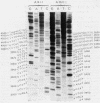
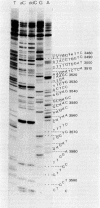
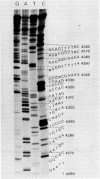
Abstract
A new method for determining nucleotide sequences in DNA is described. It is similar to the “plus and minus” method [Sanger, F. & Coulson, A. R. (1975) J. Mol. Biol. 94, 441-448] but makes use of the 2′,3′-dideoxy and arabinonucleoside analogues of the normal deoxynucleoside triphosphates, which act as specific chain-terminating inhibitors of DNA polymerase. The technique has been applied to the DNA of bacteriophage ϕX174 and is more rapid and more accurate than either the plus or the minus method.
Keywords: DNA polymerase, nucleotide sequences, bacteriophage ϕX174
Full text
PDF
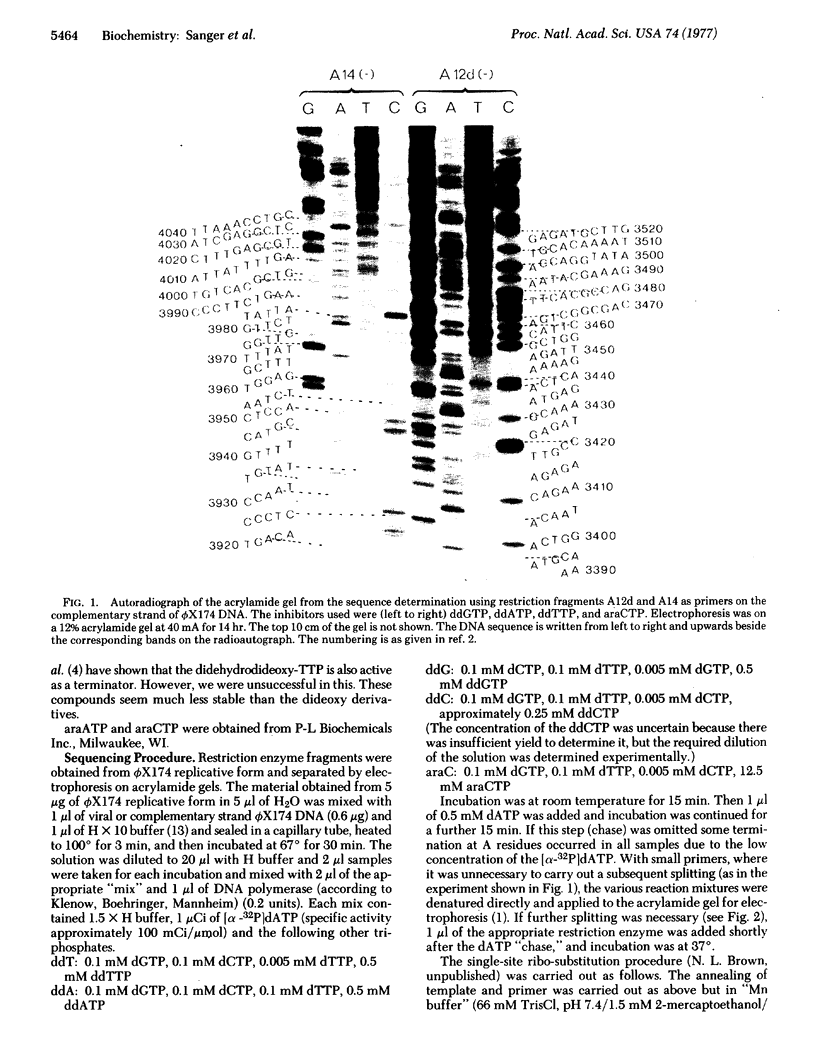


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Air G. M., Sanger F., Coulson A. R. Nucleotide and amino acid sequences of gene G of omegaX174. J Mol Biol. 1976 Dec 15;108(3):519–533. doi: 10.1016/s0022-2836(76)80134-9. [DOI] [PubMed] [Google Scholar]
- Atkinson M. R., Deutscher M. P., Kornberg A., Russell A. F., Moffatt J. G. Enzymatic synthesis of deoxyribonucleic acid. XXXIV. Termination of chain growth by a 2',3'-dideoxyribonucleotide. Biochemistry. 1969 Dec;8(12):4897–4904. doi: 10.1021/bi00840a037. [DOI] [PubMed] [Google Scholar]
- Büchi H., Khorana H. G. CV. Total synthesis of the structural gene for an alanine transfer ribonucleic acid from yeast. Chemical synthesis of an icosadeoxyribonucleotide corresponding to the nucleotide sequence 31 to 50. J Mol Biol. 1972 Dec 28;72(2):251–288. doi: 10.1016/0022-2836(72)90148-9. [DOI] [PubMed] [Google Scholar]
- Geider K. DNA synthesis in nucleotide-permeable Escherichia coli cells. The effects of nucleotide analogues on DNA synthesis. Eur J Biochem. 1972 Jun 9;27(3):554–563. doi: 10.1111/j.1432-1033.1972.tb01872.x. [DOI] [PubMed] [Google Scholar]
- HOARD D. E., OTT D. G. CONVERSION OF MONO- AND OLIGODEOXYRIBONUCLEOTIDES TO 5-TRIPHOSPHATES. J Am Chem Soc. 1965 Apr 20;87:1785–1788. doi: 10.1021/ja01086a031. [DOI] [PubMed] [Google Scholar]
- Hunter T., Francke B. In vitro polyoma DNA synthesis: inhibition by 1-beta-d-arabinofuranosyl CTP. J Virol. 1975 Apr;15(4):759–775. doi: 10.1128/jvi.15.4.759-775.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy J. R., Jr, Robins M. J., Townsend L. B., Robins R. K. Purine nucleosides. XIV. Unsaturated furanosyl adenine nucleosides prepared via base-catalyzed elimination reactions of 2'-deoxyadenosine derivatives. J Am Chem Soc. 1966 Apr 5;88(7):1549–1553. doi: 10.1021/ja00959a043. [DOI] [PubMed] [Google Scholar]
- Robins M. J., McCarthy J. R., Jr, Robins R. K. Purine nucleosides. XII. The preparation of 2',3'-dideoxyadenosine, 2',5'-dideoxyadenosine, and 2',3',5'-trideoxyadenosine from 2'-deoxyadenosine. Biochemistry. 1966 Jan;5(1):224–231. doi: 10.1021/bi00865a029. [DOI] [PubMed] [Google Scholar]
- Russell A. F., Moffatt J. G. Synthesis of some nucleotides derived from 3'-deoxythymidine. Biochemistry. 1969 Dec;8(12):4889–4896. doi: 10.1021/bi00840a036. [DOI] [PubMed] [Google Scholar]
- Sanger F., Air G. M., Barrell B. G., Brown N. L., Coulson A. R., Fiddes C. A., Hutchison C. A., Slocombe P. M., Smith M. Nucleotide sequence of bacteriophage phi X174 DNA. Nature. 1977 Feb 24;265(5596):687–695. doi: 10.1038/265687a0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R. A rapid method for determining sequences in DNA by primed synthesis with DNA polymerase. J Mol Biol. 1975 May 25;94(3):441–448. doi: 10.1016/0022-2836(75)90213-2. [DOI] [PubMed] [Google Scholar]