Abstract
The Saccharomyces sensu stricto group encompasses species ranging from the industrially ubiquitous yeast Saccharomyces cerevisiae to those that are confined to geographically limited environmental niches. The wealth of genomic data that are now available for the Saccharomyces genus is providing unprecedented insights into the genomic processes that can drive speciation and evolution, both in the natural environment and in response to human-driven selective forces during the historical “domestication” of these yeasts for baking, brewing, and winemaking.
Keywords: yeast, genomics, Saccharomyces, industrial fermentation
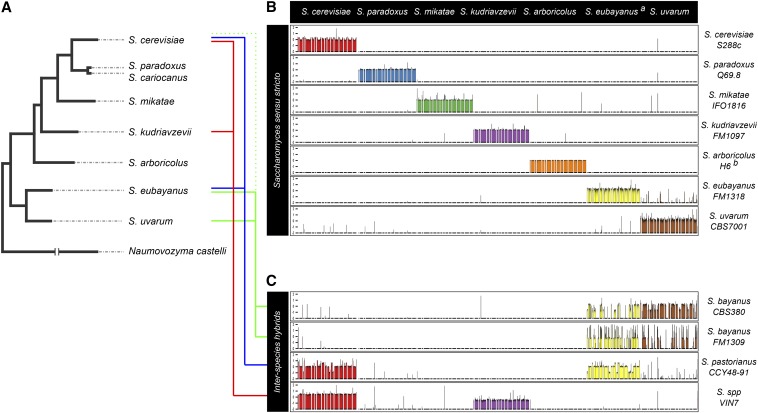
THE Saccharomyces sensu stricto complex is currently composed of at least seven distinct species with origins ∼10–20 MYA (Kellis et al. 2003). Saccharomyces uvarum and S. eubayanus are the most basal members of the Saccharomyces sensu stricto clade, while the division between S. paradoxus (encompassing S. cariocanus) and S. cerevisiae is the most recent (Figure 1A).
Figure 1.
The Saccharomyces sensu stricto clade. (A) A schematic representation of the phylogenic structure of the Saccharomyces sensu stricto members, with Naumovozyma castelli as an outgroup. (B) Genomic representation of the Saccharomyces sensu stricto clade. Short read sequencing data from individual strains (as indicated to the left of the plot) were aligned to a common reference sequence composed of ordered, chromosomal-based scaffolds for each of the seven Saccharomyces sensu stricto species (listed at the top of the plot). The log2 ratio of sequence coverage compared to the genome-wide average across this reference is shown for 10-kb sliding sequence windows. For each “pure” species, sequence reads primarily map to the expected single reference genome, with an even level of coverage indicating equal relative chromosomal copy number. Small, isolated regions of coverage may be indicative of small-scale introgression events between species in individual strains. (C) Genomic representation of Saccharomyces interspecific hybrids, including the hybrid “species” S. bayanus and S. pastorianus. Sequencing data from individual hybrid strains were analyzed as in B. Each hybrid strain displays sequence reads that map to large portions of chromosomes from multiple distinct pure species. In addition, uneven sequence coverage indicates genomic copy number variation due to aneuploidy or chromosomal rearrangement. The S. eubayanus reference genome was estimated from the S. eubayanus portion of the S. pastorianus genome and therefore lacks several genomic loci that have been lost in this strain.
Members of the Saccharomyces sensu stricto group range from the important industrial and laboratory species S. cerevisiae to those that, to date, are found in specific, geographically limited environmental ranges. However, all members share the common attributes of ease of laboratory propagation, short generation times, and small genome sizes that make them appealing for evolutionary and functional genomics studies. This has resulted in a wealth of information of the genomes of these yeasts and an unrivaled framework for comparative investigation.
S. cerevisiae Model for Fundamental Biology and Industrial Workhorse
S. cerevisiae is the most prominent of the Saccharomyces sensu stricto clade due to its historically intimate association with human activities such as brewing, baking, and winemaking. In addition to its industrial role, by way of its eukaryotic biology, ease of propagation, and well-defined genetics, S. cerevisiae represents one of the most intensively studied biological model systems and the first eukaryote for which a fully characterized genome sequence was available (Goffeau et al. 1996).
Following the introduction of next-generation sequencing, the economic importance of industrial strains of S. cerevisiae has driven the large-scale sequencing of many industrial isolates. Genome sequence information is now available for >80 strains of S. cerevisiae in some form (complete, draft, or raw data).
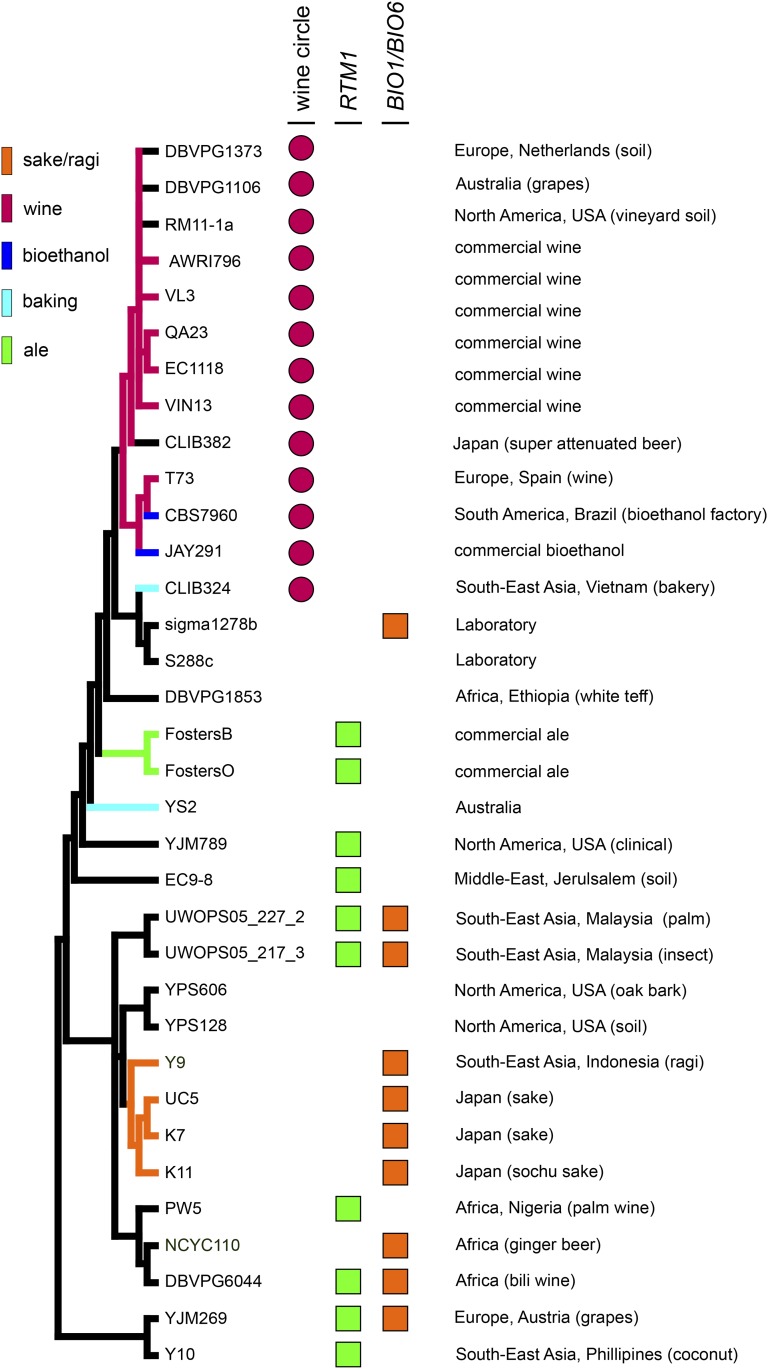
Examination of subsets of these genomic data sets has shown that the population structure of S. cerevisiae is complex, composed of both clearly defined “pure” lineages based around either strictly geographic (Africa, North America, or Southeast Asia) or industrial limits (wine or sake) and mosaic strains that appear to be the result of outcrossing between multiple pure lineages (Liti et al. 2009; Schacherer et al. 2009). The presence of a large proportion of mosaic strains is very different from the situation observed in S. paradoxus, in which outcrossing appears to be very rare (Koufopanou et al. 2006; Liti et al. 2009), and may be due to the close association of S. cerevisiae with human activity. This association with humans is almost certainly also the basis of the discovery of links between industry and geography such as that found between strains from Europe and wine and vineyard isolates from around the world (Figure 2A).
