligand availability. Further research is needed to clarify the role of any of these possible regulatory mechanisms in RORγ transcriptional activity.
Our data are most consistent with a role for CBIs, which are universal and abundant in mammalian cells, in positive regulation of RORγ activity. However, there may be multiple ligands mediating positive and negative regulation of RORγt functions in lymphoid cells, and both cholesterol biosynthetic intermediates and cholesterol catabolic products may contribute to the activity of this nuclear receptor. Further studies are needed to determine the roles of the different ligands in functions mediated by RORγt in adaptive and innate lymphoid cells and by RORγ in the non-lymphoid organs in which it is expressed. A better understanding of how these metabolites influence cellular physiology will provide greater insights into lymphocyte development and function and may facilitate development of novel therapeutics for inflammatory diseases.
Experimental Procedures
CDM
Insect CDM was comprised 40% Grace’s Medium (Gibco 11595), 10% Murashige and Skoog basal salt micronutrient solution (Sigma M0529), 1% NEAS (Gibco 11140), 1 mM sodium pyruvate, 2% Insulin-Transferin-Selenium A (ITS-A) (Gibco 51300-044) and 1% Pluronic F-68 (Sigma P5556). Semi-synthetic CDM had 2% Yeastolate Ultrafiltrate 50x (Gibco 18200).
Th17 cell polarization
Sorted CD4+CD25-CD44-CD62L+ cells were incubated overnight in IMDM with 10% FCS and anti-CD3/CD28/IFN-γ/IL-4 (mab cocktail). The following day 90% of media replaced with CDM (IMDM, ITS-X (Gibco 51500), 1% Pluronic F-68, mab cocktail, Tgfβ, IL-6) plus synthetic cholesterol (Sigma S5442), sterols, alone conjugated with recombinant ApoE3 or in FFAm (Table 3 US patent 8198084) as indicated. Cytokine expression levels were analyzed by FACS.
Transfections (transient or stable)
Full length RORγ and RORγ-gal4 fusion proteins were used to drive firefly luciferase reporters PGL4.31[luc2p/GAL4UAS/Hygro] (luc2p) (Promega C935A) for mammalian cells and luc2p with a minimal D.m.hsp70 promoter for insect cells. Renilla luciferase was used for normalization. Open Biosystems IRAV-FL060105 library was used for overexpression screening.
Tissues and embryos
Cyp51 deficiency was introduced by time mated crosses of cyp51a1fl/fl females to cyp51fl/wt EIIa cre transgenic males as described (Keber et al., 2011). Embryos were collected, processed and stained following (van de Pavert et al., 2009). The investigator performing the section analyses and cell counts was blinded to the genotype of the sections. All experiments using animals were performed following protocols approved by the NYU Institutional Animal Care and Usage Committee.
RORγ IPs
Nuclear extract from HEK293T cells transfected with 3xFlag-RORγ-LBD or CTRL vectors were IP with anti-Flag magnetic beads (Sigma M8823) and eluted with 3xFLAG peptide (Sigma F4799). IP QC by WB and lipid extracted with 2:1 Chloroform:methanol. Solvent and water phase dried with N2 and analyzed by LC-MS (Agilent 6420 Q-TOF).
Crystallization, data collection and structure determination of ROR-LBDs bound with 4ACD8
3 mg/ml RORα and RORγ-LBD were incubated with 10 μM 4ACD8 and 3-fold M excess of RIP-140498–509 peptide. Crystals grown in hanging drops at 9 °C with 200 mM MgCl2, 9–21 % PEG 3350 RORα and 0.3 M KNa tartrate RORγ. Diffraction data collected at Argonne National Laboratory APS beamline SER-CAT 22-ID and structures solved by molecular replacement with PHASER. Data collection and refinement statistics are in SEP Table I supplemental information. Structures of RORα and RORγ with 4ACD8 have pdb IDs RORγ:4S14 and RORα:4S15.
Binding assays
Competition assays 100 nM ROR-LBD loaded with 5 nM fluorescein-labeled 25-hydroxycholesterol was incubated with increasing concentrations of competitors. In peptide recruitment assays 5 nM fluorescein-NCOA2 peptide was incubated with apo or compound saturated RORγ-LBD. Fluorescence polarization was measured on FlexStation 3 and IC 50 or Kd values calculated with GraphPad Prism 5. The CD-melting was performed on AVIV circular dichroism spectrometer (Model 420) with 10–80 °C gradient (2 °C, 2 min). Melting temperatures (Tm) calculated with KaleidaGraph.
