Summary
Exercise is the archetype of physiologic demands placed on the cardiovascular system. Acute responses provide an informative assessment of cardiovascular function and fitness, while repeated exercise promotes cardiovascular health and evokes important molecular, structural, and functional changes contributing to its effects in primary and secondary prevention. Here we examine the use of exercise in murine models, both as a phenotypic assay and as a provocative intervention. We first review the advantages and limitations of exercise testing for assessing cardiac function, then highlight the cardiac structural and cellular changes elicited by chronic exercise and key molecular pathways that mediate these effects.
Acute exercise as a cardiovascular phenotyping tool
Why use exercise to phenotype the heart? The cardiac response to exercise can be viewed as a critical functional metric by which to gauge the end result of any cardiac intervention. While resting contractile function, assessed by echocardiography, is far and away the most common functional measure used in animal studies, this is often an inadequate functional readout. For examples, in heart failure with preserved ejection fraction baseline contractile function may be normal but impaired with exercise. Direct measure of cardiac function during exercise would be ideal, but in the mouse such measurements are generally impractical. Instead, exercise capacity has often been employed as a surrogate. Given the central importance of assessing cardiac function in genetically engineer mouse models, we will review the measurement of exercise capacity and the logical premises implicit in its use as a surrogate of exercise cardiac function. We pay particular attention to the experimental factors that influence its interpretation, reproducibility, and the ability to compare measurements across studies.
The essence of the cardiac response to exercise is an increase in cardiac output elicited by an increase in workload (exercise-cardiac output). Recently, Lujan and DiCarlo have succeeded in directly measuring this response in the unrestrained, unanesthetized mouse, at rest and while running on the treadmill, noting a 2-fold rise in cardiac output with exercise (Lujan and DiCarlo, 2013). Diagnosing the cause of an inadequate exercise-cardiac output can be challenging as it could arise from pathology intrinsic or extrinsic to the heart (Rowell et al., 1996). Extra-cardiac contributors to cardiac output include the skeletal muscle pump, vascular, and nervous system responses to exercise. Furthermore, it is not yet feasible to even measure all of these component responses while a mouse is exercising. The diagnostic challenge is greatly simplified when the intervention of interest specifically targets the heart. Though it remains technically challenging to measure the intervention's effect on exercise-cardiac output, in this setting a change in exercise capacity can serve as a reasonable surrogate since it directly depends on cardiac output. The measurement of exercise capacity is much more tractable from a technical perspective, but entails a number of interpretive challenges, to which we now turn.
Exercise Capacity Measurement
The measurement of exercise capacity depends on three key elements: an exercise stimulus, an assessment of exhaustion, and a measure of exercise capacity. Examples of these might be: forced treadmill running at a fixed speed, “looks exhausted”, and total distance run, respectively. To minimize the influence of volition on exercise capacity, we only consider forced exercise stimuli, in particular treadmill running. Unfortunately there are no agreed upon standards for the three assay ingredients (Booth et al., 2010). As a result, published values of exercise capacity may be difficult to reproduce and to compare across studies that employ different protocols. Perhaps surprisingly, even within a study, different exercise protocols applied to the same mice can lead to dramatically different exercise capacities. Each of the three assay elements influences exercise capacity through rich ties to the underlying physiology, connections we summarize below.
Assessing exhaustion
Given the obvious communication barrier, assessing exhaustion in a mouse can be particularly challenging. A mouse could stop exercising due to fear, distraction, depression, laziness, joint pain, heightened perception of muscle pain, or fatigue, to name a few reasons. Ideally, an exercise protocol for assessing cardiac function would define exhaustion in such a way as to automatically eliminate all reasons for stopping except fatigue. The most common practice is to conclude an animal is exhausted when, despite prodding, it stops running for 5-10 seconds, but it's unclear whether this criterion adequately discriminates among the myriad potential causes of exercise cessation (Booth et al., 2010). Though absolute confidence in the cause of exercise cessation is impossible, investigators have put forward at least four techniques that enrich for fatigue as the cause (Copp et al., 2009; Copp et al., 2010b; Knab et al., 2009). First, treadmill acclimatization and pre-test warm-up periods, together with adequate motivation during exercise, can mollify or simply overpower psychological barriers to exercise. Second, investigators can learn the behavioral patterns a mouse exhibits on the treadmill and how it looks when tiring, judging this by inescapable analogy to human experience. Using pre-specified criteria, individual exercise bouts or even entire mouse strains should be eliminated from consideration when aberrant exercise behavior is seen or lack of overt fatiguing behavior is appreciated. Third, confidence in subjective assessments can be enhanced by employing a biochemical assay of exhaustion such as elevated circulating lactate measured from a drop of tail blood. Finally, the reproducibility of the measured exercise capacity should be assessed. Poor reproducibility suggests that multiple uncontrolled factors are contributing to the exhaustion assessment.
Exercise capacity metrics
The actual measure of exercise capacity is typically expressed either in terms of the workload performed or the workload's effect on the animal's physiology (Wasserman, 2012). Examples include total mechanical work completed (Joules), peak power achieved (Joules/sec = Watts), or peak oxygen consumption (ml O2/min). Not infrequently, surrogates of workload are reported such as total distance traveled or total time on the treadmill. However, when data on the weight of the animals or incline angle of the treadmill are omitted, it is impossible to reconstruct the actual workload performed. In these cases reported exercise capacities cannot be meaningfully compared across studies. Peak oxygen consumption (VO2) is the standard physiological measure of exercise capacity in humans, often considered the gold standard. In mice, however, it is a more expensive and technically demanding measurement. For this reason, it is reported in a minority of studies.
Workload dependence of exercise capacity
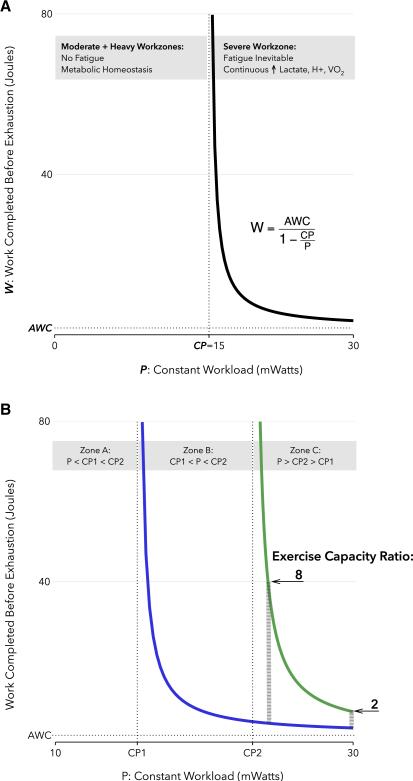
The nature of the exercise itself can have a profound influence on the measured exercise capacity, due to a nonlinearity of exercise physiology known as the critical power (CP) concept [Fig 1] (Jones et al., 2010; Morton, 2006; Poole and Jones, 2012). In treadmill running, exercise workload is determined by the incline angle and belt speed schedule, i.e. the change in treadmill speed over time. When combined with an animal's weight these protocol conditions determine its power output (in Watts). The physiology of CP is most readily illustrated by constant workload tests, wherein the treadmill speed is rapidly increased to a predetermined value and then fixed there for the duration of the test. Critical velocity (CV) is that constant speed below which exercise cessation due to fatigue does not occur. Critical power (CP) is the animal's power output at that speed. For an animal exercising below its CP, metabolic homeostasis can be maintained, as indicated by steady states of intramyocyte lactate, creatine phosphate, H+, and oxygen consumption (Jones et al., 2008; Poole et al., 1988). In theory, exertion could continue indefinitely under these conditions except for the eventual exhaustion of metabolic fuel reserves. In practice, exercise cessation below the critical power occurs due to an incompletely understood mix of influences thought unrelated to fatigue and exercise duration under these conditions is highly variable (Copp et al., 2009). In contrast, at any power output above CP fatigue will always occur. Moreover, the higher the treadmill speed is relative to the critical velocity, the fewer Joules of work that can be performed, in a hyperbolic power-work relationship (Fig 1a) (Morton, 2006). Biochemically, above the CP, intracellular metabolites such as lactate and H+ never reach a steady state, and together with oxygen consumption (VO2) they rise inexorably to some peak value at exhaustion. As illustrated in Fig 1a, a second parameter is needed to fully determine the CP curve, often termed the anaerobic work capacity (AWC). Intuitively AWC is thought to reflect the total work that can be performed using anaerobic energy stores, though precisely what it measures is an subject of ongoing investigation (Morton, 2006). These properties reflect a fundamental biochemical threshold marked by the critical power, a threshold that can be modified by exercise training as well as disease states (Jenkins and Quigley, 1992; Mezzani et al., 2010). Furthermore, while first studied in humans (Moritani et al., 1981), the critical power model has also been validated in other species (Lauderdale and Hinchcliff, 1999), including mice (Billat et al., 2005) and rats (Copp et al., 2010a).