Figure 2.
Genomic comparison of various strains of S. cerevisiae. A maximum-likelihood phylogeny was constructed for a variety of S. cerevisiae strains for which whole-genome sequence data are available. Branches involving industrial strains are shaded according to their documented use (sake/ragi, grape-based wine, bioethanol, baking, or ale production). The presence of three strain-specific genomic loci (wine circle, RTM1 cluster, and BIO1/BIO6) and their source of isolation are also indicated for each strain.
Due to their far greater demands, de novo assembled and curated genomes still represent the minority of genomic data available for S. cerevisiae. However, annotation of small numbers of industrial strains from the wine, brewing, and biofuel industries has begun to uncover the genetic variation that has accumulated in the S. cerevisiae genome. Relative to the ad hoc reference strain, S288c, there are at least 200 kb of additional DNA spread across numerous distinct genomic loci (encoding single genes to multigene clusters) present in other strains of S. cerevisiae (Table 1). Interestingly, the common theme across all of these comparisons to S288c is that this strain appears to represent an almost minimal core of common genes, displaying no ORFs that are absent in the majority of other strains (except for an extremely high number of transposon integration events), and is likely to reflect gene loss during its transition to laboratory cultivation under ideal growth conditions.
Table 1. Strain-specific genomic loci in S. cerevisiae.
| Locus chromosomal location(s) | Locus size (kb) | No. predicted ORF(s) | Strain(s) | Strain type | Source |
|---|---|---|---|---|---|
| VI (L)a | 38 | 19 | EC1118 | Wine | Novo et al. (2009) |
| Lalvin QA23 | Wine | Borneman et al. (2011) | |||
| Vin7 | Wine hybrid | ||||
| XV (R)b | 60 | >20 | EC1118 | Wine | Novo et al. (2009) |
| Lalvin QA23 | Wine | Borneman et al. (2011) | |||
| Vin13 (partial) | Wine | Borneman et al. (2011) | |||
| VL3 (partial) | Wine | Borneman et al. (2011) | |||
| Vin7 | Wine hybrid | Borneman et al. (2012) | |||
| CLIB382 | |||||
| XV | 45 | 18 | AWRI796 | Wine | Borneman et al. (2011) |
| XI, XII, XIII, XIV, often multicopy | 17 | 5 | RM11-1a | Vineyard | |
| EC1118 | Wine | Novo et al. (2009) | |||
| Lalvin QA23 | Wine | Borneman et al. (2011) | |||
| VL3 | Wine | Borneman et al. (2011) | |||
| AWRI796 | Wine | Borneman et al. (2011) | |||
| Vin13 | Wine | Borneman et al. (2011) | |||
| T73 | Wine | ||||
| CLIB382 | Ale contaminant | ||||
| JAY291 | Biofuel | Argueso et al. (2009) | |||
| CBS7960 | Biofuel | ||||
| CLIB324 | Baking | ||||
| Various, often multicopy | 4 | 2 (RTM1) | FL100 | Laboratory | |
| YJM789 | Human | Wei et al. (2007) | |||
| FostersB | Ale | Borneman et al. (2011) | |||
| FostersO | Ale | Borneman et al. (2011) | |||
| YJM269 | Wine grapes | ||||
| Y10 | Coconut | ||||
| PW5 | Palm wine | ||||
| EC9-8 | Soil | ||||
| UWOPS05_227_2 | Palm | Liti et al. (2009) | |||
| UWOPS05_217_3 | Insect | Liti et al. (2009) | |||
| NCYC110 | Ginger beer | Liti et al. (2009) | |||
| DBVPG6044 | Bili wine | Liti et al. (2009) | |||
| VI | 19 | >3 | JAY291 | Biofuel | Argueso et al. (2009) |
| PW5 | Palm Wine | ||||
| T7 | Oak | ||||
| Y10 | Coconut | ||||
| YJM269 | Wine Grapes | ||||
| EC9-8 | Soil | ||||
| YPS163 | Biofuel | ||||
| VI, X, XIV, often multicopy | <1 | 1 (MPR1 or MPR2) | Σ1278b | Laboratory | Dowell et al. (2010) |
| RM11-1a | Vineyard | ||||
| EC1118 | Wine | Novo et al. (2009) | |||
| QA23 | Wine | Borneman et al. (2011) | |||
| AWRI796 | Wine | Borneman et al. (2011) | |||
| VL3 | Wine | Borneman et al. (2011) | |||
| Vin13 | Wine | Borneman et al. (2011) | |||
| AWRI1631 | Wine | Borneman et al. (2008) | |||
| Vin7 | Wine hybrid | Borneman et al. (2012) | |||
| JAY291 | Biofuel | Argueso et al. (2009) | |||
| CBS7960 | Biofuel | ||||
| EC9-8 | Soil | ||||
| T7 | Oak | ||||
| CLIB215 | Baking | ||||
| CLIB324 | Baking | ||||
| XIV | 8 | 2 | RM11-1a | Vineyard | |
| AWRI796 | Wine | Borneman et al. (2011) | |||
| Kyokai No7 | Sake | Akao et al. (2011) | |||
| M3707 | Biofuel | ||||
| IV, VI | <1 | 1 (YJMGNAT) | YJM789 | Human | Wei et al. (2007) |
| Kyokai No7 | Sake | Akao et al. (2011) | |||
| UC5 | Sake | ||||
| FostersO | Ale | Borneman et al. (2011) | |||
| FostersB | Ale | Borneman et al. (2011) | |||
| T7 | Oak | ||||
| YPS163 | Vineyard | ||||
| YJSH1 | Biofuel | ||||
| M3707 | Biofuel | Brown et al. (2013) | |||
| EC9-8 | Soil | ||||
| IX | <1 | 1 (KHR killer toxin) | YJM789 | Human | Wei et al. (2007) |
| EC1118 | Wine | Borneman et al. (2011) | |||
| VL3 | Wine | Borneman et al. (2011) | |||
| Vin13 | Wine | Borneman et al. (2011) | |||
| FostersO | Ale | Borneman et al. (2011) | |||
| FostersB | Ale | Akao et al. (2011) | |||
| Kyokai No7 | Sake | Brown et al. (2013) | |||
| M3707 | Biofuel | ||||
| XV | 5 | 1 (AWA1) | Kyokai No7 | Sake | Akao et al. (2011) |
| I, II, VIII, IX, XII, XVI | <1 | 1 (BIO6) | Kyokai No7 | Sake | Akao et al. (2011) |
| UC5 | Sake | ||||
| CEN.PK | Laboratory | Otero et al. (2010) | |||
| Σ1278b | Laboratory | Dowell et al. (2010) | |||
| YJM269 | Wine grapes | ||||
| YJSH1 | Biofuel | ||||
| ZTW1 | Biofuel | ||||
| M3707 | Biofuel | Brown et al. (2013) | |||
| I, II, VIII, IX | <1 | 1 (BIO1) | Kyokai No7 | Sake | Akao et al. (2011) |
| UC5 | Sake | ||||
| CEN.PK | Laboratory | Otero et al. (2010) | |||
| Σ1278b | Laboratory | Dowell et al. (2010) | |||
| YJM269 | Wine grapes | ||||
| YJSH1 | Biofuel | ||||
| ZTW1 | Biofuel | ||||
| M3707 | Biofuel | Brown et al. (2013) | |||
| UWOPS05_227_2 | Palm | Liti et al. (2009) | |||
| UWOPS05_217_3 | Insect | Liti et al. (2009) | |||
| NCYC110 | Ginger beer | Liti et al. (2009) | |||
| DBVPG6044 | Bili wine | Liti et al. (2009) | |||
| I, XI | 1 | 1 (EHL) | Kyokai No7 | Sake | Akao et al. (2011) |
| UC5 | Sake | ||||
| YJSH1 | Biofuel | ||||
| ZTW1 | Biofuel | ||||
| M3707 | Biofuel | Brown et al. (2013) | |||
| UWOPS05_227_2 | Palm | Liti et al. (2009) | |||
| UWOPS05_217_3 | Insect | Liti et al. (2009) | |||