Statistical procedures
Unless otherwise stated data are averages of triplicates and error bars are standard deviations. Comparisons are two-tailed unpaired Student’s t-test with equal or unequal variance as indicated. Statistical power was calculated using aggregate data from multiple experiments.
Supplementary Material
Acknowledgments
We thank David Mangelsdorf for advice. The Scripps center for mass spectrometry and metabolomics for lc-ms and XCMS analysis; Gesine Saher for the sxlt cell line; Jun Huh for plasmids; Dr. Rene Lafont for ecdysone biosynthetic intermediates; Jialin Liu for chemical synthesis; Raghu R.V. Malapaka and Eric H. Xu for recombinant RORγ protein. Wendy Huang for experimental support. Renee de Pooter, Maria Ciofani and Natalia B. Ivanova for critical reading of the manuscript. This work was supported by NIH grant R01-AI080885-01A1 (D.R.L.), National Science Foundation grant MCB-0929212 (W.D.N), Slovenian Research Agency (ARRS) Grants J3-6804 (S.H), J4-4306 (S.H and R.K.), P1-0104 (D.R.) and graduate fellowship (G.L.), breakthrough project NGI 40-41009-98-9077 (SAvdP), Netherlands Organization for Scientific Research-Earth and Life Sciences (NWO-ALW) Top Grant 09.048 (R.E.M.) and NIH grant T32HL007151 (F.R.S.) D.R.L. is an Investigator of the Howard Hughes Medical Institute.
Footnotes
Supplementary Methods is linked to the online version of the paper.
Author contributions: F.R.S and D.R.L. designed experiments and wrote the manuscript with input from other authors. F.R.S. performed cell-based and mouse studies with help of E.D. D.J.L, B.A.H, B.N.R., W.D.N. synthesized sterols and identified thymic lanosterol oxides. A.R. isolated 4ACD8 and defined the concentration of 4AOHD7 in thymus. G.L. and D.R. studied squalene uptake and processing by cells. E.D. performed simulations of 4ACD8 concentrations. P.H. and F.R. performed RORγ binding assays and crystallography experiments. S.A. v.d.P., R.K., T.K., S.H. and R.E.M. generated, processed and stained Cyp51−/− embryos for lymph node anlagen.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bai J, Uehara Y, Montell DJ. Regulation of invasive cell behavior by taiman, a Drosophila protein related to AIB1, a steroid receptor coactivator amplified in breast cancer. Cell. 2000;103:1047–1058. doi: 10.1016/s0092-8674(00)00208-7. [DOI] [PubMed] [Google Scholar]
- Bauman DR, Bitmansour AD, McDonald JG, Thompson BM, Liang G, Russell DW. 25-Hydroxycholesterol secreted by macrophages in response to Toll-like receptor activation suppresses immunoglobulin A production. Proc Natl Acad Sci U S A. 2009;106:16764–16769. doi: 10.1073/pnas.0909142106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billheimer JT, Chamoun D, Esfahani M. Defective 3-ketosteroid reductase activity in a human monocyte-like cell line. J Lipid Res. 1987;28:704–709. [PubMed] [Google Scholar]
- Bloch K. The biological synthesis of cholesterol. Science. 1965;150:19–28. doi: 10.1126/science.150.3692.19. [DOI] [PubMed] [Google Scholar]
- Byskov AG, Andersen CY, Nordholm L, Thogersen H, Xia G, Wassmann O, Andersen JV, Guddal E, Roed T. Chemical structure of sterols that activate oocyte meiosis. Nature. 1995;374:559–562. doi: 10.1038/374559a0. [DOI] [PubMed] [Google Scholar]
- Ciofani M, Madar A, Galan C, Sellars M, Mace K, Pauli F, Agarwal A, Huang W, Parkurst CN, Muratet M, et al. A validated regulatory network for Th17 cell specification. Cell. 2012;151:289–303. doi: 10.1016/j.cell.2012.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke J, Sang JH. Utilization of sterols by larvae of Drosophila melanogaster. J Insect Physiol. 1970;16:801–812. doi: 10.1016/0022-1910(70)90214-3. [DOI] [PubMed] [Google Scholar]