Figure 1. The Power-Work relationship and its implications for exercise testing.
(A) Power-Work relationship. During constant power exercise at a workrate of P milliWatts, the amount of work that can be completed before exhaustion (W, the exercise capacity) is a function of the parameters P, CP, and AWC. For P < CP, termed the Moderate or Heavy workzones, W is theoretically unlimited. For P>CP, termed the Severe workzone, W follows the displayed power-work relation. The example value of CP was calculated for a 25g mouse with a critical velocity of 22m/min on a 10 degree treadmill incline; AWC was set at 2 Joules. (B) Workload dependence of relative exercise capacity between two groups of mice, treatment (green) and control (blue). The ratio of exercise capacities is a function of the imposed workload, P, displayed here for 2 different values within zone C (thick grey dashed lines). It also depends on the power-work parameters of each group: CP1, CP2, and AWC. AWC is assumed equal between groups. See text for details. CP, critical power; AWC, anaerobic work capacity.
The workload dependence of exercise capacity is most acutely illustrated when comparing two groups of mice without knowledge of their respective CP parameters. Fig 1b shows three workload zones demarcated by the critical power parameters of two hypothetical mouse groups, “treatment” and “control”. From the preceding discussion it's clear that if both groups are exercised at a fixed workload that lies in zone A, below the CP of both, neither group will fatigue and their measured exercise capacities will be subject to an unpredictable, and possibly irreproducible, set of factors. If the imposed workload is in zone B, the control mice will fatigue, but the treatment mice will not, theoretically leading to an unbounded relative exercise capacity. If the chosen workload lies in zone C, above the CP for both groups, then neither of these extreme and highly variable behaviors will occur. However, note that the measured effect size of treatment (e.g. exercise capacity ratio between the groups) differs dramatically depending on the relative magnitudes of the workload and the individual CP parameters. We have so far assumed the AWC parameter to be equal between groups. If we remove this simplifying assumption and group AWC values were to differ, then theoretically even the direction of the treatment effect could depend on the workload imposed. The alarming conclusion is that different choices of a preset constant workload could in principle result in wildly different relative measures of exercise capacity depending on the underlying exercise physiology (CP, AWC), which is typically unknown before testing (Whipp and Ward, 2009).
An incremental exercise test, wherein the treadmill speed increases over time, avoids some but not all of these pitfalls. Since the treadmill speed will eventually surpass the critical velocity of both groups, the unreliable results inherent in zone A and zone B cannot occur. However, the effect size of the treatment on exercise capacity will continue to be workload dependent, in this setting determined by the pace at which the treadmill speed increases (ramp slope) (Morton, 1994). Peak VO2, as an alternative measure of exercise capacity, is also workload dependent, but may be more robust than total work. In particular, as long as the workload is above CP, but not “excessive”, VO2 will rise to the same peak value no matter the workload (Gaesser and Poole, 1996). Unfortunately it is not completely invariant to workload above CP. In humans at least, an extremely high workload (in the so-called Extreme workzone) will rapidly lead to fatigue at a peak VO2 which is lower than that achieved at more modest workloads above CP (Poole and Jones, 2012).
The workload dependence of exercise capacity measurements makes it difficult to compare values across studies that employ different protocols. Moreover the parameters that determine workload, most commonly the treadmill incline angle and speed ramp slope, do in fact vary considerably between studies and often seem arbitrarily chosen. Perhaps the most protocol invariant measure of exercise capacity would be CP itself, together with the closely related AWC (in essence the ability to reconstruct the entire power-work curve). Although not a capacity in the traditional sense of measuring peak physical ability, CP is the maximum work rate at which a near biochemical steady state can be maintained. Despite its attractive invariance properties, CP has rarely been used for this purpose in animal studies to date (Mille-Hamard et al., 2012). Finally, its relationship to the exercise-cardiac response is worthy of further exploration (Mezzani et al., 2010).
Exercise Capacity as a Surrogate of Exercise Cardiac Output
If exercise capacity is reduced by a cardiac restricted intervention, is that sufficient evidence to conclude that peak exercise-cardiac output is reduced? While likely, there are at least two other explanations. First, the intervention could create an undesirable tradeoff between augmentation of cardiac output and another response that could itself cause the cessation of exercise. For example, if the intervention were to reduce cardiac compliance, then augmenting stroke volume would require higher filling pressures, which is commonly believed to cause shortness of breath (dyspnea) which could lead to cessation of exercise before cardiac output is maximized. Although peak cardiac output is reduced in this scenario, this would not be the primary cause of exercise intolerance but rather a consequence of stopping early (Houstis and Lewis, 2014). Second, a range of secondary non-cardiac changes that occur as a consequence of chronically reduced non-exercise cardiac output can impair exercise capacity. For example, changes in skeletal muscle oxygen extraction occur after myocardial infarction (Esposito et al., 2010) and could influence exercise capacity, clouding the quantitative interpretation of the cause of exercise intolerance. To be sure, neither of these examples impugn the causality of the intervention nor that its effect stems from the heart. They simply illustrate that there are more complex ways for the heart to influence exercise capacity beyond a reduction in exercise-cardiac output.
Does reduced cardiac output always cause exercise intolerance? In theory, this could only be avoided if there were a mechanism to compensate for the loss of cardiac output. In fact, the periphery is able to extract more oxygen when cardiac output is lower due to longer diffusion times. However, this compensation is not complete, and though buffered by the boost in extraction (Wagner, 2011), exercise capacity will still suffer,. Of course in practice, a particular exercise protocol might be relatively insensitive to modest changes in exercise cardiac output, especially given the dependence on exercise conditions discussed above.
When exercise intolerance is caused by interventions that are not heart-restricted determining the cardiac contribution is significantly more challenging since multiple other organ systems could be at play. It is therefore difficult in this setting to confidently ascribe exercise intolerance to a cardiac cause without directly measuring exercise-cardiac output. More commonly, an indirect inference is made by documenting a reduction in exercise capacity and cardiac abnormalities at rest, frequently by echocardiography. The potential pitfalls of using this approach are illustrated by a recent paper using two models of a cardiac-specific intervention, myocardial infarction and pressure overload (thoracic aortic constriction) (Richards et al., 2013).. The surrogate of exercise-cardiac output they used was isoproterenol-induced changes in cardiac output (CO) and ejection fraction (EF), as measured by MRI in anesthetized mice. As predicted, isoproterenol was effective at eliciting “cardiac reserve”, increasing both CO and EF. However, there was virtually no correlation between the isoproterenol-induced effects on these measures and the exercise capacity of the mice, and by inference no relationship with exercise-cardiac output. In humans it is well appreciated that pharmacologic stress can only recapitulate a subset of the cardiovascular response to exercise. As demonstrated by Richards et al's thorough study, these limitations appear to apply to mice as well.
As we have discussed, models of acute exercise can be powerful tools for assessing cardiac function. Exercise can be more than just a measurement tool though; sustained exercise can be a powerful modifier of cardiac performance. We now turn our discussion to the molecular, cellular, and architectural adaptations of the heart to chronic exercise training, with a particular focus on the beneficial effects of exercise and the mechanisms thought to underlie them..
Adaptations to chronic exercise
Exercise has well-documented clinical benefits in the prevention and treatment of cardiovascular disease (Lavie et al., 2009; Lawler et al., 2011; Taylor et al., 2004). Exercise both reduces numerous cardiovascular risk factors including diabetes mellitus (LeBrasseur et al., 2010), hyperlipidemia (Mannu et al., 2013), and hypertension (Pal et al., 2012), and benefits patients with existing heart disease, potentially with a similar degree of effect on mortality reduction as many drug interventions (Naci and Ioannidis, 2013). In patients with heart failure exercise improves measures of cardiac performance (Haykowsky et al., 2007), self-reported health status (Flynn et al., 2009), and outcomes including rates of re-infarction and mortality, both cardiac and all-cause (Lawler et al., 2011; O'Connor et al., 2009). These improvements are modest (O'Connor et al., 2009), which could be related to limited efficacy of such interventions, the specific protocols employed, and/or imperfect compliance. Of course, the sickest patients are least capable of vigorous exercise, which underscores the potential utility of identifying and exploiting the mediators of these benefits.