| NCYC110 | Ginger beer | Liti et al. (2009) | |||
| DBVPG6044 | Bili wine | Liti et al. (2009) | |||
| VI | 6 | 1 (IRC7 paralog) | Y10 | Coconut | |
| YJM450 | Human | Roncoroni et al. (2011) | |||
| IV | 8 | 3 | Kyokai No7 | Sake | Akao et al. (2011) |
| UC5 | Sake | ||||
| CEN.PK | Laboratory | Otero et al. (2010) | |||
| Σ1278b | Laboratory | Dowell et al. (2010) | |||
| YJM269 | Wine grapes | ||||
| YJSH1 | Biofuel | ||||
| ZTW1 | Biofuel | ||||
| M3707 | Biofuel | Brown et al. (2013) | |||
| PW5 | Palm wine | ||||
| T7 | Oak | ||||
| Unknown | 6 | 3 | Y10 | Coconut |
Left arm.
Right arm.
In general, the strain-specific loci reside in the subtelomeric regions of the S. cerevisiae genome, a location that appears to be the epicenter of genetic diversity in this species. This is presumably due to the presence of large numbers of subtelomeric repeats that act as the seeds for the integration, duplication, and/or loss of genomic segments between strains. The fact that these uncommon, gain-of-genome events are commonly observed in S. cerevisiae relative to the other members of the Saccharomyces sensu stricto group likely reflects the disruptive influence of human activity, whereby the selective forces imposed by the development of various industrial fermentations inadvertently selected for rare mutations with large phenotypic impact. This is highlighted by high-throughput phenotypic analysis that has shown S. cerevisiae to display a greater phenotypic plasticity than other Saccharomyces sensu stricto strains, such that industrial and wild strains of S. cerevisiae are often more distinct from each other than they are from other Saccharomyces species (which form tight, species-specific phenotypic clades) (Warringer et al. 2011).
There are three main loci that seem to define specific industrial classes of yeast, the RTM1 cluster found predominantly in ale and distilling strains and the wine strain-specific circular cluster (see below) (Novo et al. 2009; Borneman et al. 2011). The RTM1 gene was originally isolated as a subtelomeric gene, associated with the sucrose utilization (SUC) locus that was absent in laboratory strains of S. cerevisiae, which provided specific distilling strains with the ability to resist the effects of inhibitory compounds present in molasses (Ness and Aigle 1995). Subsequent genomic sequencing identified RTM1 as a member of a three-gene cluster that is present in the opportunistic human pathogen YJM789 (Wei et al. 2007), ale yeast strains (FostersB and FostersO) (Borneman et al. 2011), and several environmental isolates (Figure 2), but is absent from the genome of the biofuel strain JAY291, despite the common use of molasses for yeast propagation in brewing and biofuel production (Argueso et al. 2009). Interestingly, an ORF directly adjacent to RTM1 (SCY_1426 in YJM789, FostersB_5069 in FostersB, and FostersO_5019:5020 in FostersO) is predicted to encode a large, ∼800-amino-acid protein that has no detectable homology to proteins currently in the GenBank protein database, although it does contain sequence characteristics consistent with a role as a transcription factor. As such, the function of this protein and its relationship to RTM1, if any, remain to be determined.
The second industry-defining locus of S. cerevisiae was initially identified as one of several genomic fragments that were present in the wine strain EC1118 (Novo et al. 2009). Subsequent detailed analysis of several industrial S. cerevisiae genomes by Borneman et al. (2011) showed that while this cluster of five genes was specific to wine strains (with the exception of the biofuel strain JAY291), it displayed strain-specific differences in copy number, genomic location, and gene order. Diversity in the cluster was shown to be consistent with mobilization into, and throughout, the wine yeast genome as a circular intermediate via an unknown process that has since been proposed to also occur in both mammals and fish (Borneman et al. 2011; Fujimura et al. 2011; Durkin et al. 2012). Subsequent to this work, this genomic feature has been located in the genomes of several additional strains of S. cerevisiae that all seemingly reside in the same wine-specific phylogenic clade (Figure 2A).
The final industry-specific locus involves the evolution of biotin prototrophy in a subset of strains of S. cerevisiae. While the majority of S. cerevisiae isolates, including those used in winemaking and brewing, are biotin auxotrophs, some, such as those used for the production of sake, are able to synthesize biotin de novo, presumably due to the very low biotin content of sake mash (Wu et al. 2005). This conversion to biotin prototrophy is due to the reacquisition of two ORFs, BIO1 and BIO6, that encode the enzymatic steps that are missing in the biotin pathway of most other strains (Wu et al. 2005; Hall and Dietrich 2007). As for many of these species-specific ORFs, the donor species of these DNA sequences is also not clear; however, suggestions hint at a de novo origin in S. cerevisiae, rather than horizontal acquisition, through duplication and neofunctionalization of BIO3 (BIO6) and YJR154W (BIO1) (Hall and Dietrich 2007).
In addition to these potentially industry-defining loci there are also several strain-specific ORFs for which important phenotypes can be attributed. FSY1 was first identified as a member of the large multigenic strain-specific locus present in the EC1118 group of S. cerevisiae wine strains (Novo et al. 2009) (Table 1). Based on homology to an ORF from S. pastorianus, FSY1 was predicted to encode a H+/fructose symporter that is proposed to have been horizontally transferred into S. cerevisiae from an unidentified relative (Galeote et al. 2010). The presence of this protein is thought to enable active transport of fructose into the cell, a phenotypic trait that is lacking from the majority of S. cerevisiae strains and is expected to provide a selective advantage in the highly concentrated 1:1 mixture of glucose and fructose that is present during wine fermentation.