- Eberl G, Littman DR. Thymic origin of intestinal alphabeta T cells revealed by fate mapping of RORgammat+ cells. Science. 2004;305:248–251. doi: 10.1126/science.1096472. [DOI] [PubMed] [Google Scholar]
- Gachotte D, Barbuch R, Gaylor J, Nickel E, Bard M. Characterization of the Saccharomyces cerevisiae ERG26 gene encoding the C-3 sterol dehydrogenase (C-4 decarboxylase) involved in sterol biosynthesis. Proc Natl Acad Sci U S A. 1998;95:13794–13799. doi: 10.1073/pnas.95.23.13794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gachotte D, Eckstein J, Barbuch R, Hughes T, Roberts C, Bard M. A novel gene conserved from yeast to humans is involved in sterol biosynthesis. J Lipid Res. 2001;42:150–154. [PubMed] [Google Scholar]
- Goldstein JL, Brown MS. Regulation of the mevalonate pathway. Nature. 1990;343:425–430. doi: 10.1038/343425a0. [DOI] [PubMed] [Google Scholar]
- Hamada H, Hiroi T, Nishiyama Y, Takahashi H, Masunaga Y, Hachimura S, Kaminogawa S, Takahashi-Iwanaga H, Iwanaga T, Kiyono H, et al. Identification of multiple isolated lymphoid follicles on the antimesenteric wall of the mouse small intestine. J Immunol. 2002;168:57–64. doi: 10.4049/jimmunol.168.1.57. [DOI] [PubMed] [Google Scholar]
- He M, Kratz LE, Michel JJ, Vallejo AN, Ferris L, Kelley RI, Hoover JJ, Jukic D, Gibson KM, Wolfe LA, et al. Mutations in the human SC4MOL gene encoding a methyl sterol oxidase cause psoriasiform dermatitis, microcephaly, and developmental delay. J Clin Invest. 2011;121:976–984. doi: 10.1172/JCI42650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose T, Smith RJ, Jetten AM. ROR gamma: the third member of ROR/RZR orphan receptor subfamily that is highly expressed in skeletal muscle. Biochem Biophys Res Commun. 1994;205:1976–1983. doi: 10.1006/bbrc.1994.2902. [DOI] [PubMed] [Google Scholar]
- Huang P, Chandra V, Rastinejad F. Structural overview of the nuclear receptor superfamily: insights into physiology and therapeutics. Annu Rev Physiol. 2010;72:247–272. doi: 10.1146/annurev-physiol-021909-135917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh JR, Leung MW, Huang P, Ryan DA, Krout MR, Malapaka RR, Chow J, Manel N, Ciofani M, Kim SV, et al. Digoxin and its derivatives suppress TH17 cell differentiation by antagonizing RORgammat activity. Nature. 2011;472:486–490. doi: 10.1038/nature09978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- Jin L, Martynowski D, Zheng S, Wada T, Xie W, Li Y. Structural basis for hydroxycholesterols as natural ligands of orphan nuclear receptor RORgamma. Mol Endocrinol. 2010;24:923–929. doi: 10.1210/me.2009-0507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallen J, Schlaeppi JM, Bitsch F, Delhon I, Fournier B. Crystal structure of the human RORalpha Ligand binding domain in complex with cholesterol sulfate at 2.2 A. J Biol Chem. 2004;279:14033–14038. doi: 10.1074/jbc.M400302200. [DOI] [PubMed] [Google Scholar]
- Kallen JA, Schlaeppi JM, Bitsch F, Geisse S, Geiser M, Delhon I, Fournier B. X-ray structure of the hRORalpha LBD at 1.63 A: structural and functional data that cholesterol or a cholesterol derivative is the natural ligand of RORalpha. Structure. 2002;10:1697–1707. doi: 10.1016/s0969-2126(02)00912-7. [DOI] [PubMed] [Google Scholar]
- Kanamori Y, Ishimaru K, Nanno M, Maki K, Ikuta K, Nariuchi H, Ishikawa H. Identification of novel lymphoid tissues in murine intestinal mucosa where clusters of c-kit+ IL-7R+ Thy1+ lympho-hemopoietic progenitors develop. J Exp Med. 1996;184:1449–1459. doi: 10.1084/jem.184.4.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keber R, Motaln H, Wagner KD, Debeljak N, Rassoulzadegan M, Acimovic J, Rozman D, Horvat S. Mouse knockout of the cholesterogenic cytochrome P450 lanosterol 14alpha-demethylase (Cyp51) resembles Antley-Bixler syndrome. J Biol Chem. 2011;286:29086–29097. doi: 10.1074/jbc.M111.253245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koelle MR, Talbot WS, Segraves WA, Bender MT, Cherbas P, Hogness DS. The Drosophila EcR gene encodes an ecdysone receptor, a new member of the steroid receptor superfamily. Cell. 1991;67:59–77. doi: 10.1016/0092-8674(91)90572-g. [DOI] [PubMed] [Google Scholar]