Alterations in heart structure and function induced by exercise
In animal models, where conditions can be more rigorously controlled, exercise increases resistance to and improves recovery from cardiac insults including myocardial infarction (MI), pressure overload induced by transverse aortic constriction (TAC), diabetic cardiomyopathy, and doxorubicin cardiotoxicity (Andrews Portes et al., 2009; Barboza et al., 2013; Chicco et al., 2006; Stolen et al., 2009). In this section, we will discuss the key cardiac alterations induced by exercise – from the gross tissue level down to molecular pathways (Figure 2) – and how they may contribute to the beneficial effects of exercise. For the sake of scope we will focus primarily on mechanisms intrinsic to the heart.
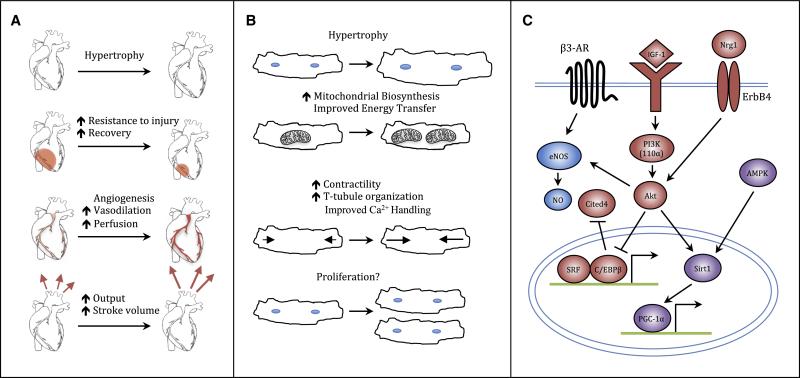
Figure 2. Responses of the heart to exercise.
(A) Organ-level responses of the heart to exercise. Exercise stimulates increases in heart size, resistance to and recovery from injury, vascularization, and output. (B) Cellular responses to exercise. Exercise stimulates cardiomyocyte hypertrophy, increases mitochondrial biosynthesis, calcium sensitivity, and contractility, and may drive cardiomyocyte proliferation. (C) Signaling pathways involved in the cardiac exercise response. Exercise activates the IGF1–PI3K–Akt cascade. Signals then converge at the level of the nucleus, resulting in inhibition of the transcription factor C/EBPβ. Down-regulation of C/EPBβ, in turn, frees serum response factor to bind target gene promoters, contributing to activation of an exercise gene set, and, ultimately, cardiac growth. Meanwhile, activation of Cited4 may drive cardiomyocyte proliferation, as does signaling through Nrg1 and ErbB4. Akt also mediates angiogenesis and vascular remodeling via eNOS and exerts beneficial metabolic effects through cross talk with AMPK, Sirt1, and PGC-1α. AMPK indicates AMP-activated kinase; β3-AR, β3 adrenergic receptor; C/EBPβ, CCAAT/enhancer binding protein β; Cited4, cbp/p300-interacting transactivator with Glu/Asp-rich carboxy-terminal domain 4; eNOS, endothelial nitric oxide synthase; IGF-1, insulin-like growth factor-1; NO, nitric oxide; Nrg1, neuregulin1; PGC-1 α, peroxisome proliferator activated receptor gamma co-activator 1α; PI3K, phosphoinositide-3 kinase; Sirt1, Sirtuin 1; SRF, serum response factor.
At the tissue level, one of the most dramatic and characteristic effects of exercise on the heart is the induction of cardiac hypertrophy, an increase in heart mass evident clinically on non-invasive imaging. In humans, exercise training can increase left ventricular mass by 20% or more (DeMaria et al., 1978). This hypertrophy is thought to result primarily from cellular hypertrophy of the cardiomyocytes themselves (Ellison et al., 2011), which may increase in size by adding new sarcomeres either in parallel (concentric hypertrophy), thus increasing cardiac wall thickness, or in series (eccentric hypertrophy), thus increasing chamber volume. Which form of hypertrophy occurs is influenced by the type of exercise: the volume challenge caused by endurance exercise characteristically results in eccentric hypertrophy, while concentric hypertrophy is more typically induced by the increased pressure against which the heart must pump during strength training.
The cardiac hypertrophy induced by exercise appears part of a beneficial remodeling process, in contrast to the cardiac hypertrophy observed in disease states such as long-standing hypertension or after myocardial infarction. Disease-associated “pathological hypertrophy” is part of an adverse remodeling process including cardiac fibrosis, electrical remodeling, and activation of a fetal gene program (Dirkx et al., 2013; Molkentin et al., 1998). In contrast exercised-induced “physiological hypertrophy”, despite morphological similarities, generally shows none of these features and is widely believed to be cardioprotective except perhaps in extreme cases such as ultra-elite athletes (La Gerche and Prior, 2007; O'Keefe et al., 2012).
This distinction between physiological and pathological hypertrophy raises the interesting question of why stress placed on the heart by exercise produces beneficial effects, while a stress such as hypertension produces adverse ones. One long-standing line of thought regarding this discordance has been that it is simply a question of degree: pathological stresses such as hypertension are generally constant and chronic, whereas physiological stresses such as exercise are only intermittent. However, pressure overload in animals produces a more pathological pattern of gene expression than exercise even when only intermittent (Perrino et al., 2006). These studies are consistent with more recent work demonstrating that distinct signaling pathways mediate cardiac growth in physiological and pathological states and are in some cases antagonistic (Boström et al., 2010; DeBosch et al., 2006; McMullen et al., 2007).
As noted above, cardiac output increases during exercise to accommodate the increased perfusion requirements of exercising muscle. This rise in output increases the three main determinants of myocardial oxygen demand: heart rate, myocardial contractility, and ventricular work. As a consequence, intense exercise can increase the left ventricular oxygen requirement by up to six-fold, which is met primarily by increased coronary blood flow and to a lesser extent by increased oxygen extraction (Duncker and Bache, 2008). While a complete discussion of the vascular effects of exercise is beyond the scope of this Perspective, it is worth highlighting the importance of key components of this vascular response. Exercise concordantly increases endothelium-dependent coronary vasodilation (Hambrecht et al., 2000), cardiac VEGF expression (Iemitsu et al., 2006), capillary angiogenesis, and vessel diameters, resulting in increased total vascular cross-sectional area (White et al., 1998) and myocardial perfusion (Hambrecht et al., 2000). In response to exercise training following MI, these improvements are associated with improved cardiac function and decreased adverse remodeling (Leosco et al., 2008).
Cellular alterations induced by exercise.
The primary function of cardiomyocytes is to rhythmically contract and relax, a process controlled at the cellular level largely by cyclically varying Ca2+ levels. Exercise improves cardiomyocyte contractility and Ca2+ sensitivity (Wisloff et al., 2001). In animals, exercise training can increase cardiomyocyte fractional shortening by up to 40-50%, while increasing the rates of both contraction and relaxation (Kemi and Wisløff, 2010). Some of these improvements are explained by an increase in the activity of the sarcoplasmic reticulum Ca2+ ATPase 2a (SERCA2a), which is primarily responsible for re-uptake of cytoplasmic calcium into the sarcoplasmic reticulum, leading to cardiomyocyte relaxation. This increase in activity results from both increased SERCA2a expression and increased phosphorylation (inhibition) of phospholambam, which binds and inhibits SERCA2a, and is in direct contrast to the decreased SERCA2a activity characteristically observed in pathological cardiac remodeling and heart failure (Fargnoli et al., 2013; Kemi et al., 2007a). These benefits are seen even in the context of cardiac injury and heart failure, where aerobic endurance training can restore contractile function, intracellular Ca2+-handling, and Ca2+-sensitivity in cardiomyocytes from rats with myocardial infarction (Wisloff et al., 2002). Exercise can also affect transverse (T)-tubules, invaginations of the plasma membrane that allow depolarization and Ca2+ entry to occur more rapidly and uniformly across the cardiomyocyte. The organization, density, or both of T-tubules have been reported to decrease in pathologic hypertrophy and cardiac dysfunction (Guo et al., 2013; Kemi et al., 2011; Louch et al., 2006; Song et al., 2006), potentially impairing excitation–contraction (EC) coupling efficiency and contractile function (Guo et al., 2013; Song et al., 2006), and these decreases can be at least partially reversed by exercise training (Kemi et al., 2011).
Powering this contractile activity requires an enormous amount of chemical energy, derived primarily from the oxidation of fatty acids. During pathological remodeling of the heart cardiomyocytes revert to a more fetal metabolic state, reducing metabolism of fatty acids and shifting back towards a reliance on glycolysis as the primary pathway for energy production (Lehman and Kelly, 2002). Pathological remodeling is also associated with decreased expression of the key mitochondrial regulator PGC-1α and its targets (Barger et al., 2000). Experiments with PGC-1α deficient mice suggest this downregulation is maladaptive, leading to reduced mitochondrial function and defects in the ability of the heart to respond to increased demand (Arany et al., 2005; Arany et al., 2006). In contrast, physiological hypertrophy is not associated with a shift to glucose utilization, and exercise upregulates PGC-1α, AMPK, and mitochondrial biogenesis (Vettor et al., 2014). Interestingly, exercising rats after MI restored cardiac energy metabolism, mainly at the level of energy transfer: exercise training corrected depressed expression of adenylate kinase, restored creatine kinase activity levels, and partially restored citrate synthase activity (Kemi et al., 2007b).