MPR1 and MPR2 were first identified as almost identical paralogous ORFs specific to the S. cerevisiae strain Σ1278b and were responsible for providing resistance to L-azetidine-2-carboxylic acid (Takagi et al. 2000). Subsequent studies have shown that this gene family provides general stress resistance by decreasing the toxic effects of reactive oxygen species (Nishimura et al. 2010; Sasano et al. 2010). Like RTM1, MPR-family paralogs are also found in the telomeric regions and can be present in multiple copies within a strain. While they are absent from the laboratory strain S288c, sequencing has identified MPR-family ORFs in many industrial strains, including those from winemaking, baking, and biofuel backgrounds, where they presumably provide resistance to stresses imposed by industrial fermentation (Table 1).
The IRC7 gene encodes a β-lyase that is responsible for the release of volatile thiols that are especially important during winemaking (Thibon et al. 2008; Roncoroni et al. 2011). While the genomes of all strains of S. cerevisiae, including S288c, appear to contain IRC7, a highly diverse homolog of this gene (88% DNA identity to S288c IRC7) was identified in the human clinical isolate YJM540. This new IRC7-family member was subsequently shown to be highly active at thiol release, providing YJM450 with the ability to produce enhanced levels of these aroma compounds compared to other yeast strains (Roncoroni et al. 2011). Subsequent genome sequencing has identified this ortholog in only one other strain of S. cerevisiae (Y10), isolated from coconut in the Philippines. During its initial characterization, this divergent ortholog was suggested to have been introgressed from S. paradoxus. However, given that this gene has been identified only in one strain of S. paradoxus (UWOPS91-917.1 isolated in Hawaii), the actual origin of this particular version of IRC7 may lie outside of both of these species or be a result of rapid sequence divergence of the common S. cerevisiae gene (Liti et al. 2009; Roncoroni et al. 2011).
S. paradoxus and S. cariocanus
S. paradoxus represents the closest known relative to S. cerevisiae (Figure 1A). Despite this phylogenetic relationship, while S. cerevisiae is intimately associated with human industry, there is very little, if any, evidence of an industrial role for S. paradoxus, which is instead generally limited to environmental niches where it is associated with trees of the Quercus (Oak) genus (and possibly related genera).
Genomic data for S. paradoxus suggest that the species comprises two very distinct populations, represented by the Americas and Eurasia, with strains of European and Asian origin also being readily separated into subpopulations within this larger clade (Liti et al. 2006, 2009). Across these subpopulations, the levels of nucleotide divergence between the most distant clades (∼4.6%) are far higher than has been observed in S. cerevisiae and may be due to an apparent lack of interbreeding in S. pastorianus (Liti et al. 2009). This lack of interbreeding even extends to populations found on the same tree branches, with no evidence of heterozygous offspring between genetically distinct neighbors observed (Koufopanou et al. 2006). This high level of sequence variation has also led to the development of partial reproductive barriers between the strains, with spore viability approaching as little as 30% for interclade crosses and possibly representing the early stages of biological concept speciation for the three subpopulations (Sniegowski et al. 2002; Liti et al. 2006).
In addition to reproductive isolation imposed by sequence divergence between S. paradoxus populations, examples of reciprocal translocations that affect reproductive success between strains have also been recorded for this species. This is highlighted by the designation of S. cariocanus as a separate Saccharomyces spp. due to its extremely low spore viability (∼5%) when mated to S. paradoxus (Naumov et al. 2000). However, subsequent genomic analysis has shown that the level of sequence divergence between S. cariocanus and S. paradoxus strains of the Americas is within the range observed across S. paradoxus, with the ultimate cause of the reproductive isolation being due to four reciprocal translocations (II and XVI, IX and XV, XII and XIV, and IV and XI) present in the genomes of the S. cariocanus strains (compared to both S. paradoxus and S. cerevisiae that are colinear) (Fischer et al. 2000).
Despite S. paradoxus displaying levels of genetic variation that are far greater than those observed for S. cerevisiae, there appears to be considerably less gene content variation within this species (Bergström et al. 2014). This difference in SNP vs. gene content variation may reflect the different selective pressures observed between the natural ecological niches of S. paradoxus in contrast to the potentially sudden and disruptive pressures imposed upon S. cerevisiae during its transition toward “domestication.” This is supported by widespread phenotype comparisons that show S. cerevisiae to display higher intraspecies trait variability than S. paradoxus in spite of its lower SNP diversity (Warringer et al. 2011).
However, despite these generalizations “atypical” strains of S. paradoxus, such as the Hawaiian isolate UWOPS91-917.1, have been identified that do contain significant numbers of novel genes (e.g., MEL1 and the variant IRC7) that impart important phenotypic characteristics. One other key difference in gene content between S. paradoxus populations is the presence of an 18-kb element that has introgressed from S. cerevisiae into the genomes of European isolates of S. paradoxus relative to their American and Far Eastern counterparts. This element has resulted in the replacement of at least 12 S. paradoxus ORFs in these strains with equivalent genes from S. cerevisiae (Liti et al. 2006).
S. mikatae
Despite being included in the first genomic comparisons of the Saccharomyces sensu stricto clade, only IFO1815, the type strain of S. mikatae, has been sequenced to date (Cliften et al. 2003; Kellis et al. 2003; Scannell et al. 2011). While IFO1815 has been shown to harbor two translocations compared to S. cerevisiae (VI and VII, VII and XVI), data suggest that this may be variable across strains as the closely related S. mikatae strain IFO1816 appears to contain only a single translocation event (VI and VII) (Fischer et al. 2000; Scannell et al. 2011).
Given that there is only one strain of S. mikatae for which genomic data are available, the levels of interstrain variation within this species remain to be resolved. However, given the ease of current genome sequencing, obtaining a wider understanding of S. mikatae genomic variation will likely be limited by the very small number of strains that are currently available for this particular species (with the largest collection of S. mikatae strains being limited to a total of only 14, all which are from Japan).
S. arboricolus
S. arboricolus is the newest addition to the Saccharomyces sensu stricto clade (Wang and Bai 2008; Naumov et al. 2010) with the genome of the S. arboricolus type strain (CBS 10644) being recently completed (Liti et al. 2013). Like S. mikatae, the single representative sequence provides no insight into the diversity within the species, but provides an additional point of comparison to the other Saccharomyces sensu stricto species. When compared to the genome of S. cerevisiae S288c, the S. arboricolus genome harbors one reciprocal translocation between the right arms of chromosomes IV and XIII that is unique to this species, as well as two small inversions (chromosome VI encompassing YFR008W through YFR017C and chromosome XIV from YNL034W through YNL041C) that are shared with S. uvarum and S. kudriavzevii (Liti et al. 2013).
Liti et al. (2013) have estimated S. arboricolus contains at least 44 and up to 210 genes that are not found in S. cerevisiae. However, for some of these ORFs, including MEL1, BIO1, and BIO6 and two ancestral paralogs of the S. cerevisiae SIR1 gene, this is due to widespread loss specifically in S. cerevisiae rather than gain in S. arboricolus, as they are also found in S. uvarum and S. kudriavzevii (Hall and Dietrich 2007; Zill et al. 2010; Warringer et al. 2011).