- Kurebayashi S, Ueda E, Sakaue M, Patel DD, Medvedev A, Zhang F, Jetten AM. Retinoid-related orphan receptor gamma (RORgamma) is essential for lymphoid organogenesis and controls apoptosis during thymopoiesis. Proc Natl Acad Sci U S A. 2000;97:10132–10137. doi: 10.1073/pnas.97.18.10132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee PP, Fitzpatrick DR, Beard C, Jessup HK, Lehar S, Makar KW, Perez-Melgosa M, Sweetser MT, Schlissel MS, Nguyen S, et al. A critical role for Dnmt1 and DNA methylation in T cell development, function, and survival. Immunity. 2001;15:763–774. doi: 10.1016/s1074-7613(01)00227-8. [DOI] [PubMed] [Google Scholar]
- Medvedev A, Yan ZH, Hirose T, Giguere V, Jetten AM. Cloning of a cDNA encoding the murine orphan receptor RZR/ROR gamma and characterization of its response element. Gene. 1996;181:199–206. doi: 10.1016/s0378-1119(96)00504-5. [DOI] [PubMed] [Google Scholar]
- Mialoundama AS, Jadid N, Brunel J, Di Pascoli T, Heintz D, Erhardt M, Mutterer J, Bergdoll M, Ayoub D, Van Dorsselaer A, et al. Arabidopsis ERG28 tethers the sterol C4-demethylation complex to prevent accumulation of a biosynthetic intermediate that interferes with polar auxin transport. Plant Cell. 2013;25:4879–4893. doi: 10.1105/tpc.113.115576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo C, Bard M. Erg28p is a key protein in the yeast sterol biosynthetic enzyme complex. J Lipid Res. 2005;46:1991–1998. doi: 10.1194/jlr.M500153-JLR200. [DOI] [PubMed] [Google Scholar]
- Mo C, Valachovic M, Randall SK, Nickels JT, Bard M. Protein-protein interactions among C-4 demethylation enzymes involved in yeast sterol biosynthesis. Proc Natl Acad Sci U S A. 2002;99:9739–9744. doi: 10.1073/pnas.112202799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motola DL, Cummins CL, Rottiers V, Sharma KK, Li T, Li Y, Suino-Powell K, Xu HE, Auchus RJ, Antebi A, et al. Identification of ligands for DAF-12 that govern dauer formation and reproduction in C. elegans. Cell. 2006;124:1209–1223. doi: 10.1016/j.cell.2006.01.037. [DOI] [PubMed] [Google Scholar]
- Nes WD. Biosynthesis of cholesterol and other sterols. Chem Rev. 2011;111:6423–6451. doi: 10.1021/cr200021m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz MA, Piedrafita FJ, Pfahl M, Maki R. TOR: a new orphan receptor expressed in the thymus that can modulate retinoid and thyroid hormone signals. Mol Endocrinol. 1995;9:1679–1691. doi: 10.1210/mend.9.12.8614404. [DOI] [PubMed] [Google Scholar]
- Rahier A, Darnet S, Bouvier F, Camara B, Bard M. Molecular and enzymatic characterizations of novel bifunctional 3beta-hydroxysteroid dehydrogenases/C-4 decarboxylases from Arabidopsis thaliana. J Biol Chem. 2006;281:27264–27277. doi: 10.1074/jbc.M604431200. [DOI] [PubMed] [Google Scholar]
- Saher G, Quintes S, Mobius W, Wehr MC, Kramer-Albers EM, Brugger B, Nave KA. Cholesterol regulates the endoplasmic reticulum exit of the major membrane protein P0 required for peripheral myelin compaction. J Neurosci. 2009;29:6094–6104. doi: 10.1523/JNEUROSCI.0686-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberkang M, Havel CM, Friend DS, McCarthy BJ, Watson JA. Isoprene synthesis in isolated embryonic Drosophila cells. I. Sterol-deficient eukaryotic cells. J Biol Chem. 1983;258:8503–8511. [PubMed] [Google Scholar]