In addition to improving the function of existing cardiomyocytes, recent work raises the possibility that exercise may also stimulate cardiomyogenesis in the adult heart. There is a measureable, albeit low, rate of formation of new cardiomyocytes in adult mammalian hearts (Ali et al., 2014; Bergmann et al., 2009; Senyo et al., 2013), which appears to increase after MI, particularly in the peri-infarct region (Senyo et al., 2013; Smart et al., 2011). We found that an intensive swimming protocol in mice not only induced cardiomyocyte and cardiac hypertrophy but also increased markers of proliferation in cardiomyocytes including PCNA expression, positivity for Ki67, pHH3, and Aurora B kinase staining, and BrdU incorporation, while others have reported that swimming or treadmill running increases the number and activation of c-kit+ and Sca-1+ cells, putative cardiac progenitor/stem cell populations, and induces the formation of small, mononuclear BrdU+ cardiomyocytes (Boström et al., 2010; Waring et al., 2014; Xiao et al., 2013). Exercise has also been found to increase cardiac mRNA and protein levels of neuregulin and periostin, which have been reported by some to induce cardiomyocytes to re-enter the cell cycle, although these findings have not been consistently confirmed (Bersell et al., 2009; Kühn et al., 2007; Lorts et al., 2009; Waring et al., 2014). The number, origin, and fate of any new cardiomyocytes formed in response to exercise remains unclear and will require more detailed cell fate-mapping studies to address.
Molecular mechanisms mediating the benefits of exercise
Exercise triggers increased IGF-1 and neuregulin production in a variety of tissues, including the heart, leading to increased autocrine and paracrine signaling (Frystyk, 2009; Neri Serneri et al., 2001). Increased cardiac IGF-1 signaling appears to be a strong driver of physiological hypertrophy, as cardiac-specific IGF-1 transgenic mice develop larger hearts during adulthood with a greater number of cardiomyocytes and cardiac-specific overexpression of IGF-1's tyrosine kinase receptor IGF-1R results in increased chamber weight, increased myocyte size, and improved systolic function without histological signs of pathology (McMullen et al., 2004; Reiss et al., 1996). IGF-1 overexpression also protects mice against a broad range of cardiac insults including diabetic cardiomyopathy, angiotensin II–mediated oxidative stress, chronic ischemia, and ischemia-reperfusion injury (Kajstura et al., 2001; Li et al., 1999; Yamashita et al., 2001). Neuregulin-1 (NRG-1) signaling through the ErbB family of tyrosine kinase receptors produces a similar spectrum of cardioprotective effects, which has prompted multiple clinical trials evaluating the use of recombinant NRG-1 fragments for heart failure treatment (Odiete et al., 2012). In addition to being sufficient to recapitulate many of the cardiac benefits of exercise, IGF-1 signaling is necessary for exercise-induced cardiac hypertrophy as demonstrated by swim training of cardiac-specific IGF-1R knockout mice (Kim et al., 2008).
IGF-1R activates PI3-kinase (PI3K), a family of heterodimeric kinases composed of regulatory and catalytic subunits, of which the PI3K (110α) isoform appears necessary and sufficient for mediating the cardiac effects of exercise and IGF-1 signaling (Matsui et al., 2002; McMullen et al., 2004). Cardiac-specific expression of dominant-negative PI3K (110α) (dnPI3K) inhibited cardiac growth in response to exercise but did not affect pathological hypertrophy in response to TAC (McMullen et al., 2003). Interestingly, dnPI3K also abolished exercise-induced protection against pressure overload, while expression of constitutively-active PI3K (110α) protected the mice regardless of exercise status, suggesting that PI3K (110α) is necessary and sufficient for exercise-induced protection against adverse remodeling in this setting (Weeks et al., 2012).
The serine-threonine protein kinase Akt1 functions downstream of PI3K to mediate physiological hypertrophy and many of the cardiac benefits of exercise. Interestingly, germline Akt1 knockout mice are resistant to swimming-induced cardiac growth while exhibiting an exaggerated hypertrophic response to TAC, suggesting Akt1 is necessary for physiological cardiac hypertrophy but antagonizes pathological hypertrophy (DeBosch et al., 2006). Akt1 is also sufficient to protect cardiomyocytes against apoptosis and preserve function in vitro and in vivo (Matsui et al., 1999; Matsui et al., 2001).
With regard to the effects of exercise, an important factor downstream of Akt1 appears to the transcription factor C/EBPβ, as we found when performing a genome-wide expression analysis of all transcriptional components in exercised hearts compared to hearts post-TAC (Boström et al., 2010). Exercise downregulated cardiomyocyte C/EBPβ expression, as did overexpression of Akt1 in vitro, while C/EBPβ overexpression blocked Akt1-induced expression of genes characteristic of physiological hypertrophy. Mimicking this reduction in C/EBPβ genetically resulted in a phenocopy of endurance exercise, and mice heterozygous for C/EBPβ were also resistant to heart failure after pressure overload (Boström et al., 2010). These effects of C/EBPβ appear to be at least in part through regulating expression of CBP/p300-interacting transactivator with ED-rich carboxy-terminal domain 4 (CITED4), though the mechanism downstream of Cited4 is as yet unknown (Boström et al., 2010).
Sirtuins, a family of nicotinamide adenine dinucleotide (NAD)-dependent deacetylases, have also been implicated in mediating the cardiac effects of exercise. Of the seven sirtuins found in mammals, Sirt1 and Sirt3 are the best studied with regard to the cardiac effects of exercise. Both are upregulated in the heart by exercise (Lai et al., 2014; Sundaresan et al., 2009). Sirt1 activates Akt1 and PGC-1α among other pathways and protects mice against cardiac ischemia/reperfusion injury, while Sirt3 protects against cardiac oxidative stress in a Foxo3a-dependent manner, regulates cardiomyocyte metabolism through activation of AMPK and PGC-1α, and causes resistance to angiotensin-II driven cardiac hypertrophy and fibrosis (Ferrara et al., 2008; Hsu et al., 2010; Pillai et al., 2010; Sundaresan et al., 2009; Sundaresan et al., 2010).
The protection which exercise training provides against ischemic injury also appears to occur through regulation of endothelial nitric oxide synthase (eNOS). Exercise results in increased circulating catecholamines such as epinephrine and norepinephrine, which act on β3-adrenergic receptors to increase eNOS phosphorylation and activity, in turn increasing the bioavailability of the NO metabolites nitrite and nitrosothiol (Calvert et al., 2011). Phosphorylation of eNOS at serine reside 1177, which increases its activity, is also promoted by Akt and AMPK in response to treadmill running (Zhang et al., 2009). Increased eNOS expression attenuates congestive heart failure in mice, while mice with heterozygous or homozygous knockout of eNOS do not experience the cardiac benefits of exercise after MI observed in wild-type mice (Calvert et al., 2011), although the later result is potentially confounded by a reduction in the exercise levels achieved by the knockout mice (Bezzerides and Rosenzweig, 2011; de Waard et al., 2010; Jones et al., 2003).
Interesting results have also begun to emerge implicating miRNAs in the regulation of cardiac hypertrophy and response to exercise. Both cardiac and circulating miRNA levels are regulated in response to exercise (Baggish et al., 2014; Ma et al., 2013), and in vitro screening has identified miRNAs with pro- and anti-hypertrophic effects on neonatal rat cardiomyocytes (Jentzsch et al., 2012). However, experiments testing the necessity and sufficiency of the observed changes in miRNA levels for the various phenotypic effects of exercise have so far been scarce, and this will likely be a productive area for future work.
Conclusion
As we have highlighted, the study of exercise has yielded many insights into cardiac biology, particularly with regard to the mechanisms by which the heart responds to stress. Exercise studies complement studies of disease, and when used as platforms for unbiased screening, exercise models will likely generate candidate gene lists largely distinct from those produced by studies of cardiac pathologies. To maximize the reliability of future exercise studies and the extent to which their results can be productively compared to other data sets, we urge detailed reporting of exercise protocols and validation, taking into account the design criteria and limits discussed above. Ultimately a deeper understanding of how the beneficial cardiac responses to physiological stress differ from and protect against pathological stressors may provide a foundation for new therapeutic approaches for the treatment of heart disease. While the multifactorial nature of the exercise response suggests that developing a simple “exercise in a pill” treatment may not be feasible, even partial recapitulation of the benefits of exercise by pharmacological intervention could provide an important new component of treatment for patients too sick to exercise.