S. kudriavzevii
Like S. mikatae, S. kudriavzevii was first isolated in Japan from decaying leaves (Naumov et al. 2000). However, unlike S. mikatae, in which the limited number of strains are all from Japan, studies have also isolated S. kudriavzevii from Europe (from the bark of Quercus spp. in Portugal). In the European environment S. kudriavzevii is found in sympatric association with both S. cerevisiae and S. paradoxus, but displays a more cryotolerant phenotype than either of these other species, thereby providing S. kudriavzevii with a competitive niche (Sampaio and Gonçalves 2008).
While the first S. kudriavzevii genome (IFO 1802) was produced in 2003 by two independent groups (Cliften et al. 2003; Kellis et al. 2003), additional refinement of the IFO 1802 genome, as well as de novo sequencing and assembly of a representative of the Portuguese S. kudriavzevii population (ZP591), has now also been completed (Scannell et al. 2011, p. 3). Like S. paradoxus, the genomes of both S. kudriavzevii IFO 1802 and ZP591 are colinear with S. cerevisiae (Fischer et al. 2000; Scannell et al. 2011)
Interestingly, comparative resequencing of 18 currently available S. kudriavzevii isolates (4 Japanese and 14 European) showed that while all the Japanese isolates of S. mikatae were incapable of assimilating galactose due to the concerted degeneration of the entire multigenic galactose utilization (GAL) pathway, all of the European strains carry a fully functional metabolic route and an associated galactose positive phenotype (Hittinger et al. 2004, 2010). The origin or selective advantage provided by this balanced polymorphism between the two geographically isolated groups remain to be determined.
S. eubayanus
Due to its contribution to the genome of the lager hybrid S. pastorianus, the existence of S. eubayanus was long predicted without a representative of the species having been identified (Martini and Kurtzman 1985; Rainieri et al. 2006; Dunn and Sherlock 2008; Nakao et al. 2009; Nguyen et al. 2011). This was finally resolved through the isolation and genomic analysis of an entirely new Saccharomyces species, S. eubayanus, although it remains the only Saccharomyces species for which a de novo assembly is not available (Libkind et al. 2011).
Surprisingly, rather than the S. eubayanus parent of S. pastorianus being of European origin, as is the case for the S. cerevisiae portion of the S. pastorianus genome, it appears that S. eubayanus may have been imported into Europe. As yet, pure S. eubayanus has not been isolated from Europe; however, a diverse number of strains have been readily found associated with southern beech (Nothofagus spp.) in Patagonia, where they form at least two distinct and diverse populations (Libkind et al. 2011; Peris et al. 2014). Furthermore, it appears that, in addition to being imported into Europe, these two distinct types of S. eubayanus may have both been imported into North America where they underwent admixture to produce a hybrid population (Peris et al. 2014).
In addition to being a parent of S. pastorianus, the identification of S. eubayanus as a pure species also affected the species definition of S. bayanus, as it appears that many members of this species complex are actually hybrids of S. eubayanus and S. uvarum (Nguyen et al. 2011).
S. uvarum and S. bayanus
The classification of the S. uvarum and S. bayanus species remains one of the more contentious issues in the classification of the Saccharomyces sensu stricto group. Initially composed of five species (S. bayanus, S. globosus, S. heterogenicus, S. inusitatus, and S. uvarum), these were subsequently merged into S. bayanus on the basis of DNA:DNA hybridization (Martini and Kurtzman 1985). However, the recent detailed examinations of the S. pastorianus and S. eubayanus genomes have shown that, from a genomic viewpoint, there are two clearly defined groups within the S. bayanus species defined by Martini and Kurtzman that relate back to the original S. bayanus and S. uvarum subspecies (Libkind et al. 2011; Nguyen et al. 2011).
In this genome-centric division, S. uvarum (S. bayanus var. uvarum) strains represent a pure lineage that contains very little genetic input from other Saccharomyces species (Figure 1B). While strains of this species are readily isolated from natural environments and low-temperature industrial fermentations, a de novo assembly exists for only the type strain of S. uvarum, CBS7001 (isolated from an insect in Spain and originally identified as S. bayanus) (Martini and Kurtzman 1985). This strain differs from S. cerevisiae by four translocations (XIII and XV, VI and X, V and VII, and II and IV) (Fischer et al. 2000; Scannell et al. 2011).
Interspecies comparison has shown that S. uvarum is the only Saccharomyces sensu stricto species to retain the budding yeast Dicer homolog that composes part of the RNAi machinery and the paralog of S. cerevisiae GAL80 that was present following the whole-genome duplication event. Both these genes are found in more distantly related yeast species such as Naumovozyma (formerly S. castelli) but have been lost from the rest of the Saccharomyces sensu stricto lineage (Hittinger et al. 2004; Cliften et al. 2006; Drinnenberg et al. 2009; Scannell et al. 2011).
In contrast, S. bayanus (S. bayanus var. bayanus) strains such as CBS380T (S. bayanus type strain) or NBRC1948 represent highly recombined, interspecific hybrids that comprise almost equal genomic contributions from S. eubayanus and S. uvarum, with a minor (70–80 kb) input from S. cerevisiae (Figure 1C). The S. cerevisiae portion of the S. bayanus genome encodes a number of genes, but the main phenotypic consequence is likely to relate to the ability to of S. bayanus to metabolize maltose and maltotriose, a phenotype that is lacking in S. uvarum. While the phylogeny of two of these genes suggests that they originated from a European wine strain of S. cerevisiae (Libkind et al. 2011; Nguyen et al. 2011), the presence of the RTM1 cluster in these strains adjacent to these genes is more consistent with the fragment originating from a European ale or distilling strain of S. cerevisiae.
While the formation of S. bayanus may have occurred as a result of the environmental sympatric association of S. eubayanus and S. uvarum (Libkind et al. 2011), S. bayanus strains have been isolated only from “artificial” brewery environments and may therefore share their origin with S. pastorianus in European breweries during the Middle Ages. Furthermore, it has been suggested that CBS380T-type strains may be the result of additional hybridization events between S. uvarum (e.g., CBS7001) and S. bayanus (e.g., NBRC1948) as these strains are interfertile and produce progeny with chromosomal content similar to that of CBS380T (Nguyen et al. 2011). This intercrossing ability may therefore have accelerated the recombination and consolidation of parental chromosomes in S. bayanus compared to those in S. pastorianus, leading to a highly composite genomic arrangement of S. bayanus when compared to S. pastorianus, which still displays many of the chromosomal hallmarks and copy number effects of the original hybridization event.
Other Interspecific Hybrids
The integrity of species within the Saccharomyces sensu stricto complex is the result of postzygotic reproductive barriers (<1% viable meiotic spores) that appear to be driven primarily by sequence divergence, rather than chromosomal rearrangements, as engineering colinear genomes between divergent species does not produce efficient intraspecific fertility (Naumov 1987; Chambers et al. 1996; Hunter et al. 1996; Delneri et al. 2003; Greig et al. 2003). However, as this barrier is postzygotic, diploid or polyploid hybrids that do form via interspecific mating events are able to reproduce indefinitely via mitotic division. This phenomenon is not limited to laboratory experimentation and there are numerous reports of Saccharomyces interspecific hybrids being associated with cold fermentative environments, such as those observed in winemaking and beer brewing in Northern European countries (Sipiczki 2008).