- Skarnes WC, Rosen B, West AP, Koutsourakis M, Bushell W, Iyer V, Mujica AO, Thomas M, Harrow J, Cox T, et al. A conditional knockout resource for the genome-wide study of mouse gene function. Nature. 2011;474:337–342. doi: 10.1038/nature10163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski AT, Kim TK, Takeda Y, Janjetovic Z, Brozyna AA, Skobowiat C, Wang J, Postlethwaite A, Li W, Tuckey RC, et al. RORalpha and ROR gamma are expressed in human skin and serve as receptors for endogenously produced noncalcemic 20-hydroxy- and 20, 23-dihydroxyvitamin D. FASEB J. 2014 doi: 10.1096/fj.13-242040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soroosh P, Wu J, Xue X, Song J, Sutton SW, Sablad M, Yu J, Nelen MI, Liu X, Castro G, et al. Oxysterols are agonist ligands of RORgammat and drive Th17 cell differentiation. Proc Natl Acad Sci U S A. 2014 doi: 10.1073/pnas.1322807111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spann NJ, Garmire LX, McDonald JG, Myers DS, Milne SB, Shibata N, Reichart D, Fox JN, Shaked I, Heudobler D, et al. Regulated accumulation of desmosterol integrates macrophage lipid metabolism and inflammatory responses. Cell. 2012;151:138–152. doi: 10.1016/j.cell.2012.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stehlin-Gaon C, Willmann D, Zeyer D, Sanglier S, Van Dorsselaer A, Renaud JP, Moras D, Schule R. All-trans retinoic acid is a ligand for the orphan nuclear receptor ROR beta. Nat Struct Biol. 2003;10:820–825. doi: 10.1038/nsb979. [DOI] [PubMed] [Google Scholar]
- Stehlin C, Wurtz JM, Steinmetz A, Greiner E, Schule R, Moras D, Renaud JP. X-ray structure of the orphan nuclear receptor RORbeta ligand-binding domain in the active conformation. EMBO J. 2001;20:5822–5831. doi: 10.1093/emboj/20.21.5822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Z, Unutmaz D, Zou YR, Sunshine MJ, Pierani A, Brenner-Morton S, Mebius RE, Littman DR. Requirement for RORgamma in thymocyte survival and lymphoid organ development. Science. 2000;288:2369–2373. doi: 10.1126/science.288.5475.2369. [DOI] [PubMed] [Google Scholar]
- van de Pavert SA, Olivier BJ, Goverse G, Vondenhoff MF, Greuter M, Beke P, Kusser K, Hopken UE, Lipp M, Niederreither K, et al. Chemokine CXCL13 is essential for lymph node initiation and is induced by retinoic acid and neuronal stimulation. Nat Immunol. 2009;10:1193–1199. doi: 10.1038/ni.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos Q, Jones LA, Kruisbeek AM. Mice deprived of exogenous antigenic stimulation develop a normal repertoire of functional T cells. J Immunol. 1992;149:1204–1210. [PubMed] [Google Scholar]
- Wang Y, Kumar N, Crumbley C, Griffin PR, Burris TP. A second class of nuclear receptors for oxysterols: Regulation of RORalpha and RORgamma activity by 24S-hydroxycholesterol (cerebrosterol) Biochim Biophys Acta. 2010a;1801:917–923. doi: 10.1016/j.bbalip.2010.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Kumar N, Solt LA, Richardson TI, Helvering LM, Crumbley C, Garcia-Ordonez RD, Stayrook KR, Zhang X, Novick S, et al. Modulation of retinoic acid receptor-related orphan receptor alpha and gamma activity by 7-oxygenated sterol ligands. J Biol Chem. 2010b;285:5013–5025. doi: 10.1074/jbc.M109.080614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassif CA, Brownson KE, Sterner AL, Forlino A, Zerfas PM, Wilson WK, Starost MF, Porter FD. HEM dysplasia and ichthyosis are likely laminopathies and not due to 3beta-hydroxysterol Delta14-reductase deficiency. Hum Mol Genet. 2007;16:1176–1187. doi: 10.1093/hmg/ddm065. [DOI] [PubMed] [Google Scholar]
- Wostmann BS, Pleasants JR, Bealmear P, Kincade PW. Serum proteins and lymphoid tissues in germ-free mice fed a chemically defined, water soluble, low molecular weight diet. Immunology. 1970;19:443–448. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.