Acknowledgements
This work was supported by grants from the NIH (AR[R01HL110733, R01HL122987], XL[T32HL073734], and CP[T32GM007226]). NH was supported by a LaDue Fellowship Award from Harvard Medical School. AR is a principal faculty member of the Harvard Stem Cell Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ali SR, Hippenmeyer S, Saadat LV, Luo L, Weissman IL, Ardehali R. Existing cardiomyocytes generate cardiomyocytes at a low rate after birth in mice. Proc Natl Acad Sci U S A. 2014;111:8850–8855. doi: 10.1073/pnas.1408233111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews Portes L, Magalhaes Saraiva R, Alberta Dos Santos A, Tucci PJ. Swimming training attenuates remodeling, contractile dysfunction and congestive heart failure in rats with moderate and large myocardial infarctions. Clin Exp Pharmacol Physiol. 2009;36:394–399. doi: 10.1111/j.1440-1681.2008.05070.x. [DOI] [PubMed] [Google Scholar]
- Arany Z, He H, Lin J, Hoyer K, Handschin C, Toka O, Ahmad F, Matsui T, Chin S, Wu P-HH, et al. Transcriptional coactivator PGC-1 alpha controls the energy state and contractile function of cardiac muscle. Cell metabolism. 2005;1:259–271. doi: 10.1016/j.cmet.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Arany Z, Novikov M, Chin S, Ma Y, Rosenzweig A, Spiegelman BM. Transverse aortic constriction leads to accelerated heart failure in mice lacking PPAR-gamma coactivator 1alpha. Proc Natl Acad Sci U S A. 2006;103:10086–10091. doi: 10.1073/pnas.0603615103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baggish AL, Park J, Min PK, Isaacs S, Parker BA, Thompson PD, Troyanos C, D'Hemecourt P, Dyer S, Thiel M, et al. Rapid upregulation and clearance of distinct circulating microRNAs after prolonged aerobic exercise. Journal of applied physiology. 2014;116:522–531. doi: 10.1152/japplphysiol.01141.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barboza CA, Rocha LY, Mostarda CT, Figueroa D, Caperuto EC, De Angelis K, Irigoyen MC, Rodrigues B. Ventricular and autonomic benefits of exercise training persist after detraining in infarcted rats. Eur J Appl Physiol. 2013;113:1137–1146. doi: 10.1007/s00421-012-2533-3. [DOI] [PubMed] [Google Scholar]
- Barger PM, Brandt JM, Leone TC, Weinheimer CJ, Kelly DP. Deactivation of peroxisome proliferator-activated receptor-alpha during cardiac hypertrophic growth. J Clin Invest. 2000;105:1723–1730. doi: 10.1172/JCI9056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnabe-Heider F, Walsh S, Zupicich J, Alkass K, Buchholz BA, Druid H, et al. Evidence for cardiomyocyte renewal in humans. Science. 2009;324:98–102. doi: 10.1126/science.1164680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bersell K, Arab S, Haring B, Kühn B. Neuregulin1/ErbB4 signaling induces cardiomyocyte proliferation and repair of heart injury. Cell. 2009;138:257–270. doi: 10.1016/j.cell.2009.04.060. [DOI] [PubMed] [Google Scholar]
- Bezzerides V, Rosenzweig A. Saying yes to exercise and NO to cardiac injury. Circ Res. 2011;108:1414–1416. doi: 10.1161/CIRCRESAHA.111.247122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billat VL, Mouisel E, Roblot N, Melki J. Inter- and intrastrain variation in mouse critical running speed. Journal of applied physiology. 2005;98:1258–1263. doi: 10.1152/japplphysiol.00991.2004. [DOI] [PubMed] [Google Scholar]
- Booth FW, Laye MJ, Spangenburg EE. Gold standards for scientists who are conducting animal-based exercise studies. Journal of applied physiology. 2010;108:219–221. doi: 10.1152/japplphysiol.00125.2009. [DOI] [PubMed] [Google Scholar]
- Boström P, Mann N, Wu J, Quintero PA, Plovie ER, Panáková D, Gupta RK, Xiao C, MacRae CA, Rosenzweig A, et al. C/EBPβ controls exercise-induced cardiac growth and protects against pathological cardiac remodeling. Cell. 2010;143:1072–1083. doi: 10.1016/j.cell.2010.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvert JW, Condit ME, Aragon JP, Nicholson CK, Moody BF, Hood RL, Sindler AL, Gundewar S, Seals DR, Barouch LA, et al. Exercise protects against myocardial ischemia-reperfusion injury via stimulation of beta(3)-adrenergic receptors and increased nitric oxide signaling: role of nitrite and nitrosothiols. Circ Res. 2011;108:1448–1458. doi: 10.1161/CIRCRESAHA.111.241117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chicco AJ, Hydock DS, Schneider CM, Hayward R. Low-intensity exercise training during doxorubicin treatment protects against cardiotoxicity. Journal of applied physiology (Bethesda, Md : 1985) 2006;100:519–527. doi: 10.1152/japplphysiol.00148.2005. [DOI] [PubMed] [Google Scholar]
- Copp SW, Davis RT, Poole DC, Musch TI. Reproducibility of endurance capacity and VO2peak in male Sprague-Dawley rats. Journal of applied physiology. 2009;106:1072–1078. doi: 10.1152/japplphysiol.91566.2008. [DOI] [PubMed] [Google Scholar]
- Copp SW, Hirai DM, Musch TI, Poole DC. Critical speed in the rat: implications for hindlimb muscle blood flow distribution and fibre recruitment. The Journal of physiology. 2010a;588:5077–5087. doi: 10.1113/jphysiol.2010.198382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copp SW, Poole DC, Musch TI. Valid and reproducible endurance protocols underlie data interpretation, integration, and application. Journal of applied physiology. 2010b;108:224–225. doi: 10.1152/japplphysiol.01233.2009. author reply 226. [DOI] [PubMed] [Google Scholar]
- de Waard MC, van Haperen R, Soullié T, Tempel D, de Crom R, Duncker DJ. Beneficial effects of exercise training after myocardial infarction require full eNOS expression. Journal of molecular and cellular cardiology. 2010;48:1041–1049. doi: 10.1016/j.yjmcc.2010.02.005. [DOI] [PubMed] [Google Scholar]
- DeBosch B, Treskov I, Lupu TS, Weinheimer C, Kovacs A, Courtois M, Muslin AJ. Akt1 is required for physiological cardiac growth. Circulation. 2006;113:2097–2104. doi: 10.1161/CIRCULATIONAHA.105.595231. [DOI] [PubMed] [Google Scholar]
- DeMaria AN, Neumann A, Lee G, Fowler W, Mason DT. Alterations in ventricular mass and performance induced by exercise training in man evaluated by echocardiography. Circulation. 1978;57:237–244. doi: 10.1161/01.cir.57.2.237. [DOI] [PubMed] [Google Scholar]
- Dirkx E, da Costa Martins PA, De Windt LJ. Regulation of fetal gene expression in heart failure. Biochim Biophys Acta. 2013;1832:2414–2424. doi: 10.1016/j.bbadis.2013.07.023. [DOI] [PubMed] [Google Scholar]
- Duncker DJ, Bache RJ. Regulation of coronary blood flow during exercise. Physiol Rev. 2008;88:1009–1086. doi: 10.1152/physrev.00045.2006. [DOI] [PubMed] [Google Scholar]
- Ellison GM, Waring CD, Vicinanza C, Torella D. Physiological cardiac remodelling in response to endurance exercise training: cellular and molecular mechanisms. Heart (British Cardiac Society) 2011;98:5–10. doi: 10.1136/heartjnl-2011-300639. [DOI] [PubMed] [Google Scholar]
- Esposito F, Mathieu-Costello O, Shabetai R, Wagner PD, Richardson RS. Limited maximal exercise capacity in patients with chronic heart failure: partitioning the contributors. Journal of the American College of Cardiology. 2010;55:1945–1954. doi: 10.1016/j.jacc.2009.11.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fargnoli AS, Katz MG, Yarnall C, Isidro A, Petrov M, Steuerwald N, Ghosh S, Richardville KC, Hillesheim R, Williams RD, et al. Cardiac surgical delivery of the sarcoplasmic reticulum calcium ATPase rescues myocytes in ischemic heart failure. The Annals of thoracic surgery. 2013;96:586–595. doi: 10.1016/j.athoracsur.2013.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara N, Rinaldi B, Corbi G, Conti V, Stiuso P, Boccuti S, Rengo G, Rossi F, Filippelli A. Exercise training promotes SIRT1 activity in aged rats. Rejuvenation research. 2008;11:139–150. doi: 10.1089/rej.2007.0576. [DOI] [PubMed] [Google Scholar]