S. pastorianus
As early as the 1980s the lager yeast S. pastorianus was suggested to be the result of a relatively recent (15th–16th century) interspecific hybridization event between S. cerevisiae and at least one other Saccharomyces spp. (Martini and Kurtzman 1985). Initial genomic analysis of several lager yeast genomes, using microarray-based comparative genome hybridization (aCGH), subsequently confirmed via genome sequencing, suggested that there were two distinct S. pastorianus sublineages, which could be roughly categorized by their geographic origin and Saaz and Frohberg types (Dunn and Sherlock 2008; Nakao et al. 2009; Walther et al. 2014), and that the non-S. cerevisiae parent of both types of S. pastorianus was more similar to but not entirely the same as S. bayanus (S. bayanus var. bayanus), which itself was considered to be a possible hybrid strain (see below) (Nakao et al. 2009; Walther et al. 2014).
The mystery surrounding the non-S. cerevisiae parent of S. pastorianus was finally resolved, as discussed above, by the work of Libkind et al. (2011) via the isolation and identification of an entirely new Saccharomyces species, S. eubayanus (Figure 1). The genomic sequence of S. eubayanus was highly homologous to the non-S. cerevisiae portions of the S. pastorianus genome, suggesting that the hybridization event that gave rise to S. pastorianus occurred between S. cerevisiae and S. eubayanus, presumably following the incidental importation of S. eubayanus into Europe, although the exact source of the S. eubayanus parent remains controversial (Libkind et al. 2011; Bing et al. 2014).
Regardless of the true geographical origin of the parental strain, the increased data afforded by genome sequencing also accurately showed that the Saaz-type strains of S. pastorianus (e.g., former S. carlsbergensis strains) are generally triploid (2n S. eubayanus, 1n S. cerevisiae), but with ∼3.5 Mb of DNA missing from the S. cerevisiae contribution (including the entirety of chromosomes VI, XI, and XII), while the Frohberg-type S. pastorianus strains (e.g., Weihenstephan strain WS34/70) are primarily tetraploid (2n, 2n) with limited loss of contributions from either parent.
In addition, despite the different chromosomal content of the two S. pastorianus groups suggesting independent origins, the analysis of the genetic variation present across the S. eubayanus portion of the genome, combined with the presence of several common genetic rearrangements between S. cerevisiae and S. eubayanus chromosomes in the Saaz and Frohberg lineages, suggests that both these groups arose from a single, common hybridization event (Dunn and Sherlock 2008; Peris et al. 2014; Walther et al. 2014). Under this model, differential mitotic recombination, unequal parental chromosomal loss, and recombination between homeologous parental chromosomes are proposed to have divergently acted on the same common ancestor to produce the two S. pastorianus groups, with differences in cryotolerance being a suspected phenotypic selective driver (Rainieri et al. 2006; Dunn and Sherlock 2008; Nakao et al. 2009; Libkind et al. 2011; Walther et al. 2014).
Wine yeast hybrids
Like the situation observed in brewing, wine fermentations performed at warm temperatures (>20°) are naturally dominated by S. cerevisiae. However, it is becoming increasingly evident that wine fermentations performed at lower temperature ranges are readily dominated by naturally occurring interspecific hybrids, including those formed between S. cerevisiae and either S. uvarum (González et al. 2006; Le Jeune et al. 2007) or S. kudriavzevii (González et al. 2006; Erny et al. 2012). In addition to naturally occurring interspecific hybrids, hybrids of S. cerevisiae and either S. paradoxus, S. kudriavzevii, or S. mikatae have been artificially induced for commercialization purposes (Bellon et al. 2011, 2013). Like the situation observed for S. pastorianus, these hybrid strains are often not complete and contain varying amounts of each parental genome (Dunn et al. 2012; Erny et al. 2012).
The only assembled genome sequence available for a hybrid wine yeast strain is that of the commercial strain VIN7 (Borneman et al. 2012). Analysis showed that VIN7 was an allotriploid, resulting from hybridization between a heterozygous diploid wine-like strain of S. cerevisiae and a haploid, European isolate of S. kudriavzevii (Figure 1C). Unlike lager yeast, and many other hybrid wine strains, VIN7 appears to be an almost complete hybrid and with limited genetic rearrangement between the two parental genomes (as few as three cases of recombination between homeologous chromosome pairs).
However, rather than providing only a cold-tolerant growth advantage, it appears that the presence of the S. kudriavzevii genome may have fortuitously allowed for VIN7 to release much larger amounts (often over double) of the fruity volatile thiol 4-mercapto-4- methylpentan-2-one (4MMP) from grape-derived, nonvolatile precursors during fermentation than S. cerevisiae wine yeast strains, providing a basis for ongoing genetic selection in a winemaking environment via human intervention (González et al. 2007; King et al. 2008; Swiegers et al. 2009).
Concluding Remarks
The wealth of genomic data that are available for the Saccharomyces genus provides an unprecedented insight into the evolution of this important group of microorganisms. However, advances in long-read genome sequencing assembly techniques are set to allow for even greater ease of de novo assembly, rather than genomic resequencing, of large numbers of strains. This will provide a detailed estimation of the breadth of the pan-genome of the Saccharomyces sensu stricto clade and how this relates to the high levels of diversity that are observed across the many varied phenotypic characteristics inherent in the Saccharomyces sensu stricto group.
Acknowledgments
Research at The Australian Wine Research Institute (AWRI) is supported by Australia’s grape growers and winemakers through their investment body the Grape and Wine Research and Development Corporation, with matching funds from the Australian Government. The AWRI is part of the Wine Innovation Cluster in the Waite Precinct, South Australia. I.S.P. is supported by an internal grant from Macquarie University.
Footnotes
Communicating editor: J. Rine
Literature Cited
- Akao T., Yashiro I., Hosoyama A., Kitagaki H., Horikawa H., et al. , 2011. Whole-genome sequencing of sake yeast Saccharomyces cerevisiae Kyokai no. 7. DNA Res. Int. J. Rapid Publ. Rep. Genes Genomes 18: 423–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argueso J. L., Carazzolle M. F., Mieczkowski P. A., Duarte F. M., Netto O. V. C., et al. , 2009. Genome structure of a Saccharomyces cerevisiae strain widely used in bioethanol production. Genome Res. 19: 2258–2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellon J. R., Eglinton J. M., Siebert T. E., Pollnitz A. P., Rose L., et al. , 2011. Newly generated interspecific wine yeast hybrids introduce flavour and aroma diversity to wines. Appl. Microbiol. Biotechnol. 91: 603–612. [DOI] [PubMed] [Google Scholar]
- Bellon J. R., Schmid F., Capone D. L., Dunn B. L., Chambers P. J., 2013. Introducing a new breed of wine yeast: interspecific hybridisation between a commercial Saccharomyces cerevisiae wine yeast and Saccharomyces mikatae. PLoS ONE 8: e62053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergström A., Simpson J. T., Salinas F., Barré B., Parts L., et al. , 2014. A high-definition view of functional genetic variation from natural yeast genomes. Mol. Biol. Evol. 31: 872–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bing J., Han P.-J., Liu W.-Q., Wang Q.-M., Bai F.-Y., 2014. Evidence for a Far East Asian origin of lager beer yeast. Curr. Biol. 24: R380–R381. [DOI] [PubMed] [Google Scholar]
- Borneman A. R., Forgan A. H., Pretorius I. S., Chambers P. J., 2008. Comparative genome analysis of a Saccharomyces cerevisiae wine strain. FEMS Yeast Res. 8: 1185–1195. [DOI] [PubMed] [Google Scholar]
- Borneman A. R., Desany B. A., Riches D., Affourtit J. P., Forgan A. H., et al. , 2011. Whole-genome comparison reveals novel genetic elements that characterize the genome of industrial strains of Saccharomyces cerevisiae. PLoS Genet. 7: e1001287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borneman A. R., Desany B. A., Riches D., Affourtit J. P., Forgan A. H., et al. , 2012. The genome sequence of the wine yeast VIN7 reveals an allotriploid hybrid genome with Saccharomyces cerevisiae and Saccharomyces kudriavzevii origins. FEMS Yeast Res. 12: 88–96. [DOI] [PubMed] [Google Scholar]
- Brown S. D., Klingeman D. M., Johnson C. M., Clum A., Aerts A., et al. , 2013. Genome sequences of industrially relevant Saccharomyces cerevisiae strain M3707, isolated from a sample of distillers yeast and four haploid derivatives. Genome Announc. 1: e00323-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers S. R., Hunter N., Louis E. J., Borts R. H., 1996. The mismatch repair system reduces meiotic homeologous recombination and stimulates recombination-dependent chromosome loss. Mol. Cell. Biol. 16: 6110–6120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cliften P., Sudarsanam P., Desikan A., Fulton L., Fulton B., et al. , 2003. Finding functional features in Saccharomyces genomes by phylogenetic footprinting. Science 301: 71–76. [DOI] [PubMed] [Google Scholar]
- Cliften P. F., Fulton R. S., Wilson R. K., Johnston M., 2006. After the duplication: gene loss and adaptation in Saccharomyces genomes. Genetics 172: 863–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delneri D., Colson I., Grammenoudi S., Roberts I. N., Louis E. J., et al. , 2003. Engineering evolution to study speciation in yeasts. Nature 422: 68–72. [DOI] [PubMed] [Google Scholar]
- Dowell R. D., Ryan O., Jansen A., Cheung D., Agarwala S., et al. , 2010. Genotype to phenotype: a complex problem. Science 328: 469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drinnenberg I. A., Weinberg D. E., Xie K. T., Mower J. P., Wolfe K. H., et al. , 2009. RNAi in budding yeast. Science 326: 544–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn B., Sherlock G., 2008. Reconstruction of the genome origins and evolution of the hybrid lager yeast Saccharomyces pastorianus. Genome Res. 18: 1610–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn B., Richter C., Kvitek D. J., Pugh T., Sherlock G., 2012. Analysis of the Saccharomyces cerevisiae pan-genome reveals a pool of copy number variants distributed in diverse yeast strains from differing industrial environments. Genome Res. 22: 908–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durkin K., Coppieters W., Drögemüller C., Ahariz N., Cambisano N., et al. , 2012. Serial translocation by means of circular intermediates underlies colour sidedness in cattle. Nature 482: 81–84. [DOI] [PubMed] [Google Scholar]
- Erny C., Raoult P., Alais A., Butterlin G., Delobel P., et al. , 2012. Ecological success of a group of Saccharomyces cerevisiae/Saccharomyces kudriavzevii hybrids in the Northern European wine-making environment. Appl. Environ. Microbiol. 78: 3256–3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer G., James S. A., Roberts I. N., Oliver S. G., Louis E. J., 2000. Chromosomal evolution in Saccharomyces. Nature 405: 451–454. [DOI] [PubMed] [Google Scholar]
- Fujimura K., Conte M. A., Kocher T. D., 2011. Circular DNA intermediate in the duplication of Nile Tilapia vasa genes. PLoS ONE 6: e29477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galeote V., Novo M., Salema-Oom M., Brion C., Valério E., et al. , 2010. FSY1, a horizontally transferred gene in the Saccharomyces cerevisiae EC1118 wine yeast strain, encodes a high-affinity fructose/H+ symporter. Microbiology 156: 3754–3761. [DOI] [PubMed] [Google Scholar]
- Goffeau A., Barrell B. G., Bussey H., Davis R. W., Dujon B., et al. , 1996. Life with 6000 genes. Science 274(546): 563–567. [DOI] [PubMed] [Google Scholar]
- González S. S., Barrio E., Gafner J., Querol A., 2006. Natural hybrids from Saccharomyces cerevisiae, Saccharomyces bayanus and Saccharomyces kudriavzevii in wine fermentations. FEMS Yeast Res. 6: 1221–1234. [DOI] [PubMed] [Google Scholar]
- González S. S., Gallo L., Climent M. A. D., Barrio E., Querol A., 2007. Enological characterization of natural hybrids from Saccharomyces cerevisiae and S. kudriavzevii. Int. J. Food Microbiol. 116: 11–18. [DOI] [PubMed] [Google Scholar]
- Greig D., Travisano M., Louis E. J., Borts R. H., 2003. A role for the mismatch repair system during incipient speciation in Saccharomyces. J. Evol. Biol. 16: 429–437. [DOI] [PubMed] [Google Scholar]
- Hall C., Dietrich F. S., 2007. The reacquisition of biotin prototrophy in Saccharomyces cerevisiae involved horizontal gene transfer, gene duplication and gene clustering. Genetics 177: 2293–2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hittinger C. T., Rokas A., Carroll S. B., 2004. Parallel inactivation of multiple GAL pathway genes and ecological diversification in yeasts. Proc. Natl. Acad. Sci. USA 101: 14144–14149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hittinger C. T., Gonçalves P., Sampaio J. P., Dover J., Johnston M., et al. , 2010. Remarkably ancient balanced polymorphisms in a multi-locus gene network. Nature 464: 54–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter N., Chambers S. R., Louis E. J., Borts R. H., 1996. The mismatch repair system contributes to meiotic sterility in an interspecific yeast hybrid. EMBO J. 15: 1726–1733. [PMC free article] [PubMed] [Google Scholar]
- Le Jeune C., Lollier M., Demuyter C., Erny C., Legras J.-L., et al. , 2007. Characterization of natural hybrids of Saccharomyces cerevisiae and Saccharomyces bayanus var. uvarum. FEMS Yeast Res. 7: 540–549. [DOI] [PubMed] [Google Scholar]
- Kellis M., Patterson N., Endrizzi M., Birren B., Lander E. S., 2003. Sequencing and comparison of yeast species to identify genes and regulatory elements. Nature 423: 241–254. [DOI] [PubMed] [Google Scholar]
- King E. S., Swiegers J. H., Travis B., Francis I. L., Bastian S. E. P., et al. , 2008. Coinoculated fermentations using Saccharomyces yeasts affect the volatile composition and sensory properties of Vitis vinifera L. cv. Sauvignon Blanc wines. J. Agric. Food Chem. 56: 10829–10837. [DOI] [PubMed] [Google Scholar]