- Flynn KE, Piña IL, Whellan DJ, Lin L, Blumenthal JA, Ellis SJ, Fine LJ, Howlett JG, Keteyian SJ, Kitzman DW, et al. Effects of exercise training on health status in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA : the journal of the American Medical Association. 2009;301:1451–1459. doi: 10.1001/jama.2009.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frystyk J. Exercise and the growth hormone-insulin-like growth factor axis. Medicine and science in sports and exercise. 2009;42:58–66. doi: 10.1249/MSS.0b013e3181b07d2d. [DOI] [PubMed] [Google Scholar]
- Gaesser GA, Poole DC. The slow component of oxygen uptake kinetics in humans. Exercise and sport sciences reviews. 1996;24:35–71. [PubMed] [Google Scholar]
- Guo A, Zhang C, Wei S, Chen B, Song LS. Emerging mechanisms of T-tubule remodelling in heart failure. Cardiovasc Res. 2013;98:204–215. doi: 10.1093/cvr/cvt020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hambrecht R, Wolf A, Gielen S, Linke A, Hofer J, Erbs S, Schoene N, Schuler G. Effect of exercise on coronary endothelial function in patients with coronary artery disease. The New England journal of medicine. 2000;342:454–460. doi: 10.1056/NEJM200002173420702. [DOI] [PubMed] [Google Scholar]
- Haykowsky MJ, Liang Y, Pechter D, Jones LW, McAlister FA, Clark AM. A meta-analysis of the effect of exercise training on left ventricular remodeling in heart failure patients: the benefit depends on the type of training performed. Journal of the American College of Cardiology. 2007;49:2329–2336. doi: 10.1016/j.jacc.2007.02.055. [DOI] [PubMed] [Google Scholar]
- Houstis NE, Lewis GD. Causes of exercise intolerance in heart failure with preserved ejection fraction: searching for consensus. Journal of cardiac failure. 2014;20:762–778. doi: 10.1016/j.cardfail.2014.07.010. [DOI] [PubMed] [Google Scholar]
- Hsu C-PP, Zhai P, Yamamoto T, Maejima Y, Matsushima S, Hariharan N, Shao D, Takagi H, Oka S, Sadoshima J. Silent information regulator 1 protects the heart from ischemia/reperfusion. Circulation. 2010;122:2170–2182. doi: 10.1161/CIRCULATIONAHA.110.958033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iemitsu M, Maeda S, Jesmin S, Otsuki T, Miyauchi T. Exercise training improves aging-induced downregulation of VEGF angiogenic signaling cascade in hearts. Am J Physiol Heart Circ Physiol. 2006;291:H1290–1298. doi: 10.1152/ajpheart.00820.2005. [DOI] [PubMed] [Google Scholar]
- Ingwall JS, Weiss RG. Is the failing heart energy starved? On using chemical energy to support cardiac function. Circulation research. 2004 doi: 10.1161/01.RES.0000137170.41939.d9. [DOI] [PubMed] [Google Scholar]
- Jenkins DG, Quigley BM. Endurance training enhances critical power. Medicine and science in sports and exercise. 1992;24:1283–1289. [PubMed] [Google Scholar]
- Jentzsch C, Leierseder S, Loyer X, Flohrschutz I, Sassi Y, Hartmann D, Thum T, Laggerbauer B, Engelhardt S. A phenotypic screen to identify hypertrophy-modulating microRNAs in primary cardiomyocytes. J Mol Cell Cardiol. 2012;52:13–20. doi: 10.1016/j.yjmcc.2011.07.010. [DOI] [PubMed] [Google Scholar]
- Jones AM, Vanhatalo A, Burnley M, Morton RH, Poole DC. Critical power: implications for determination of V O2max and exercise tolerance. Medicine and science in sports and exercise. 2010;42:1876–1890. doi: 10.1249/MSS.0b013e3181d9cf7f. [DOI] [PubMed] [Google Scholar]
- Jones AM, Wilkerson DP, DiMenna F, Fulford J, Poole DC. Muscle metabolic responses to exercise above and below the “critical power” assessed using 31P-MRS. American journal of physiology Regulatory, integrative and comparative physiology. 2008;294:R585–593. doi: 10.1152/ajpregu.00731.2007. [DOI] [PubMed] [Google Scholar]
- Jones SP, Greer JJ, van Haperen R, Duncker DJ, de Crom R, Lefer DJ. Endothelial nitric oxide synthase overexpression attenuates congestive heart failure in mice. Proc Natl Acad Sci U S A. 2003;100:4891–4896. doi: 10.1073/pnas.0837428100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajstura J, Fiordaliso F, Andreoli AM, Li B, Chimenti S, Medow MS, Limana F, Nadal-Ginard B, Leri A, Anversa P. IGF-1 overexpression inhibits the development of diabetic cardiomyopathy and angiotensin II-mediated oxidative stress. Diabetes. 2001;50:1414–1424. doi: 10.2337/diabetes.50.6.1414. [DOI] [PubMed] [Google Scholar]
- Kemi OJ, Ellingsen O, Ceci M, Grimaldi S, Smith GL, Condorelli G, Wisloff U. Aerobic interval training enhances cardiomyocyte contractility and Ca2+ cycling by phosphorylation of CaMKII and Thr-17 of phospholamban. J Mol Cell Cardiol. 2007a;43:354–361. doi: 10.1016/j.yjmcc.2007.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemi OJ, Hoydal MA, Haram PM, Garnier A, Fortin D, Ventura-Clapier R, Ellingsen O. Exercise training restores aerobic capacity and energy transfer systems in heart failure treated with losartan. Cardiovasc Res. 2007b;76:91–99. doi: 10.1016/j.cardiores.2007.06.008. [DOI] [PubMed] [Google Scholar]
- Kemi OJ, Hoydal MA, Macquaide N, Haram PM, Koch LG, Britton SL, Ellingsen O, Smith GL, Wisloff U. The effect of exercise training on transverse tubules in normal, remodeled, and reverse remodeled hearts. Journal of cellular physiology. 2011;226:2235–2243. doi: 10.1002/jcp.22559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemi OJ, Wisløff U. Mechanisms of exercise-induced improvements in the contractile apparatus of the mammalian myocardium. Acta physiologica (Oxford, England) 2010;199:425–439. doi: 10.1111/j.1748-1716.2010.02132.x. [DOI] [PubMed] [Google Scholar]
- Kim J, Wende AR, Sena S, Theobald HA, Soto J, Sloan C, Wayment BE, Litwin SE, Holzenberger M, LeRoith D, et al. Insulin-like growth factor I receptor signaling is required for exercise-induced cardiac hypertrophy. Molecular endocrinology. 2008;22:2531–2543. doi: 10.1210/me.2008-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knab AM, Bowen RS, Moore-Harrison T, Hamilton AT, Turner MJ, Lightfoot JT. Repeatability of exercise behaviors in mice. Physiology & behavior. 2009;98:433–440. doi: 10.1016/j.physbeh.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn B, del Monte F, Hajjar RJ, Chang Y-SS, Lebeche D, Arab S, Keating MT. Periostin induces proliferation of differentiated cardiomyocytes and promotes cardiac repair. Nature medicine. 2007;13:962–969. doi: 10.1038/nm1619. [DOI] [PubMed] [Google Scholar]
- La Gerche A, Prior DL. Exercise--is it possible to have too much of a good thing? Heart, lung & circulation. 2007;16(Suppl 3):S102–104. doi: 10.1016/j.hlc.2007.03.014. [DOI] [PubMed] [Google Scholar]
- Lai CH, Ho TJ, Kuo WW, Day CH, Pai PY, Chung LC, Liao PH, Lin FH, Wu ET, Huang CY. Exercise training enhanced SIRT1 longevity signaling replaces the IGF1 survival pathway to attenuate aging-induced rat heart apoptosis. Age. 2014;36:9706. doi: 10.1007/s11357-014-9706-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauderdale MA, Hinchcliff KW. Hyperbolic relationship between time-to-fatigue and workload. Equine veterinary journal. 1999;(Supplement):586–590. doi: 10.1111/j.2042-3306.1999.tb05289.x. [DOI] [PubMed] [Google Scholar]
- Lavie CJ, Thomas RJ, Squires RW, Allison TG, Milani RV. Exercise training and cardiac rehabilitation in primary and secondary prevention of coronary heart disease. Mayo Clin Proc. 2009;84:373–383. doi: 10.1016/S0025-6196(11)60548-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawler PR, Filion KB, Eisenberg MJ. Efficacy of exercise-based cardiac rehabilitation post-myocardial infarction: a systematic review and meta-analysis of randomized controlled trials. American heart journal. 2011;162:571–58400. doi: 10.1016/j.ahj.2011.07.017. [DOI] [PubMed] [Google Scholar]
- LeBrasseur NK, Walsh K, Arany Z. Metabolic benefits of resistance training and fast glycolytic skeletal muscle. American journal of physiology Endocrinology and metabolism. 2010;300:10. doi: 10.1152/ajpendo.00512.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman JJ, Kelly DP. Transcriptional activation of energy metabolic switches in the developing and hypertrophied heart. Clin Exp Pharmacol Physiol. 2002;29:339–345. doi: 10.1046/j.1440-1681.2002.03655.x. [DOI] [PubMed] [Google Scholar]
- Leosco D, Rengo G, Iaccarino G, Golino L, Marchese M, Fortunato F, Zincarelli C, Sanzari E, Ciccarelli M, Galasso G, et al. Exercise promotes angiogenesis and improves beta-adrenergic receptor signalling in the post-ischaemic failing rat heart. Cardiovasc Res. 2008;78:385–394. doi: 10.1093/cvr/cvm109. [DOI] [PubMed] [Google Scholar]
- Li B, Setoguchi M, Wang X, Andreoli AM, Leri A, Malhotra A, Kajstura J, Anversa P. Insulin-like growth factor-1 attenuates the detrimental impact of nonocclusive coronary artery constriction on the heart. Circ Res. 1999;84:1007–1019. doi: 10.1161/01.res.84.9.1007. [DOI] [PubMed] [Google Scholar]
- Lorts A, Schwanekamp JA, Elrod JW, Sargent MA, Molkentin JD. Genetic manipulation of periostin expression in the heart does not affect myocyte content, cell cycle activity, or cardiac repair. Circ Res. 2009;104:e1–7. doi: 10.1161/CIRCRESAHA.108.188649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louch WE, Mork HK, Sexton J, Stromme TA, Laake P, Sjaastad I, Sejersted OM. T-tubule disorganization and reduced synchrony of Ca2+ release in murine cardiomyocytes following myocardial infarction. The Journal of physiology. 2006;574:519–533. doi: 10.1113/jphysiol.2006.107227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lujan HL, DiCarlo SE. Cardiac output, at rest and during exercise, before and during myocardial ischemia, reperfusion, and infarction in conscious mice. American journal of physiology Regulatory, integrative and comparative physiology. 2013;304:R286–295. doi: 10.1152/ajpregu.00517.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z, Qi J, Meng S, Wen B, Zhang J. Swimming exercise training-induced left ventricular hypertrophy involves microRNAs and synergistic regulation of the PI3K/AKT/mTOR signaling pathway. European journal of applied physiology. 2013;113:2473–2486. doi: 10.1007/s00421-013-2685-9. [DOI] [PubMed] [Google Scholar]
- Mannu GS, Zaman MJ, Gupta A, Rehman HU, Myint PK. Evidence of lifestyle modification in the management of hypercholesterolemia. Current cardiology reviews. 2013;9:2–14. doi: 10.2174/157340313805076313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui T, Li L, MonteF d., Fukui Y, Franke TF, Hajjar RJ, Rosenzweig A. Adenoviral gene transfer of activated phosphatidylinositol 3'-kinase and Akt inhibits apoptosis of hypoxic cardiomyocytes in vitro. Circulation. 1999;100:2373–2379. doi: 10.1161/01.cir.100.23.2373. [DOI] [PubMed] [Google Scholar]
- Matsui T, Nagoshi T, Rosenzweig A. Akt and PI 3-kinase signaling in cardiomyocyte hypertrophy and survival. Cell cycle (Georgetown, Tex) 2002;2:220–223. [PubMed] [Google Scholar]
- Matsui T, Tao J, del Monte F, Lee K-H, Li L, Picard M, Force TL, Franke TF, Hajjar RJ, Rosenzweig A. Akt Activation Preserves Cardiac Function and Prevents Injury After Transient Cardiac Ischemia In Vivo. Circulation. 2001;104:330–335. doi: 10.1161/01.cir.104.3.330. [DOI] [PubMed] [Google Scholar]
- McMullen JR, Amirahmadi F, Woodcock EA, Schinke-Braun M, Bouwman RD, Hewitt KA, Mollica JP, Zhang L, Zhang Y, Shioi T, et al. Protective effects of exercise and phosphoinositide 3-kinase(p110alpha) signaling in dilated and hypertrophic cardiomyopathy. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:612–617. doi: 10.1073/pnas.0606663104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMullen JR, Shioi T, Huang W-YY, Zhang L, Tarnavski O, Bisping E, Schinke M, Kong S, Sherwood MC, Brown J, et al. The insulin-like growth factor 1 receptor induces physiological heart growth via the phosphoinositide 3-kinase(p110alpha) pathway. The Journal of biological chemistry. 2004;279:4782–4793. doi: 10.1074/jbc.M310405200. [DOI] [PubMed] [Google Scholar]
- McMullen JR, Shioi T, Zhang L, Tarnavski O, Sherwood MC, Kang PM, Izumo S. Phosphoinositide 3-kinase(p110alpha) plays a critical role for the induction of physiological, but not pathological, cardiac hypertrophy. Proc Natl Acad Sci U S A. 2003;100:12355–12360. doi: 10.1073/pnas.1934654100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezzani A, Corra U, Giordano A, Colombo S, Psaroudaki M, Giannuzzi P. Upper intensity limit for prolonged aerobic exercise in chronic heart failure. Medicine and science in sports and exercise. 2010;42:633–639. doi: 10.1249/MSS.0b013e3181bdc69d. [DOI] [PubMed] [Google Scholar]
- Mille-Hamard L, Billat VL, Henry E, Bonnamy B, Joly F, Benech P, Barrey E. Skeletal muscle alterations and exercise performance decrease in erythropoietin-deficient mice: a comparative study. BMC medical genomics. 2012;5:29. doi: 10.1186/1755-8794-5-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molkentin JD, Lu JR, Antos CL, Markham B, Richardson J, Robbins J, Grant SR, Olson EN. A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell. 1998;93:215–228. doi: 10.1016/s0092-8674(00)81573-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moritani T, Nagata A, deVries HA, Muro M. Critical power as a measure of physical work capacity and anaerobic threshold. Ergonomics. 1981;24:339–350. doi: 10.1080/00140138108924856. [DOI] [PubMed] [Google Scholar]
- Morton RH. Critical power test for ramp exercise. European journal of applied physiology and occupational physiology. 1994;69:435–438. doi: 10.1007/BF00865408. [DOI] [PubMed] [Google Scholar]
- Morton RH. The critical power and related whole-body bioenergetic models. European journal of applied physiology. 2006;96:339–354. doi: 10.1007/s00421-005-0088-2. [DOI] [PubMed] [Google Scholar]
- Naci H, Ioannidis JP. Comparative effectiveness of exercise and drug interventions on mortality outcomes: metaepidemiological study. Bmj. 2013;347:f5577. doi: 10.1136/bmj.f5577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neri Serneri GG, Boddi M, Modesti PA, Cecioni I, Coppo M, Padeletti L, Michelucci A, Colella A, Galanti G. Increased cardiac sympathetic activity and insulin-like growth factor-I formation are associated with physiological hypertrophy in athletes. Circulation research. 2001;89:977–982. doi: 10.1161/hh2301.100982. [DOI] [PubMed] [Google Scholar]
- O'Connor CM, Whellan DJ, Lee KL, Keteyian SJ, Cooper LS, Ellis SJ, Leifer ES, Kraus WE, Kitzman DW, Blumenthal JA, et al. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA : the journal of the American Medical Association. 2009;301:1439–1450. doi: 10.1001/jama.2009.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Keefe JH, Patil HR, Lavie CJ, Magalski A, Vogel RA, McCullough PA. Potential adverse cardiovascular effects from excessive endurance exercise. Mayo Clin Proc. 2012;87:587–595. doi: 10.1016/j.mayocp.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odiete O, Hill MF, Sawyer DB. Neuregulin in cardiovascular development and disease. Circulation research. 2012;111:1376–1385. doi: 10.1161/CIRCRESAHA.112.267286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal S, Radavelli-Bagatini S, Ho S. Potential benefits of exercise on blood pressure and vascular function. Journal of the American Society of Hypertension : JASH. 2012;7:494–506. doi: 10.1016/j.jash.2013.07.004. [DOI] [PubMed] [Google Scholar]