- Koufopanou V., Hughes J., Bell G., Burt A., 2006. The spatial scale of genetic differentiation in a model organism: the wild yeast Saccharomyces paradoxus. Philos. Trans. R. Soc. Lond. B Biol. Sci. 361: 1941–1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libkind D., Hittinger C. T., Valério E., Gonçalves C., Dover J., et al. , 2011. Microbe domestication and the identification of the wild genetic stock of lager-brewing yeast. Proc. Natl. Acad. Sci. USA 108: 14539–14544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liti G., Barton D. B. H., Louis E. J., 2006. Sequence diversity, reproductive isolation and species concepts in Saccharomyces. Genetics 174: 839–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liti G., Carter D. M., Moses A. M., Warringer J., Parts L., et al. , 2009. Population genomics of domestic and wild yeasts. Nature 458: 337–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liti G., Nguyen Ba A. N., Blythe M., Müller C. A., Bergström A., et al. , 2013. High quality de novo sequencing and assembly of the Saccharomyces arboricolus genome. BMC Genomics 14: 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martini A. V., Kurtzman C. P., 1985. Deoxyribonucleic acid relatedness among species of the genus Saccharomyces sensu stricto. Int. J. Syst. Bacteriol. 35: 508–511. [Google Scholar]
- Nakao Y., Kanamori T., Itoh T., Kodama Y., Rainieri S., et al. , 2009. Genome sequence of the lager brewing yeast, an interspecies hybrid. DNA Res. 16: 115–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naumov G. I., 1987. Genetic basis for classification and identification of the Ascomycetous yeasts. Stud. Mycol. 30: 469–475. [Google Scholar]
- Naumov G. I., James S. A., Naumova E. S., Louis E. J., Roberts I. N., 2000. Three new species in the Saccharomyces sensu stricto complex: Saccharomyces cariocanus, Saccharomyces kudriavzevii and Saccharomyces mikatae. Int. J. Syst. Evol. Microbiol. 50(Pt 5): 1931–1942. [DOI] [PubMed] [Google Scholar]
- Naumov G. I., Naumova E. S., Masneuf-Pomarède I., 2010. Genetic identification of new biological species Saccharomyces arboricolus Wang et Bai. Antonie van Leeuwenhoek 98: 1–7. [DOI] [PubMed] [Google Scholar]
- Ness F., Aigle M., 1995. RTM1: a member of a new family of telomeric repeated genes in yeast. Genetics 140: 945–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen H.-V., Legras J.-L., Neuvéglise C., Gaillardin C., 2011. Deciphering the hybridisation history leading to the Lager lineage based on the mosaic genomes of Saccharomyces bayanus strains NBRC1948 and CBS380. PLoS ONE 6: e25821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura A., Kotani T., Sasano Y., Takagi H., 2010. An antioxidative mechanism mediated by the yeast N-acetyltransferase Mpr1: oxidative stress-induced arginine synthesis and its physiological role. FEMS Yeast Res. 10: 687–698. [DOI] [PubMed] [Google Scholar]
- Novo M., Bigey F., Beyne E., Galeote V., Gavory F., et al. , 2009. Eukaryote-to-eukaryote gene transfer events revealed by the genome sequence of the wine yeast Saccharomyces cerevisiae EC1118. Proc. Natl. Acad. Sci. USA 106: 16333–16338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otero J. M., Vongsangnak W., Asadollahi M. A., Olivares-Hernandes R., Maury J., et al. , 2010. Whole genome sequencing of Saccharomyces cerevisiae: from genotype to phenotype for improved metabolic engineering applications. BMC Genomics 11: 723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peris D., Sylvester K., Libkind D., Gonçalves P., Sampaio J. P., et al. , 2014. Population structure and reticulate evolution of Saccharomyces eubayanus and its lager-brewing hybrids. Mol. Ecol. 23: 2031–2045. [DOI] [PubMed] [Google Scholar]
- Rainieri S., Kodama Y., Kaneko Y., Mikata K., Nakao Y., et al. , 2006. Pure and mixed genetic lines of Saccharomyces bayanus and Saccharomyces pastorianus and their contribution to the lager brewing strain genome. Appl. Environ. Microbiol. 72: 3968–3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roncoroni M., Santiago M., Hooks D. O., Moroney S., Harsch M. J., et al. , 2011. The yeast IRC7 gene encodes a β-lyase responsible for production of the varietal thiol 4-mercapto-4-methylpentan-2-one in wine. Food Microbiol. 28: 926–935. [DOI] [PubMed] [Google Scholar]
- Sampaio J. P., Gonçalves P., 2008. Natural populations of Saccharomyces kudriavzevii in Portugal are associated with oak bark and are sympatric with S. cerevisiae and S. paradoxus. Appl. Environ. Microbiol. 74: 2144–2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasano Y., Takahashi S., Shima J., Takagi H., 2010. Antioxidant N-acetyltransferase Mpr1/2 of industrial baker’s yeast enhances fermentation ability after air-drying stress in bread dough. Int. J. Food Microbiol. 138: 181–185. [DOI] [PubMed] [Google Scholar]
- Scannell D. R., Zill O. A., Rokas A., Payen C., Dunham M. J., et al. , 2011. The awesome power of yeast evolutionary genetics: new genome sequences and strain resources for the Saccharomyces sensu stricto genus. G3(Bethesda) 1: 11–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacherer J., Shapiro J. A., Ruderfer D. M., Kruglyak L., 2009. Comprehensive polymorphism survey elucidates population structure of Saccharomyces cerevisiae. Nature 458: 342–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipiczki M., 2008. Interspecies hybridization and recombination in Saccharomyces wine yeasts. FEMS Yeast Res. 8: 996–1007. [DOI] [PubMed] [Google Scholar]
- Sniegowski P. D., Dombrowski P. G., Fingerman E., 2002. Saccharomyces cerevisiae and Saccharomyces paradoxus coexist in a natural woodland site in North America and display different levels of reproductive isolation from European conspecifics. FEMS Yeast Res. 1: 299–306. [DOI] [PubMed] [Google Scholar]
- Swiegers J. H., Kievit R. L., Siebert T., Lattey K. A., Bramley B. R., et al. , 2009. The influence of yeast on the aroma of Sauvignon Blanc wine. Food Microbiol. 26: 204–211. [DOI] [PubMed] [Google Scholar]
- Takagi H., Shichiri M., Takemura M., Mohri M., Nakamori S., 2000. Saccharomyces cerevisiae sigma 1278b has novel genes of the N-acetyltransferase gene superfamily required for L-proline analogue resistance. J. Bacteriol. 182: 4249–4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibon C., Marullo P., Claisse O., Cullin C., Dubourdieu D., et al. , 2008. Nitrogen catabolic repression controls the release of volatile thiols by Saccharomyces cerevisiae during wine fermentation. FEMS Yeast Res. 8: 1076–1086. [DOI] [PubMed] [Google Scholar]
- Walther, A., A. Hesselbart, and J. Wendland, 2014 Genome sequence of Saccharomyces carlsbergensis, the world’s first pure culture lager yeast. G3 4: 783–793. [DOI] [PMC free article] [PubMed]
- Wang S.-A., Bai F.-Y., 2008. Saccharomyces arboricolus sp. nov., a yeast species from tree bark. Int. J. Syst. Evol. Microbiol. 58: 510–514. [DOI] [PubMed] [Google Scholar]
- Warringer J., Zörgö E., Cubillos F. A., Zia A., Gjuvsland A., et al. , 2011. Trait variation in yeast is defined by population history. PLoS Genet. 7: e1002111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W., McCusker J. H., Hyman R. W., Jones T., Ning Y., et al. , 2007. Genome sequencing and comparative analysis of Saccharomyces cerevisiae strain YJM789. Proc. Natl. Acad. Sci. USA 104: 12825–12830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H., Ito K., Shimoi H., 2005. Identification and characterization of a novel biotin biosynthesis gene in Saccharomyces cerevisiae. Appl. Environ. Microbiol. 71: 6845–6855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zill O. A., Scannell D., Teytelman L., Rine J., 2010. Co-evolution of transcriptional silencing proteins and the DNA elements specifying their assembly. PLoS Biol. 8: e1000550. [DOI] [PMC free article] [PubMed] [Google Scholar]