- Perrino C, Naga Prasad SV, Mao L, Noma T, Yan Z, Kim H-SS, Smithies O, Rockman HA. Intermittent pressure overload triggers hypertrophy-independent cardiac dysfunction and vascular rarefaction. The Journal of clinical investigation. 2006;116:1547–1560. doi: 10.1172/JCI25397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai VB, Sundaresan NR, Jeevanandam V, Gupta MP. Mitochondrial SIRT3 and heart disease. Cardiovascular research. 2010;88:250–256. doi: 10.1093/cvr/cvq250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole DC, Jones AM. Oxygen uptake kinetics. Comprehensive Physiology. 2012;2:933–996. doi: 10.1002/cphy.c100072. [DOI] [PubMed] [Google Scholar]
- Poole DC, Ward SA, Gardner GW, Whipp BJ. Metabolic and respiratory profile of the upper limit for prolonged exercise in man. Ergonomics. 1988;31:1265–1279. doi: 10.1080/00140138808966766. [DOI] [PubMed] [Google Scholar]
- Reiss K, Cheng W, Ferber A, Kajstura J, Li P, Li B, Olivetti G, Homcy CJ, Baserga R, Anversa P. Overexpression of insulin-like growth factor-1 in the heart is coupled with myocyte proliferation in transgenic mice. Proc Natl Acad Sci U S A. 1996;93:8630–8635. doi: 10.1073/pnas.93.16.8630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards DA, Bao W, Rambo MV, Burgert M, Jucker BM, Lenhard SC. Examining the relationship between exercise tolerance and isoproterenol-based cardiac reserve in murine models of heart failure. Journal of applied physiology. 2013;114:1202–1210. doi: 10.1152/japplphysiol.00556.2012. [DOI] [PubMed] [Google Scholar]
- Rowell LB, Shepherd JT, American Physiological Society (1887- ) Exercise : regulation and integration of multiple systems. Published for the American Physiological Society by Oxford University Press; New York: 1996. Ch. 17. [Google Scholar]
- Senyo SE, Steinhauser ML, Pizzimenti CL, Yang VK, Cai L, Wang M, Wu T-DD, Guerquin-Kern J-LL, Lechene CP, Lee RT. Mammalian heart renewal by pre-existing cardiomyocytes. Nature. 2013;493:433–436. doi: 10.1038/nature11682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart N, Bollini S, Dube KN, Vieira JM, Zhou B, Davidson S, Yellon D, Riegler J, Price AN, Lythgoe MF, et al. De novo cardiomyocytes from within the activated adult heart after injury. Nature. 2011;474:640–644. doi: 10.1038/nature10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song LS, Sobie EA, McCulle S, Lederer WJ, Balke CW, Cheng H. Orphaned ryanodine receptors in the failing heart. Proc Natl Acad Sci U S A. 2006;103:4305–4310. doi: 10.1073/pnas.0509324103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolen TO, Hoydal MA, Kemi OJ, Catalucci D, Ceci M, Aasum E, Larsen T, Rolim N, Condorelli G, Smith GL, et al. Interval training normalizes cardiomyocyte function, diastolic Ca2+ control, and SR Ca2+ release synchronicity in a mouse model of diabetic cardiomyopathy. Circ Res. 2009;105:527–536. doi: 10.1161/CIRCRESAHA.109.199810. [DOI] [PubMed] [Google Scholar]
- Sundaresan NR, Gupta M, Kim G, Rajamohan SB, Isbatan A, Gupta MP. Sirt3 blocks the cardiac hypertrophic response by augmenting Foxo3a-dependent antioxidant defense mechanisms in mice. The Journal of clinical investigation. 2009;119:2758–2771. doi: 10.1172/JCI39162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaresan NR, Pillai VB, Wolfgeher D, Samant S, Vasudevan P, Parekh V, Raghuraman H, Cunningham JM, Gupta M, Gupta MP. The deacetylase SIRT1 promotes membrane localization and activation of Akt and PDK1 during tumorigenesis and cardiac hypertrophy. Science signaling. 2010;4 doi: 10.1126/scisignal.2001465. [DOI] [PubMed] [Google Scholar]
- Taylor RS, Brown A, Ebrahim S, Jolliffe J, Noorani H, Rees K, Skidmore B, Stone JA, Thompson DR, Oldridge N. Exercise-based rehabilitation for patients with coronary heart disease: systematic review and meta-analysis of randomized controlled trials. The American journal of medicine. 2004;116:682–692. doi: 10.1016/j.amjmed.2004.01.009. [DOI] [PubMed] [Google Scholar]
- Vettor R, Valerio A, Ragni M, Trevellin E, Granzotto M, Olivieri M, Tedesco L, Ruocco C, Fossati A, Fabris R, et al. Exercise training boosts eNOS-dependent mitochondrial biogenesis in mouse heart: role in adaptation of glucose metabolism. American journal of physiology Endocrinology and metabolism. 2014;306:28. doi: 10.1152/ajpendo.00617.2013. [DOI] [PubMed] [Google Scholar]
- Wagner PD. Modeling O(2) transport as an integrated system limiting (.)V(O(2)MAX). Computer methods and programs in biomedicine. 2011;101:109–114. doi: 10.1016/j.cmpb.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waring CD, Vicinanza C, Papalamprou A, Smith AJ, Purushothaman S, Goldspink DF, Nadal-Ginard B, Torella D, Ellison GM. The adult heart responds to increased workload with physiologic hypertrophy, cardiac stem cell activation, and new myocyte formation. European heart journal. 2014;35:2722–2731. doi: 10.1093/eurheartj/ehs338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman K. Principles of exercise testing and interpretation : including pathophysiology and clinical applications. 5th edn Wolters Kluwer Health/Lippincott Williams & Wilkins; Philadelphia: 2012. [Google Scholar]
- Weeks KL, Gao X, Du X-JJ, Boey EJ, Matsumoto A, Bernardo BC, Kiriazis H, Cemerlang N, Tan JW, Tham YK, et al. Phosphoinositide 3-kinase p110α is a master regulator of exercise-induced cardioprotection and PI3K gene therapy rescues cardiac dysfunction. Circulation Heart failure. 2012;5:523–534. doi: 10.1161/CIRCHEARTFAILURE.112.966622. [DOI] [PubMed] [Google Scholar]
- Whipp BJ, Ward SA. Quantifying intervention-related improvements in exercise tolerance. The European respiratory journal. 2009;33:1254–1260. doi: 10.1183/09031936.00110108. [DOI] [PubMed] [Google Scholar]
- White FC, Bloor CM, McKirnan MD, Carroll SM. Exercise training in swine promotes growth of arteriolar bed and capillary angiogenesis in heart. Journal of applied physiology. 1998;85:1160–1168. doi: 10.1152/jappl.1998.85.3.1160. [DOI] [PubMed] [Google Scholar]
- Wisloff U, Loennechen JP, Currie S, Smith GL, Ellingsen O. Aerobic exercise reduces cardiomyocyte hypertrophy and increases contractility, Ca2+ sensitivity and SERCA-2 in rat after myocardial infarction. Cardiovasc Res. 2002;54:162–174. doi: 10.1016/s0008-6363(01)00565-x. [DOI] [PubMed] [Google Scholar]
- Wisloff U, Loennechen JP, Falck G, Beisvag V, Currie S, Smith G, Ellingsen O. Increased contractility and calcium sensitivity in cardiac myocytes isolated from endurance trained rats. Cardiovasc Res. 2001;50:495–508. doi: 10.1016/s0008-6363(01)00210-3. [DOI] [PubMed] [Google Scholar]
- Xiao J, Xu T, Li J, Lv D, Chen P, Zhou Q, Xu J. Exercise-induced physiological hypertrophy initiates activation of cardiac progenitor cells. International journal of clinical and experimental pathology. 2013;7:663–669. [PMC free article] [PubMed] [Google Scholar]
- Yamashita K, Kajstura J, Discher DJ, Wasserlauf BJ, Bishopric NH, Anversa P, Webster KA. Reperfusion-activated Akt kinase prevents apoptosis in transgenic mouse hearts overexpressing insulin-like growth factor-1. Circ Res. 2001;88:609–614. doi: 10.1161/01.res.88.6.609. [DOI] [PubMed] [Google Scholar]
- Zhang QJ, McMillin SL, Tanner JM, Palionyte M, Abel ED, Symons JD. Endothelial nitric oxide synthase phosphorylation in treadmill-running mice: role of vascular signalling kinases. The Journal of physiology. 2009;587:3911–3920. doi: 10.1113/jphysiol.2009.172916. [DOI] [PMC free article] [PubMed] [Google Scholar]